Involvement of HIF-1α in the Detection, Signaling, and Repair of DNA Double-Strand Breaks after Photon and Carbon-Ion Irradiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Hypoxic Conditions
2.3. Irradiations
2.4. Transient Transfections
2.5. Immunofluorescence
2.6. Microscopy
2.7. Protein Studies by Western-Blot
2.8. Statistical Analysis
3. Results
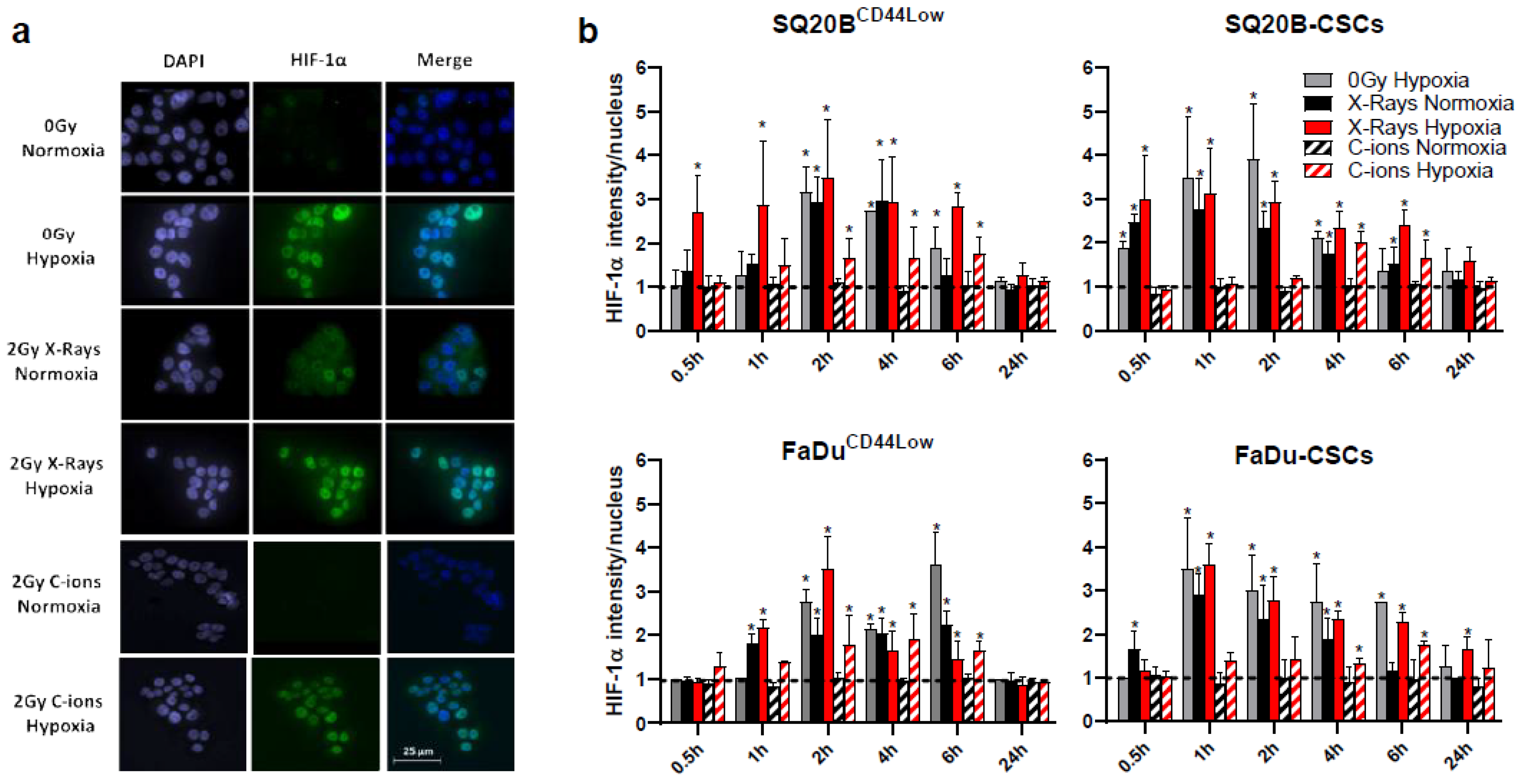
3.1. HIF-1α Nucleoshuttling in Response to X-ray and C-Ion Irradiation
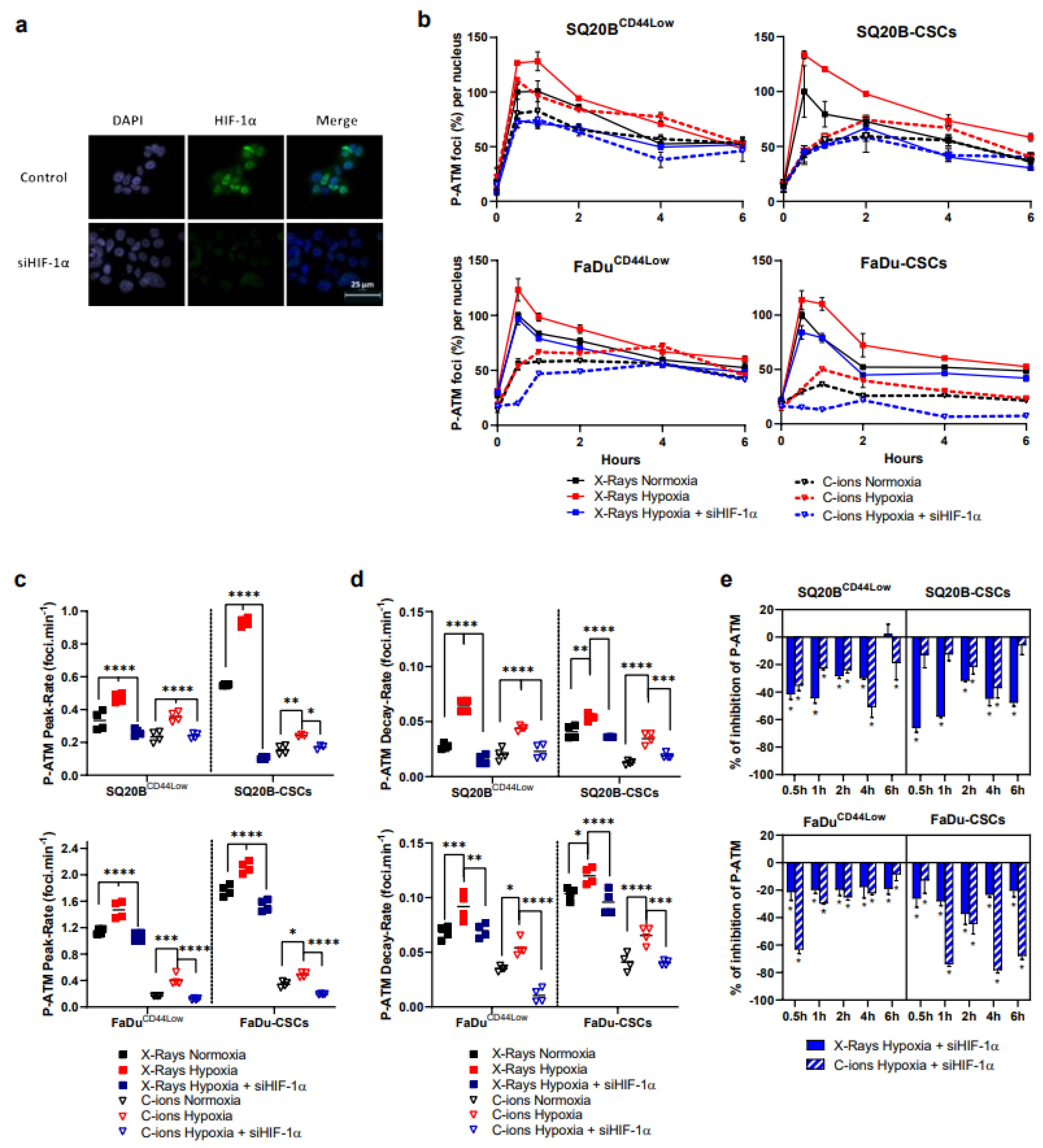
3.2. Silencing HIF-1α under Hypoxia Decreases the Initiation of the Signaling of DSBs after Photon or C-Ion Exposure
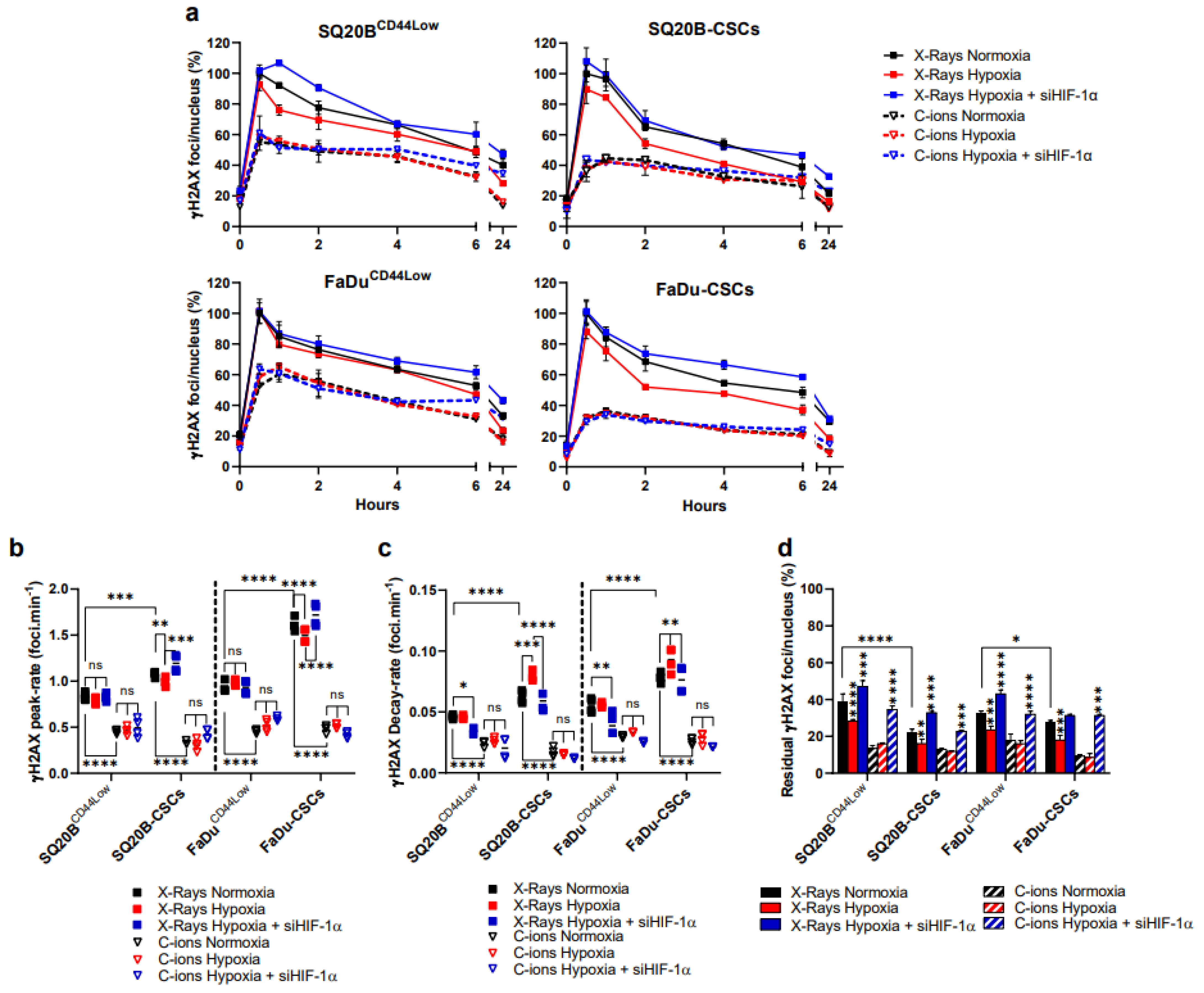
3.3. Silencing HIF-1α Modulates the Detection of DSBs in Response to Both Exposure, Particularly by Increasing Residual γH2AX Foci
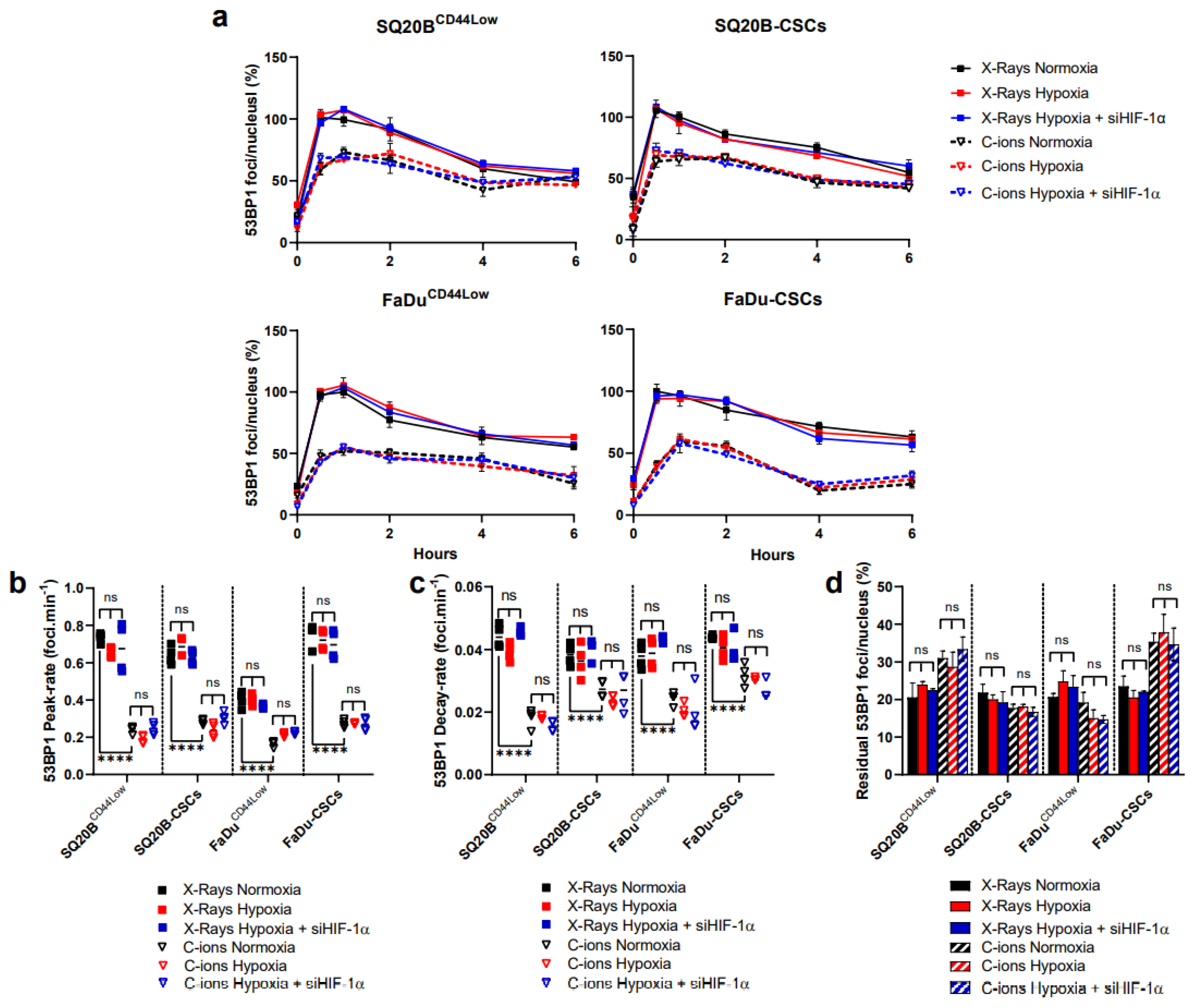
3.4. 53BP1 Is Not Significantly Modulated by HIF-1α Expression
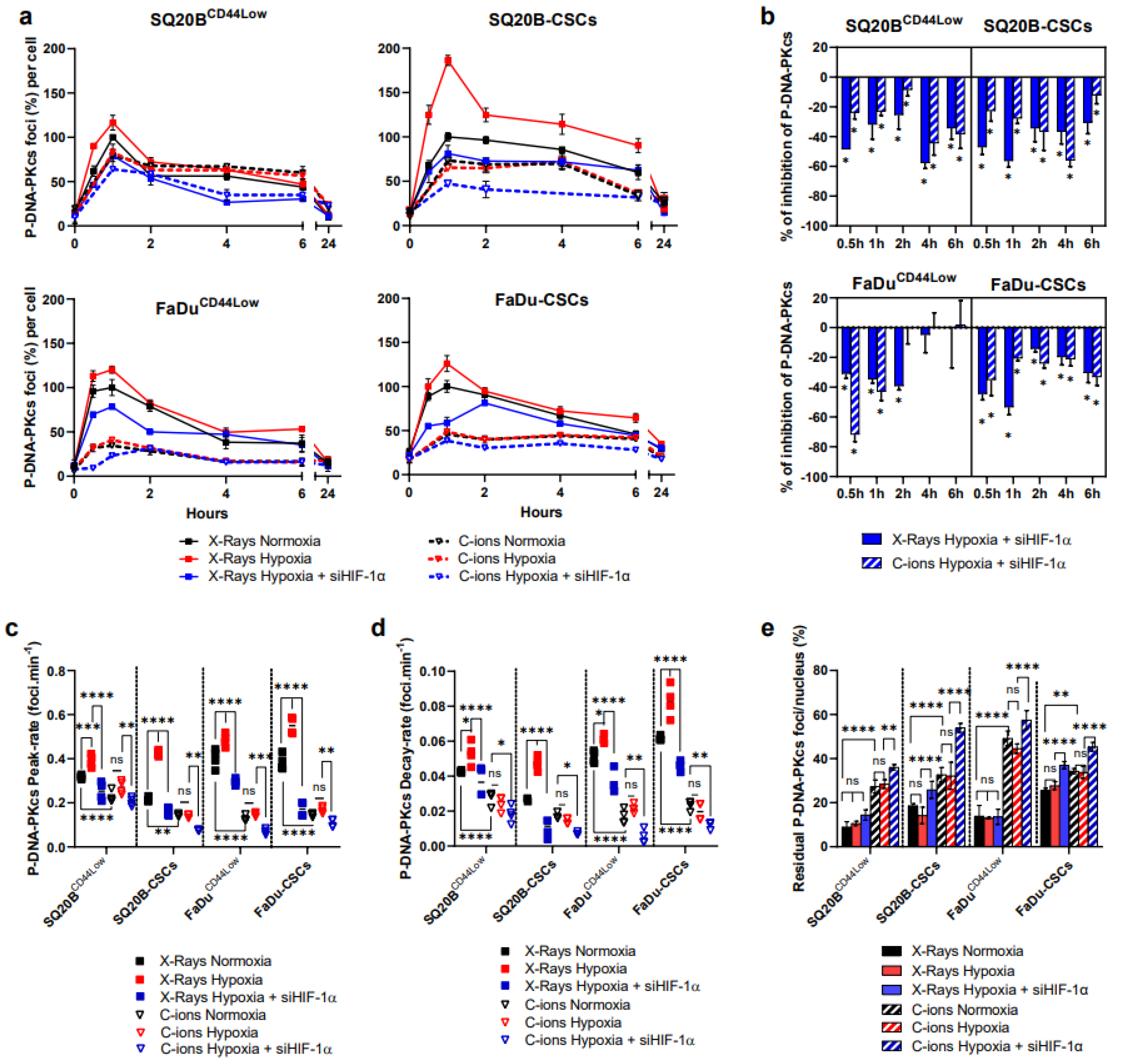
3.5. Silencing HIF-1α Decreases the NHEJ-c Pathway through DNA-PKcs in Response to Photons and C-Ions
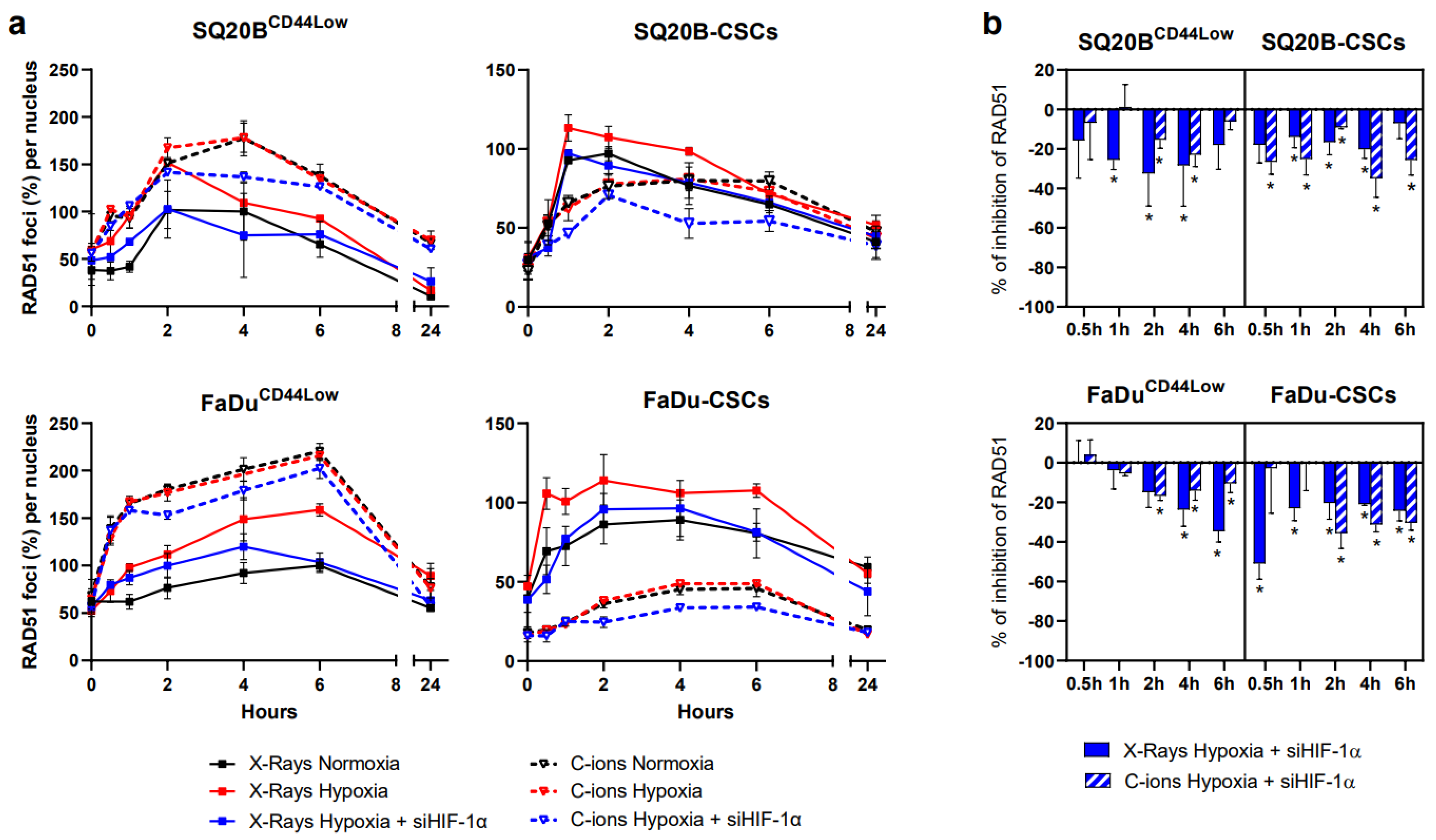
3.6. Silencing HIF-1α Decreases HR in Response to Photon and C-Ion Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bristow, R.G.; Hill, R.P. Hypoxia and Metabolism: Hypoxia, DNA Repair and Genetic Instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.-Y.; Tinganelli, W.; Maier, A.; Durante, M.; Kraft-Weyrather, W. Influence of Chronic Hypoxia and Radiation Quality on Cell Survival. J. Radiat. Res. 2013, 54 (Suppl. S1), i13–i22. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the Extracellular Matrix: Drivers of Tumour Metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hompland, T.; Fjeldbo, C.S.; Lyng, H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers 2021, 13, 499. [Google Scholar] [CrossRef]
