Oligometastatic Adenocarcinoma of the Esophagus: Current Understanding, Diagnosis, and Therapeutic Strategies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Discussion
3.1. Diagnostic Approaches
3.2. Molecular Mechanisms
3.3. Current Management
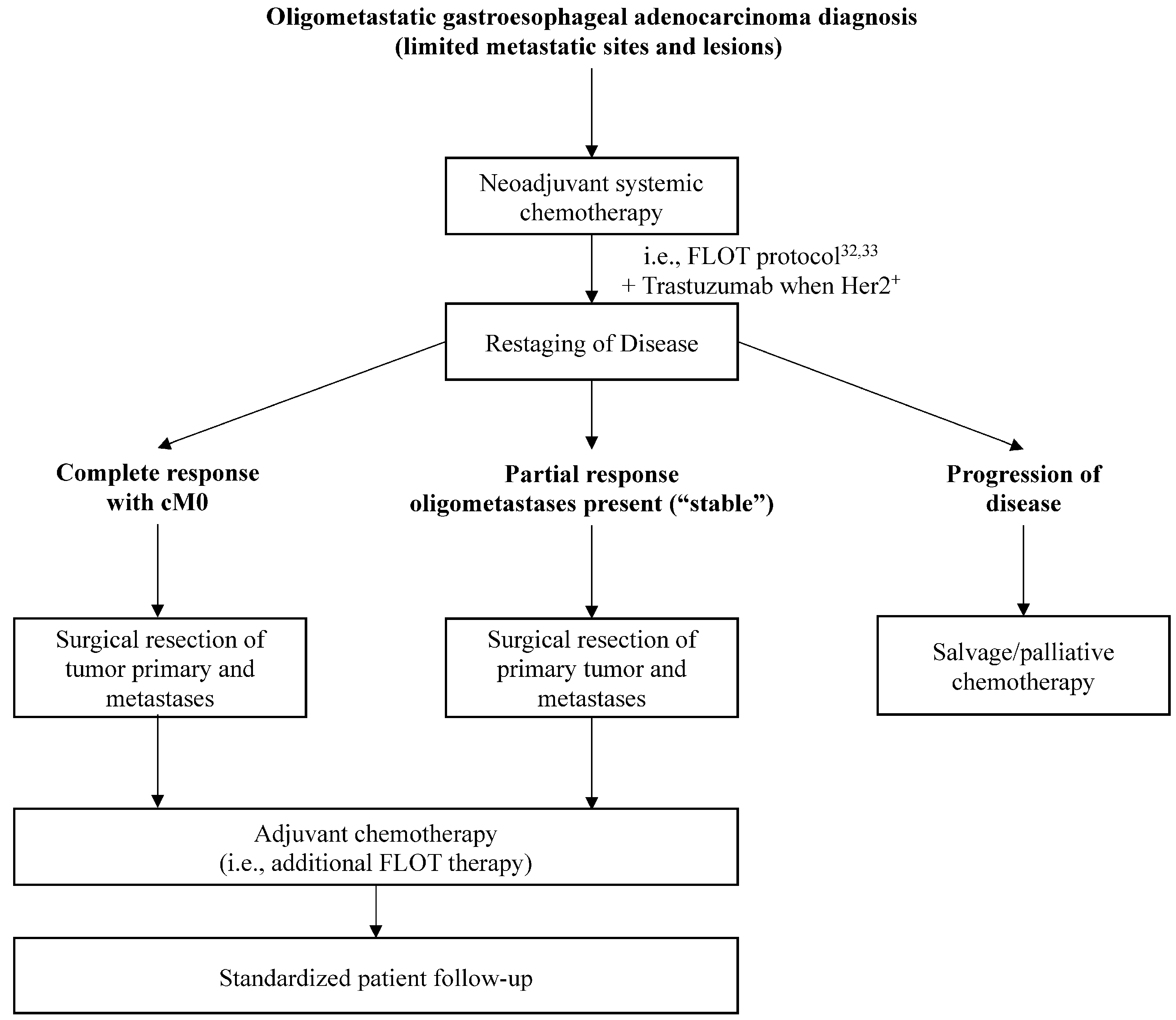
3.4. Evidence for Management of Oligometastasis
3.5. Future Projections
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Napier, K.J.; Scheerer, M.; Misra, S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J. Gastrointest. Oncol. 2014, 6, 112–120. [Google Scholar] [CrossRef]
- Derakhshan, M.H.; Arnold, M.; Brewster, D.H.; Going, J.J.; Mitchell, D.R.; Forman, D.; McColl, K.E. Worldwide Inverse Association between Gastric Cancer and Esophageal Adenocarcinoma Suggesting a Common Environmental Factor Exerting Opposing Effects. Am. J. Gastroenterol. 2016, 111, 228–239. [Google Scholar] [CrossRef]
- Howlader, N.N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2018; National Cancer Institute: Bethesda, MD, USA, 2021. [Google Scholar]
- Medical Research Council Oesophageal Cancer Working, G. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar]
- Lee, D.H.; Kim, H.R.; Kim, D.K.; Park, S.I.; Kim, Y.H. Outcomes of cervical lymph node recurrence in patients with esophageal squamous cell carcinoma after esophagectomy with 2-field lymph node dissection. J. Thorac. Cardiovasc. Surg. 2013, 146, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Abate, E.; DeMeester, S.R.; Zehetner, J.; Oezcelik, A.; Ayazi, S.; Costales, J.; Banki, F.; Lipham, J.C.; Hagen, J.A. DeMeester TR. Recurrence after esophagectomy for adenocarcinoma: Defining optimal follow-up intervals and testing. J. Am. Coll. Surg. 2010, 210, 428–435. