Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies
Abstract
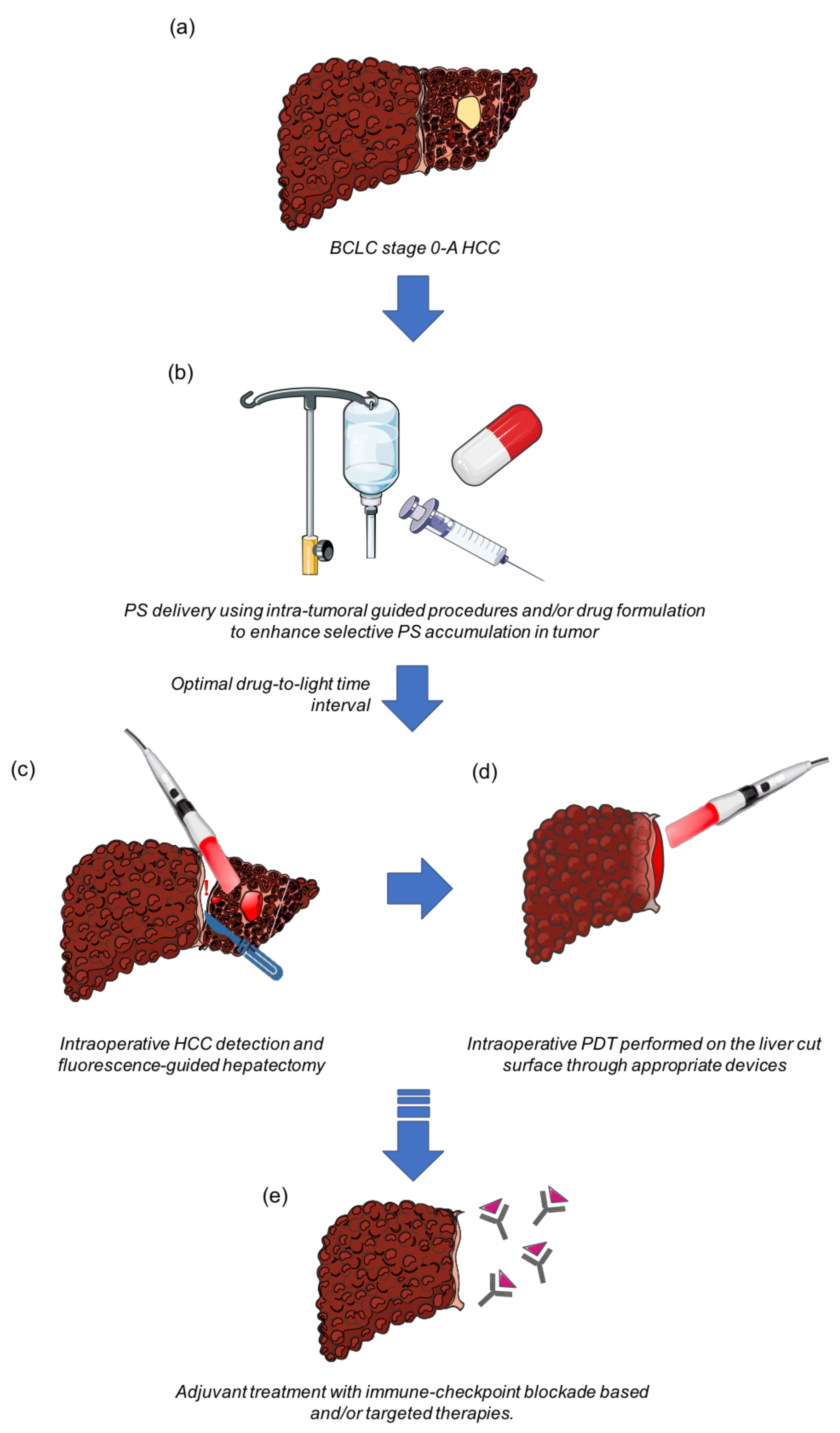
Simple Summary
Abstract
1. Introduction
2. Development of Photodynamic Therapy as an Anti-Cancer Treatment
3. Is Photodynamic Therapy Applicable in Patients with HCC?
4. Photodynamic Therapy May Induce an Anti-Tumor Immunity
5. PDT and Immune Response in HCC
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.M.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, M.S.; Kim, A.K.; Ronnekleiv-Kelly, S.; Kamel, I.R.; Ghasebeh, M.A.; Pawlik, T.M. Hepatocellular carcinoma: From diagnosis to treatment. Surg. Oncol. 2016, 25, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Schlachterman, A. Current and future treatments for hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 8478–8491. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Tabrizian, P.; Park, J.-W.; Yang, J.; Yan, L.; Schwartz, M.; Han, G.; Izzo, F.; Chen, M.; et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015, 62, 440–451. [Google Scholar] [CrossRef]
- Ramsey, D.E.; Kernagis, L.Y.; Soulen, M.C.; Geschwind, J.-F.H. Chemoembolization of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2002, 13, S211–S221. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.M.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Lee, J.M.; Jang, B.K.; Lee, Y.J.; Choi, W.Y.; Choi, S.M.; Chung, W.J.; Hwang, J.S.; Kang, K.J.; Kim, Y.H.; Chauhan, A.K.; et al. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin. Mol. Hepatol. 2016, 22, 160–167. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef]
- Wahl, D.R.; Stenmark, M.H.; Tao, Y.; Pollom, E.L.; Caoili, E.M.; Lawrence, T.S.; Schipper, M.J.; Feng, M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J. Clin. Oncol. 2016, 34, 452–459. [Google Scholar] [CrossRef]
- Salem, R.; Gabr, A.; Riaz, A.; Mora, R.; Ali, R.; Abecassis, M.; Hickey, R.; Kulik, L.; Ganger, D.; Flamm, S.; et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1000-patient 15-year experience. Hepatology 2017, 68, 1429–1440. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Huang, Z. A Review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic Therapy for Treatment of Solid Tumors—Potential and Technical Challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef]
- Von Tappeiner, H. Therapeutische Versuche Mit Fluoreszierenden Stoffen. Munch. Med. Wochenschr. 1903, 1, 2042–2044. [Google Scholar]
- Wen, X.; Li, Y.; Hamblin, M.R. Photodynamic therapy in dermatology beyond non-melanoma cancer: An update. Photodiagnosis Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Bergh, H.V.D.; Sickenberg, M.; Koh, A.H. Photodynamic therapy for polypoidal choroidal vasculopathy. Prog. Retin. Eye Res. 2013, 37, 182–199. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar]
- Bonnett, R.; Martínez, G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- Juzeniene, A.; Peng, Q.; Moan, J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef]
- Bolze, F.; Jenni, S.; Sour, A.; Heitz, V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 12857–12877. [Google Scholar] [CrossRef]
