Keratinocyte Carcinoma and Photoprevention: The Protective Actions of Repurposed Pharmaceuticals, Phytochemicals and Vitamins
Abstract
Simple Summary
Abstract
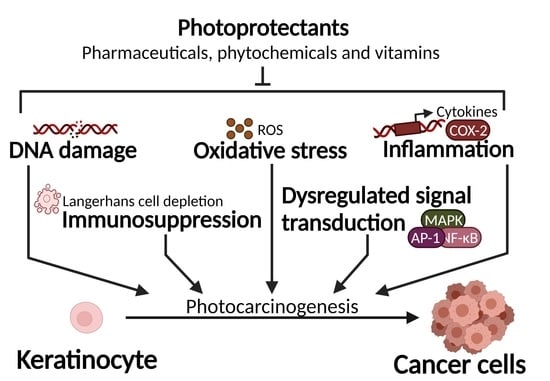
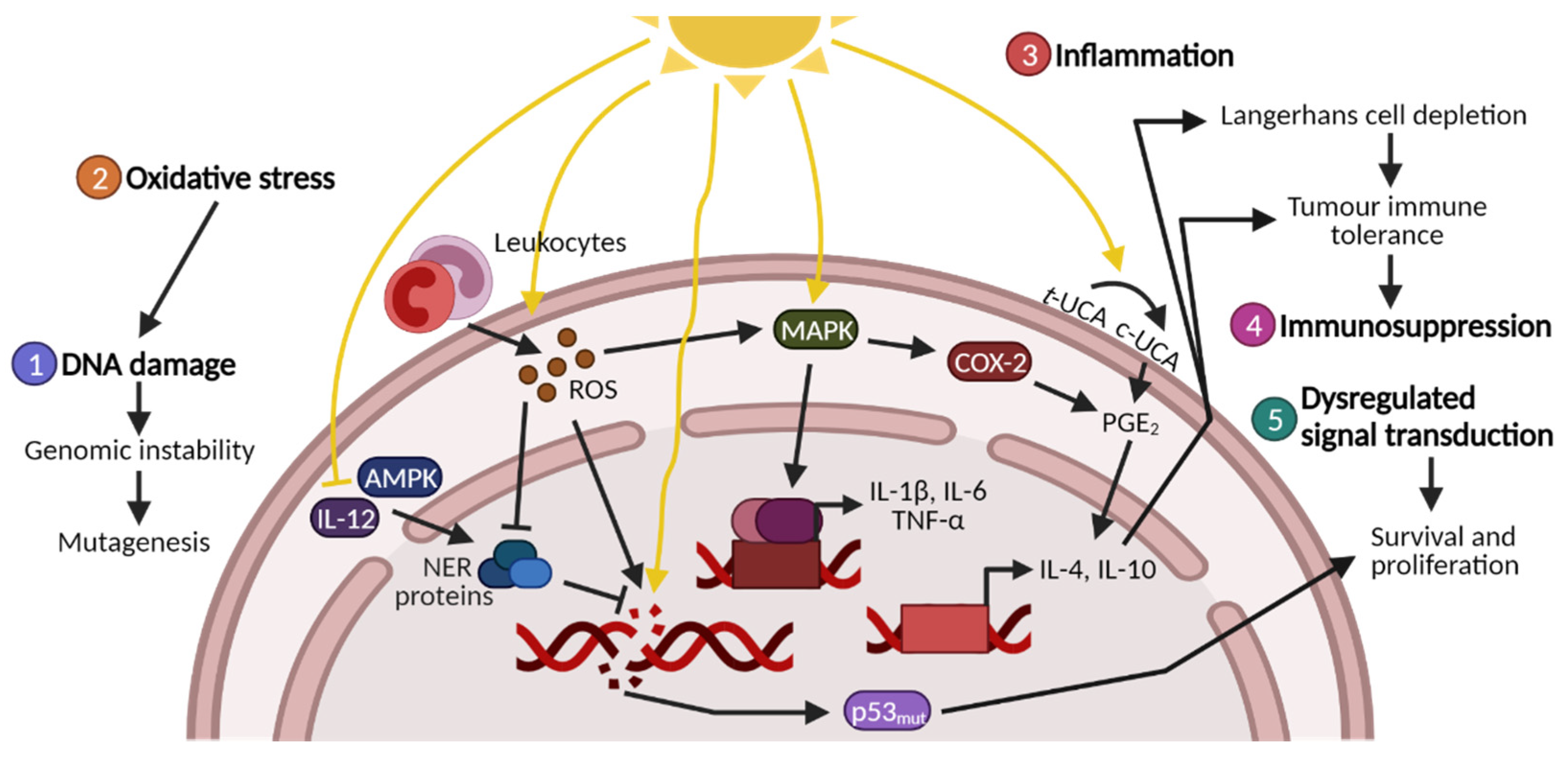
1. Introduction to Photocarcinogenesis
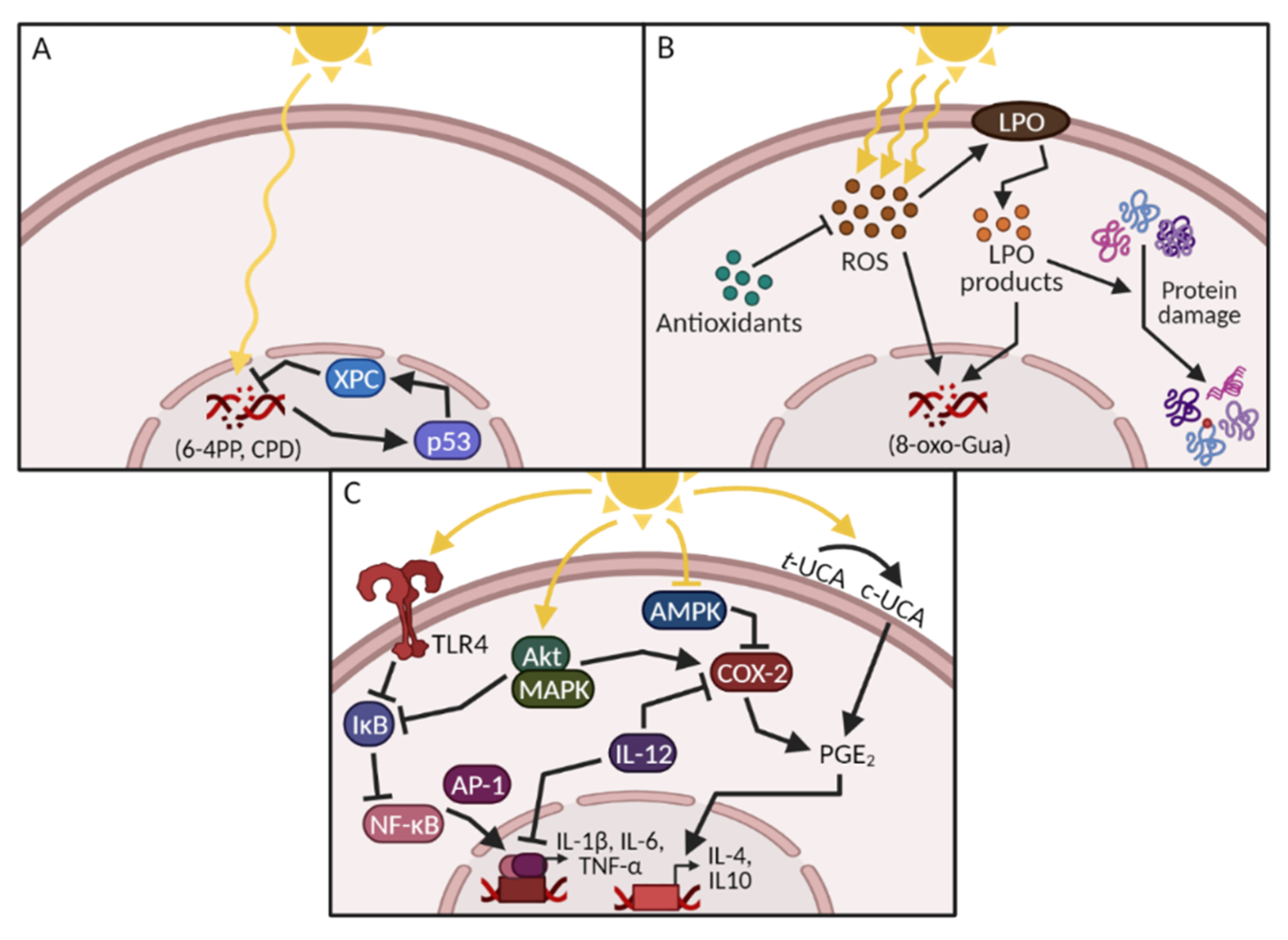
1.1. UV-Induced DNA Damage
1.2. UV-Induced Oxidative Stress and Protein Damage
| Reactive Species | Antioxidants | ||
|---|---|---|---|
| Hydrogen peroxide (H2O2) | Hydroperoxyl (HO2) | Catalase | Glutathione |
| Hydroxyl radical (OH) | Nitric oxide (NO) | Glutathione peroxidase | Superoxide dismutase |
| Singlet oxygen (1O2) | Superoxide (O–2) | Vitamin C | Vitamin E |
1.3. UV-Induced Inflammation, Immunosuppression and Signal Transduction
1.4. Photocarcinogenesis and Photoprotectants
2. Pharmaceuticals: From Repurposing to Prevention
2.1. Non-Steroidal Anti-Inflammatory Drugs
2.2. AMPK Activators: Metformin
2.3. Toll-Like Receptor 4 Antagonism: Resatorvid
2.4. Oestrogen Receptor Signalling: Erb041, 17β-Oestradiol and Phytoestrogens
2.5. Recent Discoveries in Pre-Clinical Studies: Carvedilol and Bucillamine
| Compound | Model | Mechanism of Action | (Pre-)Clinical Results | Ref |
|---|---|---|---|---|
| 17β-oestradiol | Mice | Activates ER signalling, and reduces immunosuppression | [83] | |
| Bucillamine | Hairless mice, keratinocytes | Reduces proliferation, cell cycle arrest and apoptosis while preventing leukocyte infiltration. | [95,96] | |
| Carvedilol | Hairless mice, epidermal cells, ex vivo skin | Induces DNA repair while reducing the inflammatory response via AP-1 and NF-κB inhibition. | Mice: Delays tumour onset and reduces tumour incidence and multiplicity. | [89,90,91] |
| Erb-041 | Hairless mice, keratinocytes | Activates the ERβ receptor which inhibits proliferation, angiogenesis and EMT in tumour tissue. Reduces signal transduction and the inflammatory response. | Mice: Delays tumour onset, reduces tumour incidence, volume and multiplicity and prevents SCC progression. | [82] |
| Metformin | Hairless mice, Xenografted (A431) mice, keratinocytes | Activates AMPK signalling which facilitates DNA repair, reduces the inflammatory response and induces tumour cell apoptosis. | Mice: Delays tumour onset and reduces tumour incidence and multiplicity. | [41,60,61] |
| NSAIDs | AK-affected individuals, hairless mice | Prevents DNA damage, reduces COX-2 induction and the inflammatory response via AP-1 and NF-κB inhibition. | Human: Reduces keratinocyte carcinomas (SCCs and BCCs) and promotes AK regression.Mice: Delays tumour onset and reduces tumour incidence, progression and multiplicity. | [47,48,49] |
| Resatorvid | Hairless mice, keratinocytes | Inhibits TLR4 signalling which reduces MAPK, AP-1 and NF-κB signalling and the inflammatory response. | Mice: Delays tumour onset and reduces tumour incidence and multiplicity. | [72,76] |
3. Dietary and Non-Dietary Phytochemicals
3.1. Green Tea and Polyphenols
3.2. Grapes and Related Polyphenols: Proanthocyanidins, Resveratrol and Pterostilbene
3.3. Polypodium Leucotomos
3.4. Berries: Pomegranate, Raspberries and Blackberries
3.5. Cocoa Flavanols
| Compound | Model | Mechanism of Action | (Pre-)Clinical Results | Ref |
|---|---|---|---|---|
| Blackberries | Hairless mice, keratinocytes, ex vivo skin | Reduces oxidative stress and the inflammatory response. | [146,147] | |
| Cocoa flavanols | Healthy volunteers, hairless mice | Reduces mutagenesis of p53, inflammatory markers and degradation of the extracellular matrix. | Mice: Reduces invasive SCCs. | [141,150,151,152,153,154] |
| Grape seeds | Healthy volunteers, hairless mice, keratinocytes | Increases antioxidant activity, reduces the inflammatory response and proliferation and promotes DNA repair. | Mice: Reduces tumour incidence, size, multiplicity and progression. | [114,115,116,117,118,119,120] |
| Green tea | Healthy volunteers, hairless mice, keratinocytes | Prevents DNA hypomethylation, stimulates IL-12 (facilitating DNA repair) and reduces the inflammatory response and oxidative stress. | Mice: Delays tumour onset and reduces tumour incidence, multiplicity and progression. | [99,100,101,102,103,106] |
| Polypodium leucotomos | Healthy volunteers, hairless mice, keratinocytes, fibroblasts | Prevents photoisomerisation of trans-UCA (counteracting immunosuppression) and increases antioxidant activity. Reduces DNA lesions, proliferation and the inflammatory response. | Mice: Reduces AK occurrence, delays tumour onset and reduces tumour incidence. | [124,125,126,127,128,129,130,131,132,133,134,135,136] |
| Pomegranate | Healthy volunteers, hairless mice | Reduces DNA lesions, oxidative damage, the inflammatory response and signal transduction. | Mice: Reduces SCC occurrence. | [139,140,141,142] |
| Pterostilbene | Hairless mice, keratinocytes | Reduces oxidative stress via increased antioxidant capacity. | Mice: Reduces tumour incidence and multiplicity. | [122] |
| Raspberries | Hairless mice, fibroblasts | Reduces oxidative DNA damage and stress via increased antioxidant activity. Reduces the inflammatory response and AP-1 and NF-κB activity. | Mice: Reduces tumour size and multiplicity. | [143,144,145] |
4. Vitamins and Derived Compounds
4.1. Vitamin A: The Retinoids
4.2. Vitamin B3: Nicotinamide
4.3. Vitamin C
4.4. Vitamin D3
4.5. Vitamin E: α-Tocopherol
| Compound | Model | Mechanism of Action | (Pre-)Clinical Results | Ref |
|---|---|---|---|---|
| Vitamin A: Retinoids | AK- and KC-affected patients, healthy volunteers, hairless mice, fibroblasts | Reduces DNA damage and the inflammatory response via AP-1 and NF-κB inhibition. Reduces oxidative stress by inducing Nrf2. | Human: Reduces SCC risk *. Mice: Delays tumour onset and reduces tumour incidence **. | [160,161,162,163,164,167,169,172,174,177,178] |
| Vitamin B3: Nicotinamide | AK-affected and high-risk patients, hairless mice, keratinocytes | Induces DNA repair by acting as an NAD+ precursor. Reduces immunosuppression and the inflammatory response. | Human: Reduces AK occurrence and rate of new KCs and SCCs Mice: Reduces tumour incidence and multiplicity. | [186,187,188,189,190,191,192,193,194,195] |
| Vitamin C | Healthy volunteers, hairless mice, keratinocytes | Increases antioxidant capacity and reduces ROS formation, DNA lesions and LPO. Reduces the inflammatory response. | Mice: Delays tumour onset and reduces tumour incidence **. | [202,203,204,205,206,207,208] |
| Vitamin D3 | Healthy volunteers, hairless mice, keratinocytes, ex vivo skin | Non-genomic signalling via the VDR facilitates protection against DNA lesions and cell cycle arrest while reducing immunosuppression. | Mice: Reduces tumour incidence, multiplicity and progression. | [221,222,223,224,225,226,227] |
| Vitamin E: α-tocopherol | Healthy volunteers, hairless mice, keratinocytes | Prevents oxidative damage by increasing antioxidant activity. Reduces DNA damage, immunosuppression, proliferation, apoptosis and the inflammatory response via AP-1 and NF-κB inhibition. | Mice: Delays tumour onset and reduces tumour incidence, multiplicity and size **. | [233,234,235,236,237,238,239,240] |
5. Perspectives and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A Systematic Review of Worldwide Incidence of Nonmelanoma Skin Cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; He, Y.-Y. Ultraviolet Radiation-Induced Non-Melanoma Skin Cancer: Regulation of DNA Damage Repair and Inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef]
- Dale Wilson, B.; Moon, S.; Armstrong, F. Comprehensive Review of Ultraviolet Radiation and the Current Status on Sunscreens. J. Clin. Aesthet. Dermatol. 2012, 5, 18–23. [Google Scholar]
- Jans, J.; Schul, W.; Sert, Y.-G.; Rijksen, Y.; Rebel, H.; Eker, A.P.M.; Nakajima, S.; van Steeg, H.; de Gruijl, F.R.; Yasui, A.; et al. Powerful Skin Cancer Protection by a CPD-Photolyase Transgene. Curr. Biol. CB 2005, 15, 105–115. [Google Scholar] [CrossRef]