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Tepper, J.; Krasna, M.J.; Niedzwiecki, D.; Hollis, D.; Reed, C.E.; Goldberg, R.; Kiel, K.; Willett, C.; Sugarbaker, D.; Mayer, R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 2008, 26, 1086–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamel, S.; Tukanova, K.; Markar, S. Detection and management of oligometastatic disease in oesophageal cancer and identification of prognostic factors: A systematic review. World J. Gastrointest. Oncol. 2019, 11, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Weichselbaum, R.R.; Hellman, S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 2011, 8, 378–382. [Google Scholar] [CrossRef]
- Reyes, D.K.; Pienta, K.J. The biology and treatment of oligometastatic cancer. Oncotarget 2015, 6, 8491–8524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, T.K., Jr.; Hofstetter, W.L.; Rizk, N.P.; Low, D.E.; Darling, G.E.; Watson, T.J.; Mitchell, J.D.; Krasna, M.J. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann. Thorac. Surg. 2013, 96, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Sequeiros, E.; Norton, I.D.; Clain, J.E.; Wang, K.K.; Affi, A.; Allen, M.; Deschamps, C.; Miller, D.; Salomao, D.; Wiersema, M.J. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest. Endosc. 2001, 53, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Saab, J.; Zia, H.; Mathew, S.; Kluk, M.; Narula, N.; Fernandes, H. Utility of Genomic Analysis in Differentiating Synchronous and Metachronous Lung Adenocarcinomas from Primary Adenocarcinomas with Intrapulmonary Metastasis. Transl. Oncol. 2017, 10, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Xiao, L.; Hayashi, Y.; Macapinlac, H.A.; Welsh, J.; Lin, S.H.; Lee, J.H.; Bhutani, M.S.; Maru, D.M.; Hofstetter, W.L.; et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer 2011, 117, 4823–4833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers. Available online: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (accessed on 23 May 2021).