- Champeau, M.; Vignoud, S.; Mortier, L.; Mordon, S. Photodynamic therapy for skin cancer: How to enhance drug penetration? J. Photochem. Photobiol. B: Biol. 2019, 197, 111544. [Google Scholar] [CrossRef]
- Azaïs, H.; Delhem, N.; Frochot, C.; Colombeau, L.; Grabarz, A.; Moralès, O.; Mordon, S.; Collinet, P. Photodynamic therapy of peritoneal metastases of ovarian cancer to improve microscopic cytoreduction and to enhance antitumoral immunity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, e181. [Google Scholar] [CrossRef]
- Quilbe, A.; Moralès, O.; Baydoun, M.; Kumar, A.; Mustapha, R.; Murakami, T.; Leroux, B.; De Schutter, C.; Thecua, E.; Ziane, L.; et al. An Efficient Photodynamic Therapy Treatment for Human Pancreatic Adenocarcinoma. J. Clin. Med. 2020, 9, 192. [Google Scholar] [CrossRef]
- Mordon, S.; Cochrane, C.; Tylcz, J.B.; Betrouni, N.; Mortier, L.; Koncar, V. Light emitting fabric technologies for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Thecua, E.; Ziane, L.; Baert, G.; Deleporte, P.; Leroux, B.; Kumar, A.; Baydoun, M.; Moralès, O.; Delhem, N.; Mordon, S.R. Devices based on light emitting fabrics dedicated to PDT preclinical studies. In Proceedings of the 17th International Photodynamic Association World Congress, Cambridge, MA, USA, 28 June–4 July 2019. [Google Scholar] [CrossRef]
- De Haas, E.R.; Kruijt, B.; Sterenborg, H.; Neumann, H.M.; Robinson, D.J. Fractionated Illumination Significantly Improves the Response of Superficial Basal Cell Carcinoma to Aminolevulinic Acid Photodynamic Therapy. J. Investig. Dermatol. 2006, 126, 2679–2686. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef]
- Folkman, J. The role of angiogenesis in tumor growth. Semin. Cancer Biol. 1992, 3, 65–71. [Google Scholar]
- Bhuvaneswari, R.; Yuen, G.Y.; Chee, S.K.; Olivo, M. Hypericin-mediated photodynamic therapy in combination with Avastin (bevacizumab) improves tumor response by downregulating angiogenic proteins. Photochem. Photobiol. Sci. 2007, 6, 1275–1283. [Google Scholar] [CrossRef]
- Gomer, C.J.; Ferrario, A.; Luna, M.; Rucker, N.; Wong, S. Photodynamic therapy: Combined modality approaches targeting the tumor microenvironment. Lasers Surg. Med. 2006, 38, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, R.E.; Allison, R.R.; Sibata, C.; Downie, G.H. Breast Cancer with Chest Wall Progression: Treatment With Photodynamic Therapy. Ann. Surg. Oncol. 2004, 11, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Moole, H.; Tathireddy, H.; Dharmapuri, S.; Moole, V.; Boddireddy, R.; Yedama, P.; Dharmapuri, S.; Uppu, A.; Bondalapati, N.; Duvvuri, A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Carmona, M.A.; Bolch, M.; Jansen, C.; Vogt, A.; Sampels, M.; Mohr, R.U.; Van Beekum, K.; Mahn, R.; Praktiknjo, M.; Nattermann, J.; et al. Combined photodynamic therapy with systemic chemotherapy for unresectable cholangiocarcinoma. Aliment. Pharmacol. Ther. 2019, 49, 437–447. [Google Scholar] [CrossRef]
- Khan, S.A.; Dougherty, T.J.; Mang, T.S. An evaluation of photodynamic therapy in the management of cutaneous metastases of breast cancer. Eur. J. Cancer 1993, 29, 1686–1690. [Google Scholar] [CrossRef]
