Characterization of Permeability Barrier Dysfunction in a Murine Model of Cutaneous Field Cancerization Following Chronic UV-B Irradiation: Implications for the Pathogenesis of Skin Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
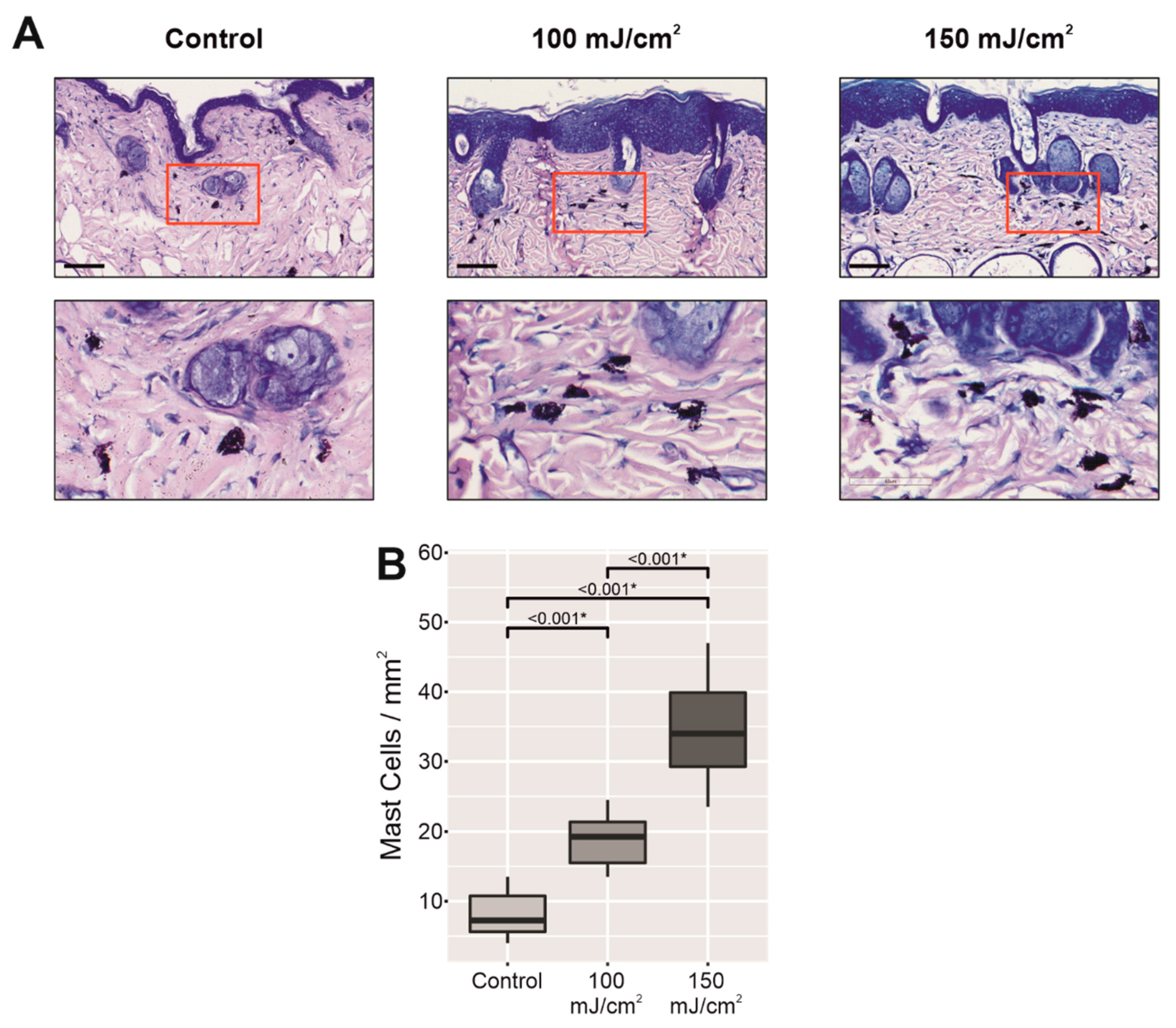
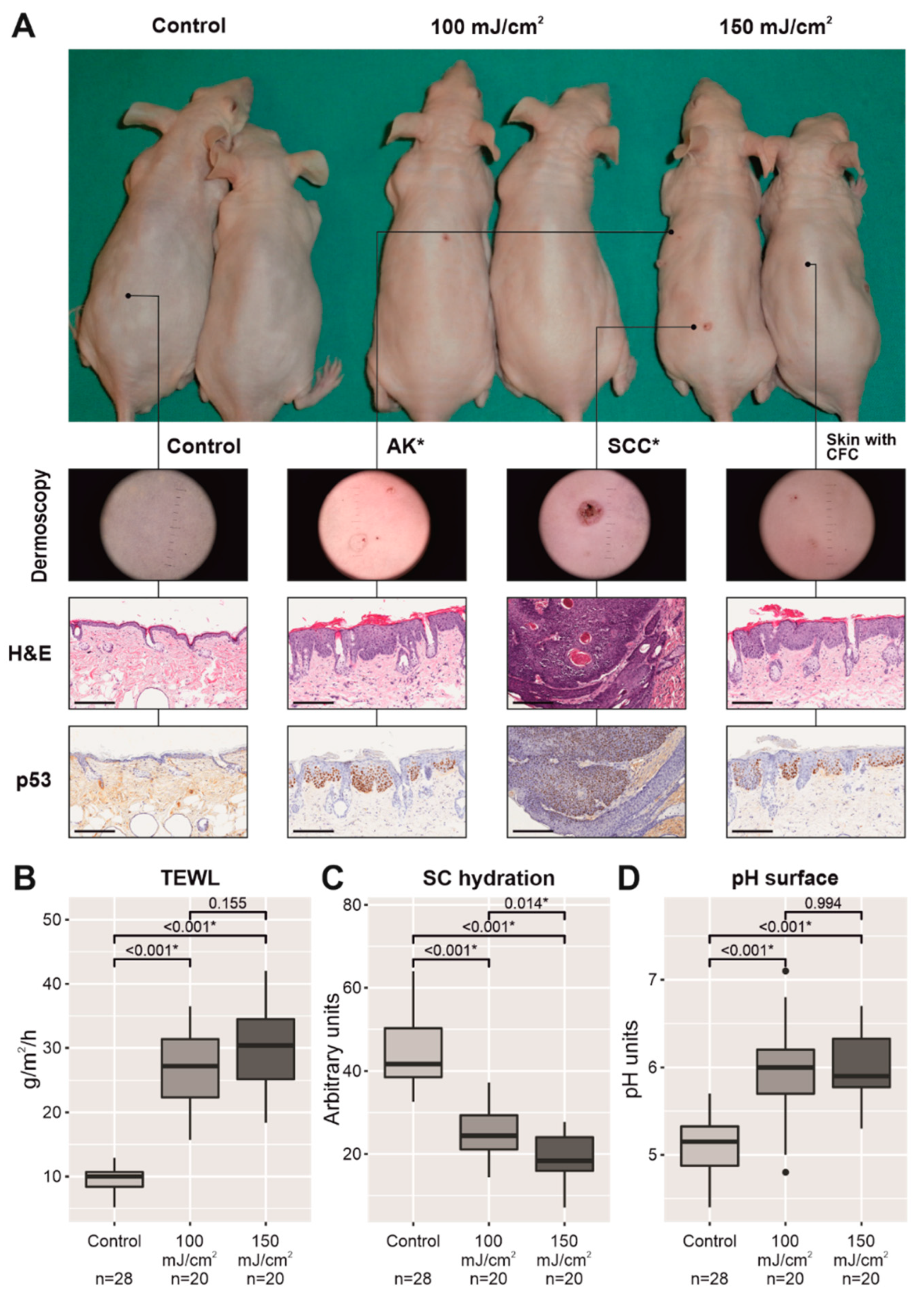
