Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment Planning and Delivery
2.3. Follow-Up Evaluation
2.4. Endpoints and Statistical Analysis
3. Results
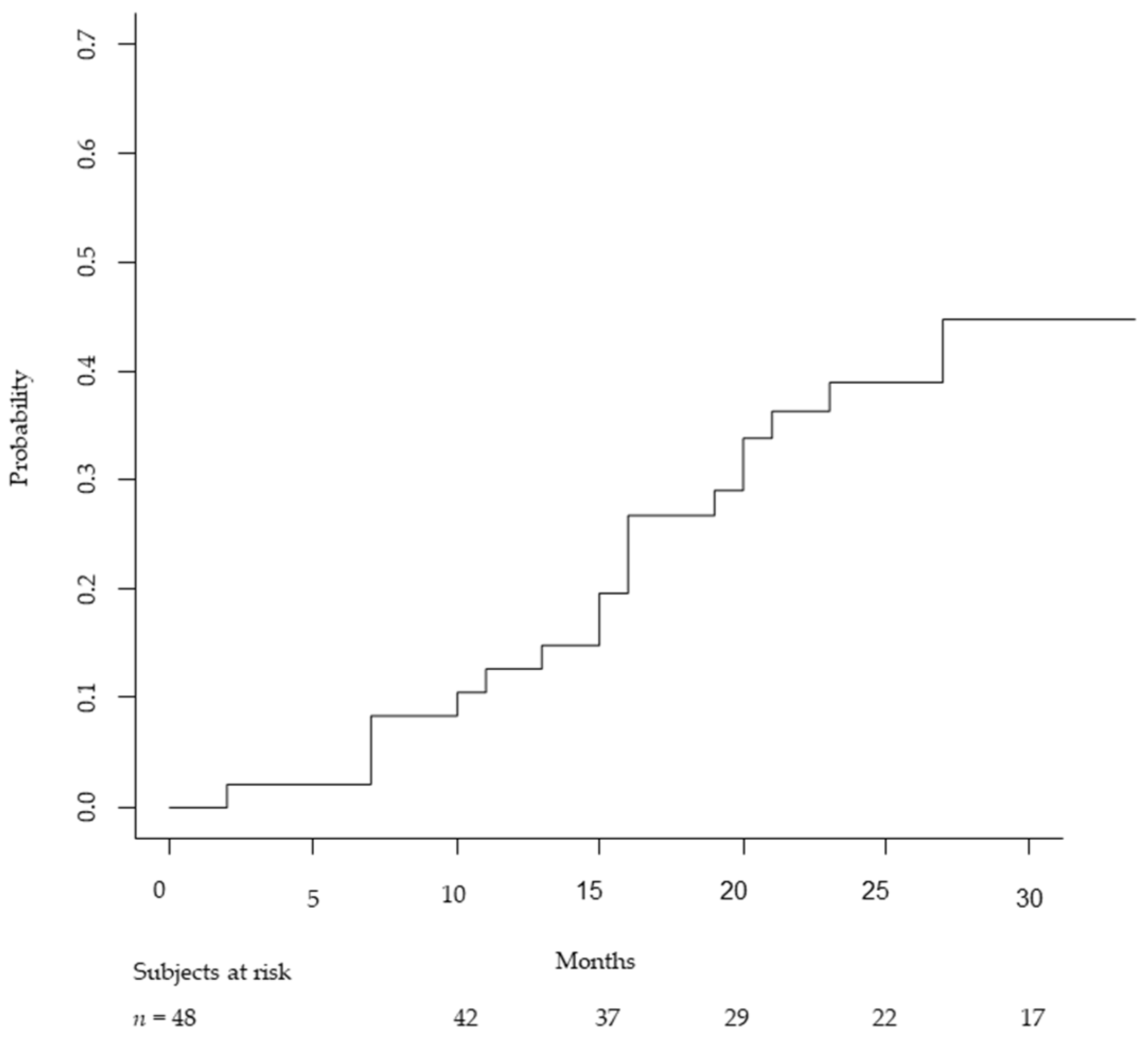
3.1. Local Control
3.2. Acute Toxicity
3.3. Late Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, B.B.; Karnell, L.H.; Hoffman, H.T.; Apostolakis, L.W.; Robinson, R.A.; Zhen, W.; Menck, H.R. National cancer database report on chondrosarcoma of the head and neck. Head Neck 2000, 22, 408–425. [Google Scholar] [CrossRef]
- Bloch, O.G.; Jian, B.J.; Yang, I.; Han, S.J.; Aranda, D.; Ahn, B.J.; Parsa, A.T. A systematic review of intracranial chondrosarcoma and survival. J. Clin. Neurosci. 2009, 16, 1547–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdegaal, S.H.; Bovée, J.V.; Pansuriya, T.C.; Grimer, R.J.; Ozger, H.; Jutte, P.C.; San Julian, M.; Biau, D.J.; van der Geest, I.C.; Leithner, A.; et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: An international multicenter study of 161 patients. Oncologist 2011, 16, 1771–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, H.L.; Ayala, A.G.; Romsdahl, M.M. Prognostic factors in chondrosarcoma of bone: A clinicopathologic analysis with emphasis on histologic grading. Cancer 1977, 40, 818–831. [Google Scholar] [CrossRef]
- Hogendoorn, P.C.W.; Bovée, J.V.M.G.; Nielsen, G.P. Chondrosarcoma. In WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; Fletcher, C.D.M., Brigde, J.A., Hogendoorn, P.C.W., Mertens, F., Eds.; IAR: Lyon, France, 2013; Volume 5, p. 265. [Google Scholar]
- Rosenberg, A.E.; Brown, G.A.; Bhan, A.K.; Lee, J.M. Chondroid chordoma—A variant of chordoma. A morphologic and immunohistochemical study. Am. J. Clin. Pathol. 1994, 101, 36–41. [Google Scholar] [CrossRef]
- Polychronidou, G.; Karavasilis, V.; Pollack, S.M.; HHuang, P.H.; Lee, A.; Jones, R.L. Novel therapeutic approaches in chondrosarcoma. Future Oncol. 2017, 13, 637–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, G.; Gondi, V. Proton therapy for tumors of the base of the skull. Chin. Clin. Oncol. 2016, 5, 51. [Google Scholar] [CrossRef]
- Frank, G.; Sciarretta, V.; Calbucci, F.; Farneti, G.; Mazzatenta, D.; Pasquini, E. The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery 2006, 59 (Suppl. 1), ONS-50–ONS-57. [Google Scholar] [CrossRef] [PubMed]
- Mercado, C.E.; Holtzman, A.L.; Rotondo, R.; Rutenberg, M.S.; Mendenhall, W.M. Proton therapy for skull base tumors: A review of clinical outcomes for chordomas and chondrosarcomas. Head Neck 2019, 41, 536–541. [Google Scholar] [CrossRef]
- De Jong, Y.; Ingola, M.; Briaire-de Bruijn, I.H.; Kruisselbrink, A.B.; Venneker, S.; Palubeckaite, I.; Heijs, B.P.; Cleton-Jansen, A.M.; Haas, R.L.; Bovée, J.V. 2019. Radiotherapy resistance in chondrosarcoma cells; a possible correlation with alterations in cell cycle related genes. Clin. Sarcoma Res. 2019, 9, 9. [Google Scholar] [CrossRef]
- Lechler, P.; Renkawitz, T.; Campean, V.; Balakrishnan, S.; Tingart, M.; Grifka, J.; Schaumburger, J. The antiapoptotic gene survivin is highly expressed in human chondrosarcoma and promotes drug resistance in chondrosarcoma cells in vitro. BMC Cancer 2011, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Hug, E.B.; Loredo, L.N.; Slater, J.D.; DeVries, A.; Grove, R.I.; Schaefer, R.A.; Rosenberg, A.E.; Slater, J.M. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J. Neurosurg. 1999, 91, 432–439. [Google Scholar] [CrossRef]