- Löning, M.; Diddens, H.; Küpker, W.; Diedrich, K.; Hüttmann, G. Laparoscopic fluorescence detection of ovarian carcinoma metastases using 5-aminolevulinic acid-induced protoporphyrin IX. Cancer 2004, 100, 1650–1656. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.-A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neuro-Oncol. 2021, 152, 501–514. [Google Scholar] [CrossRef]
- Dupont, C.; Mordon, S.; Deleporte, P.; Reyns, N.; Vermandel, M. A novel device for intraoperative photodynamic therapy dedicated to glioblastoma treatment. Futur. Oncol. 2017, 13, 2441–2454. [Google Scholar] [CrossRef]
- Thécua, E.; Ziane, L.; Grolez, G.P.; Fagart, A.; Kumar, A.; Leroux, B.; Baert, G.; Deleporte, P.; Vermandel, M.; Vignion-Dewalle, A.-S.; et al. A Warp-Knitted Light-Emitting Fabric-Based Device for In Vitro Photodynamic Therapy: Description, Characterization, and Application on Human Cancer Cell Lines. Cancers 2021, 13, 4109. [Google Scholar] [CrossRef]
- Rovers, J.P.; Saarnak, A.E.; Molina, A.; Schuitmaker, J.J.; Sterenborg, H.J.C.M.; Terpstra, O.T. Effective treatment of liver metastases with photodynamic therapy, using the second-generation photosensitizer meta-tetra(hydroxyphenyl)chlorin (mTHPC), in a rat model. Br. J. Cancer 1999, 81, 600–608. [Google Scholar] [CrossRef][Green Version]
- Vogl, T.J.; Eichler, K.; Mack, M.G.; Zangos, S.; Herzog, C.; Thalhammer, A.; Engelmann, K. Interstitial photodynamic laser therapy in interventional oncology. Eur. Radiol. 2004, 14, 1063–1073. [Google Scholar] [CrossRef]
- Rovers, J.P.; de Jode, M.L.; Grahn, M.F. Significantly increased lesion size by using the near-infrared photosensitizer 5,10,15,20-tetrakis (m-hydroxyphenyl)bacteriochlorin in interstitial photodynamic therapy of normal rat liver tissue. Lasers Surg. Med. 2000, 27, 235–240. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Zhang, T.; Wan, G.; Chen, B.; Xiong, Q.; Zhang, J.; Zhang, W.; Wang, Y. Pullulan-coated phospholipid and Pluronic F68 complex nanoparticles for carrying IR780 and paclitaxel to treat hepatocellular carcinoma by combining photothermal therapy/photodynamic therapy and chemotherapy. Int. J. Nanomed. 2017, 12, 8649–8670. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, A.; Li, J.; Wu, M.; Wu, L.; Wei, Z.; Liao, N.; Zhang, X.; Cai, Z.; Yang, H.; et al. Smart Cu(II)-aptamer complexes based gold nanoplatform for tumor micro-environment triggered programmable intracellular prodrug release, photodynamic treatment and aggregation induced photothermal therapy of hepatocellular carcinoma. Theranostics 2017, 7, 164–179. [Google Scholar] [CrossRef]
- Tsuda, T.; Kaibori, M.; Hishikawa, H.; Nakatake, R.; Okumura, T.; Ozeki, E.; Hara, I.; Morimoto, Y.; Yoshii, K.; Kon, M. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosome has antineoplastic effects for hepatocellular carcinoma. PLoS ONE 2017, 12, e0183527. [Google Scholar] [CrossRef]
- Nishimura, M.; Murayama, Y.; Harada, K.; Kamada, Y.; Morimura, R.; Ikoma, H.; Ichikawa, D.; Fujiwara, H.; Okamoto, K.; Otsuji, E. Photodynamic Diagnosis of Hepatocellular Carcinoma Using 5-Aminolevulinic Acid. Anticancer. Res. 2016, 36, 4569–4574. [Google Scholar] [CrossRef]
