Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
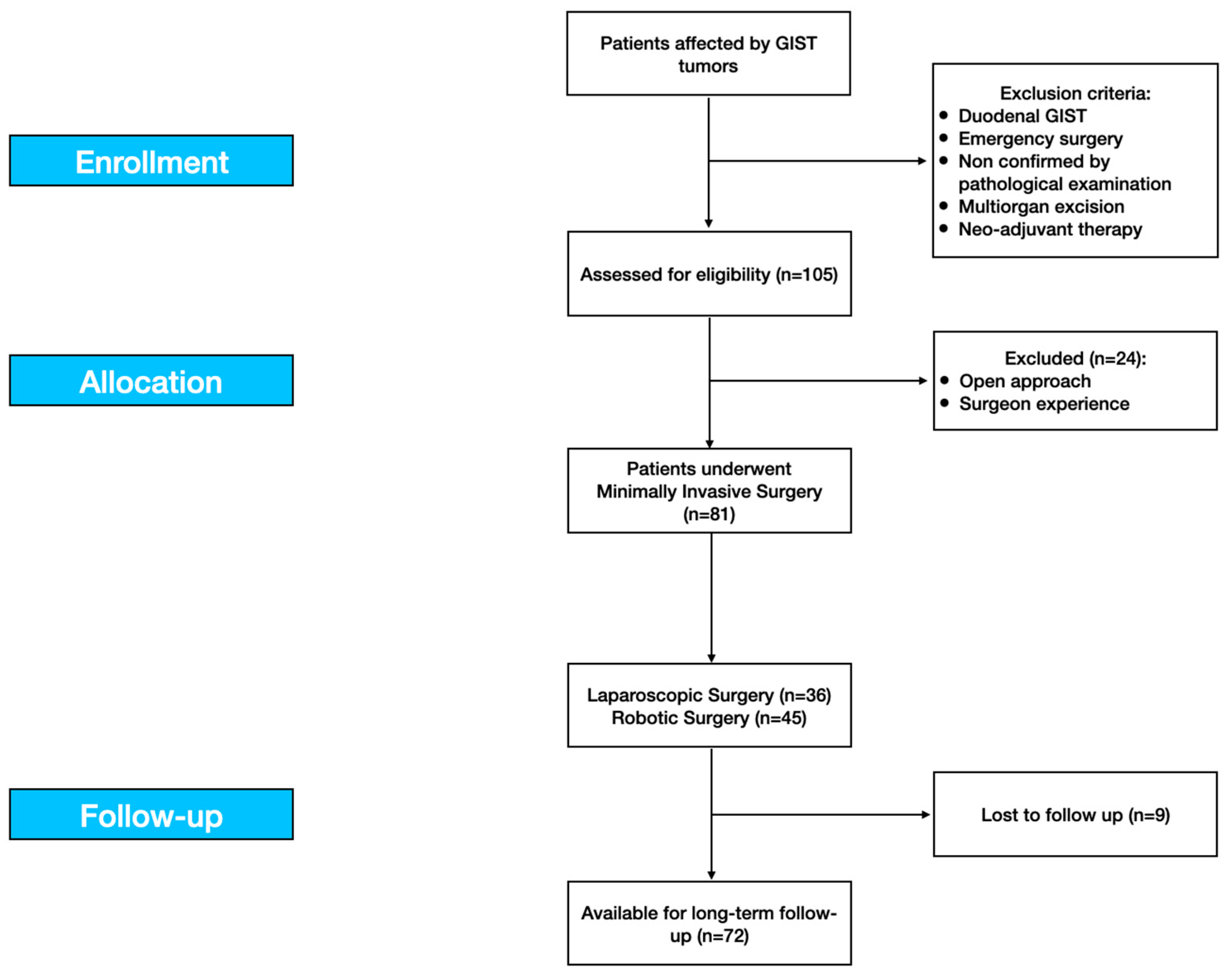
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Variables and Definitions
2.4. The Tumor Location
2.5. Follow up Program
2.6. Technical Notes
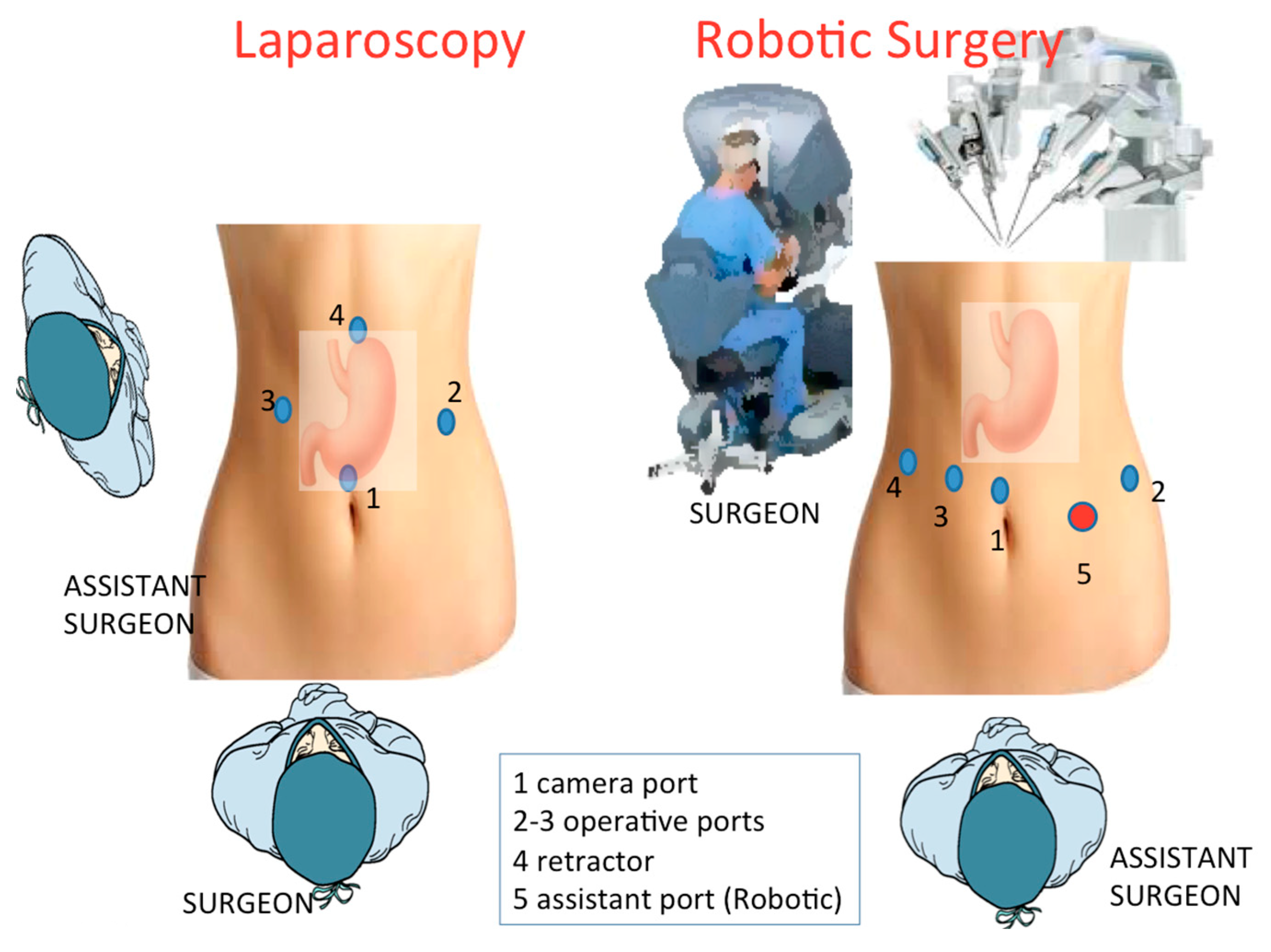
2.6.1. Laparoscopic Approach
2.6.2. Robotic-Assisted Surgery
2.7. Statistical Analysis
3. Results
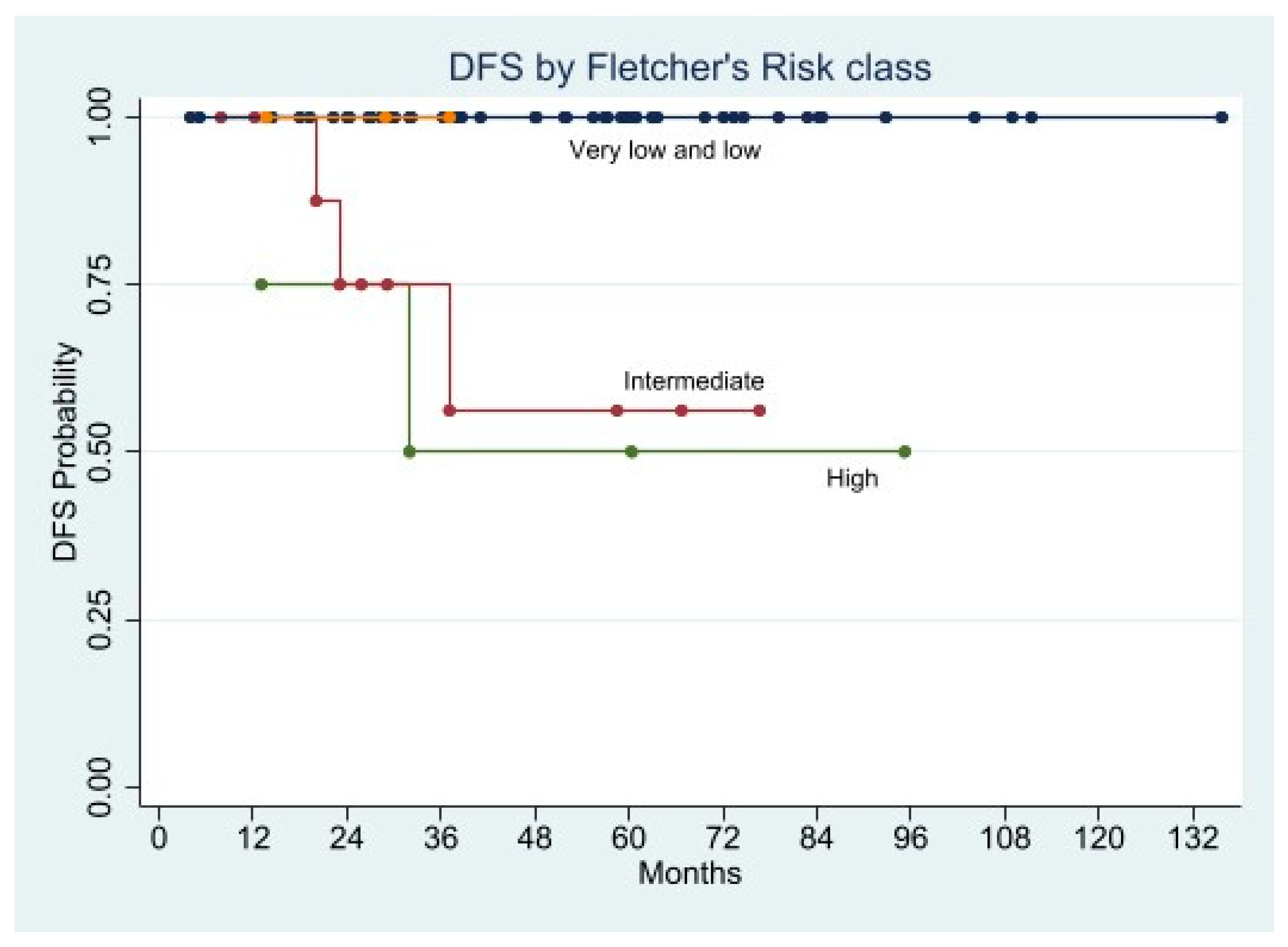
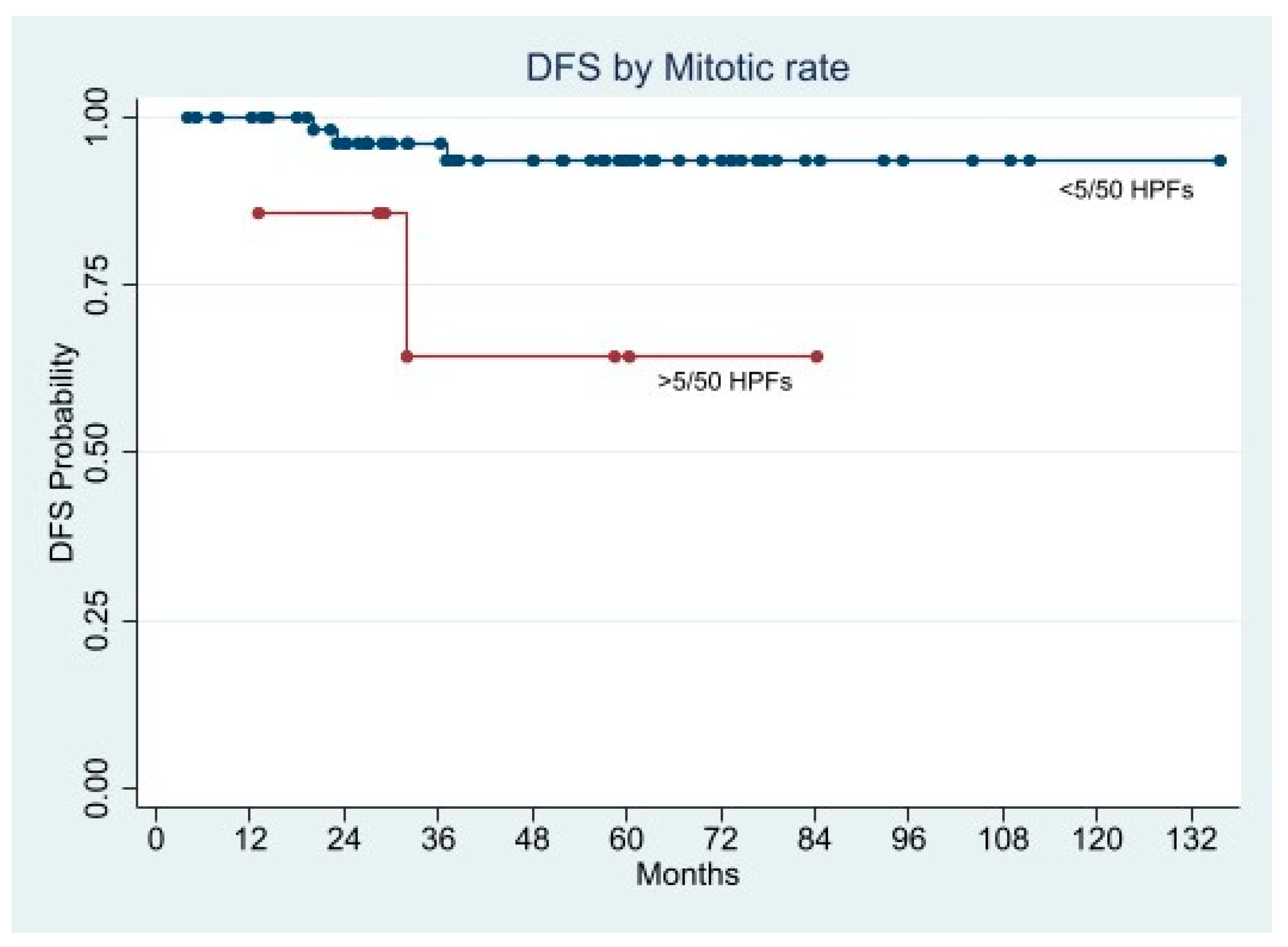
Long-Term Oncological Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raut, C.P.; Morgan, J.A.; Ashley, S.W. Current issues in gastrointestinal stromal tumors: Incidence, molecular biology, and contemporary treatment of localized and advanced disease. Curr. Opin. Gastroenterol. 2007, 23, 149–158. [Google Scholar] [CrossRef]
- Fudalej, M.M.; Badowska-Kozakiewicz, A.M. Improved understanding of gastrointestinal stromal tumors biology as a step for developing new diagnostic and therapeutic schemes. Oncol. Lett. 2021, 21, 417. [Google Scholar] [CrossRef]
- Khoshnood, A. Gastrointestinal stromal tumor—A review of clinical studies. J. Oncol. Pharm. Pract. 2019, 25, 1473–1485. [Google Scholar] [CrossRef]
- Joensuu, H.; Vehtari, A.; Riihimäki, J.; Nishida, T.; Steigen, S.E.; Brabec, P.; Plank, L.; Nilsson, B.; Cirilli, C.; Braconi, C.; et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012, 13, 265–274. [Google Scholar] [CrossRef]
- Sorour, M.A.; Kassem, M.I.; Ghazal Ael, H.; El-Riwini, M.T.; Abu Nasr, A. Gastrointestinal stromal tumors (GIST) related emergencies. Int. J. Surg. 2014, 12, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Bucher, P.; Villiger, P.; Egger, J.F.; Buhler, L.H.; Morel, P. Management of gastrointestinal stromal tumors: From diagnosis to treatment. Swiss Med. Wkly. 2004, 134, 145–153. [Google Scholar] [PubMed]
- Kawanowa, K.; Sakuma, Y.; Sakurai, S.; Hishima, T.; Iwasaki, Y.; Saito, K.; Hosoya, Y.; Nakajima, T.; Funata, N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum. Pathol. 2006, 37, 1527–1535. [Google Scholar] [CrossRef]
- Miettinen, M.; Kopczynski, J.; Makhlouf, H.R.; Sarlomo-Rikala, M.; Gyorffy, H.; Burke, A.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: A clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am. J. Surg. Pathol. 2003, 27, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Biasco, G.; Velo, D.; Angriman, I.; Astorino, M.; Baldan, A.; Baseggio, M.; Basso, U.; Battaglia, G.; Bertin, M.; Bertorelle, R.; et al. Gastrointestinal stromal tumors: Report of an audit and review of the literature. Eur. J. Cancer Prev. 2009, 18, 106–116. [Google Scholar] [CrossRef]
- Nguyen, S.Q.; Divino, C.M.; Wang, J.L.; Dikman, S.H. Laparoscopic management of gastrointestinal stromal tumors. Surg. Endosc. 2006, 20, 713–716. [Google Scholar] [CrossRef]
- De Vogelaere, K.; Van Loo, I.; Peters, O.; Hoorens, A.; Haentjens, P.; Delvaux, G. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg. Endosc. 2012, 26, 2339–2345. [Google Scholar] [CrossRef]
- Eight Edition AJCC Cancer Staging Manual A.C.S. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O’Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002, 33, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Gao, Z.; Shen, K.; Cao, J.; Shen, Z.; Jiang, K.; Wang, S.; Ye, Y. Safety and efficiency of endoscopic resection versus laparoscopic resection in gastric gastrointestinal stromal tumours: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, IV68–IV78. [Google Scholar] [CrossRef]
- Correa-Cote, J.; Morales-Uribe, C.; Sanabria, A. Laparoscopic management of gastric gastrointestinal stromal tumors. World J. Gastrointest. Endosc. 2014, 6, 296–303. [Google Scholar] [CrossRef]