- Weber, D.C.; Malyapa, R.; Albertini, F.; Bolsi, A.; Kliebsch, U.; Walser, M.; Pica, A.; Combescure, C.; Lomax, A.J.; Schneider, R. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother. Oncol. 2016, 120, 169–174. [Google Scholar] [CrossRef]
- DeLaney, T.F.; Liebsch, N.J.; Pedlow, F.X. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J. Surg. Oncol. 2014, 110, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef]
- Tinganelli, W.; Durante, M. Carbon Ion Radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef]
- Kawashiro, S.; Mori, S.; Yamada, S.; Miki, K.; Nemoto, K.; Tsuji, H.; Kamada, T. Dose escalation study with respiratory-gated carbon-ion scanning radiotherapy using a simultaneous integrated boost for pancreatic cancer: Simulation with four-dimensional computed tomography. Br. J. Radiol. 2017, 90, 20160790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, C.M.; Oei, A.L.; Crezee, J.; Bel, A.; Franken, N.A.P.; Stalpers, L.J.A. The alfa and beta of tumours: A review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat. Oncol. 2018, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Mayo, C.; Yorke, E.; Merchant, T.E. Radiation Associated Brainstem Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S36–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debus, J.; Hug, E.B.; Liebsch, N.J.; O’farrel, D.; Finkelstein, D.; Efird, J.; Munzenrider, J.E. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 967–975. [Google Scholar] [CrossRef]
- Mayo, C.; Martel, M.K.; Marks, L.B.; Flickinger, J.; Nam, J.; Kirkpatrick, J. Radiation dose–volume effects of optic nerves and chiasm. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, S.; Bonora, M.; Magro, G.; Casale, S.; Dale, J.E.; Fossati, P.; Hasegawa, A.; Mirandola, A.; Ronchi, S.; Russo, S.; et al. RBE-weighted dose in carbon ion therapy for ACC patients: Impact of the RBE model translation on treatment outcomes. Radiother. Oncol. 2019, 141, 227–233. [Google Scholar] [CrossRef]
- Dale, J.D.; Molinelli, S.; Vischioni, B. Brainstem NTCP and Dose Constraints for Carbon Ion RT-Application and Translation from Japanese to European RBE-Weighted Dose. Front. Oncol. 2020, 10, 2051. [Google Scholar] [CrossRef]
- Hasegawa, A.; Mizoe, J.E.; Mizota, A.; Tsujii, H. Outcomes of visual acuity in carbon ion radiotherapy: Analysis of dose–volume histograms and prognostic factors. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, B.; Ares, C.; Lomax, A.J.; Stadelmann, O.; Goitein, G.; Timmermann, B.; Schneider, R.A.; Hug, E.B. Temporal lobe toxicity analysis after proton radiation therapy for skull base tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Koto, M.; Demizu, Y.; Saitoh, J.I.; Suefuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Takagi, R.; Ikawa, H.; et al. A retrospective multicenter study of carbon-ion radiotherapy for major salivary gland carcinomas: Subanalysis of J-CROS 1402 HN. Cancer Sci. 2018, 109, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 28 June 2021).