- Chan, D.A.; Krieg, A.J.; Turcotte, S.; Giaccia, A.J. HIF Gene Expression in Cancer Therapy. Meth. Enzymol. 2007, 435, 323–345. [Google Scholar] [CrossRef]
- Semenza, G.L. Defining the Role of Hypoxia-Inducible Factor 1 in Cancer Biology and Therapeutics. Oncogene 2010, 29, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Rohwer, N.; Zasada, C.; Kempa, S.; Cramer, T. The Growing Complexity of HIF-1α’s Role in Tumorigenesis: DNA Repair and Beyond. Oncogene 2013, 32, 3569–3576. [Google Scholar] [CrossRef] [Green Version]
- LaGory, E.L.; Giaccia, A.J. The Ever Expanding Role of HIF in Tumour and Stromal Biology. Nat. Cell Biol. 2016, 18, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Semenza, G.L. Purification and Characterization of Hypoxia-Inducible Factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh, F.; Okoye, I.; Esmaily, M.; Ghasemi Chaleshtari, M.; Masjedi, A.; Azizi, G.; Irandoust, M.; Ghalamfarsa, G.; Jadidi-Niaragh, F. Hypoxia Inducible Factors in the Tumor Microenvironment as Therapeutic Targets of Cancer Stem Cells. Life Sci. 2019, 237, 116952. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen Sensing, Homeostasis, and Disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greijer, A.E.; van der Groep, P.; Kemming, D.; Shvarts, A.; Semenza, G.L.; Meijer, G.A.; van de Wiel, M.A.; Belien, J.A.M.; van Diest, P.J.; van der Wall, E. Up-Regulation of Gene Expression by Hypoxia Is Mediated Predominantly by Hypoxia-Inducible Factor 1 (HIF-1). J. Pathol. 