- Otake, M.; Nishiwaki, M.; Kobayashi, Y.; Baba, S.; Kohno, E.; Kawasaki, T.; Fujise, Y.; Nakamura, H. Selective accumulation of ALA-induced PpIX and photodynamic effect in chemically induced hepatocellular carcinoma. Br. J. Cancer 2003, 89, 730–736. [Google Scholar] [CrossRef][Green Version]
- Zhang, N.-Z.; Bai, S.; Cai, X.-J.; Li, L.-B. Inhibitory and immunological effects induced by the combination of photodynamic therapy and dendritic cells on mouse transplanted hepatoma. Photodiagnosis Photodyn. Ther. 2015, 13, 201–204. [Google Scholar] [CrossRef]
- Tang, P.M.-K.; Bui-Xuan, N.-H.; Wong, C.-K.; Fong, W.-P.; Fung, K.-P. Pheophorbide a-Mediated Photodynamic Therapy Triggers HLA Class I-Restricted Antigen Presentation in Human Hepatocellular Carcinoma. Transl. Oncol. 2010, 3, 114–122. [Google Scholar] [CrossRef]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504. [Google Scholar] [CrossRef]
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J. Hepatol. 2016, 8, 355–367. [Google Scholar] [CrossRef]
- Giraudeau, C.; Moussaron, A.; Stallivieri, A.; Mordon, S.; Frochot, C. Indocyanine Green: Photosensitizer or Chromophore? Still a Debate. Curr. Med. Chem. 2014, 21, 1871–1897. [Google Scholar] [CrossRef]
- Curnow, A.; Pye, A. The importance of iron chelation and iron availability during PpIX-induced photodynamic therapy. Photon- Lasers Med. 2015, 4. [Google Scholar] [CrossRef][Green Version]
- Chang, S.-C.; MacRobert, A.J.; Porter, J.B.; Brown, S.G. The efficacy of an iron chelator (CP94) in increasing cellular protoporphyrin IX following intravesical 5-aminolaevulinic acid administration: An in vivo study. J. Photochem. Photobiol. B Biol. 1997, 38, 114–122. [Google Scholar] [CrossRef]
- Sato, N.; Moore, B.W.; Keevey, S.; Drazba, J.A.; Hasan, T.; Maytin, E.V. Vitamin D Enhances ALA-Induced Protoporphyrin IX Production and Photodynamic Cell Death in 3-D Organotypic Cultures of Keratinocytes. J. Investig. Dermatol. 2007, 127, 925–934. [Google Scholar] [CrossRef]
- Vignion-Dewalle, A.; Baert, G.; Thecua, E.; Lecomte, F.; Vicentini, C.; Abi-Rached, H.; Mortier, L.; Mordon, S. Comparison of 10 efficient protocols for photodynamic therapy of actinic keratosis: How relevant are effective light dose and local damage in predicting the complete response rate at 3 months? Lasers Surg. Med. 2018, 50, 576–589. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, Y.-J.; Park, K.-M.; Hwang, D.-W.; Lee, J.H.; Song, K.B. Anatomical Resection But Not Surgical Margin Width Influence Survival Following Resection for HCC, A Propensity Score Analysis. World J. Surg. 2016, 40, 1429–1439. [Google Scholar] [CrossRef]
- Aoki, T.; Kubota, K.; Hasegawa, K.; Kubo, S.; Izumi, N.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Nakashima, O.; et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. BJS 2019, 107, 113–120. [Google Scholar] [CrossRef]