- Meng, X.; Wang, L.; Zhu, B.; Sun, T.; Guo, S.; Wang, Y.; Zhang, J.; Yang, D.; Zheng, G.; Zhang, T.; et al. Totally laparoscopic gastrectomy versus laparoscopic-assisted gastrectomy for gastric cancer: A systematic review and meta-analysis. J. Laparoendosc. Adv. Surg. Tech. A 2020, 31, 676–691. [Google Scholar] [CrossRef]
- de’Angelis, N.; Brunetti, F.; Felli, E.; Mehdaoui, D.; Memeo, R.; Carra, M.C.; Zuddas, V.; Azoulay, D. Laparoscopic versus open gastric wedge resection for primary gastrointestinal tumors: Clinical outcomes and health care costs analysis. Surg. Laparosc. Endosc. Percutan. Tech. 2015, 25, 143–146. [Google Scholar] [CrossRef]
- Vicente, E.; Quijano, Y.; Ielpo, B.; Duran, H.; Diaz, E.; Fabra, I.; Malave, L.; Ferri, V.; Ferronetti, A.; Caruso, R. Robot-assisted resection of gastrointestinal stromal tumors (GIST): A single center case series and literature review. Int. J. Med. Robot. Comput. Assist. Surg. 2016, 12, 718–723. [Google Scholar] [CrossRef]
- Desiderio, J.; Trastulli, S.; Cirocchi, R.; Boselli, C.; Noya, G.; Parisi, A.; Cavaliere, D. Robotic gastric resection of large gastrointestinal stromal tumors. Int. J. Surg. 2013, 11, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Parisi, A.; Reim, D.; Borghi, F.; Nguyen, N.T.; Qi, F.; Coratti, A.; Cianchi, F.; Cesari, M.; Bazzocchi, F.; Alimoglu, O.; et al. Minimally invasive surgery for gastric cancer: A comparison between robotic, laparoscopic and open surgery. World J. Gastroenterol. 2017, 23, 2376–2384. [Google Scholar] [CrossRef]
- Maeso, S.; Reza, M.; Mayol, J.A.; Blasco, J.A.; Guerra, M.; Andradas, E.; Plana, M.N. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: A systematic review and meta-analysis. Ann. Surg. 2010, 252, 254–262. [Google Scholar] [CrossRef]
- Ishida, Y.; Kanaya, S.; Uyama, I. Robotic surgery for gastric cancer with da Vinci SHD Surgical System]. Nihon Rinsho Jpn. J. Clin. Med. 2010, 68, 1212–1214. [Google Scholar]
- Hur, H.; Kim, J.Y.; Cho, Y.K.; Han, S.U. Technical feasibility of robot-sewn anastomosis in robotic surgery for gastric cancer. J. Laparoendosc. Adv. Surg. Tech. 2010, 20, 693–697. [Google Scholar] [CrossRef]
- Maggioni, C.; Shida, A.; Mancini, R.; Ioni, L.; Pernazza, G. Safety profile and oncological outcomes of gastric gastrointestinal stromal tumors (GISTs) robotic resection: Single center experience. Int. J. Med. Robot. Comput. Assist. Surg. 2019, 15, e2031. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.; Corless, C.L.; Duensing, A.; Hornick, J.L.; Oliveira, A.M.; Heinrich, M.C.; Fletcher, J.A.; Fletcher, C.D. KIT-negative gastrointestinal stromal tumors: Proof of concept and therapeutic implications. Am. J. Surg. Pathol. 2004, 28, 889–894. [Google Scholar] [CrossRef]
- Arseneaux, M.; Yarbrough, D.; Nagamoto, T. Robotic-assisted free-handed, full-thickness gastric GIST resection with primary repair in unfavorable locations. J. Robot. Surg. 2019, 13, 491–494. [Google Scholar] [CrossRef]
- Associazione Italiana di Oncologia Medica. Linee Guida per Sarcomi dei Tessuti Molli e GIST. Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Sarcomi-1.pdf (accessed on 15 May 2021).
- Buchs, N.C.; Bucher, P.; Pugin, F.; Hagen, M.E.; Morel, P. Robot-assisted oncologic resection for large gastric gastrointestinal stromal tumor: A preliminary case series. J. Laparoendosc. Adv. Surg. Tech. A 2010, 20, 411–415. [Google Scholar] [CrossRef]
- de’Angelis, N.; Genova, P.; Amiot, A.; Charpy, C.; Disabato, M.; Belgaumkar, A.P.; Chahrour, A.; Legou, F.; Azoulay, D.; Brunetti, F. Robotic versus laparoscopic gastric resection for primary gastrointestinal stromal tumors >5 cm: A size-matched and location-matched comparison. Surg. Laparosc. Endosc. Percutan. Tech. 2017, 27, 65–71. [Google Scholar] [CrossRef]