2005, 206, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Huang, T.; Hou, F.; Yao, L.; Wang, X.; Wu, X. The Prognostic Value of Hypoxia-Inducible Factor-1α in Advanced Cancer Survivors: A Meta-Analysis with Trial Sequential Analysis. Ther. Adv. Med. Oncol. 2019, 11, 1758835919875851. [Google Scholar] [CrossRef] [PubMed]
- Wozny, A.-S.; Lauret, A.; Battiston-Montagne, P.; Guy, J.-B.; Beuve, M.; Cunha, M.; Saintigny, Y.; Blond, E.; Magne, N.; Lalle, P.; et al. Differential Pattern of HIF-1α Expression in HNSCC Cancer Stem Cells after Carbon Ion or Photon Irradiation: One Molecular Explanation of the Oxygen Effect. Br. J. Cancer 2017, 116, 1340–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, G.; Maalouf, M.; Boivin, A.; Battiston-Montagne, P.; Beuve, M.; Levy, A.; Jalade, P.; Fournier, C.; Ardail, D.; Magné, N.; et al. Targeting Head and Neck Cancer Stem Cells to Overcome Resistance to Photon and Carbon Ion Radiation. Stem Cell Rev. 2014, 10, 114–126. [Google Scholar] [CrossRef]
- Subtil, F.S.B.; Wilhelm, J.; Bill, V.; Westholt, N.; Rudolph, S.; Fischer, J.; Scheel, S.; Seay, U.; Fournier, C.; Taucher-Scholz, G.; et al. Carbon Ion Radiotherapy of Human Lung Cancer Attenuates HIF-1 Signaling and Acts with Considerably Enhanced Therapeutic Efficiency. FASEB J. 2014, 28, 1412–1421. [Google Scholar] [CrossRef]
- Moncharmont, C.; Guy, J.-B.; Wozny, A.-S.; Gilormini, M.; Battiston-Montagne, P.; Ardail, D.; Beuve, M.; Alphonse, G.; Simoëns, X.; Rancoule, C.; et al. Carbon Ion Irradiation Withstands Cancer Stem Cells’ Migration/Invasion Process in Head and Neck Squamous Cell Carcinoma (HNSCC). Oncotarget 2016, 7, 47738–47739. [Google Scholar] [CrossRef] [Green Version]
