Urological Melanoma: A Comprehensive Review of a Rare Subclass of Mucosal Melanoma with Emphasis on Differential Diagnosis and Therapeutic Approaches
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Kidney
4.2. Ureter
4.3. Urinary Bladder
4.4. Urethra and Penis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Classification of Skin Tumours; IARC: Lyon, France, 2018. [Google Scholar]
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal Melanoma: A Literature Review. Curr. Oncol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef]
- Sánchez, R.B.; Bustos, B.D.U.; Mira, M.N.; Estrada, R.B. Mucosal Melanoma: An Update. Actas Dermo-Sifiliográficas 2015, 106, 96–103. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 15, 1664–1678. [Google Scholar] [CrossRef]
- Gru, A.A.; Becker, N.; Dehner, L.P.; Pfeifer, J.D. Mucosal melanoma: Correlation of clinicopathologic, prognostic, and molecular features. Melanoma Res. 2014, 24, 360–370. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Kavolius, J.P.; Russo, P.; Grimaldi, G.; Katz, J.; Brady, M.S. Primary genitourinary melanoma. Urology 2001, 57, 633–638. [Google Scholar] [CrossRef]
- Acikalin, A.; Bagir, E.; Karim, S.; Bisgin, A.; Izol, V.; Erdogan, S. Primary melanoma of the urinary tract; Clinicopathologic and molecular review of a case series. Pathol. Res. Pract. 2020, 216, 153095. [Google Scholar] [CrossRef] [PubMed]
- Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence. Available online: http://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf (accessed on 19 September 2020).
- Fujimoto, H.; Chitose, K.; Tobisu, K.; Yamazaki, N.; Sakamoto, M.; Kakizoe, T. Solitary renal melanoma? A case with long survival after initial treatment. J. Urol. 1995, 153, 1887–1889. [Google Scholar] [CrossRef]
- Frasier, B.L.; Wachs, B.H.; Watson, L.R.; Tomasulo, J.P. Malignant Melanoma of the Renal Pelvis Presenting as a Primary Tumor. J. Urol. 1988, 140, 812–814. [Google Scholar] [CrossRef]
- Tajima, K.; Saito, K.; Umeda, Y.; Murata, T.; Satani, H. Malignant Melanoma of the Kidney Presenting as a Primary Tumor. Int. J. Urol. 1997, 4, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Hor Bayazit, Y.; Aridoğan, I.A.; Zeren, S.; Gönlüşen, G.; Tansug, Z. Primary malignant melanoma of the kidney. Scand. J. Urol. Nephrol. 2002, 36, 77–79. [Google Scholar] [CrossRef]
- Tasdemir, C.; Samdanci, E.T.; Dogan, M.; Elmali, C.; Sargin, S.Y. Primer malignant melanoma of kidney: A case report. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 971–972. [Google Scholar] [PubMed]
- Agnew, C.H. Metastatic malignant melanoma of the kidney simulating a primary neoplasm: A case report. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1958, 80, 813–816. [Google Scholar] [PubMed]
- Cunningham, J.A.; Fendler, J.-P.; Nichols, P.J.; Skinner, D.G. Metastatic malignant melanoma: An unusual case presentation. Urology 1994, 44, 924–926. [Google Scholar] [CrossRef]
- Boughan, K.M.; Setrakian, S.; Lee, C.H.; Spiro, T.P.; Daw, H.A. A Renal Mass in a Patient with Melanoma. Clin. Genitourin. Cancer 2009, 7, E98–E100. [Google Scholar] [CrossRef]
- Klatte, T.; Rao, J.Y.; Ribas, A.; Pantuck, A.J. Metastatic Melanoma to the Kidney Presenting with Renal Vein Tumor Thrombus. Urology 2007, 69, 982.e7–982.e9. [Google Scholar] [CrossRef]
- Levin, B.M.; Boulos, F.I.; Herrell, S.D. Metastatic ocular melanoma to the kidney 20 years after initial diagnosis. Urology 2005, 66, 658.e11–658.e12. [Google Scholar] [CrossRef]
- Judd, R.L. Melanoma of the Ureter: A Case Report. J. Urol. 1962, 87, 805–807. [Google Scholar] [CrossRef]
- Garcia, A.; Monserrat, J.M.; Martin, G.G. Melanoma of the ureter. Rev. Argent. Urol. Nefrol. 1969, 38, 58–61. [Google Scholar]
- Khan, M.; O’Kane, D.; Du Plessis, J.; Hoag, N.; Lawrentschuk, N. Primary malignant melanoma of the urinary bladder and ureter. Can. J. Urol. 2016, 23, 8171–8175. [Google Scholar]
- Gakis, G.; Merseburger, A.S.; Sotlar, K.; A Kuczyk, M.; Sievert, K.-D.; Stenzl, A. Metastasis of malignant melanoma in the ureter: Possible algorithms for a therapeutic approach. Int. J. Urol. 2009, 16, 407–409. [Google Scholar] [CrossRef]
- MacNeil, J.; Hossack, T. A Case of Metastatic Melanoma in the Ureter. Case Rep. Urol. 2016, 2016, 1853015. [Google Scholar] [CrossRef]