- Al-Thani, H.; El-Menyar, A.; Mekkodathil, A.; Elgohary, H.; Tabeb, A.H. Robotic management of gastric stromal tumors (GIST): A single Middle Eastern center experience. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, e1729. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.; Jiang, Z.; Jiang, C.; Liu, J.; Zhou, J.; Li, J. Robotic gastrotomy with intracorporeal suture for patients with gastric gastrointestinal stromal tumors located at cardia and subcardiac region. Surg. Laparosc. Endosc. Percutan. Tech. 2018, 28, e1–e7. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Li, Y.; Pan, Y.; Sun, Q.; Wang, G.; Yu, T.; Shi, C.; Xia, H.; She, J. Clinical feasibility and safety of third space robotic and endoscopic cooperative surgery for gastric gastrointestinal stromal tumors dissection: A new surgical technique for treating gastric GISTs. Surg. Endosc. 2019, 33, 4192–4200. [Google Scholar] [CrossRef] [Green Version]
- Furbetta, N.; Palmeri, M.; Guadagni, S.; Di Franco, G.; Gianardi, D.; Latteri, S.; Marciano, E.; Moglia, A.; Cuschieri, A.; Di Candio, G.; et al. Gastrointestinal stromal tumours of stomach: Robot-assisted excision with the da Vinci Surgical System regardless of size and location site. J. Minim. Access Surg. 2018, 15, 142–147. [Google Scholar] [CrossRef]
- Solaini, L.; Cavaliere, D.; Fico, V.; Milone, M.; De Pascale, S.; Desiderio, J.; Vitali, G.; Parisi, A.; Fumagalli Romario, U.; De Palma, G.D.; et al. Open versus laparoscopic versus robotic gastric gastrointestinal stromal tumour resections: A multicentre cohort study. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2198. [Google Scholar] [CrossRef]
- Winder, A.; Strauss, D.C.; Jones, R.L.; Benson, C.; Messiou, C.; Chaudry, M.A.; Smith, M.J. Robotic surgery for gastric gastrointestinal stromal tumors: A single center case series. J. Surg. Oncol. 2020, 122, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Iorio, N.; Sawaya, R.A.; Friedenberg, F.K. Review article: The biology, diagnosis and management of gastrointestinal stromal tumours. Aliment. Pharmacol. Ther. 2014, 39, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, M.; Takifuji, K.; Ojima, T.; Nakamura, M.; Nakamori, M.; Nakatani, Y.; Ueda, K.; Ishida, K.; Naka, T.; Ono, K.; et al. Surgical management of small gastrointestinal stromal tumors of the stomach. World J. Surg. 2006, 30, 28–35. [Google Scholar] [CrossRef]
- Kumar, M.; Goel, M.M.; Singh, D. Rare case of gastrointestinal stromal tumor of the anal canal. J. Cancer Res. Ther. 2013, 9, 736–738. [Google Scholar] [CrossRef] [PubMed]
- De Vogelaere, K.; Aerts, M.; Haentjens, P.; De Grève, J.; Delvaux, G. Gastrointestinal stromal tumor of the stomach: Progresses in diagnosis and treatment. Acta Gastroenterol. Belg. 2013, 76, 403–406. [Google Scholar]
- Chiang, N.J.; Chen, L.T.; Tsai, C.R.; Chang, J.S. The epidemiology of gastrointestinal stromal tumors in Taiwan, 1998–2008: A nation-wide cancer registry-based study. BMC Cancer 2014, 14, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agaimy, A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: More questions than answers? A review emphasizing the need for a standardized GIST reporting. Int. J. Clin. Exp. Pathol. 2010, 3, 461–471. [Google Scholar]