- Wozny, A.-S.; Vares, G.; Alphonse, G.; Lauret, A.; Monini, C.; Magné, N.; Cuerq, C.; Fujimori, A.; Monboisse, J.-C.; Beuve, M.; et al. ROS Production and Distribution: A New Paradigm to Explain the Differential Effects of X-ray and Carbon Ion Irradiation on Cancer Stem Cell Migration and Invasion. Cancers 2019, 11, 468. [Google Scholar] [CrossRef] [Green Version]
- Prise, K.M.; Folkard, M.; Newman, H.C.; Michael, B.D. Effect of Radiation Quality on Lesion Complexity in Cellular DNA. Int. J. Radiat. Biol. 1994, 66, 537–542. [Google Scholar] [CrossRef]
- Schipler, A.; Iliakis, G. DNA Double-Strand-Break Complexity Levels and Their Possible Contributions to the Probability for Error-Prone Processing and Repair Pathway Choice. Nucleic Acids Res. 2013, 41, 7589–7605. [Google Scholar] [CrossRef] [Green Version]
- Friedland, W.; Schmitt, E.; Kundrát, P.; Dingfelder, M.; Baiocco, G.; Barbieri, S.; Ottolenghi, A. Comprehensive Track-Structure Based Evaluation of DNA Damage by Light Ions from Radiotherapy-Relevant Energies down to Stopping. Sci. Rep. 2017, 7, 45161. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Z.; Pei, S.; Song, L.; Wang, C.; Ma, J.; Jin, L.; Ma, Y.; He, R.; Zhong, J.; et al. PATM and ΓH2AX Are Effective Radiation Biomarkers in Assessing the Radiosensitivity of 12C6+ in Human Tumor Cells. Cancer Cell Int. 2017, 17, 49. [Google Scholar] [CrossRef] [Green Version]
- Maalouf, M.; Granzotto, A.; Devic, C.; Bodgi, L.; Ferlazzo, M.; Peaucelle, C.; Bajard, M.; Giraud, J.-Y.; Balosso, J.; Hérault, J.; et al. Influence of Linear Energy Transfer on the Nucleo-Shuttling of the ATM Protein: A Novel Biological Interpretation Relevant for Particles and Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 709–718. [Google Scholar] [CrossRef]