- Michelakos, T.; Kontos, F.; Sekigami, Y.; Qadan, M.; Cai, L.; Catalano, O.; Deshpande, V.; Patel, M.S.; Yamada, T.; Elias, N.; et al. Hepatectomy for Solitary Hepatocellular Carcinoma: Resection Margin Width Does Not Predict Survival. J. Gastrointest. Surg. 2020, 25, 1727–1735. [Google Scholar] [CrossRef]
- Nitta, H.; Allard, M.-A.; Sebagh, M.; Golse, N.; Ciacio, O.; Pittau, G.; Vibert, E.; Cunha, A.S.; Cherqui, D.; Castaing, D.; et al. Ideal Surgical Margin to Prevent Early Recurrence After Hepatic Resection for Hepatocellular Carcinoma. World J. Surg. 2021, 45, 1159–1167. [Google Scholar] [CrossRef]
- Paradis, V.; Zalinski, S.; Chelbi, E.; Guedj, N.; Degos, F.; Vilgrain, V.; Bedossa, P.; Belghiti, J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology 2008, 49, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Liu, B.; Bhuket, T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: A cross-sectional analysis of 2011-2014 National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 2017, 46, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Matroule, J.-Y.; Bonizzi, G.; Morlière, P.; Paillous, N.; Santus, R.; Bours, V.; Piette, J. Pyropheophorbide-a Methyl Ester-mediated Photosensitization Activates Transcription Factor NF-κB through the Interleukin-1 Receptor-dependent Signaling Pathway. J. Biol. Chem. 1999, 274, 2988–3000. [Google Scholar] [CrossRef]
- Pizova, K.; Tománková, K.; Daskova, A.; Binder, S.; Bajgar, R.; Kolarova, H. Photodynamic therapy for enhancing antitumour immunity. Biomed. Pap. 2012, 156, 93–102. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Garg, A.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef]
- Garg, A.; Krysko, D.; Verfaillie, T.; Kaczmarek, A.; Ferreira, G.B.; Marysael, T.; Rubio, N.; Firczuk, M.; Mathieu, C.; Roebroek, A.J.M.; et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012, 31, 1062–1079. [Google Scholar] [CrossRef]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol. Immunother. 2011, 61, 215–221. [Google Scholar] [CrossRef]
- Korbelik, M.; Cecic, I. Enhancement of tumour response to photodynamic therapy by adjuvant mycobacterium cell-wall treatment. J. Photochem. Photobiol. B Biol. 1998, 44, 151–158. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Evans, S.S.; Baumann, H.; Owczarczak, B.; Maier, P.; Vaughan, L.; Wang, W.C.; Unger, E.; Henderson, B.W. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer 2003, 88, 1772–1779. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Liu, X.; Owczarczak, B.; A Musser, D.; Henderson, B.W. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997, 57, 3904–3909. [Google Scholar]
- Korbelik, M.; Dougherty, G.J. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999, 59, 1941–1946. [Google Scholar]
- Kabingu, E.; Vaughan, L.; Owczarczak, B.; Ramsey, K.D.; O Gollnick, S. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br. J. Cancer 2007, 96, 1839–1848. [Google Scholar] [CrossRef]
- Delhem, N.; Carpentier, A.; Morales, O.; Miroux, C.; Groux, H.; Auriault, C.; Pancré, V. [Regulatory T-cells and hepatocellular carcinoma: Implication of the regulatory T lymphocytes in the control of the immune response]. Bull. Cancer 2008, 95. [Google Scholar] [CrossRef]