- Schwameis, K.; Fochtmann, A.; Schwameis, M.; Asari, R.; Schur, S.; Köstler, W.; Birner, P.; Ba-Ssalamah, A.; Zacherl, J.; Wrba, F.; et al. Surgical treatment of GIST—An institutional experience of a high-volume center. Int. J. Surg. 2013, 11, 801–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Miettinen, M.; Furlong, M.; Sarlomo-Rikala, M.; Burke, A.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: A clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am. J. Surg. Pathol. 2001, 25, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Bylina, E.; Wozniak, A.; Nowecki, Z.I.; Osuch, C.; Matlok, M.; Switaj, T.; Michej, W.; Wroński, M.; Głuszek, S.; et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour—The impact of tumour rupture on patient outcomes. Eur. J. Surg. Oncol. 2011, 37, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Nowecki, Z.I.; Michej, W.; Debiec-Rychter, M.; Woźniak, A.; Limon, J.; Siedlecki, J.; Grzesiakowska, U.; Kakol, M.; Osuch, C.; et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann. Surg. Oncol. 2007, 14, 2018–2027. [Google Scholar] [CrossRef]
- Rutkowski, P.; Hompes, D. Combined therapy of gastrointestinal stromal tumors. Surg. Oncol. Clin. N. Am. 2016, 25, 735–759. [Google Scholar] [CrossRef]
- Demetri, G.D.; Benjamin, R.S.; Blanke, C.D.; Blay, J.Y.; Casali, P.; Choi, H.; Corless, C.L.; Debiec-Rychter, M.; DeMatteo, R.P.; Ettinger, D.S.; et al. NCCN Task Force report: Management of patients with gastrointestinal stromal tumor (GIST)—Update of the NCCN clinical practice guidelines. J. Natl. Compr. Cancer Netw. 2007, 5 (Suppl. 2), S1–S29. [Google Scholar] [CrossRef]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Casper, E.S.; Conrad, E.U., III; DeLaney, T.F.; Ganjoo, K.N.; George, S.; et al. Gastrointestinal stromal tumors, version 2.2014. J. Natl. Compr. Cancer Netw. 2014, 12, 853–862. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Kodera, Y.; Fujiwara, M.; Ito, S.; Yamamura, Y.; Sawaki, A.; Yamao, K.; Kato, T. Laparoscopic wedge resection for gastrointestinal stromal tumors of the stomach: Initial experience. Surg. Today 2006, 36, 341–347. [Google Scholar] [CrossRef]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Conrad, E.U., III; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; et al. Soft tissue sarcoma, version 2.2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 758–786. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.W.; Zheng, Z.C.; Zhang, J.J.; Zhang, T.; Zhao, Y.; Yang, W.; Liu, Y.Q. Laparoscopic versus open gastric resections for gastric gastrointestinal stromal tumors: A meta-analysis. Surg. Laparosc. Endosc. Percutan. Tech. 2013, 23, 378–387. [Google Scholar] [CrossRef]
- Landi, B.; Blay, J.Y.; Bonvalot, S.; Brasseur, M.; Coindre, J.M.; Emile, J.F.; Hautefeuille, V.; Honore, C.; Lartigau, E.; Mantion, G.; et al. Gastrointestinal stromal tumours (GISTs): French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. 2019, 51, 1223–1231. [Google Scholar] [CrossRef]
- Ye, X.; Kang, W.M.; Yu, J.C.; Ma, Z.Q.; Xue, Z.G. Comparison of short- and long-term outcomes of laparoscopic vs open resection for gastric gastrointestinal stromal tumors. World J. Gastroenterol. 2017, 23, 4595–4603. [Google Scholar] [CrossRef]
- Lin, J.; Huang, C.; Zheng, C.; Li, P.; Xie, J.; Wang, J.; Lu, J. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): A size-matched comparison. Surg. Endosc. 2014, 28, 2577–2583. [Google Scholar] [CrossRef]
- De Vogelaere, K.; Hoorens, A.; Haentjens, P.; Delvaux, G. Laparoscopic versus open resection of gastrointestinal stromal tumors of the stomach. Surg. Endosc. 2013, 27, 1546–1554. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncologicy (NCNN Guidelines): Soft Tissue Sarcoma. Version 2. 2016. Available online: https://jnccn.org/view/journals/jnccn/16/5/article-p536.xml (accessed on 27 June 2021).