- March, B.; Calopedos, R.J.S.; Latif, E.; Ouyang, R. Ureteric Obstruction from Malignant Melanoma in Both Right Double Moiety and Left Single Moiety Ureters. Urology 2017, 103, e7–e8. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.J.; Huang, A.H.; Carroll, P. Primary Melanoma of the Bladder: A Case Report and Review. J. Urol. 1980, 123, 278–281. [Google Scholar] [CrossRef]
- Anichkov, N.M.; Nikonov, A.A. Primary Malignant Melanomas of the Bladder. J. Urol. 1982, 128, 813–815. [Google Scholar] [CrossRef]
- Goldschmidt, P.; Py, J.M.; Kostakopoulos, A.; Jacqmin, D.; Grosshans, E.; Bollack, C. Primary Malignant Melanomas of the Urinary Bladder. BJU Int. 1988, 61, 359. [Google Scholar] [CrossRef] [PubMed]
- Van Ahlen, H.; Nicolas, V.; Lenz, W.; Boldth, I.; Bockisch, A.; Vahlensieck, W. Primary melanoma of urinary bladder. Urology 1992, 40, 550–554. [Google Scholar] [CrossRef]
- Lange-Welker, U.; Papadopoulos, I.; Wacker, H. Primary Malignant Melanoma of the Bladder. A case report and literature review. Urol. Int. 1993, 50, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Pacella, M.; Gallo, F.; Gastaldi, C.; Ambruosi, C.; Carmignani, G. Primary malignant melanoma of the bladder. Int. J. Urol. 2006, 13, 635–637. [Google Scholar] [CrossRef]
- El Ammari, J.E.; Ahallal, Y.; El Fassi, M.J.; Farih, M.H. Primary malignant melanoma of the urinary bladder. Case Rep. Urol. 2011, 2011, 932973. [Google Scholar] [CrossRef][Green Version]
- Schindler, K.; Schicher, N.; Kunstfeld, R.; Pehamberger, H.; Toepker, M.; Haitel, A.; Hoeller, C.; Harmankaya, K. A rare case of primary rhabdoid melanoma of the urinary bladder treated with ipilimumab, an anti-CTLA 4 monoclonal antibody. Melanoma Res. 2012, 22, 320–325. [Google Scholar] [CrossRef]
- Truong, H.; Sundi, D.; Sopko, N.; Xing, D.; Lipson, E.J.; Bivalacqua, T.J. A Case Report of Primary Recurrent Malignant Mel-anoma of the Urinary Bladder. Urol. Case Rep. 2013, 12, 2–4. [Google Scholar] [CrossRef]
- Karabulut, Y.Y.; Erdogan, S.; Sayar, H.; Ergen, A.; Baydar, D.E. Primary malignant melanoma of the urinary bladder: Clinical, morphological, and molecular analysis of five cases. Melanoma Res. 2016, 26, 616–624. [Google Scholar] [CrossRef]
- Laudisio, A.; Giua, R.; Papalia, R.; Taffon, C.; Muto, G.; Incalzi, R.A. An Unusual Cause of Hematuria: Primary Bladder Melanoma in an Older Man. J. Am. Geriatr. Soc. 2016, 64, e122–e123. [Google Scholar] [CrossRef]
- Buscarini, M.; Conforti, C.; Incalzi, R.A.; Falavolti, C.; Taffon, C.; Muto, G.; Dianzani, C. Primary Malignant Melanoma of the Bladder. Skinmed 2017, 15, 395–397. [Google Scholar]
- Singh, V.; Gupta, K.; Dewana, S.; Mandal, A.K. Spotting the pigmented ‘Monster’: Primary melanoma in urinary bladder. BMJ Case Rep. 2019, 23, e231950. [Google Scholar] [CrossRef] [PubMed]
- Kirigin, M.; Lež, C.; Šarčević, B.; Šoipi, Š.; Jaić, G.; Ulamec, M.; Krušlin, B. Primary Malignant Melanoma of the Urinary Bladder: Case Report. Acta Clin. Croat. 2019, 58, 180–182. [Google Scholar] [CrossRef]
- Snajdar, E.; Ajo, A.R.; Rosen, K.; Miller, R.; Mohammed, S.; Gordon, C.; Pui, J.C.; McIntosh, G. Primary Malignant Melanoma of the Urinary Bladder. Cureus 2021, 23, e14067. [Google Scholar]
- Maeda, T.; Uchida, Y.; Kouda, T.; Matsui, H.; Kawahara, Y.; Sato, T.; Nakajima, F. Metastatic malignant melanoma of the urinary bladder presenting with hematuria: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2008, 54, 787–790. [Google Scholar]
- Nohara, T.; Sakai, A.; Fuse, H.; Imamura, Y. Metastatic Malignant Melanoma of the Urinary Bladder: A Case Report. Jpn. J. Urol. 2009, 100, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Siroy, A.E.; MacLennan, G.T. Primary Melanoma of the Bladder. J. Urol. 2011, 185, 1096–1097. [Google Scholar] [CrossRef]
- Efesoy, O.; Çayan, S. Bladder metastasis of malignant melanoma: A case report and review of literature. Med. Oncol. 2010, 28, 667–669. [Google Scholar] [CrossRef]
- Meunier, R.; Pareek, G.; Amin, A. Metastasis of Malignant Melanoma to Urinary Bladder: A Case Report and Review of the Literature. Case Rep. Pathol. 2015, 2015, 173870. [Google Scholar] [CrossRef] [PubMed]
- Theocharides, C.; Chatzopoulos, K.; Papanikolaou, D.; Siokas, V.; Amplianitis, I.; Papanikolaou, A. Metastatic Melanoma to the Urinary Bladder of Ocular Origin Accompanied with Primary Cutaneous Melanoma: Diagnostic Challenge—A Report of a Case. Case Rep. Pathol. 2017, 2017, 4818537. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.; Sut, M.; Kaul, A.; Altieri, V.; Mutch, F.; Patel, J.; Sharma, H. Metastatic malignant melanoma of the urinary bladder: Case report and literature review. Cent. Eur. J. Urol. 2012, 65, 232–234. [Google Scholar] [CrossRef]