- Klungboonkrong, V.; Das, D.; McLennan, G. Molecular Mechanisms and Targets of Therapy for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2017, 28, 949–955. [Google Scholar] [CrossRef]
- Fu, J.; Xu, D.; Liu, Z.; Shi, M.; Zhao, P.; Fu, B.; Zhang, Z.; Yang, H.; Zhang, H.; Zhou, C.; et al. Increased Regulatory T Cells Correlate With CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology 2007, 132, 2328–2339. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Aikata, H.; Izumi, N.; Yamasaki, T.; Hino, K.; Kuzuya, T.; Isoda, N.; et al. TACTICS: Final overall survival (OS) data from a randomized, open label, multicenter, phase II trial of transcatheter arterial chemoembolization (TACE) therapy in combination with sorafenib as compared with TACE alone in patients (pts) with hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 270. [Google Scholar] [CrossRef]
| Photosensitizer | Cancer | n | Conclusion | Ref |
|---|---|---|---|---|
| Hematoporphyrin Derivatives | Carcinomas of the breast, colon, prostate, squamous cell, basal cell, and endometrium; malignant melanoma; mycosis fungoides; chondrosarcoma; and angiosarcoma | 24 | Highly pigmented and larger sub-cutaneous tumors require a stronger dose (5 mg/kg of PS) than non-pigmented and superficial tumors (2.5 mg/kg of PS); skin damage reduced by reducing illumination dose; maximum tumor necrosis observed till 2 cm. | [16] |
| Hypericin | Bladder carcinoma | (pre-clinical) | PDT in combination with anti-VEGF (bevacizumab) increases tumor responsiveness and reduced VEGF expression along with downregulation of other angiogenic proteins. | [31] |
| Photofrin® | Breast cancer with chest wall progression | 14 | A low dose of Photofrin® (0.8 mg/kg) mediated PDT induced tumor necrosis with lesions >2 cm in thickness; initial regression of untreated lesion was observed; Wound care-related difficulties were observed. | [33] |
| Photofrin®, Photogem, Photosan-3, or Temoporfin | Unresectable cholangiocarcinoma | 402 (meta-analysis) | PDT with biliary stenting could significantly improve patient survival period. | [34] |
| Photosan®, Photofrin®, or Foscan® | Unresectable extrahepatic cholangiocarcinoma | 96 | Combination of PDT with systemic chemotherapy showed significantly longer overall survival than chemotherapy alone with higher median survival than control groups. The therapy was well tolerated. | [35] |
| Photofrin II | Metastatic breast cancer | 37 | PDT yields the best results in patients with asymptomatic lesions; reductions in Photofrin® dose with reciprocal increases in light dose did not impair treatment efficacy. | [36] |
| 5-ALA | Ovarian carcinoma metastases | 29 | Laparoscopic fluorescence detection of PpIX after intraperitoneal application of 5-ALA; histological assessment of the biopsy specimens proved that strong red fluorescence had a sensitivity of 92% for detecting tumor tissue on specimens. | [37] |
| 5-ALA | Glioblastoma | 10 | Intraoperative PDT following PpIX fluorescence-guided maximal resection and adjuvant therapy resulted in an increased overall survival rate; no adverse effects were observed. | [38] |