- Nickoloff, J.A. Paths from DNA Damage and Signaling to Genome Rearrangements via Homologous Recombination. Mutat. Res. 2017, 806, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Daley, J.M.; Sung, P. 53BP1, BRCA1, and the Choice between Recombination and End Joining at DNA Double-Strand Breaks. Mol. Cell. Biol. 2014, 34, 1380–1388. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Bai, Y.; Zhao, M.; Zhou, M.; Shen, Q.; Yun, C.-H.; Zhang, H.; Zhu, W.-G.; Wang, J. Acetylation of 53BP1 Dictates the DNA Double Strand Break Repair Pathway. Nucleic Acids Res. 2018, 46, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Bindra, R.S.; Schaffer, P.J.; Meng, A.; Woo, J.; Måseide, K.; Roth, M.E.; Lizardi, P.; Hedley, D.W.; Bristow, R.G.; Glazer, P.M. Down-Regulation of Rad51 and Decreased Homologous Recombination in Hypoxic Cancer Cells. Mol. Cell. Biol. 2004, 24, 8504–8518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouquet, F.; Ousset, M.; Biard, D.; Fallone, F.; Dauvillier, S.; Frit, P.; Salles, B.; Muller, C. A DNA-Dependent Stress Response Involving DNA-PK Occurs in Hypoxic Cells and Contributes to Cellular Adaptation to Hypoxia. J. Cell. Sci. 2011, 124, 1943–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauth, F.; Toulany, M.; Zips, D.; Menegakis, A. Cell-Line Dependent Effects of Hypoxia Prior to Irradiation in Squamous Cell Carcinoma Lines. Clin. Transl. Radiat. Oncol. 2017, 5, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Murata, Y.; Urushihara, Y.; Shiga, S.; Takeda, K.; Hosoi, Y. Severe Hypoxia Increases Expression of ATM and DNA-PKcs and It Increases Their Activities through Src and AMPK Signaling Pathways. Biochem. Biophys. Res. Commun. 