- Yang, Z.; Li, P.; Hu, Y. Laparoscopic versus open wedge resection for gastrointestinal stromal tumors of the stomach: A meta-analysis. Wideochir Inne Tech. Maloinwazyjne 2019, 14, 149–159. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, W.; Zhou, D.; Lu, L.; Yao, L. Laparoscopic versus open resection for gastric gastrointestinal stromal tumors: A meta-analysis. Am. Surg. 2014, 80, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Di Maria Grimaldi, S.; Marano, A.; Pellegrino, L.; Geretto, P.; Palagi, S.; Borghi, F. Robotic wedge resection for unfavorably located gastric gastrointestinal stromal tumors: Perioperative and long-term oncological outcomes. J. Laparoendosc. Adv. Surg. Tech. A 2021, 31, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Stanciulea, O.; Ionescu, M.I.; Blanita, D.; Lacatus, M.; Gheorghe, C.; Vasilescu, C. Minimal Access surgery for the treatment of gastric gastrointestinal stromal tumours—A single centre experience. Chirurgia 2020, 115, 726–734. [Google Scholar] [CrossRef]
- Hiki, N.; Yamamoto, Y.; Fukunaga, T.; Yamaguchi, T.; Nunobe, S.; Tokunaga, M.; Miki, A.; Ohyama, S.; Seto, Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg. Endosc. 2008, 22, 1729–1735. [Google Scholar] [CrossRef]
- Hiki, N.; Nunobe, S.; Matsuda, T.; Hirasawa, T.; Yamamoto, Y.; Yamaguchi, T. Laparoscopic endoscopic cooperative surgery. Dig. Endosc. 2015, 27, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niimi, K.; Ishibashi, R.; Mitsui, T.; Aikou, S.; Kodashima, S.; Yamashita, H.; Yamamichi, N.; Hirata, Y.; Fujishiro, M.; Seto, Y.; et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal tumor. Ann. Transl. Med. 2017, 5, 187. [Google Scholar] [CrossRef] [Green Version]
- Nunobe, S.; Hiki, N.; Gotoda, T.; Murao, T.; Haruma, K.; Matsumoto, H.; Hirai, T.; Tanimura, S.; Sano, T.; Yamaguchi, T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer 2012, 15, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ikeda, H.; Hosoya, T.; Yoshida, A.; Onimaru, M.; Suzuki, M.; Kudo, S.E. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: Full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg. Oncol. Clin. N. Am. 2012, 21, 129–140. [Google Scholar] [CrossRef]
- Kim, C.G.; Yoon, H.M.; Lee, J.Y.; Cho, S.J.; Kook, M.C.; Eom, B.W.; Ryu, K.W.; Kim, Y.W.; Choi, I.J. Nonexposure endolaparoscopic full-thickness resection with simple suturing technique. Endoscopy 2015, 47, 1171–1174. [Google Scholar] [CrossRef]
- Cao, L.; Zheng, K.; Wang, H.; Zhao, Y.; Yang, Z.; Li, W. Laparoscopic and endoscopic cooperative dissection for small gastric gastrointestinal stromal tumor without causing injury to the mucosa. Gastroenterol. Res. Pract. 2019, 2019, 7376903. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, S.; Nishizaki, M.; Kuroda, S.; Tanabe, S.; Noma, K.; Kagawa, S.; Shirakawa, Y.; Kato, H.; Okada, H.; Fujiwara, T. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer 2017, 20, 553–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, T.; Nunobe, S.; Kosuga, T.; Kawahira, H.; Inaki, N.; Kitashiro, S.; Abe, N.; Miyashiro, I.; Nagao, S.; Nishizaki, M.; et al. Laparoscopic and luminal endoscopic cooperative surgery can be a standard treatment for submucosal tumors of the stomach: A retrospective multicenter study. Endoscopy 2017, 49, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Andolfi, E.; Fontani, A.; Calise, F.; Rocca, A.; Giuliani, A. Robot-assisted liver surgery in a general surgery unit with a ”Referral Centre Hub&Spoke Learning Program”. Early outcomes after our first 70 consecutive patients. Minerva Chirurgica 2018, 73, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, P.C.; Coratti, A.; Angelini, M.; Sbrana, F.; Cecconi, S.; Balestracci, T.; Caravaglios, G. Robotics in general surgery: Personal experience in a large community hospital. Arch. Surg. 2003, 138, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, M.; Sugimachi, K. Robot-assisted gastric surgery. Surg. Clin. N. Am. 2003, 83, 1429–1444. [Google Scholar] [CrossRef]
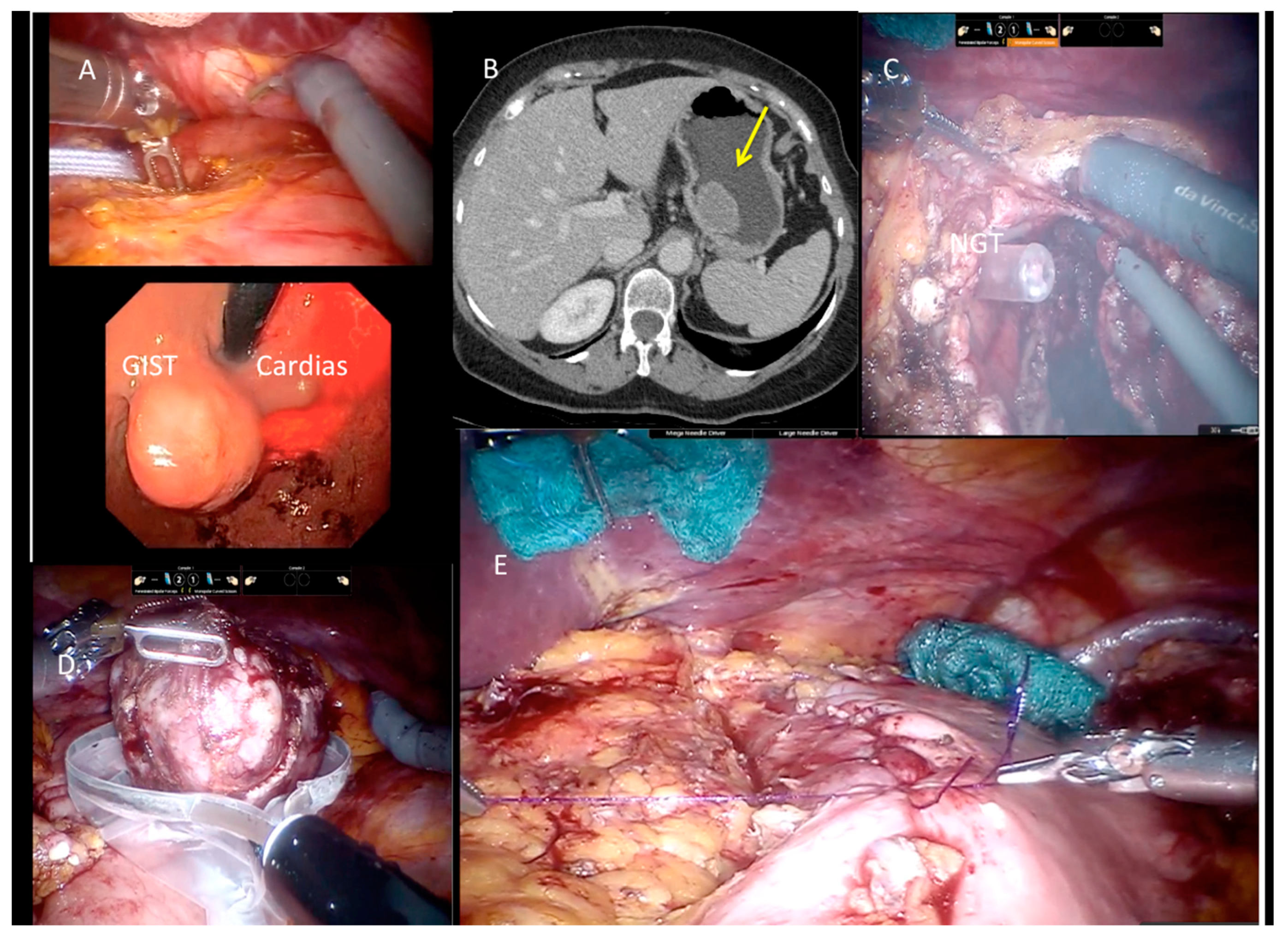
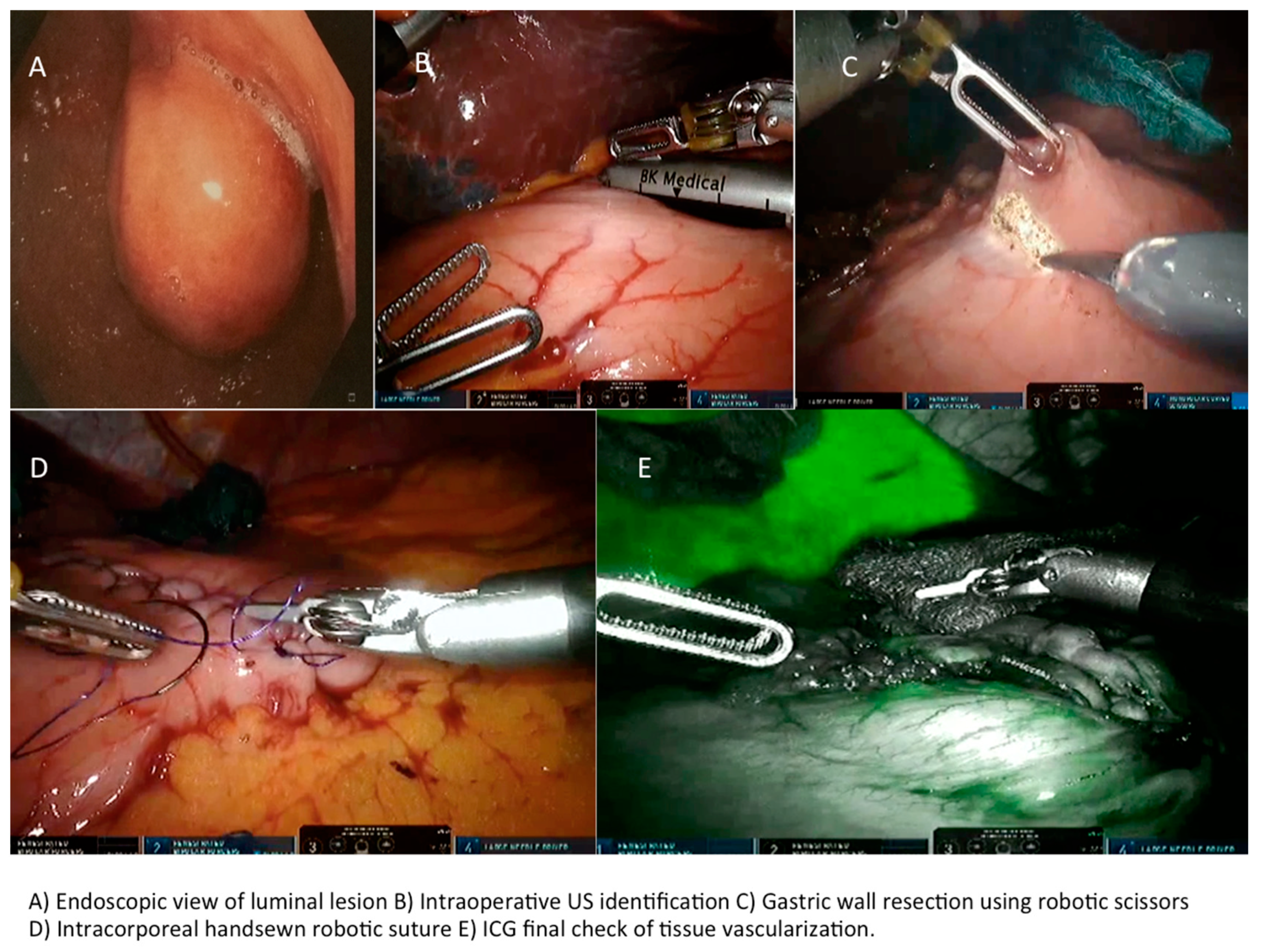