- Munzenrider, J.E.; Liebsch, N.J. Proton Therapy for Tumors of the Skull Base. Strahlenther. Onkol. 1999, 175 (Suppl. 2), 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ares, C.; Hug, E.B.; Lomax, A.J.; Bolsi, A.; Timmermann, B.; Rutz, H.P.; Schuller, J.C.; Pedroni, E.; Goitein, G. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: First long-term report. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Fuji, H.; Nakasu, Y.; Ishida, Y.; Horiguchi, S.; Mitsuya, K.; Kashiwagi, H.; Murayama, S. Feasibility of Proton Beam Therapy for Chordoma and Chondrosarcoma of the Skull Base. Skull Base 2011, 21, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Mattke, M.; Vogt, K.; Bougatf, N.; Welzel, T.; Oelmann-Avendano, J.; Hauswald, H.; Jensen, A.; Ellerbrock, M.; Jäkel, O.; Haberer, T.; et al. High control rates of proton-and carbon-ion–beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer 2018, 124, 2036–2044. [Google Scholar] [CrossRef]
- Holtzman, A.L.; Rotondo, R.L.; Rutenberg, M.S.; Indelicato, D.J.; Mercado, C.E.; Rao, D.; Tavanaiepour, D.; Morris, C.G.; Louis, D.; Flampouri, S.; et al. Proton therapy for skull-base chondrosarcoma, a single-institution outcomes study. J. Neuro-Oncol. 2019, 142, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Iannalfi, A.; D’Ippolito, E.; Riva, G.; Molinelli, S.; Gandini, S.; Viselner, G.; Fiore, M.R.; Vischioni, B.; Vitolo, V.; Bonora, M.; et al. Proton and carbon ion radiotherapy in skull base chordomas: A prospective study based on a dual particle and a patient-customized treatment strategy. Neuro-Oncology 2020, 22, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Mattke, M.; Welzel, T.; Roeder, F.; Oelmann, J.; Habl, G.; Jensen, A.; Ellerbrock, M.; Jäkel, O.; Haberer, T.; et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: First long-term results. Cancer 2014, 120, 3410–3417. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, S.; Magro, G.; Mairani, A. Dose prescription in carbon ion radiotherapy: How to compare two different RBE-weighted dose calculation systems. Radiother. Oncol. 2016, 120, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Viselner, G.; Farina, L.; Lucev, F.; Turpini, E.; Lungarotti, L.; Bacila, A.; Iannalfi, A.; D’Ippolito, E.; Vischioni, B.; Ronchi, S.; et al. Brain MR findings in patients treated with particle therapy for skull base tumors. Insights Imaging 2019, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Feuvret, L.; Bracci, S.; Calugaru, V. Efficacy and Safety of Adjuvant Proton Therapy Combined with Surgery for Chondrosarcoma of the Skull Base: A Retrospective, Population-Based Study. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 312–321. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Value | Number of Patients n = 48 | % |
|---|---|---|---|
| Sex | F | 29 | 60 |
| M | 19 | 40 | |
| Genetic syndrome | no | 44 | 92 |
| yes | 4 | 8 | |
| Hypertension | no | 34 | 71 |
| yes | 14 | 29 | |
| Diabetes | no | 44 | 92 |
| yes | 4 | 8 | |
| Histology grade according WHO | G1 | 19 | 40 |
| G2 | 27 | 56 | |
| G3 | 2 | 4 | |
| Outcome of surgery | gross total resection | 3 | 6 |