- Testa, U.; Castelli, G.; Pelosi, E. Esophageal Cancer: Genomic and Molecular Characterization, Stem Cell Compartment and Clonal Evolution. Medicines 2017, 4, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulak, A.M.; Stojanov, P.; Peng, S.; Lawrence, M.S.; Fox, C.; Stewart, C.; Bandla, S.; Imamura, Y.; Schumacher, S.E.; Shefler, E.; et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 2013, 45, 478–486. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, L.; Thomas, D.G.; Nadal, E.; Chang, A.C.; Beer, D.G.; Lin, J. The role of Dickkopf-3 overexpression in esophageal adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2015, 150, 377–385 e2. [Google Scholar] [CrossRef] [Green Version]
- Helminen, O.; Huhta, H.; Leppanen, J.; Kauppila, J.H.; Takala, H.; Lehenkari, P.P.; Saarnio, J.; Karttunen, T.J. Nuclear localization of Toll-like receptor 5 in Barrett’s esophagus and esophageal adenocarcinoma is associated with metastatic behavior. Virchows Arch. 2016, 469, 465–470. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Wei, L.; Lai, S.; Zheng, W.; Wu, F. Serum tumor-associated glycoprotein 72, a helpful predictor of lymph nodes invasion in esophagogastric junction adenocarcinoma. Biochem. Biophys. Res. Commun. 2019, 509, 133–137. [Google Scholar] [CrossRef]
- Jung, J.O.; Nienhuser, H.; Schleussner, N.; Schmidt, T. Oligometastatic Gastroesophageal Adenocarcinoma: Molecular Pathophysiology and Current Therapeutic Approach. Int. J. Mol. Sci. 2020, 21, 951. [Google Scholar] [CrossRef] [Green Version]
- Castano-Rodriguez, N.; Kaakoush, N.O.; Mitchell, H.M. Pattern-recognition receptors and gastric cancer. Front Immunol. 2014, 5, 336. [Google Scholar] [PubMed]
- Barghash, A.; Golob-Schwarzl, N.; Helms, V.; Haybaeck, J.; Kessler, S.M. Elevated expression of the IGF2 mRNA binding protein 2 (IGF2BP2/IMP2) is linked to short survival and metastasis in esophageal adenocarcinoma. Oncotarget 2016, 7, 49743–49750. [Google Scholar] [CrossRef] [Green Version]
- Haussler, U.; Bitzer, M.; Bosmuller, H.; Clasen, S.; Gotz, M.; Malek, N.P.; Plentz, R.R. AFP-producing adenocarcinoma of the esophagogastric junction: Report of a case with atypical immunohistochemical findings responding to palliative chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT regime). Z. Gastroenterol. 2016, 54, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Sohda, M.; Sakai, M.; Kumakura, Y.; Yoshida, T.; Kuriyama, K.; Yokobori, T.; Miyazaki, M.; Hirato, J.; Okumura, T.; et al. Multimodality Therapy Including Proton Beam Therapy for AFP Producing Esophageal Cancer with Multiple Liver Metastases. Intern. Med. 2018, 57, 2333–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, Y.; Kato, T.; Harano, M.; Satoh, D.; Choda, Y.; Tokumoto, N.; Kanazawa, T.; Matsukawa, H.; Ojima, Y.; Idani, H.; et al. [A case of AFP-producing esophagogastric junction cancer with liver metastases with a good response to chemotherapy]. Gan Kagaku Ryoho 2014, 41, 2349–2351. [Google Scholar]
- Siewert, J.R.; Stein, H.J. Carcinoma of the gastroesophageal junction—classification, pathology and extent of resection. Dis. Esophagus 1996, 9, 173–182. [Google Scholar]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef] [Green Version]