- Brash, D.E. Sunlight and the Onset of Skin Cancer. Trends Genet. TIG 1997, 13, 410–414. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- De Feraudy, S.; Ridd, K.; Richards, L.M.; Kwok, P.-Y.; Revet, I.; Oh, D.; Feeney, L.; Cleaver, J.E. The DNA Damage-Binding Protein XPC Is a Frequent Target for Inactivation in Squamous Cell Carcinomas. Am. J. Pathol. 2010, 177, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Pontén, J. A Role for Sunlight in Skin Cancer: UV-Induced P53 Mutations in Squamous Cell Carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Vousden, K.H. Mutant P53 in Cancer: New Functions and Therapeutic Opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef]
- Deliconstantinos, G.; Villiotou, V.; Stavrides, J.C. Alterations of Nitric Oxide Synthase and Xanthine Oxidase Activities of Human Keratinocytes by Ultraviolet B Radiation. Potential Role for Peroxynitrite in Skin Inflammation. Biochem. Pharmacol. 1996, 51, 1727–1738. [Google Scholar] [CrossRef]
- Brem, R.; Macpherson, P.; Guven, M.; Karran, P. Oxidative Stress Induced by UVA Photoactivation of the Tryptophan UVB Photoproduct 6-Formylindolo[3,2-b]Carbazole (FICZ) Inhibits Nucleotide Excision Repair in Human Cells. Sci. Rep. 2017, 7, 4310. [Google Scholar] [CrossRef]
- Podda, M.; Traber, M.G.; Weber, C.; Yan, L.-J.; Packer, L. UV-Irradiation Depletes Antioxidants and Causes Oxidative Damage in a Model of Human Skin. Free Radic. Biol. Med. 1998, 24, 55–65. [Google Scholar] [CrossRef]
- Kawanishi, S.; Hiraku, Y.; Oikawa, S. Mechanism of Guanine-Specific DNA Damage by Oxidative Stress and Its Role in Carcinogenesis and Aging. Mutat. Res. Mutat. Res. 2001, 488, 65–76. [Google Scholar] [CrossRef]
- Hattorinakakuki, Y.; Nishigori, C.; Okamoto, K.; Imamura, S.; Hiai, H.; Toyokuni, S. Formation of 8-Hydroxy-2′-Deoxyguanosine in Epidermis of Hairless Mice Exposed to Near-UV. Biochem. Biophys. Res. Commun. 1994, 201, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of Specific Bases during DNA Synthesis Past the Oxidation-Damaged Base 8-OxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The Ascorbate-Glutathione-α-Tocopherol Triad in Abiotic Stress Response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef]
- Ramana, K.V.; Srivastava, S.; Singhal, S.S. Lipid Peroxidation Products in Human Health and Disease 2014. Oxid. Med. Cell. Longev. 2014, 2014, 162414. [Google Scholar] [CrossRef] [PubMed]
- Schaur, R.J. Basic Aspects of the Biochemical Reactivity of 4-Hydroxynonenal. Mol. Asp. Med. 2003, 24, 149–159. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet Oxygen-Mediated Damage to Proteins and Its Consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein Carbonylation in Human Diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Petrov, D.; Zagrovic, B. Microscopic Analysis of Protein Oxidative Damage: Effect of Carbonylation on Structure, Dynamics, and Aggregability of Villin Headpiece. J. Am. Chem. Soc. 2011, 133, 7016–7024. [Google Scholar] [CrossRef]
- Zimmerman, R.; Cerutti, P. Active Oxygen Acts as a Promoter of Transformation in Mouse Embryo C3H/10T1/2/C18 Fibroblasts. Proc. Natl. Acad. Sci. USA 1984, 81, 2085. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Bartnicka, D.; Rapala-Kozik, M. UVA and UVB Radiation Induce the Formation of Neutrophil Extracellular Traps by Human Polymorphonuclear Cells. J. Photochem. Photobiol. B 2019, 196, 111511. [Google Scholar] [CrossRef]
- Kato, T.; Delhase, M.; Hoffmann, A.; Karin, M. CK2 Is a C-Terminal IκB Kinase Responsible for NF-ΚB Activation during the UV Response. Mol. Cell 2003, 12, 829–839. [Google Scholar] [CrossRef]
- May, M.J.; Ghosh, S. Rel/NF-ΚB and IκB Proteins: An Overview. Semin. Cancer Biol. 1997, 8, 63–73. [Google Scholar] [CrossRef]
- Abeyama, K.; Eng, W.; Jester, J.V.; Vink, A.A.; Edelbaum, D.; Cockerell, C.J.; Bergstresser, P.R.; Takashima, A. A Role for NF-ΚB–Dependent Gene Transactivation in Sunburn. J. Clin. Investig. 2000, 105, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Bachelor, M.A.; Cooper, S.J.; Sikorski, E.T.; Bowden, G.T. Inhibition of P38 Mitogen-Activated Protein Kinase and Phosphatidylinositol 3-Kinase Decreases UVB-Induced Activator Protein-1 and Cyclooxygenase-2 in a SKH-1 Hairless Mouse Model. Mol. Cancer Res. MCR 2005, 3, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Nagamachi, M.; Honda, T.; Nishigori, C.; Miyachi, Y.; Tokura, Y.; Narumiya, S. Prostaglandin E2 Is Required for Ultraviolet B-Induced Skin Inflammation via EP2 and EP4 Receptors. Lab. Investig. 2007, 87, 49–55. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid Receptors: Structures, Properties, and Functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [PubMed]
- Stingl, L.A.; Sauder, D.N.; Iijima, M.; Wolff, K.; Pehamberger, H.; Stingl, G. Mechanism of UV-B-Induced Impairment of the Antigen-Presenting Capacity of Murine Epidermal Cells. J. Immunol. Baltim. 1983, 130, 1586–1591. [Google Scholar]
- Aberer, W.; Schuler, G.; Stingl, G.; Hönigsmann, H.; Wolff, K. Ultraviolet Light Depletes Surface Markers of Langerhans Cells. J. Investig. Dermatol. 1981, 76, 202–210. [Google Scholar] [CrossRef]
- Fukunaga, A.; Khaskhely, N.M.; Ma, Y.; Sreevidya, C.S.; Taguchi, K.; Nishigori, C.; Ullrich, S.E. Langerhans Cells Serve as Immunoregulatory Cells by Activating NKT Cells. J. Immunol. 2010, 185, 4633. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Simpson, T.J.; Ross, J.A. Urocanic Acid and Immunosuppression. Photochem. Photobiol. 1989, 50, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Noonan, F.P.; De Fabo, E.C. Immunosuppression by Ultraviolet B Radiation: Initiation by Urocanic Acid. Immunol. Today 1992, 13, 250–254. [Google Scholar] [CrossRef]
- Kaneko, K.; Smetana-Just, U.; Matsui, M.; Young, A.R.; John, S.; Norval, M.; Walker, S.L. Cis-Urocanic Acid Initiates Gene Transcription in Primary Human Keratinocytes. J. Immunol. 2008, 181, 217. [Google Scholar] [CrossRef]
- Shreedhar, V.; Giese, T.; Sung, V.W.; Ullrich, S.E. A Cytokine Cascade Including Prostaglandin E2, IL-4, and IL-10 Is Responsible for UV-Induced Systemic Immune Suppression. J. Immunol. 1998, 160, 3783. [Google Scholar]
- Rivas, J.M.; Ullrich, S.E. Systemic Suppression of Delayed-Type Hypersensitivity by Supernatants from UV-Irradiated Keratinocytes. An Essential Role for Keratinocyte-Derived IL-10. J. Immunol. 1992, 149, 3865–3871. [Google Scholar]
- Mittal, S.K.; Cho, K.-J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-Mediated Immunosuppression: March-I Induction Regulates Antigen Presentation by Macrophages but Not Dendritic Cells. J. Biol. Chem. 2015, 290, 27158–27167. [Google Scholar] [CrossRef]
- Noske, K. Secreted Immunoregulatory Proteins in the Skin. J. Dermatol. Sci. 2018, 89, 3–10. [Google Scholar] [CrossRef]
- Wu, C.L.; Qiang, L.; Han, W.; Ming, M.; Viollet, B.; He, Y.Y. Role of AMPK in UVB-Induced DNA Damage Repair and Growth Control. Oncogene 2013, 32, 2682–2689. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. Mitogen-Activated Protein Kinase Activation in UV-Induced Signal Transduction. Sci. STKE Signal Transduct. Knowl. Environ. 2003, 2003, RE2. [Google Scholar] [CrossRef]
- De Gruijl, F.R.; Forbes, P.D. UV-induced Skin Cancer in a Hairless Mouse Model. BioEssays 1995, 17, 651–660. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655–683. [Google Scholar] [PubMed]
- Tang, X.; Kim, A.L.; Kopelovich, L.; Bickers, D.R.; Athar, M. Cyclooxygenase-2 Inhibitor Nimesulide Blocks Ultraviolet B-Induced Photocarcinogenesis in SKH-1 Hairless Mice. Photochem. Photobiol. 2008, 84, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Reeve, V.E.; Matheson, M.J.; Bosnic, M.; Boehm-Wilcox, C. The Protective Effect of Indomethacin on Photocarcinogenesis in Hairless Mice. Cancer Lett. 1995, 95, 213–219. [Google Scholar] [CrossRef]
- Rahman, H.; Kumar, D.; Liu, T.; Okwundu, N.; Lum, D.; Florell, S.R.; Burd, C.E.; Boucher, K.M.; VanBrocklin, M.W.; Grossman, D. Aspirin Protects Melanocytes and Keratinocytes against UVB-Induced DNA Damage In Vivo. J. Investig. Dermatol. 2021, 141, 132–141.e3. [Google Scholar] [CrossRef] [PubMed]
- Pentland, A.P.; Schoggins, J.W.; Scott, G.A.; Khan, K.N.; Han, R. Reduction of UV-Induced Skin Tumors in Hairless Mice by Selective COX-2 Inhibition. Carcinogenesis 1999, 20, 1939–1944. [Google Scholar] [CrossRef]
- Athar, M.; An, K.P.; Tang, X.; Morel, K.D.; Kim, A.L.; Kopelovich, L.; Bickers, D.R. Photoprotective Effects of Sulindac against Ultraviolet B-Induced Phototoxicity in the Skin of SKH-1 Hairless Mice. Toxicol. Appl. Pharmacol. 2004, 195, 370–378. [Google Scholar] [CrossRef]