- Ikeda, A.; Miyagawa, T.; Kurobe, M.; Uchida, M.; Kojima, T.; Tsutsumi, M.; Ito, S.; Sugita, S.; Nishiyama, H. Case of metastatic malignant melanoma of the urinary bladder. Hinyokika Kiyo Acta Urol. Jpn. 2013, 59, 579–582. [Google Scholar]
- Topal, C.; Kır, G.; Daş, T.; Sarbay, B.; Tosun, M.I. Metastatic malignant melanoma of the urinary bladder: A case report and review of the literature. Indian J. Pathol. Microbiol. 2016, 59, 532–534. [Google Scholar] [CrossRef]
- Patil, R.V.; Woldu, S.L.; Lucas, E.; Quinn, A.M.; Francis, F.; Margulis, V. Metastatic Melanoma to the Bladder: Case Report and Review of the Literature. Urol. Case Rep. 2017, 11, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Barillaro, F.; Camilli, M.; Dessanti, P.; Chiesa, F.G.N.; Villa, A.; Pastorino, A.; Aschele, C.; Conti, E. Primary melanoma of the bladder: Case report and review of the literature. Arch. Ital. Urol. Androl. 2018, 30, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.C.; Williams, N.C.; Cui, C.; Summers, D.; Mastrangelo, M.J.; Hubosky, S.G.; Shields, C.L.; Shields, J.A.; Sato, T. Con-junctival melanoma: Bladder and upper urinary tract metastases. J. Clin. Oncol. 2011, 20, e216–e219. [Google Scholar] [CrossRef] [PubMed]
- Chaus, F.M.; Craig, M.; Bracamonte, E.; Sundararajan, S.; Lee, B.R. Primary Malignant Melanoma of the Bladder Treated by Robotic Partial Cystectomy and Immunotherapy. J. Endourol. Case Rep. 2019, 5, 151–153. [Google Scholar] [CrossRef]
- Mercimek, M.N.; Ozden, E. Bladder-sparing Approach in a Woman with Muscle-invasive Primary Bladder Melanoma. J. Coll. Physicians Surg. Pak. 2019, 29, S154–S156. [Google Scholar] [CrossRef]
- Moez, R.; Khaireddine, M.D.; Sahnoun, W.; Zehani, A.; Bibi, M.; Ouannes, Y.; Sellami, A.; Ben Rhouma, S.; Nouira, Y. Symptomatic bladder metastasis of malignant melanoma: A case report. J. Surg. Case Rep. 2020, 2020, rjaa509. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, S.; Bada, M.; Polara, A.; Crocetto, F.; Creta, M.; Chiancone, F.; Occhipinti, M.; Bertoloni, R.; Marciano, A.; Aresu, L.; et al. Conservative management of primary malignant melanoma of the bladder: A case report. J. Med. Case Rep. 2021, 15, 1–4. [Google Scholar] [CrossRef]
- Konigsberg, H.A.; Gray, G.F. Benign melanosis and malignant melanoma of penis and male urethra. Urology 1976, 7, 323–326. [Google Scholar] [CrossRef]
- Kokotas, N.S.; Kallis, E.G.; Fokitis, P.J. Primary malignant melanoma of male urethra. Urology 1981, 18, 392–394. [Google Scholar] [CrossRef]
- Barbagli, G.; Natali, A.; Urso, C.; Barbanti, G.; Menchi, I.; Moroni, F. Primary Malignant Melanoma of the Female Urethra: A Case Report with Immunohistochemical Findings. Urol. Int. 1988, 43, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Maeda, S.; Takeuchi, T.; Tokuyama, H.; Kanematsu, M.; Kuriyama, M.; Ban, Y.; Kawada, Y.; Mizoguchi, Y.; Kasahara, M. Malignant melanoma of male urethra: A case report. Hinyokika Kiyo Acta Urol. Jpn. 1989, 35, 121–126. [Google Scholar]
- Primus, G.; Soyer, H.P.; Smolle, J.; Mertl, G.; Pummer, K.; Kerl, H. Early ‘Invasive’ Malignant Melanoma of the Glans penis and the Male Urethra. Report of a case and review of the literature. Eur. Urol. 1990, 18, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, L.; Casarico, A.; Bandelloni, R.; Gambini, C. Primary malignant melanoma of male urethra. Urology 1991, 37, 366–368. [Google Scholar] [CrossRef]
- Fujimoto, N.; Oda, M.; Shimoe, S. Primary Malignant Melanoma of the Male Urethra. Urol. Int. 1991, 47, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Ander, H.; Esen, T.; Tellaloğlu, S.; Uysal, V. Successful management of malignant melanoma of male urethra with local excision and adjuvant radiochemotherapy. Prog. Clin. Biol. Res. 1991, 370, 379–383. [Google Scholar]
- Arai, K.; Joko, M.; Kagebayashi, Y.; Tsumatani, K.; Kimura, S.; Sasaki, K.; Samma, S.; Okajima, E.; Nakaoka, S. Primary ma-lignant melanoma of the female urethra: A case report. Jpn. J. Clin. Oncol. 1993, 23, 74–77. [Google Scholar] [PubMed]
- Kim, C.J.; Pak, K.; Hamaguchi, A.; Ishida, A.; Arai, Y.; Konishi, T.; Okada, Y.; Tomoyoshi, T. Primary malignant melanoma of the female urethra. Cancer 1993, 71, 448–451. [Google Scholar] [CrossRef]