- Herskovic, A.; Martz, K.; al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients with Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Yoshida, M.; Ohtsu, A.; Boku, N.; Miyata, Y.; Shirao, K.; Shimada, Y.; Hyodo, I.; Koizumi, W.; Kurihara, M.; Yoshida, S.; et al. Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn. J. Clin. Oncol. 2004, 34, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Al-Batran, S.E.; Goetze, T.O.; Mueller, D.W.; Vogel, A.; Winkler, M.; Lorenzen, S.; Novotny, A.; Pauligk, C.; Homann, N.; Jungbluth, T.; et al. The RENAISSANCE (AIO-FLOT5) trial: Effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction—a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer 2017, 17, 893. [Google Scholar]
- Chen, F.; Sato, K.; Sakai, H.; Miyahara, R.; Bando, T.; Okubo, K.; Hirata, T. Date H Pulmonary resection for metastasis from esophageal carcinoma. Interact Cardiovasc. Thorac. Surg. 2008, 7, 809–812. [Google Scholar] [CrossRef] [Green Version]
- Ghaly, G.; Harrison, S.; Kamel, M.K.; Rahouma, M.; Nasar, A.; Port, J.L.; Stiles, B.M.; Altorki, N.K. Predictors of Survival After Treatment of Oligometastases After Esophagectomy. Ann. Thorac. Surg. 2018, 105, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiono, S.; Kawamura, M.; Sato, T.; Nakagawa, K.; Nakajima, J.; Yoshino, I.; Ikeda, N.; Horio, H.; Akiyama, H.; Kobayashi, K.; et al. Disease-free interval length correlates to prognosis of patients who underwent metastasectomy for esophageal lung metastases. J. Thorac. Oncol. 2008, 3, 1046–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozu, Y.; Sato, H.; Tsubosa, Y.; Ogawa, H.; Yasui, H.; Kondo, H. Surgical treatment for pulmonary metastases from esophageal carcinoma after definitive chemoradiotherapy: Experience from a single institution. J. Cardiothorac. Surg. 2011, 6, 135. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, H.; Kosugi, S.; Nakagawa, S.; Kanda, T.; Tsuchida, M.; Koike, T.; Tanaka, O.; Hatakeyama, K. Operative treatment for metachronous pulmonary metastasis from esophageal carcinoma. Surgery 2011, 149, 164–170. [Google Scholar] [CrossRef]
- Takemura, M.; Sakurai, K.; Takii, M.; Yoshida, K. Metachronous pulmonary metastasis after radical esophagectomy for esophageal cancer: Prognosis and outcome. J. Cardiothorac. Surg. 2012, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.; Alldinger, I.; Blank, S.; Klose, J.; Springfeld, C.; Dreikhausen, L.; Weichert, W.; Grenacher, L.; Bruckner, T.; Lordick, F.; et al. Surgery in oesophago-gastric cancer with metastatic disease: Treatment, prognosis and preoperative patient selection. Eur. J. Surg. Oncol. 2015, 41, 1340–1347. [Google Scholar] [CrossRef]
- Ichida, H.; Imamura, H.; Yoshimoto, J.; Sugo, H.; Kajiyama, Y.; Tsurumaru, M.; Suzuki, K.; Ishizaki, Y.; Kawasaki, S. Pattern of postoperative recurrence and hepatic and/or pulmonary resection for liver and/or lung metastases from esophageal carcinoma. World J. Surg. 2013, 37, 398–407. [Google Scholar] [CrossRef]
- Huddy, J.R.; Thomas, R.L.; Worthington, T.R.; Karanjia, N.D. Liver metastases from esophageal carcinoma: Is there a role for surgical resection? Dis. Esophagus. 2015, 28, 483–487. [Google Scholar] [CrossRef]