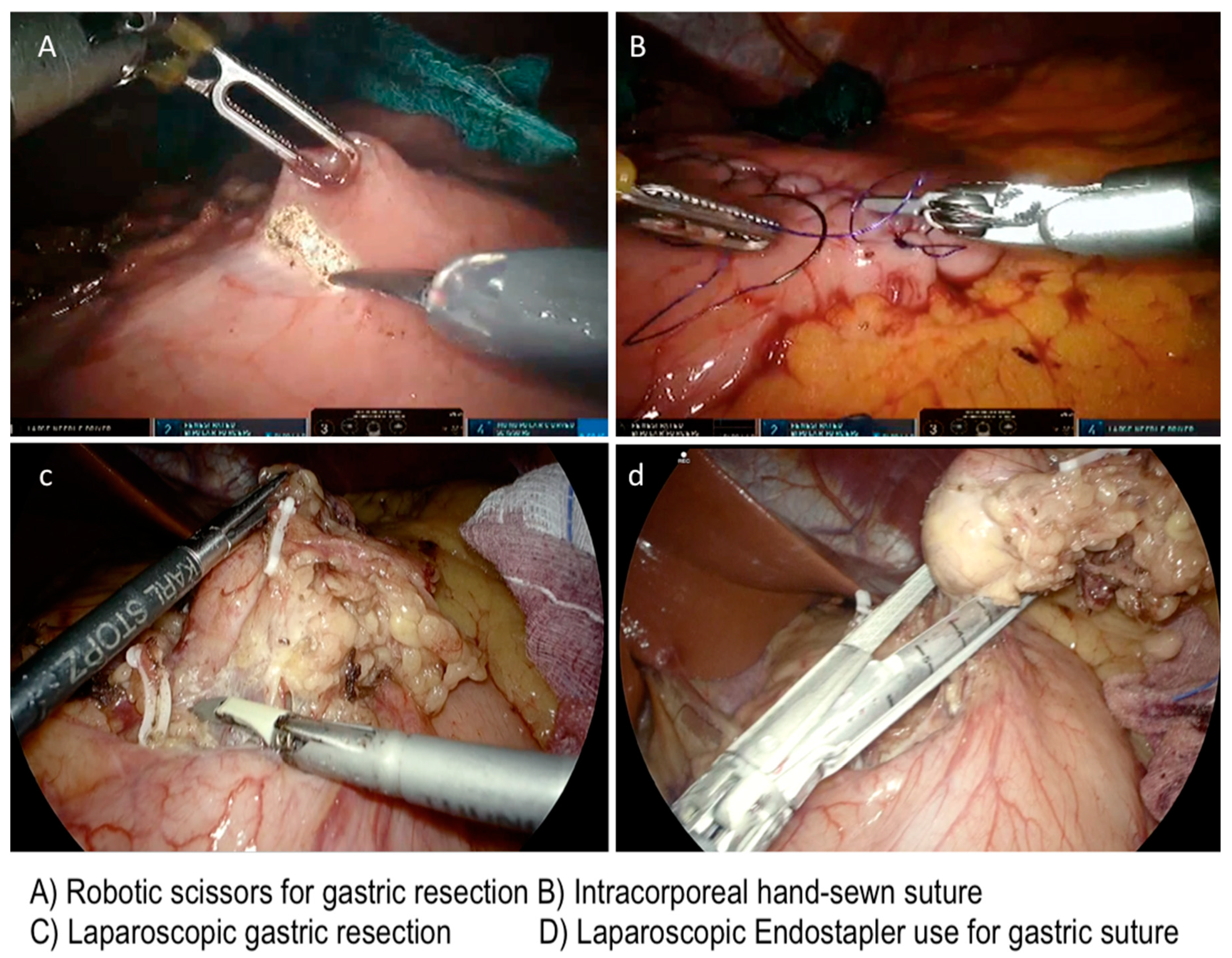



| Variable | Tot | Robotic | Laparoscopy | p-Value |
|---|---|---|---|---|
| 45 (55.5) | 36 (44.5) | |||
| Mitotic rate (50 HPF) >5, n (%) | 5 (11.1) | 3 (8.3) | 0.7271 | |
| Intraoperative tumor rupture, n (%) | 45 (100) | 35 (97.2) | NA | |
| Free margins/R0 resections, (%) | (100) | (100) | 1.0000 | |
| Immunohistochemistry (pos) | ||||
| CD117, n (%) | 44 (97.7) | 36 (100) | 1.0000 | |
| CD34, n (%) | 45 (100) | 36 (100) | 1.0000 | |
| DOG-1, n (%) | 40 (88.8) | 33 (91.6) | 0.7271 | |
| S-100, n (%) | 3 (6.6) | 2 (5.5) | 1.0000 | |
| Fletcher classification, n (%) | ||||
| Very low/Low risk | 68 (83.9) | 36 (80) | 32 (88.8) | 0.3669 |
| Intermediate risk | 8 (9.9) | 6 (13.4) | 2 (5.6) | 0.2896 |
| High risk | 5 (6.2) | 3 (6.6) | 2 (5.6) | 1.0000 |
| Characteristics | Total | Robotic | Laparoscopy | p-Value |
|---|---|---|---|---|
| Total (n%) | 81 (100) | 45 (55.5) | 36 (44.5) | |
| Mean age, (range) | 66.6 (35–87) | 68.3 (38–87) | 64.3 (35–84) | 0.1767 |
| Age > 80 years n (%) | 12 (14.8) | 9/45 (20) | 3/36 (8.3) | 0.7467 |
| Sex, male n (%) | 35 (43.2) | 19 (42.2) | 16 (44.4) | 0.9800 |
| Body mass index (kg/m2) ≥30, n (%) | 9 (11,1) | 6 (13.3) | 3 (8.3) | 0.3070 |
| ASA classification 3–4, n (%) | 10 (12,3) | 6 (13.3) | 4 (11.1) | 0.5141 |
| Main clinical manifestations, n (%) ° | ||||
| GI bleeding/anemia | 23 (28.4) | 14 (31.1) | 9 (25) | 0.7202 |
| Abdominal pain/discomfort | 32 (39.5) | 21 (46.6) | 11 (30.5) | 0.2131 |
| Radiological/diagnostic exams, n (%) ° | ||||
| Endoscopy with biopsy | 81 (100) | 45 | 36 | 1.0000 |
| CT scan (with contrast enhanced) | 79 (97.5) | 44/45 (97.7) | 35/36 (97.2) | 1.0000 |
| Abdominal ultrasonography US | 81 (100) | 45/45 (100) | 36/36 (100) | 1.0000 |
| US-Endoscopy with biopsy | 19 (23.4) | 12/45 (26.6) | 7/36 (19.4) | 0.5792 |
| MRI | 7 (8.6) | 4/45 (8.8) | 3/36 (8.3) | 1.0000 |
| Comorbidities presence, n (%) | 33 (40.75) | 20 (44.4) | 13 (36.1) | 0.5955 |
| Tumor size * (cm) mean (range) | 4.4 (1.5–12) | 5.1 (1.5–12) | 3,7 (1.5–10) | 0.0078 |
| size 1–5 cm, n | 63 (77.8) | 30 (66.7) | 33 (91.7) | |
| size ≥ 5 cm, n(%) | 18 (22.2) | 15 (33.3) | 3 (8.3) | |
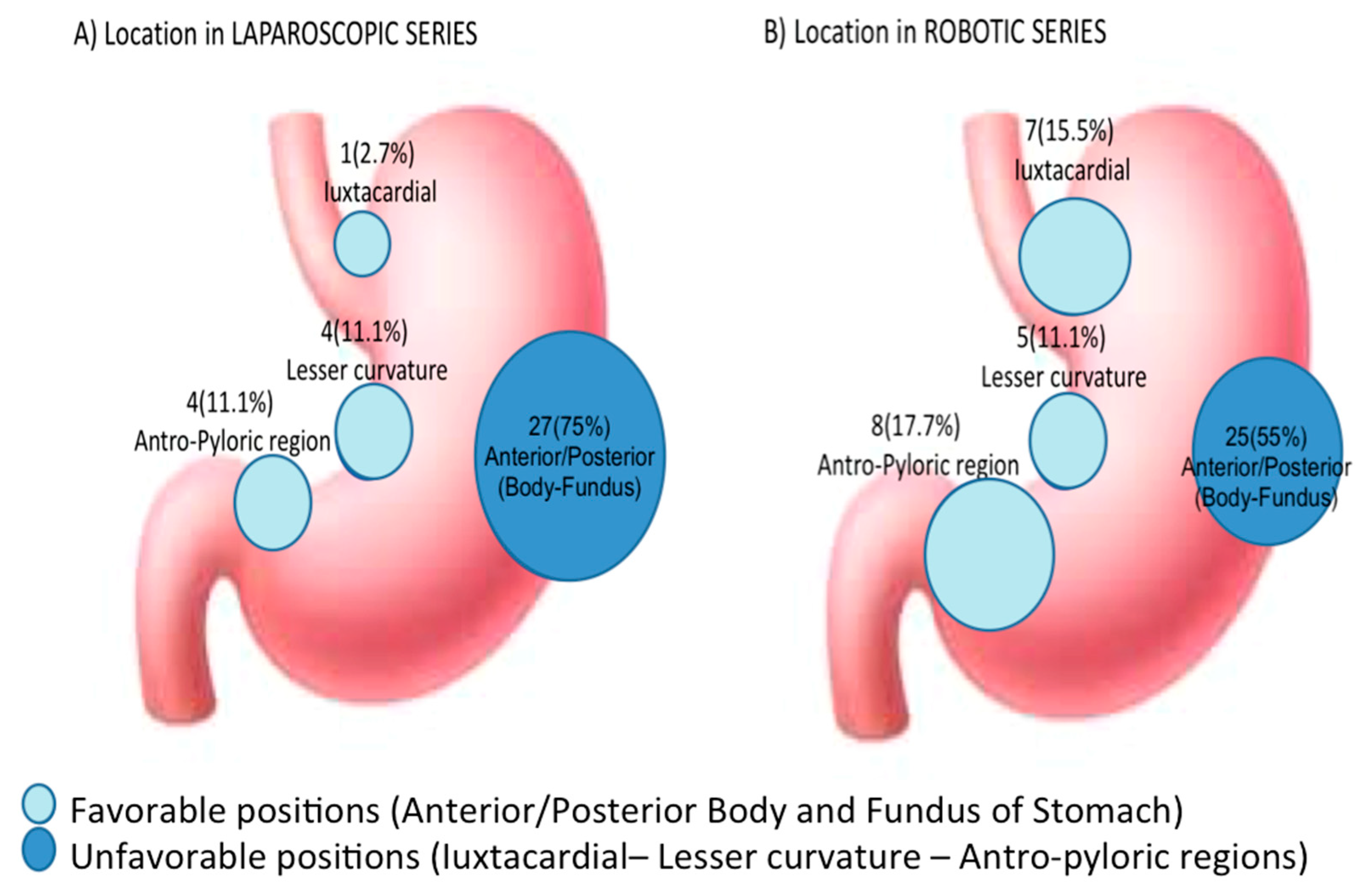
| Unfavorable gastric location, n (%) | 29 (35.8) | 20 (44.4) | 9 (25.0) | 0.1140 |
| Cardia/Juxtacardial | 8 | 7 (15.5) | 1 (2.7) | 0.0701 |
| Lesser curvature | 9 | 5 (11.1) | 4 (11.1) | 1.0000 |
| Antro-pyloric region | 12 | 8 (17.7) | 4 (11.1) | 0.5338 |
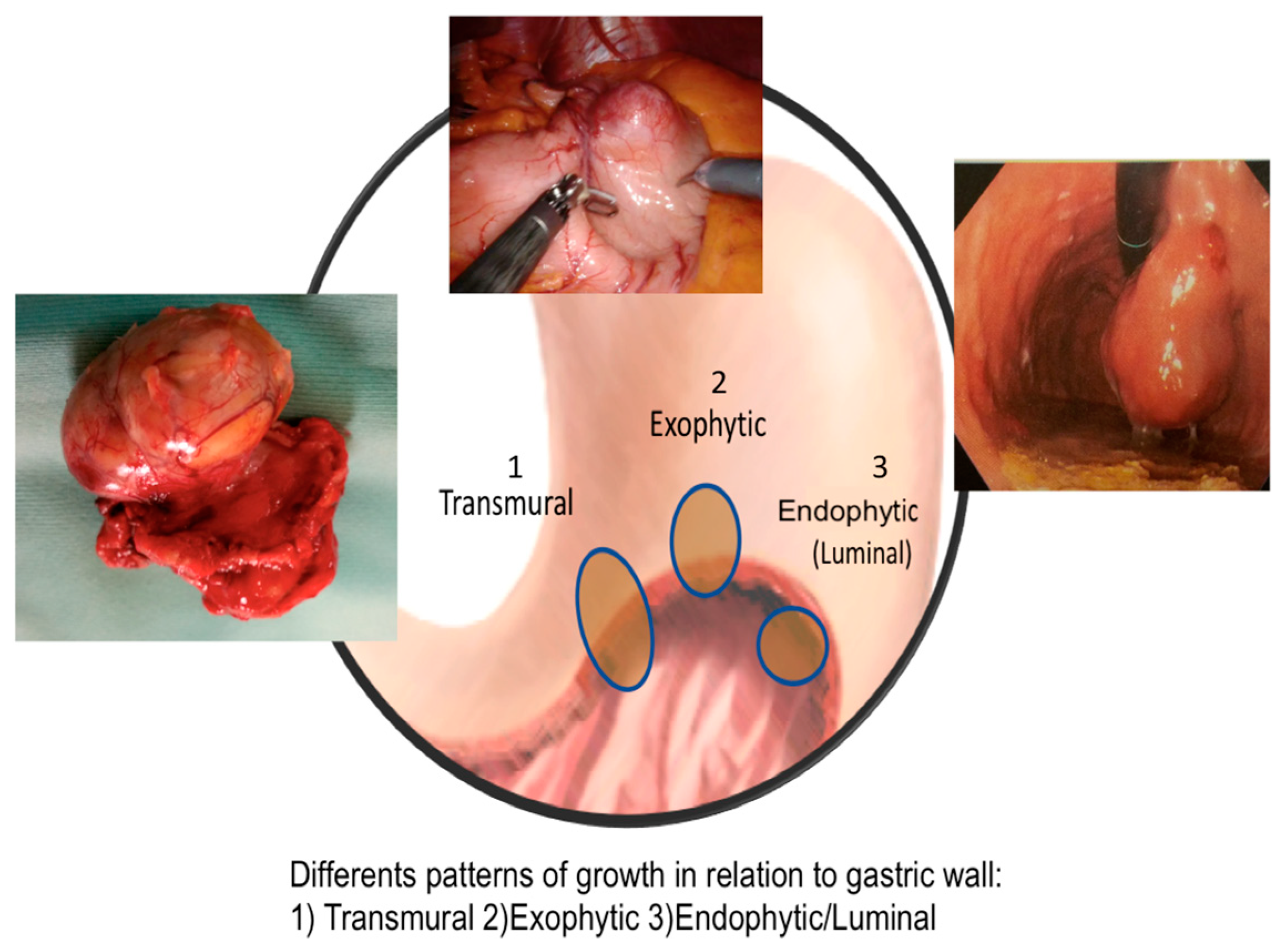
| Type of growth, n (%) | 0.8561 | |||
| Endophytic (luminal) | 29 (35.8) | 17 (37.7) | 12 (33.3) | |
| Exophytic and Transmural | 52 (64.2) | 28 (62.3) | 24 (66.7) | |
| Tot | Robotic | Laparoscopy | p-Value | |
|---|---|---|---|---|
| 81 | 45 (55.5%) | 36 (44.5%) | ||
| Type of gastric resection | 0.6511 | |||
| Wedge resections, n/tot (%) | 76 (93.8) | 43/45 (95.5) | 33/36 (91.6) | |
| Major resections (Gastrectomy), n/tot (%) | 2/45 (4.4) | 3/36 (8.3) | ||
| Conversion to open, n (%) | 5 (6.2) | 2 (4.4) | 3 (8.3) ° | 0.6511 |
| Associated abdominal surgery, n (%) | 23 (51.1) | 13 (36.1) | 0.2606 | |
| major (colon, liver, hiatal hernia, obesity) | 15 (18.5) | 10 (22.2) | 5 (13.8) | 0.3985 |
| minor (cholecystectomy, adhesions) | 21 (25.9) | 13 (28.8) | 8 (22.2) | |
| Operation time (min), median (range) °° | 151 (75–300) | 97 (35–185) | 0.0002 | |
| Effective time of resection/suture °°° | 65 (33–115) | 49 (25–110) | 0.0006 | |
| Stapler use for resection, n (%) | 2 (4.4) | 35 (97.2) | <0.0001 | |
| Intraoperative endoscopy, n (%) | 31 (38.3) | 17 (37.7) | 14 (38.9) | 0.8981 |
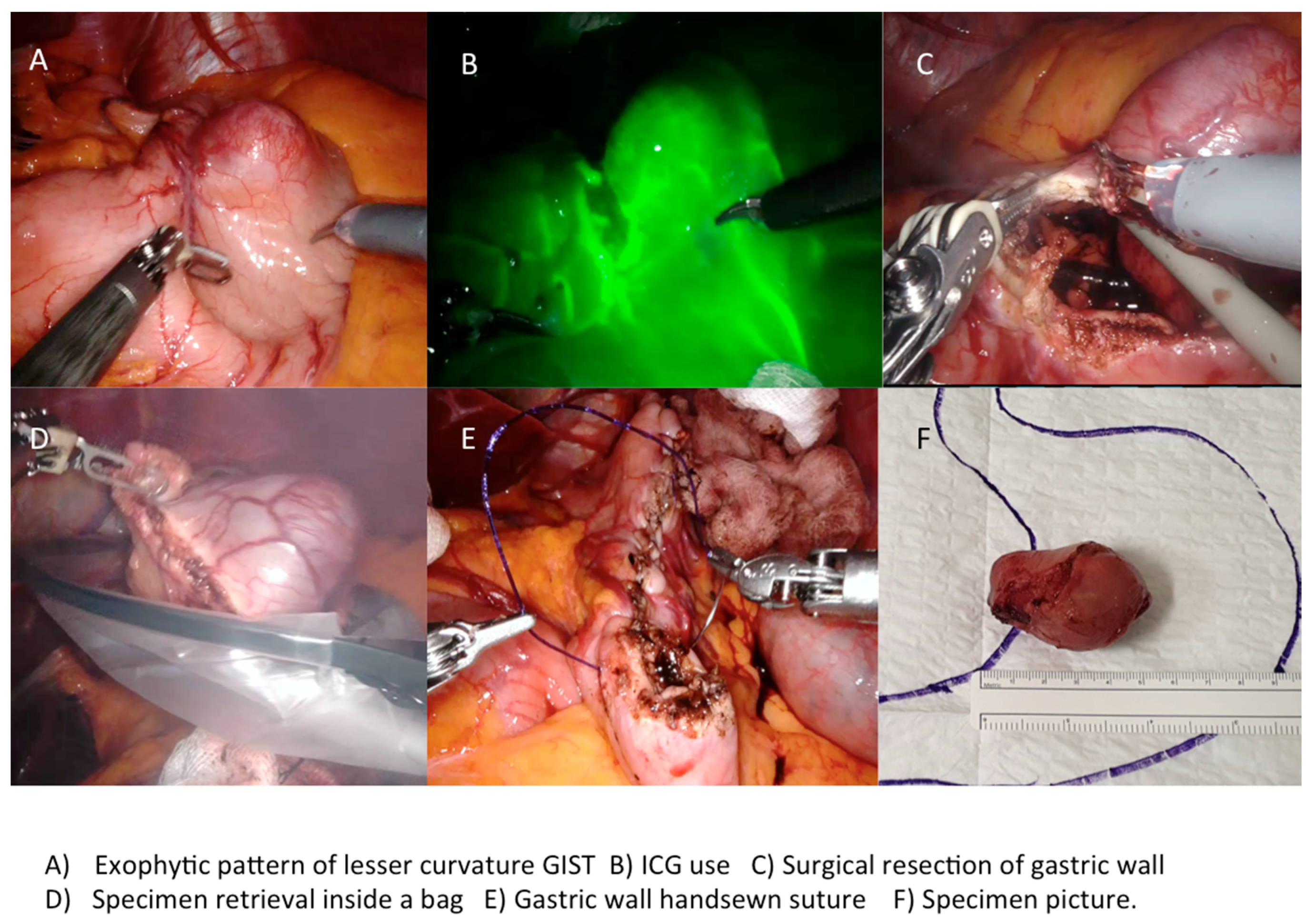
| Intraoperative ICG use, n (%) | 15 | 15 (33.3) | 0 | |