- Rashi, A.-M.; Williams, R.M.; Horton, L. Malignant melanoma of penis and male urethra Is it a difficult tumor to diagnose? Urology 1993, 41, 470–471. [Google Scholar] [CrossRef]
- Aragona, F.; Maio, G.; Piazza, R.; Salmaso, R. Primary malignant melanoma of the female urethra: A case report. Int. Urol. Nephrol. 1995, 27, 107–111. [Google Scholar] [CrossRef]
- Gincherman, Y.; Weiss, J.; Elder, D.; Hamilton, R. A unique case of long-term survival in a male patient with malignant melanoma of the distal urethra. Cutis 1996, 57, 44–46. [Google Scholar]
- Touyama, H.; Hatano, T.; Ogawa, Y. Primary malignant melanoma of the female urethra: A case report. Hinyokika Kiyo Acta Urol. Jpn. 1997, 43, 597–598. [Google Scholar] [PubMed]
- Girgin, C.; Tarhan, H.; Sezer, A.; Ermete, M.; Gürel, G. A large primary malignant melanoma of the female urethra. Urol. Int. 1999, 63, 198–200. [Google Scholar] [CrossRef]
- Watanabe, J.; Yamamoto, S.; Souma, T.; Hida, S.; Takasu, K. Primary malignant melanoma of the male urethra. Int. J. Urol. 2000, 7, 351–353. [Google Scholar] [CrossRef]
- Chitale, S.V.; Szemere, J.C.; Burgess, N.A.; Sethia, K.K.; Ball, R.Y.; Bardsley, A. Surgical technique for the conservative man-agement of distal urethral melanoma. Br. J. Plast. Surg. 2001, 54, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Miyawaki, I.; Kawagoe, M.; Kuriwaki, K.; Hatanaka, S.; Tanaka, K.; Nakagawa, M. Primary malignant melanoma of the male urethra. Int. J. Urol. 2002, 9, 268–271. [Google Scholar] [CrossRef]
- Mukai, M.; Uemura, M.; Fukuhara, S.; Kanno, N.; Nishimura, K.; Miyoshi, S.; Yoshida, K.; Kawano, K. Primary malignant melanoma of the female urethra: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2003, 49, 157–160. [Google Scholar]
- DiMarco, D.S.; DiMarco, C.S.; Zincke, H.; Webb, M.J.; Keeney, G.L.; Bass, S.; Lightner, D.J. Outcome of Surgical Treatment for Primary Malignant Melanoma of the Female Urethra. J. Urol. 2004, 171, 765–767. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, R.; Huang, S.F.; Tamboli, P.; Prieto, V.G.; Hester, G.; Pettaway, C.A. Melanoma of the penis, scrotum and male urethra: A 40-year single institution experience. J. Urol. 2005, 173, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.E.; Suzue, K.; Wille, M.A.; Krausz, T.; Rapp, D.E.; Sokoloff, M.H. Primary malignant melanoma of the urethra. Urology 2005, 65, 389. [Google Scholar] [CrossRef]
- Kato, H.; Hayashi, K.; Saida, T.; Kontani, K.; Nishizawa, O. Urethral Advancement Procedure for Reconstruction after Excision of Male Parameatal Melanoma in situ. Urol. Int. 2005, 74, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Kawata, N.; Sato, K.; Hirakata, H.; Igarashi, T.; Ichinose, T.; Yamaguchi, K.; Takahashi, S. Primary Malignant Melanoma of the Female Urethra. Urology 2007, 70, 1222.e13–1222.e16. [Google Scholar] [CrossRef]
- Nakamoto, T.; Inoue, Y.; Ueki, T.; Niimi, N.; Iwasaki, Y. Primary amelanotic malignant melanoma of the female urethra. Int. J. Urol. 2007, 14, 153–155. [Google Scholar] [CrossRef]
- Inoue, M.; Ishioka, J.-I.; Kageyama, Y.; Fukuda, H.; Higashi, Y. Primary malignant melanoma of the male urethra: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2008, 54, 305–308. [Google Scholar] [PubMed]
- Comploj, E.; Palermo, S.; Trenti, E.; Lodde, M.; Mian, C.; Carella, R.; Pycha, A. Unexpected Long Survival in Primary Malignant Melanoma of the Male Urethra. Case Rep. Dermatol. 2009, 1, 93–99. [Google Scholar] [CrossRef]
- Akbas, A.; Akman, T.; Erdem, M.R.; Antar, B.; Kilicaslan, I.; Şinasi, Y.Ö. Female Urethral Malignant Melanoma with Vesical Invasion: A Case Report. Kaohsiung J. Med. Sci. 2010, 26, 96–98. [Google Scholar] [CrossRef]
- Yoshii, T.; Horiguchi, A.; Shirotake, S.; Tobe, M.; Tasaki, S.; Hayakawa, M.; Sumitomo, M.; Asano, T. A case of primary amelanotic malignant melanoma of the female urethra. Jpn. J. Urol. 2010, 101, 734–737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, S.T.; Song, H.C.; Cho, B.; Choi, W.S.; Lee, W.K.; Lee, Y.S.; Lee, Y.G.; Kim, K.K.; Park, S.-H.; Kim, J.W. Primary Malignant Melanoma of the Female Urethra. Korean J. Urol. 2012, 53, 206–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karaman, H.; Yesil, Y. Primary melanoma of the male urethra. Turk. J. Urol. 2014, 39, 201–203. [Google Scholar] [CrossRef]