- Elmets, C.A.; Viner, J.L.; Pentland, A.P.; Cantrell, W.; Lin, H.-Y.; Bailey, H.; Kang, S.; Linden, K.G.; Heffernan, M.; Duvic, M.; et al. Chemoprevention of Nonmelanoma Skin Cancer with Celecoxib: A Randomized, Double-Blind, Placebo-Controlled Trial. JNCI J. Natl. Cancer Inst. 2010, 102, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Diluvio, L.; Paternò, E.J.; Chimenti, S. Topical Treatment of Actinic Keratoses with Piroxicam 1% Gel: A Preliminary Open-Label Study Utilizing a New Clinical Score. Am. J. Clin. Dermatol. 2010, 11, 45–50. [Google Scholar] [CrossRef]
- Trelle, S.; Reichenbach, S.; Wandel, S.; Hildebrand, P.; Tschannen, B.; Villiger, P.M.; Egger, M.; Jüni, P. Cardiovascular Safety of Non-Steroidal Anti-Inflammatory Drugs: Network Meta-Analysis. BMJ 2011, 342, c7086. [Google Scholar] [CrossRef] [PubMed]
- Straube, S.; Tramèr, M.R.; Moore, R.A.; Derry, S.; McQuay, H.J. Mortality with Upper Gastrointestinal Bleeding and Perforation: Effects of Time and NSAID Use. BMC Gastroenterol. 2009, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.; Castellsague, J.; Varas-Lorenzo, C.; García Rodríguez, L.A. Nonsteroidal Anti-Inflammatory Drugs and Risk of ARF in the General Population. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2005, 45, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zang, M.; Guo, W. AMPK as a Metabolic Tumor Suppressor: Control of Metabolism and Cell Growth. Future Oncol. Lond. Engl. 2010, 6, 457–470. [Google Scholar] [CrossRef]
- Jones, R.G.; Plas, D.R.; Kubek, S.; Buzzai, M.; Mu, J.; Xu, Y.; Birnbaum, M.J.; Thompson, C.B. AMP-Activated Protein Kinase Induces a P53-Dependent Metabolic Checkpoint. Mol. Cell 2005, 18, 283–293. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, Y.-W.; Lee, J.-H.; Zeng, S.X.; Wang, Y.V.; Luo, Z.; Dong, X.C.; Viollet, B.; Wahl, G.M.; Lu, H. AMP-Activated Protein Kinase Induces P53 by Phosphorylating MDMX and Inhibiting Its Activity. Mol. Cell. Biol. 2014, 34, 148–157. [Google Scholar] [CrossRef]
- Kirpichnikov, D.; McFarlane, S.I.; Sowers, J.R. Metformin: An Update. Ann. Intern. Med. 2002, 137, 25–33. [Google Scholar] [CrossRef] [PubMed]
- DeCensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and Cancer Risk in Diabetic Patients: A Systematic Review and Meta-Analysis. Cancer Prev. Res. 2010, 3, 1451. [Google Scholar] [CrossRef]
- Ba, W.; Xu, Y.; Yin, G.; Yang, J.; Wang, R.; Chi, S.; Wang, Y.; Li, C. Metformin Inhibits Pro-Inflammatory Responses via Targeting Nuclear Factor-ΚB in HaCaT Cells. Cell Biochem. Funct. 2019, 37, 4–10. [Google Scholar] [CrossRef]
- Chaudhary, S.C.; Kurundkar, D.; Elmets, C.A.; Kopelovich, L.; Athar, M. Metformin, an Antidiabetic Agent Reduces Growth of Cutaneous Squamous Cell Carcinoma by Targeting MTOR Signaling Pathway. Photochem. Photobiol. 2012, 88, 1149–1156. [Google Scholar] [CrossRef]
- Cui, B.; Liu, Q.; Tong, L.; Feng, X. The Effects of the Metformin on Inhibition of UVA-Induced Expression of MMPs and COL-I in Human Skin Fibroblasts. Eur. J. Inflamm. 2019, 17, 2058739219876423. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.; Powers, M.A.; Dellavalle, R.P. Therapeutic Potential of the Anti-Diabetic Agent Metformin in Targeting the Skin Cancer Stem Cell Diaspora. Exp. Dermatol. 2014, 23, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Robertson, B.A.; Coffey, D.S.; Taichman, R.S. The Cancer Diaspora: Metastasis beyond the Seed and Soil Hypothesis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5849–5855. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H. Metformin Is Associated with Decreased Skin Cancer Risk in Taiwanese Patients with Type 2 Diabetes. J. Am. Acad. Dermatol. 2018, 78, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Adalsteinsson, J.A.; Muzumdar, S.; Waldman, R.; Wu, R.; Ratner, D.; Feng, H.; Ungar, J.; Silverberg, J.I.; Olafsdottir, G.H.; Kristjansson, A.K.; et al. Metformin Is Associated with Decreased Risk of Basal Cell Carcinoma: A Whole-Population Case-Control Study from Iceland. J. Am. Acad. Dermatol. 2021, 85, 56–61. [Google Scholar] [CrossRef]
- Zhou, Q.; Kim, S.H.; Pérez-Lorenzo, R.; Liu, C.; Huang, M.; Dotto, G.P.; Zheng, B.; Wu, X. Phenformin Promotes Keratinocyte Differentiation via the Calcineurin/NFAT Pathway. J. Investig. Dermatol. 2021, 141, 152–163. [Google Scholar] [CrossRef]
- Jung, J.; Bollag, W.B. Phenformin: AMP(K)Ed for Potential Repurposing. J. Investig. Dermatol. 2021, 141, 11–14. [Google Scholar] [CrossRef]
- Chen, L.; Guo, S.; Ranzer, M.J.; DiPietro, L.A. Toll-like Receptor 4 Has an Essential Role in Early Skin Wound Healing. J. Investig. Dermatol. 2013, 133, 258–267. [Google Scholar] [CrossRef]
- Min, W.; Ahmad, I.; Chang, M.E.; Burns, E.M.; Qian, Q.; Yusuf, N. Baicalin Protects Keratinocytes from Toll-like Receptor-4 Mediated DNA Damage and Inflammation Following Ultraviolet Irradiation. Photochem. Photobiol. 2015, 91, 1435–1443. [Google Scholar] [CrossRef]
- Janda, J.; Burkett, N.B.; Blohm-Mangone, K.; Huang, V.; Curiel-Lewandrowski, C.; Alberts, D.S.; Petricoin, E.F., 3rd; Calvert, V.S.; Einspahr, J.; Dong, Z.; et al. Resatorvid-Based Pharmacological Antagonism of Cutaneous TLR4 Blocks UV-Induced NF-ΚB and AP-1 Signaling in Keratinocytes and Mouse Skin. Photochem. Photobiol. 2016, 92, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gao, J.; Cui, Y.; Li, M.; Li, R.; Cui, C.; Cui, J. Neuroprotective Effects of Resatorvid Against Traumatic Brain Injury in Rat: Involvement of Neuronal Autophagy and TLR4 Signaling Pathway. Cell. Mol. Neurobiol. 2017, 37, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Zandi, Z.; Kashani, B.; Poursani, E.M.; Bashash, D.; Kabuli, M.; Momeny, M.; Mousavi-Pak, S.H.; Sheikhsaran, F.; Alimoghaddam, K.; Mousavi, S.A.; et al. TLR4 Blockade Using TAK-242 Suppresses Ovarian and Breast Cancer Cells Invasion through the Inhibition of Extracellular Matrix Degradation and Epithelial-Mesenchymal Transition. Eur. J. Pharmacol. 2019, 853, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Blohm-Mangone, K.; Burkett, N.B.; Tahsin, S.; Myrdal, P.B.; Aodah, A.; Ho, B.; Janda, J.; McComas, M.; Saboda, K.; Roe, D.J.; et al. Pharmacological TLR4 Antagonism Using Topical Resatorvid Blocks Solar UV-Induced Skin Tumorigenesis in SKH-1 Mice. Cancer Prev. Res. 2018, 11, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.-L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of TAK-242 for the Treatment of Severe Sepsis. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X. The Role of Estrogen Receptor Beta in Breast Cancer. Biomark. Res. 2020, 8, 39. [Google Scholar] [CrossRef]
- Chang, E.C.; Frasor, J.; Komm, B.; Katzenellenbogen, B.S. Impact of Estrogen Receptor β on Gene Networks Regulated by Estrogen Receptor α in Breast Cancer Cells. Endocrinology 2006, 147, 4831–4842. [Google Scholar] [CrossRef]
- Malamas, M.S.; Manas, E.S.; McDevitt, R.E.; Gunawan, I.; Xu, Z.B.; Collini, M.D.; Miller, C.P.; Dinh, T.; Henderson, R.A.; Keith, J.C.; et al. Design and Synthesis of Aryl Diphenolic Azoles as Potent and Selective Estrogen Receptor-β Ligands. J. Med. Chem. 2004, 47, 5021–5040. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Castañeda, S.; Cutolo, M.; Herrero-Beaumont, G. Efficacy and Safety of a Selective Estrogen Receptor β Agonist, ERB-041, in Patients with Rheumatoid Arthritis: A 12-Week, Randomized, Placebo-Controlled, Phase II Study. Arthritis Care Res. 2010, 62, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.C.; Singh, T.; Talwelkar, S.S.; Srivastava, R.K.; Arumugam, A.; Weng, Z.; Elmets, C.A.; Afaq, F.; Kopelovich, L.; Athar, M. Erb-041, an Estrogen Receptor-β Agonist, Inhibits Skin Photocarcinogenesis in SKH-1 Hairless Mice by Downregulating the WNT Signaling Pathway. Cancer Prev. Res. 2014, 7, 186–198. [Google Scholar] [CrossRef]
- Hiramoto, K.; Tanaka, H.; Yanagihara, N.; Sato, E.F.; Inoue, M. Effect of 17β-Estradiol on Immunosuppression Induced by Ultraviolet B Irradiation. Arch. Dermatol. Res. 2004, 295, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic Activity, Biological Effect and Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Roca, P. Phytoestrogens for Cancer Prevention and Treatment. Biology 2020, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A Stress Response Pathway Regulates DNA Damage through Β2-Adrenoreceptors and β-Arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef]