2018, 505, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, M.; Langer, H.F.; Celeste, A.; Orlova, V.V.; Choi, E.Y.; Ma, M.; Vassilopoulos, A.; Callen, E.; Deng, C.; Bassing, C.H.; et al. Histone H2AX Is Integral to Hypoxia-Driven Neovascularization. Nat. Med. 2009, 15, 553–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cam, H.; Easton, J.B.; High, A.; Houghton, P.J. MTORC1 Signaling under Hypoxic Conditions Is Controlled by ATM-Dependent Phosphorylation of HIF-1α. Mol. Cell 2010, 40, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Wrann, S.; Kaufmann, M.R.; Wirthner, R.; Stiehl, D.P.; Wenger, R.H. HIF Mediated and DNA Damage Independent Histone H2AX Phosphorylation in Chronic Hypoxia. Biol. Chem. 2013, 394, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Gilormini, M.; Wozny, A.-S.; Battiston-Montagne, P.; Ardail, D.; Alphonse, G.; Rodriguez-Lafrasse, C. Isolation and Characterization of a Head and Neck Squamous Cell Carcinoma Subpopulation Having Stem Cell Characteristics. J. Vis. Exp. JoVE 2016. [CrossRef] [PubMed] [Green Version]
- Matsufuji, N.; Kanai, T.; Kanematsu, N.; Miyamoto, T.; Baba, M.; Kamada, T.; Kato, H.; Yamada, S.; Mizoe, J.-E.; Tsujii, H. Specification of Carbon Ion Dose at the National Institute of Radiological Sciences (NIRS). J. Radiat. Res. 2007, 48 (Suppl. A), A81–A86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozny, A.-S.; Alphonse, G.; Cassard, A.; Malesis, C.; Louati, S.; Beuve, M.; Lalle, P.; Ardail, D.; Nakajima, T.; Rodriguez-Lafrasse, C. Impact of Hypoxia on the Double-Strand Break Repair after Photon and Carbon Ion Irradiation of Radioresistant HNSCC Cells. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Semenza, G.L. Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 379–403. [Google Scholar] [CrossRef]