- Maruyama, T.; Matsui, T.; Kobayashi, Y.; Kuwae, H. Case of primary malignant melanoma of the female urethra at age 94: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2014, 60, 571–574. [Google Scholar]
- Papeš, D.; Altarac, S.; Arslani, N.; Rajković, Z.; Antabak, A.; Ćaćić, M. Melanoma of the glans penis and urethra. Urology 2014, 83, 6–11. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; Wang, A.; Zhang, Z.; Wu, J.; Wei, Q. Malignant melanoma of the penis and urethra: One case report. World J. Surg. Oncol. 2014, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Vijay, M.K.; Goel, H.; Shukla, S. Primary malignant melanoma of female urethra: A rare neoplasm. J. Cancer Res. Ther. 2014, 10, 758–760. [Google Scholar]
- Broussard, A.P.; Chaudoir, C.; Gomelsky, A. Urethral melanoma in an elderly woman. Int. Urogynecol. J. 2014, 26, 149–150. [Google Scholar] [CrossRef]
- Safadi, A.; Schwalb, S.; Ben-Shachar, I.; Katz, R. Primary malignant urethral melanoma resembling a urethral caruncle. Urol. Case Rep. 2017, 15, 28–29. [Google Scholar] [CrossRef]
- Suzuki, H.; Nakanishi, Y.; Yoshino, K.; Kataoka, M.; Fukushima, H.; Tobisu, K.; Koga, F. Primary malignant melanoma of the female urethra: A case report. Jpn. J. Urol. 2018, 109, 111–115. [Google Scholar] [CrossRef]
- Davuluri, M.; Long, B.; Semple, S.; Villanueva-Siles, E.; Aboumohamed, A. Primary Urethral Melanoma: A Case Report and Literature Review. Urology 2018, 126, 1–4. [Google Scholar] [CrossRef]
- Aoki, Y.; Soma, T.; Nakamura, Y.; Fukui, N.; Sakai, Y.; Kageyama, Y. Malignant melanoma of the male urethra with increased 5-S-cysteinyldopa: A case report. IJU Case Rep. 2019, 2, 215–217. [Google Scholar] [CrossRef]
- Tokita, T.; Kawahara, T.; Ito, Y.; Tsutsumi, S.; Abe, K.; Namura, K.; Sano, F.; Shioi, K.; Takamoto, D.; Yumura, Y.; et al. Primary amelanotic malignant melanoma of the male urethra with inguinal lymph node metastasis successfully controlled by nivolumab: A case report. Urol. Case Rep. 2018, 18, 54–56. [Google Scholar] [CrossRef]
- Maruyama, Y.; Sadahira, T.; Mitsui, Y.; Wada, K.; Tanimoto, R.; Kobayashi, Y.; Araki, M.; Watanabe, M.; Watanabe, T.; Nasu, Y. Red nodular melanoma of the penile foreskin: A case report and literature review. Mol. Clin. Oncol. 2018, 9, 449–452. [Google Scholar] [CrossRef]
- Bansal, N.; Garg, G.; Vashist, S. Primary malignant melanoma of urethra mimicking as urethral caruncle. BMJ Case Rep. 2018, 2018, bcr2018226056. [Google Scholar] [CrossRef]
- Hansen, M.F.; Abel, I.; Clasen-Linde, E. Primary malignant melanoma of the urethra in a patient with rheumatoid arthritis treated with methotrexate. BMJ Case Rep. 2019, 12, e228033. [Google Scholar] [CrossRef] [PubMed]
- Nakra, T.; Dadhwal, R.; Nayyar, R.; Rastogi, S.; Kakkar, A.; Sharma, M.C.; Yadav, R. Primary urethral small cell melanoma with neuroendocrine differentiation: A case report. J. Egypt. Natl. Cancer Inst. 2020, 32, 40. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Yu, D.; Wang, Y.; Bi, L.; Xie, D. Primary Female Urethral Malignant Melanoma: A Case Report. Urology 2020, 142, e8–e10. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Hasegawa, G.; Kashima, K.; Sato, Y.; Hara, N.; Nishiyama, T. Primary malignant melanoma of the female urethra: A case report. Urol. Case Rep. 2021, 34, 101493. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, F.A.; Ouédraogo, B.; Ido, F.A.H.A.; Hafing, T.; Karama, H.; Traoré, O. Primary malignant melanoma of the urethra in women: About a case. Urol. Case Rep. 2021, 35, 101542. [Google Scholar] [CrossRef] [PubMed]
- Zeighami, S.; Soltani, M.; Khajeh, F.; Ariafar, A.; Jahanabadi, Z.; Miladpour, B.; Naghdi-Sede, N. Primary amelanotic melanoma of the male urethra: A rare entity and diagnostic challenge. Qatar Med. J. 2020, 2020, 11. [Google Scholar] [CrossRef]
- Burity, C.R.T.; Linica, S.B.; Saade, R.D.; Ferreira, F.T.; Schultz, L.; Bezerra, E.S.; Franco, H.C.; Oliveira, R.A.; Costa, M.V.S. Lo-calized primary melanoma of male urethra with a 4-year follow up. Urol. Case Rep. 2021, 10, 101702. [Google Scholar] [CrossRef] [PubMed]
- Ribalta, T.; Lloreta, J.; Munné, A.; Serrano, S.; Cardesa, A. Malignant pigmented clear cell epithelioid tumor of the kidney:Clear cell (“SUGAR”) tumor versus malignant melanoma. Hum. Pathol. 2000, 31, 516–519. [Google Scholar] [CrossRef]
- Ainsworth, A.M.; Clark, W.H.; Mastrangelo, M.; Conger, K.B. Primary malignant melanoma of the urinary bladder. Cancer 1976, 37, 1928–1936. [Google Scholar] [CrossRef]