- Schizas, D.; Mylonas, K.S.; Kapsampelis, P.; Bagias, G.; Katsaros, I.; Frountzas, M.; Hemmati, P.; Liakakos, T. Patients undergoing surgery for oligometastatic oesophageal cancer survive for more than 2 years: Bootstrapping systematic review data. Interact Cardiovasc. Thorac. Surg. 2020, 31, 299–304. [Google Scholar] [CrossRef]
- Schmidt, T.; Monig, S.P. Therapeutic approach in oligometastatic gastric and esophageal cancer. Chirurg 2017, 88, 1024–1032. [Google Scholar] [CrossRef]
- Nakagawa, S.; Nishimaki, T.; Kosugi, S.; Ohashi, M.; Kanda, T.; Hatakeyama, K. Cervical lymphadenectomy is beneficial for patients with carcinoma of the upper and mid-thoracic esophagus. Dis. Esophagus. 2003, 16, 4–8. [Google Scholar] [CrossRef]
- Hiyoshi, Y.; Morita, M.; Kawano, H.; Otsu, H.; Ando, K.; Ito, S.; Miyamoto, Y.; Sakamoto, Y.; Saeki, H.; Oki, E.; et al. Clinical significance of surgical resection for the recurrence of esophageal cancer after radical esophagectomy. Ann. Surg. Oncol. 2015, 22, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Akkus Yildirim, B.; Guler, O.C. Outcomes of aggressive treatment in esophageal cancer patients with synchronous solitary brain metastasis. Mol. Clin. Oncol. 2017, 7, 107–112. [Google Scholar] [CrossRef]
- Inderson, A.; Slingerland, M.; Farina Sarasqueta, A.; de Steur, W.O.; Boonstra, J.J. EUS-guided radiofrequency ablation for a left adrenal oligometastasis of an esophageal adenocarcinoma. VideoGIE 2018, 3, 159–161. [Google Scholar] [CrossRef] [Green Version]
- Chai, G.; Yin, Y.; Zhou, X.; Hu, Q.; Lv, B.; Li, Z.; Shi, M.; Zhao, L. Pulmonary oligometastases treated by stereotactic body radiation therapy (SBRT): A single institution’s experience. Transl. Lung Cancer Res. 2020, 9, 1496–1506. [Google Scholar] [CrossRef]
- Wang, H.H.; Zaorsky, N.G.; Meng, M.B.; Zeng, X.L.; Deng, L.; Song, Y.C.; Zhuang, H.Q.; Li, F.T.; Zhao, L.J.; Yuan, Z.Y.; et al. Stereotactic radiation therapy for oligometastases or oligorecurrence within mediastinal lymph nodes. Oncotarget 2016, 7, 18135–18145. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lievre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef]
- Kato, K.; Sun, J.-M.; Shah, M.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.-P.; Li, Z.; Kim, S.-B.; et al. Pembrolizumab Plus Chemotherapy Versus Chemotherapy as First-Line Therapy in Patients with Advanced Esophageal Cancer: The Phase 3 KEYNOTE-590 Study. Ann. Oncol. 2020, 31, S1192–S1193. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, M.P.; DeSantis, A.J.; DuCoin, C.G. Oligometastatic Adenocarcinoma of the Esophagus: Current Understanding, Diagnosis, and Therapeutic Strategies. Cancers 2021, 13, 4352. https://doi.org/10.3390/cancers13174352
Rogers MP, DeSantis AJ, DuCoin CG. Oligometastatic Adenocarcinoma of the Esophagus: Current Understanding, Diagnosis, and Therapeutic Strategies. Cancers. 2021; 13(17):4352. https://doi.org/10.3390/cancers13174352
Chicago/Turabian StyleRogers, Michael P., Anthony J. DeSantis, and Christopher G. DuCoin. 2021. "Oligometastatic Adenocarcinoma of the Esophagus: Current Understanding, Diagnosis, and Therapeutic Strategies" Cancers 13, no. 17: 4352. https://doi.org/10.3390/cancers13174352
APA StyleRogers, M. P., DeSantis, A. J., & DuCoin, C. G. (2021). Oligometastatic Adenocarcinoma of the Esophagus: Current Understanding, Diagnosis, and Therapeutic Strategies. Cancers, 13(17), 4352. https://doi.org/10.3390/cancers13174352