| incomplete surgery | 36 | 75 | |
| biopsy | 9 | 19 | |
| Number or surgeries | 1 | 34 | 71 |
| 2 | 9 | 19 | |
| 3 | 4 | 8 | |
| 4 | 1 | 2 | |
| Surgical technique | TNS | 28 | 58 |
| craniotomy | 15 | 31 | |
| combined | 5 | 11 | |
| Brainstem involvement | no | 44 | 92 |
| yes (abutment) | 1 | 2 | |
| yes (compression) | 3 | 6 | |
| Optic pathways involvement | no | 40 | 83 |
| yes (abutment) | 6 | 13 | |
| yes (compression) | 2 | 4 | |
| Timing of treatment | first diagnosis | 37 | 77 |
| at the relapse | 11 | 23 | |
| Treatment intent | post-operative | 41 | 85 |
| primary | 7 | 15 | |
| Particle | CIRT | 16 | 33 |
| PT | 32 | 67 |
| Toxicity | CIRT n = 16 (%) | PT = 32 (%) | Total = 48 (%) | p-Value * |
|---|---|---|---|---|
| Late toxicity | ||||
| G0–G2 | 14 (87.5) | 30 (93.8) | 44 (91.7) | 0.59 |
| G3 | 2 (12.5) | 2 (6.3) | 4 (8.3) | |
| Brain injury | ||||
| G0 | 7 (43.8) | 19 (59.4) | 26 (54.2) | |
| G1–G2 | 9 (56.3) | 13 (40.6) | 22 (45.8) | 0.36 |
| Study, Year | Particle | Number of Patients | Prescription Dose (GyRBE) | Median Time of Follow-Up (Months) | LC Rate | Late Toxicity |
|---|---|---|---|---|---|---|
| Hug et al., 1999 [13] | Protons | 25 | 70.2 * (median) | 33.2 * | 3 y LC: 94% | 7% (G3–G4) |
| Munzenrider et al., 1999 [29] | Protons | 229 | 72 * (mean) | 41 * | 5 y LC: 98% | - |
| Ares et al., 2009 [30] | Protons | 22 | 68.4 (median) | 34 * | 5 y LC: 94% | 6.2% |
| Fuji et al., 2011 [31] | Protons | 8 | 63 * (median) | 42 * | 3 y LC: 86% | No G ≥3 |
| Weber et al., 2016 [14] | Protons | 71 | 72.5 * (median) | 50 * | 5 y LC: 93.6% | 8.1% (G3–G4) |
| Mattke et al., 2018 [32] | Protons | 22 | 70 (median) | 30.7 | 4 y LC: 100% | No G ≥3 |
| Carbon ions | 79 | 60 (median) | 43.7 | 4 y LC: 90.5% | ||
| Holtzman et al., 2019 [33] | Protons | 43 | 73.8 (median) | 44 | 4 y LC: 89% | 4.6% (G3) + 9% G3 expected hear loss |
| Present study, 2021 | Protons | 32 | 70 | 31 | 3 y LC: 100% | 8% (G3) |
| Carbon ions | 16 | 70.4 | 66 | 3 y LC: 94% | No G4–G5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, G.; Cavallo, I.; Gandini, S.; Ingargiola, R.; Pecorilla, M.; Imparato, S.; Rossi, E.; Mirandola, A.; Ciocca, M.; Orlandi, E.; et al. Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy. Cancers 2021, 13, 4423. https://doi.org/10.3390/cancers13174423
Riva G, Cavallo I, Gandini S, Ingargiola R, Pecorilla M, Imparato S, Rossi E, Mirandola A, Ciocca M, Orlandi E, et al. Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy. Cancers. 2021; 13(17):4423. https://doi.org/10.3390/cancers13174423
Chicago/Turabian StyleRiva, Giulia, Iacopo Cavallo, Sara Gandini, Rossana Ingargiola, Mattia Pecorilla, Sara Imparato, Eleonora Rossi, Alfredo Mirandola, Mario Ciocca, Ester Orlandi, and et al. 2021. "Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy" Cancers 13, no. 17: 4423. https://doi.org/10.3390/cancers13174423
APA StyleRiva, G., Cavallo, I., Gandini, S., Ingargiola, R., Pecorilla, M., Imparato, S., Rossi, E., Mirandola, A., Ciocca, M., Orlandi, E., & Iannalfi, A. (2021). Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy. Cancers, 13(17), 4423. https://doi.org/10.3390/cancers13174423