- Seiffert, K.; Hosoi, J.; Torii, H.; Ozawa, H.; Ding, W.; Campton, K.; Wagner, J.A.; Granstein, R.D. Catecholamines Inhibit the Antigen-Presenting Capability of Epidermal Langerhans Cells. J. Immunol. 2002, 168, 6128. [Google Scholar] [CrossRef]
- Steinkraus, V.; Steinfath, M.; Körner, C.; Mensing, H. Binding of Beta-Adrenergic Receptors in Human Skin. J. Investig. Dermatol. 1992, 98, 475–480. [Google Scholar] [CrossRef]
- Chen, M.; Liang, S.; Shahid, A.; Andresen, B.T.; Huang, Y. The β-Blocker Carvedilol Prevented Ultraviolet-Mediated Damage of Murine Epidermal Cells and 3D Human Reconstructed Skin. Int. J. Mol. Sci. 2020, 21, 798. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.M.; Liang, S.; Yeung, S.; Oiyemhonlan, E.; Cleveland, K.H.; Parsa, C.; Orlando, R.; Meyskens, F.L.; Andresen, B.T.; Huang, Y. Topically Applied Carvedilol Attenuates Solar Ultraviolet Radiation Induced Skin Carcinogenesis. Cancer Prev. Res. 2017, 10, 598. [Google Scholar] [CrossRef]
- Chen, M.; Shamim, M.A.; Shahid, A.; Yeung, S.; Andresen, B.T.; Wang, J.; Nekkanti, V.; Meyskens, F.L.J.; Kelly, K.M.; Huang, Y. Topical Delivery of Carvedilol Loaded Nano-Transfersomes for Skin Cancer Chemoprevention. Pharmaceutics 2020, 12, 1151. [Google Scholar] [CrossRef] [PubMed]
- Suda, A.; Nagaoka, S.; Ohono, S.; Ideguchi, H.; Soga, T.; Ishigatsubo, Y. The Efficacy and Safety of Bucillamine as a Second-Line DMARD in the Treatment of Rheumatoid Arthritis: A Retrospective Cohort Study. Mod. Rheumatol. 2008, 18, 609–614. [Google Scholar] [CrossRef] [PubMed]
- D’Agostini, F.; Balansky, R.M.; Camoirano, A.; De Flora, S. Modulation of Light-Induced Skin Tumors by N-Acetylcysteine and/or Ascorbic Acid in Hairless Mice. Carcinogenesis 2005, 26, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Mazor, D.; Greenberg, L.; Shamir, D.; Meyerstein, D.; Meyerstein, N. Antioxidant Properties of Bucillamine: Possible Mode of Action. Biochem. Biophys. Res. Commun. 2006, 349, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Anwar, H.; Yamauchi, T.; Tseng, R.; Agarwal, R.; Horwitz, L.D.; Zhai, Z.; Fujita, M. Bucillamine Inhibits UVB-Induced MAPK Activation and Apoptosis in Human HaCaT Keratinocytes and SKH-1 Hairless Mouse Skin. Photochem. Photobiol. 2020, 96, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Gu, M.; Brady, S.; Qamar, L.; Behbakht, K.; Shellman, Y.G.; Agarwal, R.; Norris, D.A.; Horwitz, L.D.; Fujita, M. Photoprotective Effects of Bucillamine Against UV-Induced Damage in an SKH-1 Hairless Mouse Model. Photochem. Photobiol. 2008, 84, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Paiva, L.; Rego, C.; Lima, E.; Marcone, M.; Baptista, J. Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia Sinensis Varieties Affected by Different Harvested and Processing Conditions. Antioxidants 2021, 10, 183. [Google Scholar] [CrossRef]
- Abotorabi, Z.; Khorashadizadeh, M.; Arab, M.; Hassanpour Fard, M.; Zarban, A. Jujube and Green Tea Extracts Protect Human Fibroblast Cells against UVB-Mediated Photo Damage and MMP-2 and MMP-9 Production. Avicenna J. Phytomed. 2020, 10, 287–296. [Google Scholar]
- Katiyar, S.K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-Induced Oxidative Stress-Mediated Phosphorylation of Mitogen-Activated Protein Kinase Signaling Pathways in Cultured Human Epidermal Keratinocytes by Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate. Toxicol. Appl. Pharmacol. 2001, 176, 110–117. [Google Scholar] [CrossRef]
- Song, X.; Bi, Z.; Xu, A. Green Tea Polyphenol Epigallocatechin-3-Gallate Inhibits the Expression of Nitric Oxide Synthase and Generation of Nitric Oxide Induced by Ultraviolet B in HaCaT Cells. Chin. Med. J. (Engl.) 2006, 119, 282–287. [Google Scholar] [CrossRef]
- Mittal, A.; Piyathilake, C.; Hara, Y.; Katiyar, S.K. Exceptionally High Protection of Photocarcinogenesis by Topical Application of (–)-Epigallocatechin-3-Gallate in Hydrophilic Cream in SKH-1 Hairless Mouse Model: Relationship to Inhibition of UVB-Induced Global DNA Hypomethylation. Neoplasia 2003, 5, 555–565. [Google Scholar] [CrossRef]
- Meeran, S.M.; Akhtar, S.; Katiyar, S.K. Inhibition of UVB-Induced Skin Tumor Development by Drinking Green Tea Polyphenols Is Mediated through DNA Repair and Subsequent Inhibition of Inflammation. J. Investig. Dermatol. 2009, 129, 1258–1270. [Google Scholar] [CrossRef]
- Wajed, S.A.; Laird, P.W.; DeMeester, T.R. DNA Methylation: An Alternative Pathway to Cancer. Ann. Surg. 2001, 234, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Ständer, S.; Berneburg, M.; Böhm, M.; Kulms, D.; van Steeg, H.; Grosse-Heitmeyer, K.; Krutmann, J.; Schwarz, T. Interleukin-12 Suppresses Ultraviolet Radiation-Induced Apoptosis by Inducing DNA Repair. Nat. Cell Biol. 2002, 4, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Maeda, A.; Gan, D.; Mammone, T.; Matsui, M.S.; Schwarz, T. Green Tea Phenol Extracts Reduce UVB-Induced DNA Damage in Human Cells via Interleukin-12. Photochem. Photobiol. 2008, 84, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Perez, A.; Mukhtar, H. Green Tea Polyphenol Treatment to Human Skin Prevents Formation of Ultraviolet Light B-Induced Pyrimidine Dimers in DNA. Clin. CANCER Res. 2000, 6, 3864–3869. [Google Scholar] [PubMed]
- Farrar, M.D.; Huq, R.; Mason, S.; Nicolaou, A.; Clarke, K.A.; Dew, T.P.; Williamson, G.; Watson, R.E.B.; Rhodes, L.E. Oral Green Tea Catechins Do Not Provide Photoprotection from Direct DNA Damage Induced by Higher Dose Solar Simulated Radiation: A Randomized Controlled Trial. J. Am. Acad. Dermatol. 2018, 78, 414–416. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and Safety of Green Tea Polyphenols after Multiple-Dose Administration of Epigallocatechin Gallate and Polyphenon E in Healthy Individuals. Clin. Cancer Res. 2003, 9, 3312. [Google Scholar]
- Farrar, M.D.; Nicolaou, A.; Clarke, K.A.; Mason, S.; Massey, K.A.; Dew, T.P.; Watson, R.E.; Williamson, G.; Rhodes, L.E. A Randomized Controlled Trial of Green Tea Catechins in Protection against Ultraviolet Radiation–Induced Cutaneous Inflammation1,2. Am. J. Clin. Nutr. 2015, 102, 608–615. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.-U.; Kozukue, N. Stability of Green Tea Catechins in Commercial Tea Leaves during Storage for 6 Months. J. Food Sci. 2009, 74, H47–H51. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, L.; Lee, M.J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and Urine Levels of Tea Catechins after Ingestion of Different Amounts of Green Tea by Human Volunteers. Cancer Epidemiol. Biomark. 1998, 7, 351. [Google Scholar]
- Perde-Schrepler, M.; Chereches, G.; Brie, I.; Tatomir, C.; Postescu, I.D.; Soran, L.; Filip, A. Grape Seed Extract as Photochemopreventive Agent against UVB-Induced Skin Cancer. J. Photochem. Photobiol. B 2013, 118, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Katiyar, S.K. Dietary Grape Seed Proanthocyanidins Inhibit UVB-Induced Cyclooxygenase-2 Expression and Other Inflammatory Mediators in UVB-Exposed Skin and Skin Tumors of SKH-1 Hairless Mice. Pharm. Res. 2010, 27, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Mintie, C.A.; Musarra, A.K.; Singh, C.K.; Ndiaye, M.A.; Sullivan, R.; Eickhoff, J.C.; Ahmad, N. Protective Effects of Dietary Grape on UVB-Mediated Cutaneous Damages and Skin Tumorigenesis in SKH-1 Mice. Cancers 2020, 12, 1751. [Google Scholar] [CrossRef]
- Sharma, S.D.; Meeran, S.M.; Katiyar, S.K. Dietary Grape Seed Proanthocyanidins Inhibit UVB-Induced Oxidative Stress and Activation of Mitogen-Activated Protein Kinases and Nuclear Factor-KappaB Signaling in in Vivo SKH-1 Hairless Mice. Mol. Cancer Ther. 2007, 6, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Katiyar, S.K. Dietary Grape-Seed Proanthocyanidin Inhibition of Ultraviolet B-Induced Immune Suppression Is Associated with Induction of IL-12. Carcinogenesis 2005, 27, 95–102. [Google Scholar] [CrossRef]
- Vaid, M.; Sharma, S.D.; Katiyar, S.K. Proanthocyanidins Inhibit Photocarcinogenesis through Enhancement of DNA Repair and Xeroderma Pigmentosum Group A-Dependent Mechanism. Cancer Prev. Res. 2010, 3, 1621–1629. [Google Scholar] [CrossRef]
- Oak, A.S.W.; Shafi, R.; Elsayed, M.; Bae, S.; Saag, L.; Wang, C.L.; Athar, M.; Elmets, C.A. Dietary Table Grape Protects against UV Photodamage in Humans: 1. Clinical Evaluation. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef]