- Stein, B.S.; Kendall, A.R. Malignant Melanoma of the Genitourinary Tract. J. Urol. 1984, 132, 859–868. [Google Scholar] [CrossRef]
- Makhlouf, H.R.; Ishak, K.G.; Shekar, R.; Sesterhenn, I.A.; Young, D.Y.; Fanburg-Smith, J.C. Melanoma markers in angiomyo-lipoma of the liver and kidney: A comparative study. Arch. Pathol. Lab. Med. 2002, 126, 49–55. [Google Scholar] [CrossRef]
- Esheba Gel, S.; Esheba Nel, S. Angiomyolipoma of the kidney: Clinicopathological and immunohistochemical study. J. Egypt. Natl. Canc. Inst. 2013, 25, 125–134. [Google Scholar] [CrossRef]
- Montes, F.G.; Gómez, M.F.L.; Boyd, J. Does primary melanoma of the bladder exist? Actas Urológicas Españolas 2000, 24, 433–436. [Google Scholar]
- Lee, C.S.D.; Komenaka, I.K.; Hurst-Wicker, K.S.; DeRaffele, G.; Mitcham, J.; Kaufman, H.L. Management of metastatic malignant melanoma of the bladder. Urology 2003, 62, 351. [Google Scholar] [CrossRef]
- El-Safadi, S.; Estel, R.; Mayser, P.; Muenstedt, K. Primary malignant melanoma of the urethra: A systematic analysis of the current literature. Arch. Gynecol. Obstet. 2013, 289, 935–943. [Google Scholar] [CrossRef]
- McComiskey, M.; Iavazzo, C.; Datta, M.; Slade, R.; Winter-Roach, B.; Lambe, G.; Sangar, V.K.; Smith, M. Balloon Cell Urethral Melanoma: Differential Diagnosis and Management. Case Rep. Obstet. Gynecol. 2015, 2015, 919584. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, O.; Scharpf, M.; Fend, F.; Stenzl, A.; Gakis, G. Feasibility of Penis-Preserving Surgery for Urethral Melanoma: Proposal for a Therapeutic Algorithm. Clin. Genitourin. Cancer 2015, 13, e411–e413. [Google Scholar] [CrossRef]
- Sandru, F.; Draghici, C.; Predescu, T.; Constantin, M.M.; Petca, R.-C.; Constantin, T.; Petca, A.; Dumitrașcu, M.C. Regressive melanoma in a female patient: A case report. Exp. Ther. Med. 2020, 20, 87–90. [Google Scholar] [CrossRef]
- Smith, H.G.; Bagwan, I.; Board, R.E.; Capper, S.; Coupland, S.E.; Glen, J.; Lalondrelle, S.; Mayberry, A.; Muneer, A.; Nugent, K.; et al. Ano-uro-genital mucosal melanoma UK national guidelines. Eur. J. Cancer 2020, 135, 22–30. [Google Scholar] [CrossRef]
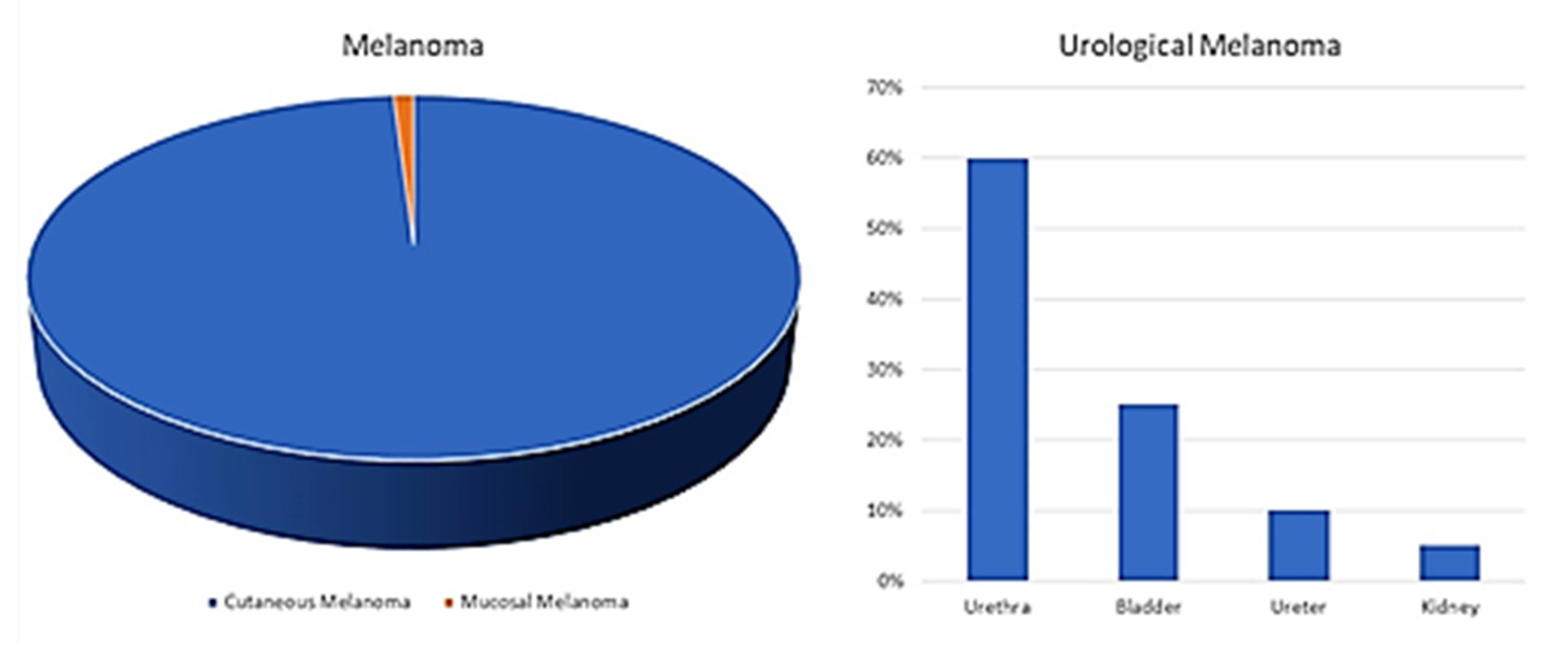
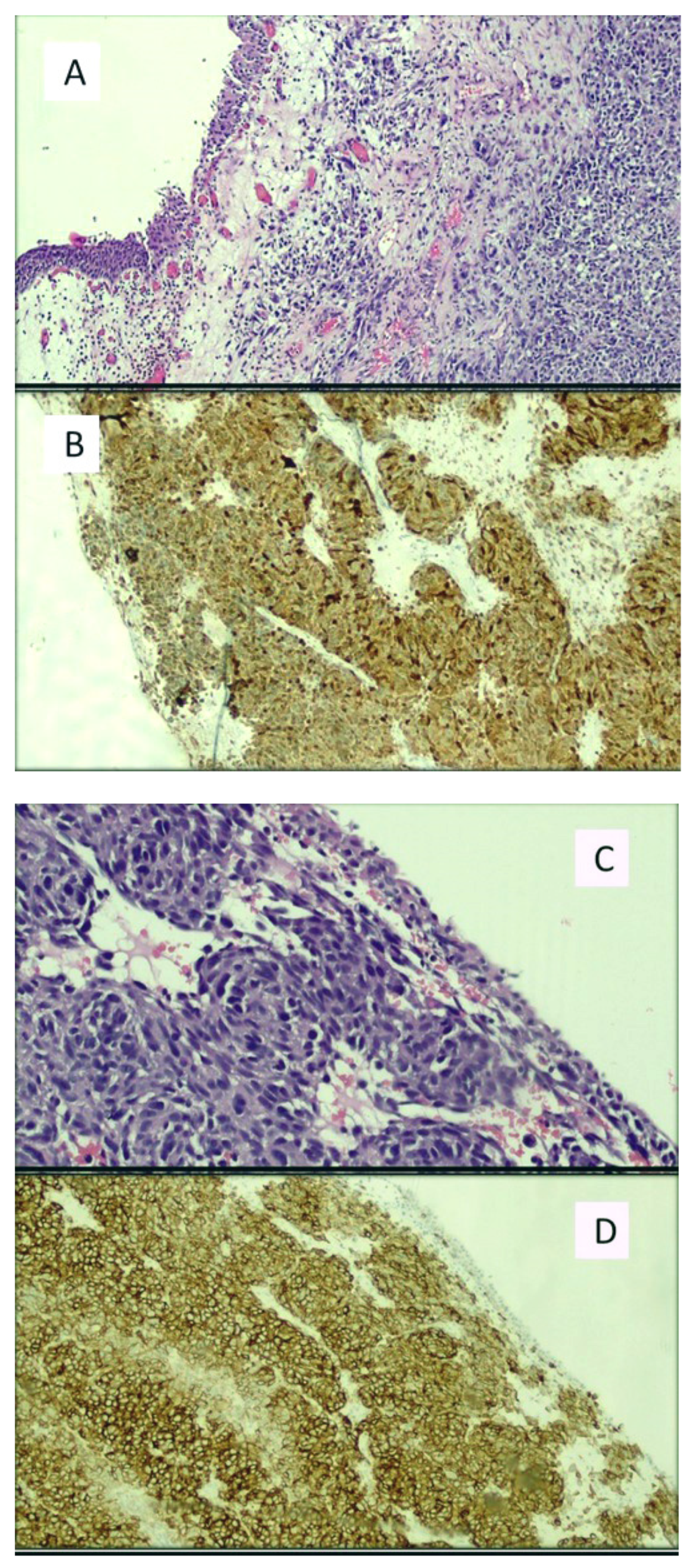
| Author(s) | Year(s) | Primary/Metastatic | Treatment | Survival |
|---|---|---|---|---|
| Fujimoto, H. et al. [9] | 1995 | Primary | PRK and CHT | 44 months |
| Frasier, B.L. et al. [10] | 1988 | Primary, renal pelvis | TRK and AI | 22 months |
| Tajima, K. [11] | 1997 | Primary | TRK | 2 years and 3 months |
| Hor Bayazit, Y. et al. [12] | 2002 | Primary | TRK and LY | 1 year |
| Tasdemir, C. et al. [13] | 2011 | ? | TRK | some months |
| Agnew, C.H. et al. [14] | 1958 | Metastatic | TRK | some months |
| Cunningham, J.A. et al. [15] | 1994 | Metastatic | CHT, HT and IT | 6 weeks |
| Boughan, K.M. et al. [16] | 2009 | Metastatic | Not reported | 10 days |
| Klatte, T. et al. [17] | 2007 | Metastatic, like renal vein thrombus | TRK | 5 months |
| Levin, B.M. et al. [18] | 2005 | Metastatic, from ocular melanoma | TRK | still alive |
| Author(s) | Year(s) | Primary/Metastatic | Treatment | Survival |
|---|---|---|---|---|
| Nguyen, A.T. et al. [6] | 2001 | Metastatic (13 patients) | CHT, IT and/or HT | median survival (43 months) |
| Acikalin, A. et al. [7] | 2020 | Metastatic (8 patients) | CHT, IT, HT and/or TT | median survival (<3 years) |
| Judd, R.L. [19] | 1962 | Primary | SH | 5 months |
| Garcia, A.E. et al. [20] | 1969 | Primary | CHT | <6 months |
| Khan, M. et al. [21] | 2016 | Primary | / | death before starting therapy |
| Gakis, G. et al. [22] | 2009 | Primary | CHT and SH | 3 months |
| Macneil, J. [23] | 2016 | Primary | ER | after 12 months still alive |
| March, B. et al. [24] | 2017 | Metastatic, obstruction | / | / |
| Author(s) | Year(s) | Primary/Metastatic | Treatment | Survival |
|---|---|---|---|---|
| Willis, A.J. et al. [25] | 1980 | Primary | CHU and CHT | <6 months |
| Anichkov, N.M. et al. [26] | 1982 | Primary (2 patients) | both CH and CHT | 1 year, lost to FU |
| Goldschmidt, P. et al. [27] | 1988 | Primary (2 patients) | both CH and CHT | (1) still alive after 6 months, (2) 7 months |
| Van Ahlen, H. et al. [28] | 1992 | Primary | CH, IT and CHT | still alive after 15 months |
| Lange-Welker, U. et al. [29] | 1993 | Primary | CHU and CHT | some months |