| Intraoperative US, n (%) | 5 (6.2) | 3 | 2 | 1.0000 |
| Estimated blood loss > 50 mL, n (%) | 7 (15.5) | 5 (13.8) | 1.0000 | |
| Perioperative transfusion request n° cases | 1 | 0 | NA | |
| Complications Clavien-Dindo 3–4/Reoperations | 0 | 0 | NA | |
| Time to return to bowel function (mean days) | 3.1 | 3.4 | 0.1543 | |
| Time to oral liquid intake (days) | 2.9 | 3.3 | 0.5484 | |
| Postop. hospital length of stay, median (range) | 6.7(4–12) | 6.3(3–9) | 0.3922 | |
| Post-operative follow-up, n.ro of patients (%) | 72 (88.9) | |||
| - Mean follow-up (months) | 47.4 | |||
| - Median follow-up (months) | 39.8 | |||
| Overall Disease-Free Survival (%) | ||||
| - 24 months | 95.1 | |||
| - 36 months | 92.9 | |||
| - 60 months | 90.6 | |||
| Author | Years | Type of Study | Period, year | N° Patients | Approach | Gender M;F | Age | BMI, kg/m2 | Tumor Size, cm | Fletcher Criteria | Resection Type | Tumor Location | Associated Procedures | Conversion, N (%) | Operative Time, min | R0 Resections | Blood Loss, mL | Length of Hospital Stay, days | Follow-Up Time, months | Clavien-Dindo >2 | Morbidity | Mortality | Costs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buchs N. et al. [32] | 2010 | Retrospective single center | 2006–2009 | 5 | Robot | 3; 2 | Mdn 39 (Ra 32–74) | NA | Mdn 5.5 | 1 L, 2 I, 2 H | 4 WR /1 TG | 2 cardia/ 3 distal antrum | No associated procedures | 1 (20) | Mdn 192 (Ra 132–285) | 5 | NA | Mdn 7 (Ra 5–10) | Mdn 18 (Ra 11–27) | 0 | 0 | 0 | NA |
| Desiderio J. et al. [20] | 2013 | Retrospective single center | 2011–2012 | 5 | Robot | 2; 3 | M 63.6 (Ra 43–76) | NA | M 5 (Ra 4–7) | 2 L, 3 I | 5 DG | 2 antrum/ 3 prepyloric | No associated procedures | 0 | Mdn 240 (Ra 210–300) | 5 | M 96 (Ra 80–120) | M 4.2 (Ra 3–5) | Mdn 13,5 (Ra 12–15) | 0 | 0 | 0 | NA |
| Vicente et al. [19] | 2015 | Retrospective single center | 2012–2014 | 6 (3) * | Robot | 0; 3 | M 55.7 (Ra 41–67) | NA | M 4 (Ra 3–5.5) | 2 L, 1 H | 1 WR/2 STG | 1 cardia/ 1 antrum/ 1 body | Intraoperative endoscopy | 0 | M 333.3 (Ra 230–540) | 3 | NA | M 7.7 (Ra 7–9) | M 16.7 (Ra 8–26) | 0 | 0 | 0 | NA |
| De Angelis et al. [33] | 2017 | Retrospective single center | 2012–2015 | 12 | Robot | 8; 4 | Mdn 62.5 (Ra 32–86) | Mdn 23.25 (Ra 21.6–28.4) | Mdn 7.25 (Ra 5.5–11.5) | 7 L, 3 I, 2 H | 11 WR/1 DG | 6 fundus (4 anterior, 2 posterior)/ 1 pylorus/ 2 posterior body/ 3 anterior body | 3 intraoperative laparoscopic ultrasound | 0 | 162.5 (Ra 140–220) | 12 | 42 (Ra 25–80) | 4 (Ra 3–7) | 16 (Ra 5–32) | 0 | 1 wound abscess | 1 | > 21.6% robotic |
| Al-Thani H. et al. [34] | 2016 | Retrospective single center | 2009–2010 | 4 | Robot | 3; 1 | M 45 (Ra 33–67) | NA | M 6 (Ra 3.5–10) | 3 L, 1 H | 4 WR | 4 posterior | 1 Intraoperative endoscopy | 0 | Mdn 360 | 4 | NA | M 8 (Ra 5–8) | Mdn 44 (Ra 0–73) | 0 | 0 | 0 | Cost unfavorable to robot assisted gastrectomy |
| Arseneaux M et al. [30] | 2018 | Retrospective single center | NA | 3 | Robot | 2; 1 | M 68.7 (Ra 59–78) | NA | M 4.8 (Ra 2.8–7) | NA | 3 WR | 1 cardia/ 1 antrum/ 1 posterior body | 3 Intraoperative endoscopy, 1 Nissen fundoplication, 1 gastric diverticulum dissection, 2 hiatal hernia repair | 0 | NA | 3 | NA | M 1.5 (Ra 1–2) | M 3.3 (Ra 1–5) | 0 | 1 acute blood loss anemia | 0 | NA |
| Zhao J. Et al [35] | 2018 | Retrospective single center | 2014–2016 | 11 | Robot | 5; 6 | M 59.5 (Ra 43–77) | M 22.1 (Ra 18.2–26.6) | M 5.3 (Ra 3–7.5) | 6 L, 4 I, 1 H | 11 WR | 11 cardia/subcardial | No associated procedures | 0 | M 82.7 (Ra 60–110) | 11 | M 30 (Ra 5–50) | M 3.3 (Ra 2–5) | M 25.5 (Ra 8–40) | 0 | 0 | 0 | NA |