- Oak, A.S.W.; Shafi, R.; Elsayed, M.; Mishra, B.; Bae, S.; Barnes, S.; Kashyap, M.P.; Slominski, A.T.; Wilson, L.S.; Athar, M.; et al. Dietary Table Grape Protects against Ultraviolet Photodamage in Humans: 2. Molecular Biomarker Studies. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, Oral Bioavailability, and Metabolic Profile of Resveratrol and Its Dimethylether Analog, Pterostilbene, in Rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical Treatment with Pterostilbene, a Natural Phytoalexin, Effectively Protects Hairless Mice against UVB Radiation-Induced Skin Damage and Carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Gombau, L.; García, F.; Lahoz, A.; Fabre, M.; Roda-Navarro, P.; Majano, P.; Alonso-Lebrero, J.L.; Pivel, J.P.; Castell, J.V.; Gómez-Lechon, M.J.; et al. Polypodium Leucotomos Extract: Antioxidant Activity and Disposition. Toxicol. Vitr. 2006, 20, 464–471. [Google Scholar] [CrossRef]
- Capote, R.; Alonso-Lebrero, J.L.; García, F.; Brieva, A.; Pivel, J.P.; González, S. Polypodium Leucotomos Extract Inhibits Trans-Urocanic Acid Photoisomerization and Photodecomposition. J. Photochem. Photobiol. B 2006, 82, 173–179. [Google Scholar] [CrossRef]
- González, S.; Pathak, M.A. Inhibition of Ultraviolet-Induced Formation of Reactive Oxygen Species, Lipid Peroxidation, Erythema and Skin Photosensitization by Polypodium Leucotomos. Photodermatol. Photoimmunol. Photomed. 1996, 12, 45–56. [Google Scholar] [CrossRef]
- Rodriguez-Yanes, E.; Juarranz, A.; Cuevas, J.; Gonzalez, S.; Mallol, J. Polypodium Leucotomos Decreases UV-Induced Epidermal Cell Proliferation and Enhances P53 Expression and Plasma Antioxidant Capacity in Hairless Mice. Exp. Dermatol. 2012, 21, 638–640. [Google Scholar] [CrossRef]
- Zattra, E.; Coleman, C.; Arad, S.; Helms, E.; Levine, D.; Bord, E.; Guillaume, A.; El-Hajahmad, M.; Zwart, E.; van Steeg, H.; et al. Polypodium Leucotomos Extract Decreases UV-Induced Cox-2 Expression and Inflammation, Enhances DNA Repair, and Decreases Mutagenesis in Hairless Mice. Am. J. Pathol. 2009, 175, 1952–1961. [Google Scholar] [CrossRef]
- Jańczyk, A.; Garcia-Lopez, M.A.; Fernandez-Peñas, P.; Alonso-Lebrero, J.L.; Benedicto, I.; López-Cabrera, M.; Gonzalez, S. A Polypodium Leucotomos Extract Inhibits Solar-Simulated Radiation-Induced TNF-Alpha and INOS Expression, Transcriptional Activation and Apoptosis. Exp. Dermatol. 2007, 16, 823–829. [Google Scholar] [CrossRef]
- Siscovick, J.R.; Zapolanski, T.; Magro, C.; Carrington, K.; Prograis, S.; Nussbaum, M.; Gonzalez, S.; Ding, W.; Granstein, R.D. Polypodium Leucotomos Inhibits Ultraviolet B Radiation-Induced Immunosuppression. Photodermatol. Photoimmunol. Photomed. 2008, 24, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Mulero, M.; Rodríguez-Yanes, E.; Nogués, M.R.; Giralt, M.; Romeu, M.; González, S.; Mallol, J. Polypodium Leucotomos Extract Inhibits Glutathione Oxidation and Prevents Langerhans Cell Depletion Induced by UVB/UVA Radiation in a Hairless Rat Model. Exp. Dermatol. 2008, 17, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Yanes, E.; Cuevas, J.; González, S.; Mallol, J. Oral Administration of Polypodium Leucotomos Delays Skin Tumor Development and Increases Epidermal P53 Expression and the Anti-Oxidant Status of UV-Irradiated Hairless Mice. Exp. Dermatol. 2014, 23, 526–528. [Google Scholar] [CrossRef]
- Alcaraz, M.V.; Pathak, M.A.; Rius, F.; Kollias, N.; González, S. An Extract of Polypodium Leucotomos Appears to Minimize Certain Photoaging Changes in a Hairless Albino Mouse Animal Model: A Pilot Study. Photodermatol. Photoimmunol. Photomed. 1999, 15, 120–126. [Google Scholar] [CrossRef]
- González, S.; Pathak, M.A.; Cuevas, J.; Villarrubia, V.G.; Fitzpatrick, T.B. Topical or Oral Administration with an Extract of Polypodium Leucotomos Prevents Acute Sunburn and Psoralen-Induced Phototoxic Reactions as Well as Depletion of Langerhans Cells in Human Skin. Photodermatol. Photoimmunol. Photomed. 1997, 13, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp-Hup, M.A.; Pathak, M.A.; Parrado, C.; Garcia-Caballero, T.; Rius-Díaz, F.; Fitzpatrick, T.B.; González, S. Orally Administered Polypodium Leucotomos Extract Decreases Psoralen-UVA-Induced Phototoxicity, Pigmentation, and Damage of Human Skin. J. Am. Acad. Dermatol. 2004, 50, 41–49. [Google Scholar] [CrossRef]
- Middelkamp-Hup, M.A.; Pathak, M.A.; Parrado, C.; Goukassian, D.; Rius-Díaz, F.; Mihm, M.C.; Fitzpatrick, T.B.; González, S. Oral Polypodium Leucotomos Extract Decreases Ultraviolet-Induced Damage of Human Skin. J. Am. Acad. Dermatol. 2004, 51, 910–918. [Google Scholar] [CrossRef]
- Kohli, I.; Shafi, R.; Isedeh, P.; Griffith, J.L.; Al-Jamal, M.S.; Silpa-Archa, N.; Jackson, B.; Athar, M.; Kollias, N.; Elmets, C.A.; et al. The Impact of Oral Polypodium Leucotomos Extract on Ultraviolet B Response: A Human Clinical Study. J. Am. Acad. Dermatol. 2017, 77, 33–41.e1. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Berman, B.; Swenson, N. Safety and Efficacy of Oral Polypodium Leucotomos Extract in Healthy Adult Subjects. J. Clin. Aesthet. Dermatol. 2015, 8, 19–23. [Google Scholar] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Pal, H.C.; Mukhtar, H.; Afaq, F. Pomegranate Fruit Extract Inhibits UVB-Induced Inflammation and Proliferation by Modulating NF-ΚB and MAPK Signaling Pathways in Mouse Skin. Photochem. Photobiol. 2012, 88, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Khan, N.; Syed, D.N.; Mukhtar, H. Oral Feeding of Pomegranate Fruit Extract Inhibits Early Biomarkers of UVB Radiation-Induced Carcinogenesis in SKH-1 Hairless Mouse Epidermis. Photochem. Photobiol. 2010, 86, 1318–1326. [Google Scholar] [CrossRef]
- Gómez-García, F.J.; López López, A.; Guerrero-Sánchez, Y.; Sánchez Siles, M.; Martínez Díaz, F.; Camacho Alonso, F. Chemopreventive Effect of Pomegranate and Cocoa Extracts on Ultraviolet Radiation-Induced Photocarcinogenesis in SKH-1 Mice. PLoS ONE 2020, 15, e0232009. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Lee, R.-P.; Huang, J.; Hsu, M.; Thames, G.; Gilbuena, I.; Long, J.; Xu, Y.; Park, E.H.; et al. Pomegranate Juice and Extract Consumption Increases the Resistance to UVB-Induced Erythema and Changes the Skin Microbiome in Healthy Women: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 14528. [Google Scholar] [CrossRef]
- Duncan, F.J.; Martin, J.R.; Wulff, B.C.; Stoner, G.D.; Tober, K.L.; Oberyszyn, T.M.; Kusewitt, D.F.; Van Buskirk, A.M. Topical Treatment with Black Raspberry Extract Reduces Cutaneous UVB-Induced Carcinogenesis and Inflammation. Cancer Prev. Res. 2009, 2, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-W.; Cheng, Y.-C.; Hung, Y.-C.; Lee, C.-H.; Fang, J.-Y.; Li, W.-T.; Wu, Y.-R.; Pan, T.-L. Red Raspberry Extract Protects the Skin against UVB-Induced Damage with Antioxidative and Anti-Inflammatory Properties. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, Y.; Hwang, E.; Lin, P.; Bae, J.; Seo, S.A.; Yan, Z.; Yi, T.-H. Rubus Idaeus L. (Red Raspberry) Blocks UVB-Induced MMP Production and Promotes Type I Procollagen Synthesis via Inhibition of MAPK/AP-1, NF-Κβ and Stimulation of TGF-β/Smad, Nrf2 in Normal Human Dermal Fibroblasts. J. Photochem. Photobiol. B 2018, 185, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.-O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P.; et al. Blackberry Extract Inhibits UVB-Induced Oxidative Damage and Inflammation through MAP Kinases and NF-ΚB Signaling Pathways in SKH-1 Mice Skin. Toxicol. Appl. Pharmacol. 2015, 284, 92–99. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.-O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-Glucoside Inhibits UVB-Induced Oxidative Damage and Inflammation by Regulating MAP Kinase and NF-ΚB Signaling Pathways in SKH-1 Hairless Mice Skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kundu, J.K.; Kim, S.O.; Chun, K.-S.; Lee, H.J.; Surh, Y.-J. Cocoa Polyphenols Inhibit Phorbol Ester-Induced Superoxide Anion Formation in Cultured HL-60 Cells and Expression of Cyclooxygenase-2 and Activation of NF-KappaB and MAPKs in Mouse Skin in Vivo. J. Nutr. 2006, 136, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Davinelli, S.; Di Renzo, L.; De Lorenzo, A.; Olarte, H.H.; Micali, G.; Cicero, A.F.; Gonzalez, S. Cocoa Bioactive Compounds: Significance and Potential for the Maintenance of Skin Health. Nutrients 2014, 6, 3202–3213. [Google Scholar] [CrossRef]