| 5-ALA | Malignant Pleural Mesothelioma | 20 | Combination of 5-ALA PDT with thoracoscopy followed by Anti-PD-1 Nivolumab immunotherapy for a maximum of 2 years; Currently ongoing. | NCT04400539 |
| Photosensitizer | Study Set-Up | Conclusion | Ref |
|---|---|---|---|
| mTHPC | In-Vivo | High tumoral accumulation of the PS was observed in rat liver metastases with respect to the normal liver; extensive tumor tissue damage upon illumination; mild and transient damage to normal tissue was observed. | [41] |
| Photofrin, mTHPC, and mTHPBC | In-Vivo | Upon illumination, near-infrared PS mTHPBC showed significantly larger necrotic areas than the others in normal rat livers; highlight the advantage of near-infrared PS activation for pigmented tissues like the liver. | [43] |
| New nanocarrier containing IR780 | In-Vitro and in-vivo | IR780 and Paclitaxel (chemotherapeutic drug) loaded nanocarriers exhibited synergistic effect by inducing cancer cell apoptosis and cell cycle arrest at the G2/M phase for HCC; the combined treatment inhibited the in-vivo tumor growth and the tumor angiogenesis. | [44] |
| Chlorin e6 containing gold nanoparticles | In-Vitro and in-vivo | PDT coupled with hypoxia-induced chemotherapy showed a synergistic anti-HCC effect. | [45] |
| ICG-loaded lactosomes | In-Vitro and in-vivo | ICG-lactosome PDT treated HCC cells have higher cytotoxicity than ICG PDT; ICG-lactosome had higher fluorescence of tumor areas than ICG alone, along with anti-neoplastic effects on these malignant implants. | [46] |
| 5-ALA | In-Vitro and in-vivo | In-Vitro and in-vivo PpIX fluorescence was detected in tumors; red fluorescence was detected in HCC patient samples who were orally administered with 5-ALA before resection. | [47] |
| 5-ALA | In-Vivo | Higher PpIX fluorescence intensity was detected in HCC than in non-tumoral tissues in Male Fisher-344 rats; PDT induced necrosis in tumoral tissue; no necrosis was evident in non-tumoral tissue. | [48] |
| Deuteporfin | In-Vivo | PDT can inhibit mouse hepatoma growth and induce an anti-tumor immune response. | [49] |
| Pheophorbide-a | In-Vitro | PDT caused tumoral cytotoxicity of HCC cell lines by induction of apoptosis; PDT-induced immunogenicity triggered phagocytic capture of HCC cell lines by human macrophages. | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Moralès, O.; Mordon, S.; Delhem, N.; Boleslawski, E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers 2021, 13, 5176. https://doi.org/10.3390/cancers13205176
Kumar A, Moralès O, Mordon S, Delhem N, Boleslawski E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers. 2021; 13(20):5176. https://doi.org/10.3390/cancers13205176
Chicago/Turabian StyleKumar, Abhishek, Olivier Moralès, Serge Mordon, Nadira Delhem, and Emmanuel Boleslawski. 2021. "Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies" Cancers 13, no. 20: 5176. https://doi.org/10.3390/cancers13205176
APA StyleKumar, A., Moralès, O., Mordon, S., Delhem, N., & Boleslawski, E. (2021). Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers, 13(20), 5176. https://doi.org/10.3390/cancers13205176