| Pacella, M. et al. [30] | 2006 | Primary | PCH and CHT | 9 months |
| El Ammari, J.E. et al. [31] | 2011 | Primary | CH | <6 months |
| Schindler, K. et al. [32] | 2012 | Primary, with rhabdoid features | CH, CHT and IT (anti CTLA4) | 12 months still alive |
| Truong, H. et al. [33] | 2013 | Primary, recurrent | CHU and IT (anti CTLA4) | still alive |
| Karabulut, Y.Y. et al. [34] | 2016 | Primary, 5 cases | CH and 2 patients IT | 2 died after some months, 3 stil alive |
| Laudisio, A. et al. [35] | 2016 | Primary, older man | Only CHT | after 12 months still alive |
| Buscarini, M. et al. [36] | 2017 | Primary | CH and IT | some months |
| Singh, V. et al. [37] | 2019 | Primary, with monster cells | CH and IT with Nivolumab | still alive |
| Kirigin, M. et al. [38] | 2019 | Primary | TURBT | death after few weeks |
| Snajdar, E. et al. [39] | 2021 | Primary | CH and adjuvant CHT | 16 months |
| Maeda, T. et al. [40] | 2008 | Metastatic | CH, CHT and IT | some months |
| Nohara, T. et al. [41] | 2009 | Metastatic | TURBT and CHT | some months |
| Siroy, A.E. et al. [42] | 2011 | Primary | TURBT and CH | some months |
| Efesoy, O. et al. [43] | 2011 | Metastatic | TURBT and CHT | 7 months |
| Meunier, R. et al. [44] | 2015 | Metastatic | TURBT, CH and CHT | some months |
| Theocharides, C. et al. [45] | 2017 | Metastatic | TURBT and IT | some months |
| Paterson, A. et al. [46] | 2012 | Metastatic | CH and CHT | <5 months |
| Ikeda, A. et al. [47] | 2013 | Metastatic | TURBT and CHT | <5 months |
| Topal, C.S. et al. [48] | 2016 | Metastatic | CH and CHT | some months |
| Patil, R.V. et al. [49] | 2017 | Metastatic | CHU, SRT and IT with anti CTLA4 | 4 months |
| Barillaro, F. et al. [50] | 2018 | Metastatic | CH and IT with Nivolumab | still alive |
| Nair, B.C. et al. [51] | 2011 | Metastatic, from conjunctival melanoma | CH, CHT and IT | some months |
| Chaus, F.M. et al. [52] | 2019 | Metastatic | RPCH and IT with Pembrolizumab | still alive after 2 years |
| Mercimek, M.N. et al. [53] | 2019 | Metastatic | TURBT, LPC and IT | still alive |
| Moez, R. et al. [54] | 2020 | Metastatic | TURBT, CH and CHT | still alive |
| Rapisarda, S. et al. [55] | 2021 | Metastatic | TURBT, CH and BI | still alive after 6 months |
| Author(s) | Year(s) | Primary/Metastatic | Gender | Treatment | Survival |
|---|---|---|---|---|---|
| Konigsberg, H.A. et al. [56] | 1976 | Primary, urethra and penis | 2 M | UTH and PH UTH and PH | 20 years <6 months |
| Kokotas, N.S. et al. [57] | 1981 | Primary, urethra | M | PH | 6 months |
| Barbagli, G. et al. [58] | 1988 | Primary, urethra | F | UTH and CH | 1 year |
| Yamamoto, N. et al. [59] | 1989 | Primary, urethra | M | PCH | 6 months |
| Primus, G. et al. [60] | 1990 | Primary, urethra and penis | M | UTH and PH | >6 months |
| Calcagno, L. et al. [61] | 1990 | Primary, urethra | M | UTH and CHT | >6 months |
| Fujimoto, N. et al. [62] | 1991 | Primary, urethra | M | UTH and CHT | 4 months |
| Ander, H. et al. [63] | 1991 | Primary, urethra | M | UTH and RT | >6 months |
| Arai, K. et al. [64] | 1993 | Primary, urethra | F | UTH and RT | 1 year |
| Kim, C.J. et al. [65] | 1993 | Primary, urethra | F | UTH and CHT | 5 years |
| Rashid, A.M. et al. [66] | 1993 | Primary, urethra and penis | 2 M | UTH UTH and CHT | <6 months <6 months |
| Aragona, F. et al. [67] | 1995 | Primary, urethra | F | UTH and CHT | 6 months |
| Gincherman, Y. et al. [68] | 1996 | Primary, distal urethra | M | UTH and CHT | 5 years |
| Touyama, H. et al. [69] | 1997 | Primary, urethra | F | UTH and CHT | After 4 months still alive |
| Girgin, C. et al. [70] | 1999 | Primary, urethra | F | UTH | <6 months |
| Watanabe, J. et al. [71] | 2000 | Primary, urethra | M | UTH and CHT | >6 months |
| Chitale, S.V. et al. [72] | 2001 | Primary, urethra | M | PUTH | >6 months |
| Kubo, H. et al. [73] | 2002 | Primary, urethra | M | PUTH and IT | >6 months |
| Mukai, M. et al. [74] | 2003 | Primary, urethra | F | PUTH, UTH and IT | >6 months |
| DiMarco, D.S. [75] | 2004 | Primary, urethra | 11 F/M | PUTH, UTH, CHT and IT | Median survival: 7 months |
| Sánchez-Ortiz, R. et al. [76] | 2005 | 10 patients: primary of urethra and/or penis 6 patients: primary of scrotum | M | PUTH and/or UTH and/or CHT and/or IT | Median survival: 9 months |
| Katz, E.E. et al. [77] | 2005 | Primary, urethra | M | UTH and CHT | 6 months |