- Kim, J.-E.; Song, D.; Kim, J.; Choi, J.; Kim, J.R.; Yoon, H.-S.; Bae, J.-S.; Han, M.; Lee, S.; Hong, J.S.; et al. Oral Supplementation with Cocoa Extract Reduces UVB-Induced Wrinkles in Hairless Mouse Skin. J. Investig. Dermatol. 2016, 136, 1012–1021. [Google Scholar] [CrossRef]
- Heinrich, U.; Neukam, K.; Tronnier, H.; Sies, H.; Stahl, W. Long-Term Ingestion of High Flavanol Cocoa Provides Photoprotection against UV-Induced Erythema and Improves Skin Condition in Women. J. Nutr. 2006, 136, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.; Calzavara-Pinton, I.; Arisi, M.; Rossi, M.T.; Scapagnini, G.; Davinelli, S.; Venturini, M. Cutaneous Photoprotective Activity of a Short-Term Ingestion of High-Flavanol Cocoa: A Nutritional Intervention Study. Photochem. Photobiol. 2019, 95, 1029–1034. [Google Scholar] [CrossRef]
- Williams, S.; Tamburic, S.; Lally, C. Eating Chocolate Can Significantly Protect the Skin from UV Light. J. Cosmet. Dermatol. 2009, 8, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Mogollon, J.A.; Boivin, C.; Lemieux, S.; Blanchet, C.; Claveau, J.; Dodin, S. Chocolate Flavanols and Skin Photoprotection: A Parallel, Double-Blind, Randomized Clinical Trial. Nutr. J. 2014, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.S.; Vine, A.L. Cancer Prevention by Retinoids and Carotenoids: Independent Action on a Common Target. Carotenoids Diet. Lipids 2005, 1740, 170–178. [Google Scholar] [CrossRef]
- Heinonen, M. Food Groups as the Source of Retinoids, Carotenoids, and Vitamin A in Finland. Int. J. Vitam. Nutr. Res. Int. Z. Vitam. Ernahr. J. Int. Vitaminol. Nutr. 1991, 61, 3–9. [Google Scholar]
- Ollilainen, V.; Heinonen, M.; Linkola, E.; Varo, P.; Koivistoinen, P. Carotenoids and Retinoids in Finnish Foods: Dairy Products and Eggs. J. Dairy Sci. 1989, 72, 2257–2265. [Google Scholar] [CrossRef]
- Balić, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef]
- Cheng, X.; Qian, W.; Chen, F.; Jin, Y.; Wang, F.; Lu, X.; Lee, S.R.; Su, D.; Chen, B. ATRA Protects Skin Fibroblasts against UV-induced Oxidative Damage through Inhibition of E3 Ligase Hrd1. Mol. Med. Rep. 2019, 20, 2294–2302. [Google Scholar] [CrossRef]
- Darwiche, N.; Bazzi, H.; El-Touni, L.; Abou-Lteif, G.; Doueiri, R.; Hatoum, A.; Maalouf, S.; Gali-Muhtasib, H. Regulation of Ultraviolet B Radiation-Mediated Activation of AP1 Signaling by Retinoids in Primary Keratinocytes. Radiat. Res. 2005, 163, 296–306. [Google Scholar] [CrossRef]
- Huang, C.; Ma, W.Y.; Dawson, M.I.; Rincon, M.; Flavell, R.A.; Dong, Z. Blocking Activator Protein-1 Activity, but Not Activating Retinoic Acid Response Element, Is Required for the Antitumor Promotion Effect of Retinoic Acid. Proc. Natl. Acad. Sci. USA 1997, 94, 5826–5830. [Google Scholar] [CrossRef]
- Bécherel, P.-A.; Mossalayi, M.; Goff, L.; Francès, C.; Chosidow, O.; Debré, P.; Arock, M. Mechanism of Anti-Inflammatory Action of Retinoids on Keratinocytes. Lancet 1994, 344, 1570–1571. [Google Scholar] [CrossRef]
- Bécherel, P.A.; Le Goff, L.; Ktorza, S.; Chosidow, O.; Francès, C.; Issaly, F.; Mencia-Huerta, J.M.; Debré, P.; Mossalayi, M.D.; Arock, M. CD23-Mediated Nitric Oxide Synthase Pathway Induction in Human Keratinocytes Is Inhibited by Retinoic Acid Derivatives. J. Investig. Dermatol. 1996, 106, 1182–1186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, B.-H. Safety Evaluation and Anti-Wrinkle Effects of Retinoids on Skin. Toxicol. Res. 2010, 26, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lerche, C.M.; Philipsen, P.A.; Sehested, M.; Wulf, H.C. Photocarcinogenesis of Topical Tazarotene and Isotretinoin Alone and in Combination with Valproic Acid in Hairless Mice. Exp. Dermatol. 2008, 17, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Sorg, O.; Iran, C.; Carraux, P.; Grand, D.; Hügin, A.; Didierjean, L.; Saurat, J.-H. Spectral Properties of Topical Retinoids Prevent DNA Damage and Apoptosis After Acute UV-B Exposure in Hairless Mice. Photochem. Photobiol. 2007, 81, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.H. Chemicals and Photocarcinogenesis*. Australas. J. Dermatol. 1977, 18, 57. [Google Scholar] [CrossRef]
- Epstein, J.H.; Grekin, D.A. Inhibition of Ultraviolet-Induced Carcinogenesis by All-Trans Retinoic Acid. J. Investig. Dermatol. 1981, 76, 178–180. [Google Scholar] [CrossRef]
- Mikkelsen, S.; Berne, B.; Staberg, B.; Vahlquist, A. Potentiating Effect of Dietary Vitamin A on Photocarcinogenesis in Hairless Mice. Carcinogenesis 1998, 19, 663–666. [Google Scholar] [CrossRef][Green Version]
- Halliday, G.M.; Robertson, B.O.; Barnetson, R.S.T.C. Topical Retinoic Acid Enhances, and a Dark Tan Protects, from Subedemal Solar-Simulated Photocarcinogenesis. J. Investig. Dermatol. 2000, 114, 923–927. [Google Scholar] [CrossRef]
- Kligman, L.H.; Crosby, M.J. Topical Tretinoin Enhances Corticosteroid-Induced Inhibition of Tumorigenesis in Hairless Mice Previously Exposed to Solar Simulating Radiation. Cancer Lett. 1996, 107, 217–222. [Google Scholar] [CrossRef]
- Kligman, L.H.; Kligman, A.M. Lack of Enhancement of Experimental Photocarcinogenesis by Topical Retinoic Acid. Arch. Dermatol. Res. 1981, 270, 453–462. [Google Scholar] [CrossRef]
- Antille, C.; Tran, C.; Sorg, O.; Carraux, P.; Didierjean, L.; Saurat, J.-H. Vitamin A Exerts a Photoprotective Action in Skin by Absorbing Ultraviolet B Radiation. J. Investig. Dermatol. 2003, 121, 1163–1167. [Google Scholar] [CrossRef]
- Smit, J.V.; de Jong, E.M.; de Jongh, G.J.; van de Kerkhof, P.C. Topical All-Trans Retinoic Acid Does Not Influence Minimal Erythema Doses for UVB Light in Normal Skin. Acta Derm. Venereol. 2000, 80, 66–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.; Park, M.K.; Li, W.-Q.; Qureshi, A.A.; Cho, E. Association of Vitamin A Intake with Cutaneous Squamous Cell Carcinoma Risk in the United States. JAMA Dermatol. 2019, 155, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Nijsten, T.E.C.; Stern, R.S. Oral Retinoid Use Reduces Cutaneous Squamous Cell Carcinoma Risk in Patients with Psoriasis Treated with Psoralen-UVA: A Nested Cohort Study. J. Am. Acad. Dermatol. 2003, 49, 644–650. [Google Scholar] [CrossRef]
- Moon, T.E.; Levine, N.; Cartmel, B.; Bangert, J.L.; Rodney, S.; Dong, Q.; Peng, Y.M.; Alberts, D.S. Effect of Retinol in Preventing Squamous Cell Skin Cancer in Moderate-Risk Subjects: A Randomized, Double-Blind, Controlled Trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1997, 6, 949–956. [Google Scholar]
- Levine, N.; Moon, T.E.; Cartmel, B.; Bangert, J.L.; Rodney, S.; Dong, Q.; Peng, Y.M.; Alberts, D.S. Trial of Retinol and Isotretinoin in Skin Cancer Prevention: A Randomized, Double-Blind, Controlled Trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1997, 6, 957–961. [Google Scholar]
- PREISS, J.; HANDLER, P. Biosynthesis of Diphosphopyridine Nucleotide. II. Enzymatic Aspects. J. Biol. Chem. 1958, 233, 493–500. [Google Scholar] [CrossRef]
- Bieganowski, P.; Brenner, C. Discoveries of Nicotinamide Riboside as a Nutrient and Conserved NRK Genes Establish a Preiss-Handler Independent Route to NAD+ in Fungi and Humans. Cell 2004, 117, 495–502. [Google Scholar] [CrossRef]
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ Metabolism in Health and Disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of Nicotinamide in DNA Damage, Mutagenesis, and DNA Repair. J. Nucleic Acids 2010, 2010, 157591. [Google Scholar] [CrossRef]
- Robu, M.; Shah, R.G.; Petitclerc, N.; Brind’Amour, J.; Kandan-Kulangara, F.; Shah, G.M. Role of Poly(ADP-Ribose) Polymerase-1 in the Removal of UV-Induced DNA Lesions by Nucleotide Excision Repair. Proc. Natl. Acad. Sci. USA 2013, 110, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.; Song, X.; Chu, W.; et al. SIRT1 Confers Protection against UVB- and H2O2-Induced Cell Death via Modulation of P53 and JNK in Cultured Skin Keratinocytes. J. Cell. Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef] [PubMed]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Nicotinamide Enhances Repair of Ultraviolet Radiation-Induced DNA Damage in Human Keratinocytes and Ex Vivo Skin. Carcinogenesis 2013, 34, 1144–1149. [Google Scholar] [CrossRef]