| Maggioni C. et al. [25] | 2019 | Retrospective single center | NA | 6 (5) ** | Robot | 2; 3 | M 77,6 (Ra 58–87) | NA | M 6,3 (Ra 3.5–8.4) | 3 L, 2 H | 5 WR | 1 lesser curvature/ 1 fundus/ 2 posterior body/ 1 anterior body | No associated procedures | 0 | M 173 (SD ± 39) | 6 | NA | M 3 (SD ± 1) | M 12 | 0 | 0 | 0 | NA |
| Shi F. et al. [36] | 2019 | Retrospective single center | 2018–2019 | 20 | Robot | 7; 13 | M 54.5 (Ra 37–80) | M 22.3 (Ra 19.5–25.2) | M 3.3 (Ra 2.4–5.0) | 19 L, 1 I | 20 WR | 2 cardias/ 2 pyloric/ 5 anterior body/ 4 posterior body/ 1 lesser curvature/ 6 greater curvature | No associted procedures | 0 | Mdn 115 (Ra 90–160) | 20 | Mdn 20 (Ra 5–100) | Mdn 6 (Ra 4–10) | Mdn 10 (Ra 3–15) | 0 | 1 pneomonia | 0 | Mdn 7793.25 (Ra 7128.8–11880.7) hospitalization expensive |
| Furbetta N. et al. [37] | 2019 | Retrospective single center | 2010–2017 | 12 | Robot | 5; 7 | M 67.4 (SD ± 2.7) | M 24.9 (SD ± 7.1) | M 3.8 (SD ± 1.4) | 8 L, 3 I, 1 H | 12 WR | 4 greater curvature/fundus/ 1 cardia/ 2 antrum/ 3 lesser curvature/ 2 posterior body | 1 liver biopsy, 1 cholecystectomy | 0 | M 149 (SD ± 16.6) | 12 | NA | M 4.8 (SD ± 1.1) | M 38.5 (Ra 3–90) | 0 | 1 atrial fibrillation | 0 | NA |
| Solaini L. et al. [38] | 2019 | Retrospective multi center | 2009–2019 | 24 | Robot | 13; 11 | 4 ≥ 75, 20 <75 | 15 ( < 30), 3 ( ≥30), 6NA | Mdn 4.0 (Ra 1.8–7) | 19 L, 3 I, 2 NA | 24 WR | 2 cardias/ 4 fundus/ 2 greater curvature/ 9 lesser curvature/ 3 anterior body/ 4 posterior body | No associted procedures | 1 (4.2) | Mdn 180 (Ra 70–390) | 23 | ≤100 | Mdn 6 (Ra 2–12) | Mdn 24 (Ra 1–87) | 0 | 0 | 0 | higher cost |
| Winder A. et al. [39] | 2020 | Retrospective single center | 2017–2019 | 12 | Robot | 6;6 | Mdn 62 (Ra 43–79) | Mdn 27.5 (Ra 20.3–35.1) | M 4.6 (Ra 2,5–8.3) | 9 L, 2 I, 1 H | 12 WR | 2 anterior body/ 1 greater curvature/ 3 antrum/ 2 posterior body/ 2 fundus/ 1 lesser curvature/ 1 gastrosplenic lig. | 1 splenectomy | 1 (8.3) | M 192 (Ra 95–250) | 12 | <50 | M 2.7 (Ra 2–6) | NA | 0 | 0 | 0 | NA |
| Ceccarelli G. et al. | 2021 | Retrospective multi center | 2010–2020 | 45 | Robot | 19;26 | M 68.3 (Ra 38–87) | 6 ( >30) | M 5.1 (Ra 1.5–12) | 36 L, 6 I, 3 H | 43 WR, 2 MG | 7 cardias, 5 lesser curvature, 8 antro pyloric, 25 favorable gastric location | 17 intraoperative endoscopy, 15 intraoperative ICG, 3 intraoperative US, 10 major (colon, liver, hiatal hernia, obesity, etc.), 13 minor (cholecystectomy, adhesions) | 2 (4.4) | Mdn 151 (Ra 75–300) | 45 | 7 pz >50 | Mdn 6.7 (Ra 4–12) | Mdn 39.8 (Ra4.1–135.69) | 0 | 0 | 0 | higher cost |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, G.; Costa, G.; De Rosa, M.; Codacci Pisanelli, M.; Frezza, B.; De Prizio, M.; Bravi, I.; Scacchi, A.; Gallo, G.; Amato, B.; et al. Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review. Cancers 2021, 13, 4351. https://doi.org/10.3390/cancers13174351
Ceccarelli G, Costa G, De Rosa M, Codacci Pisanelli M, Frezza B, De Prizio M, Bravi I, Scacchi A, Gallo G, Amato B, et al. Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review. Cancers. 2021; 13(17):4351. https://doi.org/10.3390/cancers13174351
Chicago/Turabian StyleCeccarelli, Graziano, Gianluca Costa, Michele De Rosa, Massimo Codacci Pisanelli, Barbara Frezza, Marco De Prizio, Ilaria Bravi, Andrea Scacchi, Gaetano Gallo, Bruno Amato, and et al. 2021. "Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review" Cancers 13, no. 17: 4351. https://doi.org/10.3390/cancers13174351
APA StyleCeccarelli, G., Costa, G., De Rosa, M., Codacci Pisanelli, M., Frezza, B., De Prizio, M., Bravi, I., Scacchi, A., Gallo, G., Amato, B., Bugiantella, W., Tacchi, P., Bartoli, A., Patriti, A., Cappuccio, M., Komici, K., Mariani, L., Avella, P., & Rocca, A. (2021). Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review. Cancers, 13(17), 4351. https://doi.org/10.3390/cancers13174351