- Li, M.; Xie, H.; Liu, Y.; Xia, C.; Cun, X.; Long, Y.; Chen, X.; Deng, M.; Guo, R.; Zhang, Z.; et al. Knockdown of Hypoxia-Inducible Factor-1 Alpha by Tumor Targeted Delivery of CRISPR/Cas9 System Suppressed the Metastasis of Pancreatic Cancer. J. Control. Release 2019, 304, 204–215. [Google Scholar] [CrossRef]
- Rezaeian, A.-H.; Li, C.-F.; Wu, C.-Y.; Zhang, X.; Delacerda, J.; You, M.J.; Han, F.; Cai, Z.; Jeong, Y.S.; Jin, G.; et al. A Hypoxia-Responsive TRAF6-ATM-H2AX Signalling Axis Promotes HIF1α Activation, Tumorigenesis and Metastasis. Nat. Cell Biol. 2017, 19, 38–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, S.M.; Coackley, C.; Bristow, R.G. ATM-Dependent Phosphorylation of 53BP1 in Response to Genomic Stress in Oxic and Hypoxic Cells. Radiother. Oncol. 2011, 99, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Asensio, I.; Dillon, E.T.; Lowndes, N.F.; Ceredig, R. The Transcription Factor Hif-1 Enhances the Radio-Resistance of Mouse MSCs. Front. Physiol. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, J.; Zhao, X.; Wang, X.; Zhao, Y.; Li, Y.; Zhao, R.; Cheng, K.; Li, Y.; Han, X.; Zheng, X.; et al. Targeted Co-Delivery of the Iron Chelator Deferoxamine and a HIF1α Inhibitor Impairs Pancreatic Tumor Growth. ACS Nano 2019, 13, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Afkham, A.; Aghebati-Maleki, L.; Siahmansouri, H.; Sadreddini, S.; Ahmadi, M.; Dolati, S.; Afkham, N.M.; Akbarzadeh, P.; Jadidi-Niaragh, F.; Younesi, V.; et al. Chitosan (CMD)-Mediated Co-Delivery of SN38 and Snail-Specific SiRNA as a Useful Anticancer Approach against Prostate Cancer. Pharm. Rep. 2018, 70, 418–425. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wozny, A.-S.; Gauthier, A.; Alphonse, G.; Malésys, C.; Varoclier, V.; Beuve, M.; Brichart-Vernos, D.; Magné, N.; Vial, N.; Ardail, D.; et al. Involvement of HIF-1α in the Detection, Signaling, and Repair of DNA Double-Strand Breaks after Photon and Carbon-Ion Irradiation. Cancers 2021, 13, 3833. https://doi.org/10.3390/cancers13153833
Wozny A-S, Gauthier A, Alphonse G, Malésys C, Varoclier V, Beuve M, Brichart-Vernos D, Magné N, Vial N, Ardail D, et al. Involvement of HIF-1α in the Detection, Signaling, and Repair of DNA Double-Strand Breaks after Photon and Carbon-Ion Irradiation. Cancers. 2021; 13(15):3833. https://doi.org/10.3390/cancers13153833
Chicago/Turabian StyleWozny, Anne-Sophie, Arnaud Gauthier, Gersende Alphonse, Céline Malésys, Virginie Varoclier, Michael Beuve, Delphine Brichart-Vernos, Nicolas Magné, Nicolas Vial, Dominique Ardail, and et al. 2021. "Involvement of HIF-1α in the Detection, Signaling, and Repair of DNA Double-Strand Breaks after Photon and Carbon-Ion Irradiation" Cancers 13, no. 15: 3833. https://doi.org/10.3390/cancers13153833
APA StyleWozny, A.-S., Gauthier, A., Alphonse, G., Malésys, C., Varoclier, V., Beuve, M., Brichart-Vernos, D., Magné, N., Vial, N., Ardail, D., Nakajima, T., & Rodriguez-Lafrasse, C. (2021). Involvement of HIF-1α in the Detection, Signaling, and Repair of DNA Double-Strand Breaks after Photon and Carbon-Ion Irradiation. Cancers, 13(15), 3833. https://doi.org/10.3390/cancers13153833