- Monfrecola, G.; Gaudiello, F.; Cirillo, T.; Fabbrocini, G.; Balato, A.; Lembo, S. Nicotinamide Downregulates Gene Expression of Interleukin-6, Interleukin-10, Monocyte Chemoattractant Protein-1, and Tumour Necrosis Factor-α Gene Expression in HaCaT Keratinocytes after Ultraviolet B Irradiation. Clin. Exp. Dermatol. 2013, 38, 185–188. [Google Scholar] [CrossRef]
- Monfrecola, G.; Di Caprio, R.; Balato, N.; Bevilacqua, M.A.; Iovine, B.; Lembo, S.; Balato, A. Nicotinamide Reduces Cyclooxygenase-2 Expression in HaCaT Keratinocytes after Ultraviolet-B Irradiation. Br. J. Dermatol. 2017, 176, 1402–1404. [Google Scholar] [CrossRef]
- Gensler, H.L. Prevention of Photoimmunosuppression and Photocarcinogenesis by Topical Nicotinamide. Nutr. Cancer 1997, 29, 157–162. [Google Scholar] [CrossRef]
- Gensler, H.L.; Williams, T.; Huang, A.C.; Jacobson, E.L. Oral Niacin Prevents Photocarcinogenesis and Photoimmunosuppression in Mice. Nutr. Cancer 1999, 34, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Damian, D.L.; Patterson, C.R.S.; Stapelberg, M.; Park, J.; Barnetson, R.S.C.; Halliday, G.M. UV Radiation-Induced Immunosuppression Is Greater in Men and Prevented by Topical Nicotinamide. J. Investig. Dermatol. 2008, 128, 447–454. [Google Scholar] [CrossRef]
- Sivapirabu, G.; Yiasemides, E.; Halliday, G.M.; Park, J.; Damian, D.L. Topical Nicotinamide Modulates Cellular Energy Metabolism and Provides Broad-Spectrum Protection against Ultraviolet Radiation-Induced Immunosuppression in Humans. Br. J. Dermatol. 2009, 161, 1357–1364. [Google Scholar] [CrossRef]
- Yiasemides, E.; Sivapirabu, G.; Halliday, G.M.; Park, J.; Damian, D.L. Oral Nicotinamide Protects against Ultraviolet Radiation-Induced Immunosuppression in Humans. Carcinogenesis 2009, 30, 101–105. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Martin, A.J.; Moloney, F.J.; Damian, D.L. Oral Nicotinamide Reduces Actinic Keratoses in Phase II Double-Blinded Randomized Controlled Trials. J. Investig. Dermatol. 2012, 132, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2016, 374, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Kyme, P.; Thoennissen, N.H.; Tseng, C.W.; Thoennissen, G.B.; Wolf, A.J.; Shimada, K.; Krug, U.O.; Lee, K.; Müller-Tidow, C.; Berdel, W.E.; et al. C/EBPε Mediates Nicotinamide-Enhanced Clearance of Staphylococcus Aureus in Mice. J. Clin. Investig. 2012, 122, 3316–3329. [Google Scholar] [CrossRef]
- Ciebiada-Adamiec, A.; Małafiej, E.; Ciebiada, I. Inhibitory Effect of Nicotinamide on Enzymatic Activity of Selected Fungal Strains Causing Skin Infection. Mycoses 2010, 53, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Yélamos, O.; Halpern, A.C.; Weinstock, M.A. Reply to ‘A Phase II Randomized Controlled Trial of Nicotinamide for Skin Cancer Chemoprevention in Renal Transplant Recipients’. Br. J. Dermatol. 2017, 176, 551–552. [Google Scholar] [CrossRef]
- Boyce, S.T.; Supp, A.P.; Swope, V.B.; Warden, G.D. Vitamin C Regulates Keratinocyte Viability, Epidermal Barrier, and Basement Membrane In Vitro, and Reduces Wound Contraction After Grafting of Cultured Skin Substitutes. J. Investig. Dermatol. 2002, 118, 565–572. [Google Scholar] [CrossRef]
- Smirnoff, N.; Conklin, P.L.; Loewus, F.A. Biosynthesis of Ascorbic Acid in Plants: A Renaissance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 437–467. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, H.N.; Jung, D.J.; Kim, J.E.; Mun, G.H.; Kim, Y.S.; Cho, D.; Shin, D.H.; Hwang, Y.-I.; Lee, W.J. Regulation of UVB-Induced IL-8 and MCP-1 Production in Skin Keratinocytes by Increasing Vitamin C Uptake via the Redistribution of SVCT-1 from the Cytosol to the Membrane. J. Investig. Dermatol. 2007, 127, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.S.; Cameron, G.S.; Pence, B.C. Antioxidant Nutrients Protect against UVB-Induced Oxidative Damage to DNA of Mouse Keratinocytes in Culture. J. Investig. Dermatol. 1996, 106, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, B.; Wu, S.; Geilen, C.C.; Eberle, J.; Kodelja, V.; Orfanos, C.E. L-Ascorbic Acid Inhibits UVA-Induced Lipid Peroxidation and Secretion of IL-1alpha and IL-6 in Cultured Human Keratinocytes in Vitro. J. Investig. Dermatol. 1997, 108, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Funakoshi, T.; Sato, Y.; Saito, N.; Ohsawa, H.; Kurita, K.; Nagata, K.; Yoshida, M.; Ishigami, A. Protective Effect of Pre- and Post-Vitamin C Treatments on UVB-Irradiation-Induced Skin Damage. Sci. Rep. 2018, 8, 16199. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Qin, H.; Wu, W.; He, S.; Xu, J. Vitamin C Protects against UV Irradiation-Induced Apoptosis through Reactivating Silenced Tumor Suppressor Genes P21 and P16 in a Tet-Dependent DNA Demethylation Manner in Human Skin Cancer Cells. Cancer Biother. Radiopharm. 2014, 29, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Dunham, W.B.; Zuckerkandl, E.; Reynolds, R.; Willoughby, R.; Marcuson, R.; Barth, R.; Pauling, L. Effects of Intake of L-Ascorbic Acid on the Incidence of Dermal Neoplasms Induced in Mice by Ultraviolet Light. Proc. Natl. Acad. Sci. USA 1982, 79, 7532–7536. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Effect of Ascorbic Acid on Incidence of Spontaneous Mammary Tumors and UV-Light-Induced Skin Tumors in Mice. Am. J. Clin. Nutr. 1991, 54, 1252S–1255S. [Google Scholar] [CrossRef]
- McArdle, F.; Rhodes, L.E.; Parslew, R.; Jack, C.I.A.; Friedmann, P.S.; Jackson, M.J. UVR-Induced Oxidative Stress in Human Skin in Vivo: Effects of Oral Vitamin C Supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef]
- Mireles-Rocha, H.; Galindo, I.; Huerta, M.; Trujillo-Hernández, B.; Elizalde, A.; Cortés-Franco, R. UVB Photoprotection with Antioxidants: Effects of Oral Therapy with d-Alpha-Tocopherol and Ascorbic Acid on the Minimal Erythema Dose. Acta Derm. Venereol. 2002, 82, 21–24. [Google Scholar] [CrossRef]
- Fuchs, J.; Kern, H. Modulation of UV-Light-Induced Skin Inflammation by D-Alpha-Tocopherol and L-Ascorbic Acid: A Clinical Study Using Solar Simulated Radiation. Free Radic. Biol. Med. 1998, 25, 1006–1012. [Google Scholar] [CrossRef]
- Dreher, F.; Gabard, B.; Schwindt, D.A.; Maibach, H.I. Topical Melatonin in Combination with Vitamins E and C Protects Skin from Ultraviolet-Induced Erythema: A Human Study in Vivo. Br. J. Dermatol. 1998, 139, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Dreher, F.; Denig, N.; Gabard, B.; Schwindt, D.A.; Maibach, H.I. Effect of Topical Antioxidants on UV-Induced Erythema Formation When Administered after Exposure. Dermatology 1999, 198, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Spiegelman, D.; Egan, K.M.; Giovannucci, E.; Hunter, D.J.; Willett, W.C. Vitamin and Carotenoid Intake and Risk of Squamous Cell Carcinoma of the Skin. Int. J. Cancer 2003, 103, 110–115. [Google Scholar] [CrossRef]
- Fung, T.T.; Hunter, D.J.; Spiegelman, D.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Vitamins and Carotenoids Intake and the Risk of Basal Cell Carcinoma of the Skin in Women (United States). Cancer Causes Control CCC 2002, 13, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Placzek, M.; Gaube, S.; Kerkmann, U.; Gilbertz, K.-P.; Herzinger, T.; Haen, E.; Przybilla, B. Ultraviolet B-Induced DNA Damage in Human Epidermis Is Modified by the Antioxidants Ascorbic Acid and D-α-Tocopherol. J. Investig. Dermatol. 2005, 124, 304–307. [Google Scholar] [CrossRef]
- Eberlein-König, B.; Placzek, M.; Przybilla, B. Protective Effect against Sunburn of Combined Systemic Ascorbic Acid (Vitamin C) and d-α-Tocopherol (Vitamin E). J. Am. Acad. Dermatol. 1998, 38, 45–48. [Google Scholar] [CrossRef]
- Oresajo, C.; Stephens, T.; Hino, P.D.; Law, R.M.; Yatskayer, M.; Foltis, P.; Pillai, S.; Pinnell, S.R. Protective Effects of a Topical Antioxidant Mixture Containing Vitamin C, Ferulic Acid, and Phloretin against Ultraviolet-Induced Photodamage in Human Skin. J. Cosmet. Dermatol. 2008, 7, 290–297. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism and Function in the Skin. Mol. Cell. Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef]
- Reichrath, J.; Reichrath, S.; Heyne, K.; Vogt, T.; Roemer, K. Tumor Suppression in Skin and Other Tissues via Cross-Talk between Vitamin D- and P53-Signaling. Front. Physiol. 2014, 5, 166. [Google Scholar] [CrossRef]