2.1. Chronic UV-B Irradiation Produced an Impairment in the Permeability Barrier in Skin with CFC
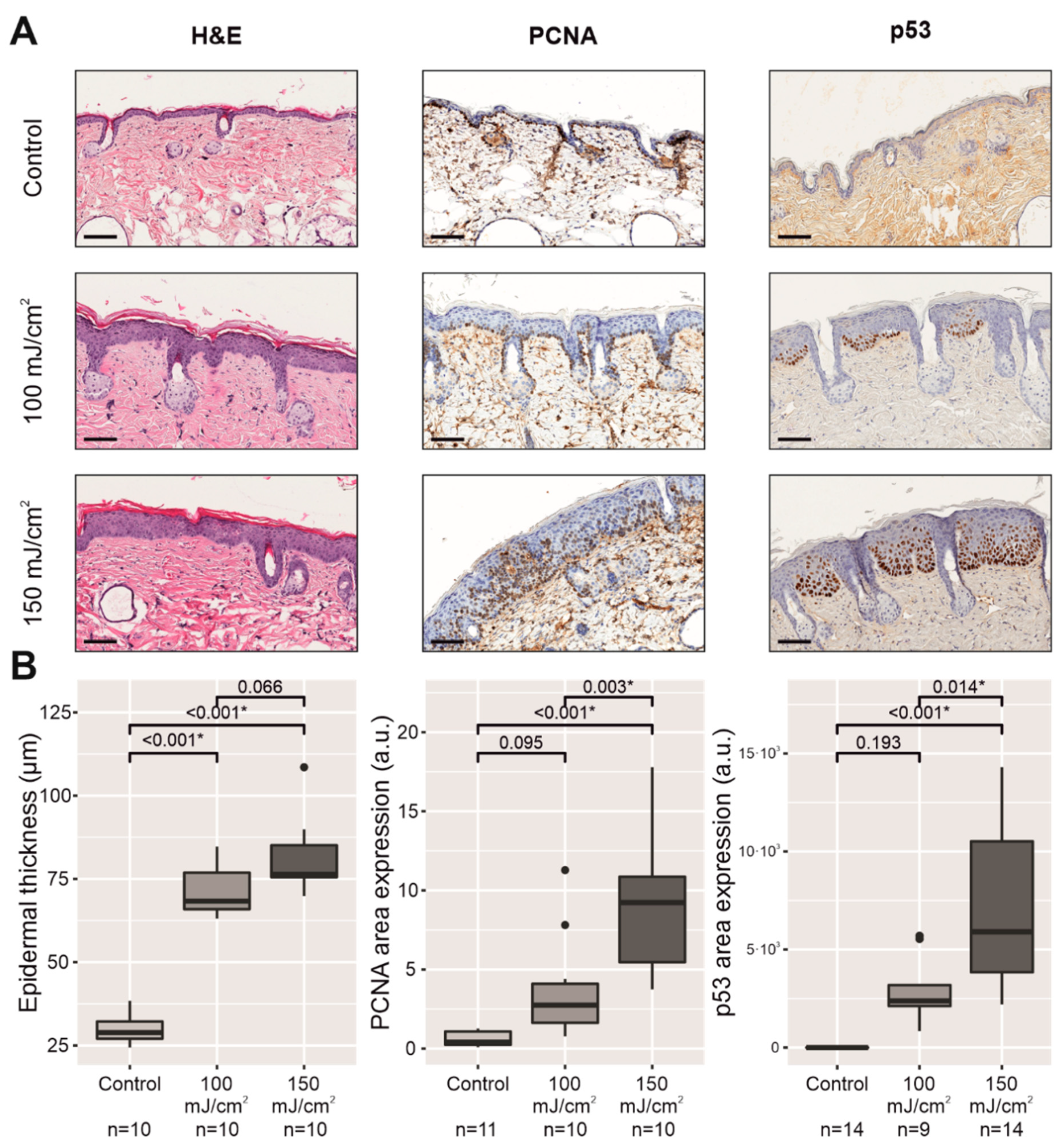