| Kato, H. et al. [78] | 2005 | Primary, urethra | M | UTH and CHT | >6 months |
| Yoshizawa, T. et al. [79] | 2007 | Primary, urethra | F | UTH, CH and CHT | 14 months |
| Nakamoto, T. et al. [80] | 2007 | Primary, urethra | F | UTH, CH and HY | >6 months |
| Inoue, M. et al. [81] | 2008 | Primary, urethra | M | PH and PRH | 7 months |
| Comploj, E. et al. [82] | 2009 | Primary, urethra | M | UTH and IT | after 5 years still alive |
| Akbas, A. et al. [83] | 2010 | Primary, urethra | F | UTH and CH | 13 months |
| Yoshii, T. et al. [84] | 2010 | Primary, urethra | F | UTH, CH and CHT | 25 months |
| Cho, S.T. et al. [85] | 2012 | Primary, urethra | F | UTH | After 6 months still alive |
| Karaman, H. et al. [86] | 2013 | Primary, urethra | M | UTH | >6 months |
| Maruyama, T. et al. [87] | 2014 | Primary, urethra | F | UTH and CH | 11 months |
| Papeš, D. et al. [88] | 2014 | Primary, urethra and penis | Review | UTH and/or CH and/or PCH and/or CHT and/or IT | Median survival: 28 months |
| Li, Y. et al. [89] | 2014 | Primary, urethra and penis | M | UTH, PH, CHT and IT | After 3 years still alive |
| Pandey, P.K. et al. [90] | 2014 | Primary, urethra | F | UTH and CHT | >6 months |
| Broussard, A.P. [91] and McComiskey, M. [88] | 2015 2015 | Primary, urethra Primary, urethra | F F | UTH and CHT UTH and CHT | >6 months >6 months |
| Safadi, A. et al. [92] | 2017 | Primary, urethra | F | UTH and CH | / |
| Suzuki, H. et al. [93] | 2018 | Primary, urethra | F | UTH, CH and CHT | 19 months |
| Davuluri, M. et al. [94] | 2019 | Primary, urethra | M | UTH, CH and CHT | 11 months |
| Aoki, Y. et al. [95] | 2019 | Primary, urethra | M | UTH and PH | After 6 months still alive |
| Tokita, T. et al. [96] | 2018 | Primary, urethra | M | UTH, PH and IT with Nivolumab | After 20 months still alive |
| Maruyama, Y. et al. [97] | 2018 | Penile foreskin | M | UTH and PH | After 2 years still alive |
| Bansal, N. et al. [98] | 2018 | Primary, urethra | F | UTH | After 3 months still alive |
| Hansen, M.F. et al. [99] | 2019 | Primary, urethra | F | UTH | 1 years and 8 months |
| Nakra, T. et al. [100] | 2020 | Primary, urethra with small cells | M | UTH and IT | >6 months |
| Sun, X. et al. [101] | 2020 | Primary, urethra | F | UTH | >6 months |
| Watanabe, K. et al. [102] | 2020 | Primary, urethra | F | UTH, HY and CH CHT and IT with nivolumab and ipilimumab | 22 months |
| Kaboré, F.A. et al. [103] | 2020 | Primary, urethra | F | UTH and CH | After 1 year still alive |
| Zeighami, S. et al. [104] | 2020 | Primary, urethra | M | UTH and CH | >6 months |
| Burity et al. [105] | 2021 | Primary, urethra | M | UTH and PH | After 6 months still alive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Colagrande, A.; Cimmino, A.; Caporusso, C.; Candance, P.M.V.; Trabucco, S.M.R.; Zingarelli, M.; Lorusso, A.; Marrone, M.; Stellacci, A.; et al. Urological Melanoma: A Comprehensive Review of a Rare Subclass of Mucosal Melanoma with Emphasis on Differential Diagnosis and Therapeutic Approaches. Cancers 2021, 13, 4424. https://doi.org/10.3390/cancers13174424
Cazzato G, Colagrande A, Cimmino A, Caporusso C, Candance PMV, Trabucco SMR, Zingarelli M, Lorusso A, Marrone M, Stellacci A, et al. Urological Melanoma: A Comprehensive Review of a Rare Subclass of Mucosal Melanoma with Emphasis on Differential Diagnosis and Therapeutic Approaches. Cancers. 2021; 13(17):4424. https://doi.org/10.3390/cancers13174424
Chicago/Turabian StyleCazzato, Gerardo, Anna Colagrande, Antonietta Cimmino, Concetta Caporusso, Pragnell Mary Victoria Candance, Senia Maria Rosaria Trabucco, Marcello Zingarelli, Alfonso Lorusso, Maricla Marrone, Alessandra Stellacci, and et al. 2021. "Urological Melanoma: A Comprehensive Review of a Rare Subclass of Mucosal Melanoma with Emphasis on Differential Diagnosis and Therapeutic Approaches" Cancers 13, no. 17: 4424. https://doi.org/10.3390/cancers13174424
APA StyleCazzato, G., Colagrande, A., Cimmino, A., Caporusso, C., Candance, P. M. V., Trabucco, S. M. R., Zingarelli, M., Lorusso, A., Marrone, M., Stellacci, A., Arezzo, F., Marzullo, A., Serio, G., Filoni, A., Bonamonte, D., Romita, P., Foti, C., Lettini, T., Loizzi, V., ... Ingravallo, G. (2021). Urological Melanoma: A Comprehensive Review of a Rare Subclass of Mucosal Melanoma with Emphasis on Differential Diagnosis and Therapeutic Approaches. Cancers, 13(17), 4424. https://doi.org/10.3390/cancers13174424