- Gupta, R.; Dixon, K.M.; Deo, S.S.; Holliday, C.J.; Slater, M.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. Photoprotection by 1,25 Dihydroxyvitamin D3 Is Associated with an Increase in P53 and a Decrease in Nitric Oxide Products. J. Investig. Dermatol. 2007, 127, 707–715. [Google Scholar] [CrossRef]
- Dixon, K.M.; Norman, A.W.; Sequeira, V.B.; Mohan, R.; Rybchyn, M.S.; Reeve, V.E.; Halliday, G.M.; Mason, R.S. 1α,25(OH)2-Vitamin D and a Nongenomic Vitamin D Analogue Inhibit Ultraviolet Radiation–Induced Skin Carcinogenesis. Cancer Prev. Res. 2011, 4, 1485. [Google Scholar] [CrossRef]
- Wong, G.; Gupta, R.; Dixon, K.M.; Deo, S.S.; Choong, S.M.; Halliday, G.M.; Bishop, J.E.; Ishizuka, S.; Norman, A.W.; Posner, G.H.; et al. 1,25-Dihydroxyvitamin D and Three Low-Calcemic Analogs Decrease UV-Induced DNA Damage via the Rapid Response Pathway. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 567–570. [Google Scholar] [CrossRef] [PubMed]
- De Haes, P.; Garmyn, M.; Verstuyf, A.; De Clercq, P.; Vandewalle, M.; Degreef, H.; Vantieghem, K.; Bouillon, R.; Segaert, S. 1,25-Dihydroxyvitamin D3 and Analogues Protect Primary Human Keratinocytes against UVB-Induced DNA Damage. J. Photochem. Photobiol. B 2005, 78, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Song, E.J.; Gordon-Thomson, C.; Cole, L.; Stern, H.; Halliday, G.M.; Damian, D.L.; Reeve, V.E.; Mason, R.S. 1α,25-Dihydroxyvitamin D-3 Reduces Several Types of UV-Induced DNA Damage and Contributes to Photoprotection. J. STEROID Biochem. Mol. Biol. 2013, 136, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.M.; Sequeira, V.B.; Deo, S.S.; Mohan, R.; Posner, G.H.; Mason, R.S. Differential Photoprotective Effects of 1,25-Dihydroxyvitamin D3 and a Low Calcaemic Deltanoid. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2012, 11, 1825–1830. [Google Scholar] [CrossRef]
- Kim, J.S.; Jung, M.; Yoo, J.; Choi, E.H.; Park, B.C.; Kim, M.H.; Hong, S.P. Protective Effect of Topical Vitamin D3 against Photocarcinogenesis in a Murine Model. Ann Derm. 2016, 28, 304–313. [Google Scholar] [CrossRef]
- Scott, J.F.; Das, L.M.; Ahsanuddin, S.; Qiu, Y.; Binko, A.M.; Traylor, Z.P.; Debanne, S.M.; Cooper, K.D.; Boxer, R.; Lu, K.Q. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J. Investig. Dermatol. 2017, 137, 2078–2086. [Google Scholar] [CrossRef]
- Ellison, T.I.; Smith, M.K.; Gilliam, A.C.; MacDonald, P.N. Inactivation of the Vitamin D Receptor Enhances Susceptibility of Murine Skin to UV-Induced Tumorigenesis. J. Investig. Dermatol. 2008, 128, 2508–2517. [Google Scholar] [CrossRef]
- Sequeira, V.B.; Rybchyn, M.S.; Tongkao-On, W.; Gordon-Thomson, C.; Malloy, P.J.; Nemere, I.; Norman, A.W.; Reeve, V.E.; Halliday, G.M.; Feldman, D.; et al. The Role of the Vitamin D Receptor and ERp57 in Photoprotection by 1α,25-Dihydroxyvitamin D3. Mol. Endocrinol. 2012, 26, 574–582. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Jurkiewicz, B.A.; Bissett, D.L.; Buettner, G.R. Effect of Topically Applied Tocopherol on Ultraviolet Radiation-Mediated Free Radical Damage in Skin. J. Investig. Dermatol. 1995, 104, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Delinasios, G.J.; Karbaschi, M.; Cooke, M.S.; Young, A.R. Vitamin E Inhibits the UVAI Induction of “Light” and “Dark” Cyclobutane Pyrimidine Dimers, and Oxidatively Generated DNA Damage, in Keratinocytes. Sci. Rep. 2018, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cheng, Y.; Dai, Y.; Chen, M.; Wang, C. A-Tocopherol Protects Keratinocytes against Ultraviolet A Irradiation by Suppressing Glutathione Depletion, Lipid Peroxidation and Reactive Oxygen Species Generation. Biomed. Rep. 2014, 2, 419–423. [Google Scholar] [CrossRef]
- Maalouf, S.; El-Sabban, M.; Darwiche, N.; Gali-Muhtasib, H. Protective Effect of Vitamin E on Ultraviolet B Light-Induced Damage in Keratinocytes. Mol. Carcinog. 2002, 34, 121–130. [Google Scholar] [CrossRef]
- Jin, G.-H.; Liu, Y.; Jin, S.-Z.; Liu, X.-D.; Liu, S.-Z. UVB Induced Oxidative Stress in Human Keratinocytes and Protective Effect of Antioxidant Agents. Radiat. Environ. Biophys. 2007, 46, 61–68. [Google Scholar] [CrossRef]
- Trevithick, J.R.; Xiong, H.; Lee, S.; Shum, D.T.; Sanford, S.E.; Karlik, S.J.; Norley, C.; Dilworth, G.R. Topical Tocopherol Acetate Reduces Post-UVB, Sunburn-Associated Erythema, Edema, and Skin Sensitivity in Hairless Mice. Arch. Biochem. Biophys. 1992, 296, 575–582. [Google Scholar] [CrossRef]
- Roshchupkin, D.I.; Pistsov, M.Y.; Potapenko, A.Y. Inhibition of Ultraviolet Light-Induced Erythema by Antioxidants. Arch. Dermatol. Res. 1979, 266, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Gensler, H.L.; Magdaleno, M. Topical Vitamin E Inhibition of Immunosuppression and Tumorigenesis Induced by Ultraviolet Irradiation. Nutr. Cancer 1991, 15, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kuchide, M.; Tokuda, H.; Takayasu, J.; Enjo, F.; Ishikawa, T.; Ichiishi, E.; Naito, Y.; Yoshida, N.; Yoshikawa, T.; Nishino, H. Cancer Chemopreventive Effects of Oral Feeding Alpha-Tocopherol on Ultraviolet Light B Induced Photocarcinogenesis of Hairless Mouse. Cancer Lett. 2003, 196, 169–177. [Google Scholar] [CrossRef]
- Burns, E.M.; Tober, K.L.; Riggenbach, J.A.; Kusewitt, D.F.; Young, G.S.; Oberyszyn, T.M. Differential Effects of Topical Vitamin E and C E Ferulic® Treatments on Ultraviolet Light B-Induced Cutaneous Tumor Development in Skh-1 Mice. PLoS ONE 2013, 8, e63809. [Google Scholar] [CrossRef]
- Werninghaus, K.; Meydani, M.; Bhawan, J.; Margolis, R.; Blumberg, J.B.; Gilchrest, B.A. Evaluation of the Photoprotective Effect of Oral Vitamin E Supplementation. Arch. Dermatol. 1994, 130, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- McArdle, F.; Rhodes, L.E.; Parslew, R.A.G.; Close, G.L.; Jack, C.I.A.; Friedmann, P.S.; Jackson, M.J. Effects of Oral Vitamin E and Beta-Carotene Supplementation on Ultraviolet Radiation-Induced Oxidative Stress in Human Skin. Am. J. Clin. Nutr. 2004, 80, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Selim, M.A.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.A.; Pinnell, S.R. UV Photoprotection by Combination Topical Antioxidants Vitamin C and Vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef]
- Lin, F.-H.; Lin, J.-Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Angelica Selim, M.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic Acid Stabilizes a Solution of Vitamins C and E and Doubles Its Photoprotection of Skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef]
- Sugiyama, T.; Sadzuka, Y. Enhancing Effects of Green Tea Components on the Antitumor Activity of Adriamycin against M5076 Ovarian Sarcoma. Cancer Lett. 1998, 133, 19–26. [Google Scholar] [CrossRef]
- Mehendale, S.; Aung, H.; Wang, A.; Yin, J.-J.; Wang, C.-Z.; Xie, J.-T.; Yuan, C.-S. American Ginseng Berry Extract and Ginsenoside Re Attenuate Cisplatin-Induced Kaolin Intake in Rats. Cancer Chemother. Pharmacol. 2005, 56, 63–69. [Google Scholar] [CrossRef]
- Sak, K. Chemotherapy and Dietary Phytochemical Agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pihl, C.; Togsverd-Bo, K.; Andersen, F.; Haedersdal, M.; Bjerring, P.; Lerche, C.M. Keratinocyte Carcinoma and Photoprevention: The Protective Actions of Repurposed Pharmaceuticals, Phytochemicals and Vitamins. Cancers 2021, 13, 3684. https://doi.org/10.3390/cancers13153684
Pihl C, Togsverd-Bo K, Andersen F, Haedersdal M, Bjerring P, Lerche CM. Keratinocyte Carcinoma and Photoprevention: The Protective Actions of Repurposed Pharmaceuticals, Phytochemicals and Vitamins. Cancers. 2021; 13(15):3684. https://doi.org/10.3390/cancers13153684
Chicago/Turabian StylePihl, Celina, Katrine Togsverd-Bo, Flemming Andersen, Merete Haedersdal, Peter Bjerring, and Catharina Margrethe Lerche. 2021. "Keratinocyte Carcinoma and Photoprevention: The Protective Actions of Repurposed Pharmaceuticals, Phytochemicals and Vitamins" Cancers 13, no. 15: 3684. https://doi.org/10.3390/cancers13153684
APA StylePihl, C., Togsverd-Bo, K., Andersen, F., Haedersdal, M., Bjerring, P., & Lerche, C. M. (2021). Keratinocyte Carcinoma and Photoprevention: The Protective Actions of Repurposed Pharmaceuticals, Phytochemicals and Vitamins. Cancers, 13(15), 3684. https://doi.org/10.3390/cancers13153684