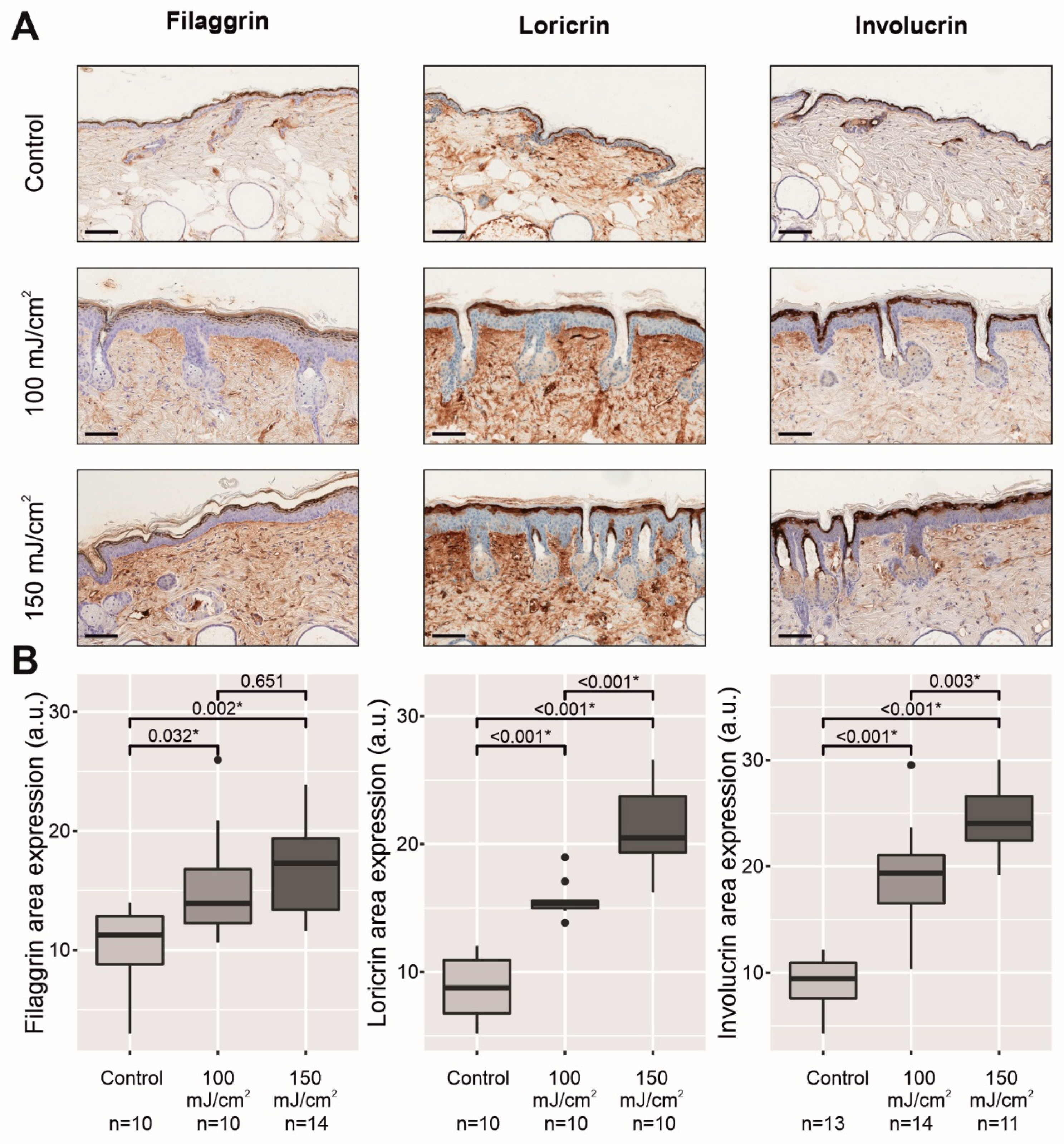
2.2. Chronic UV-B Irradiation Caused Epidermal Hyperplasia, PCNA Overexpression, and PIPs, Linked to the Skin with CFC
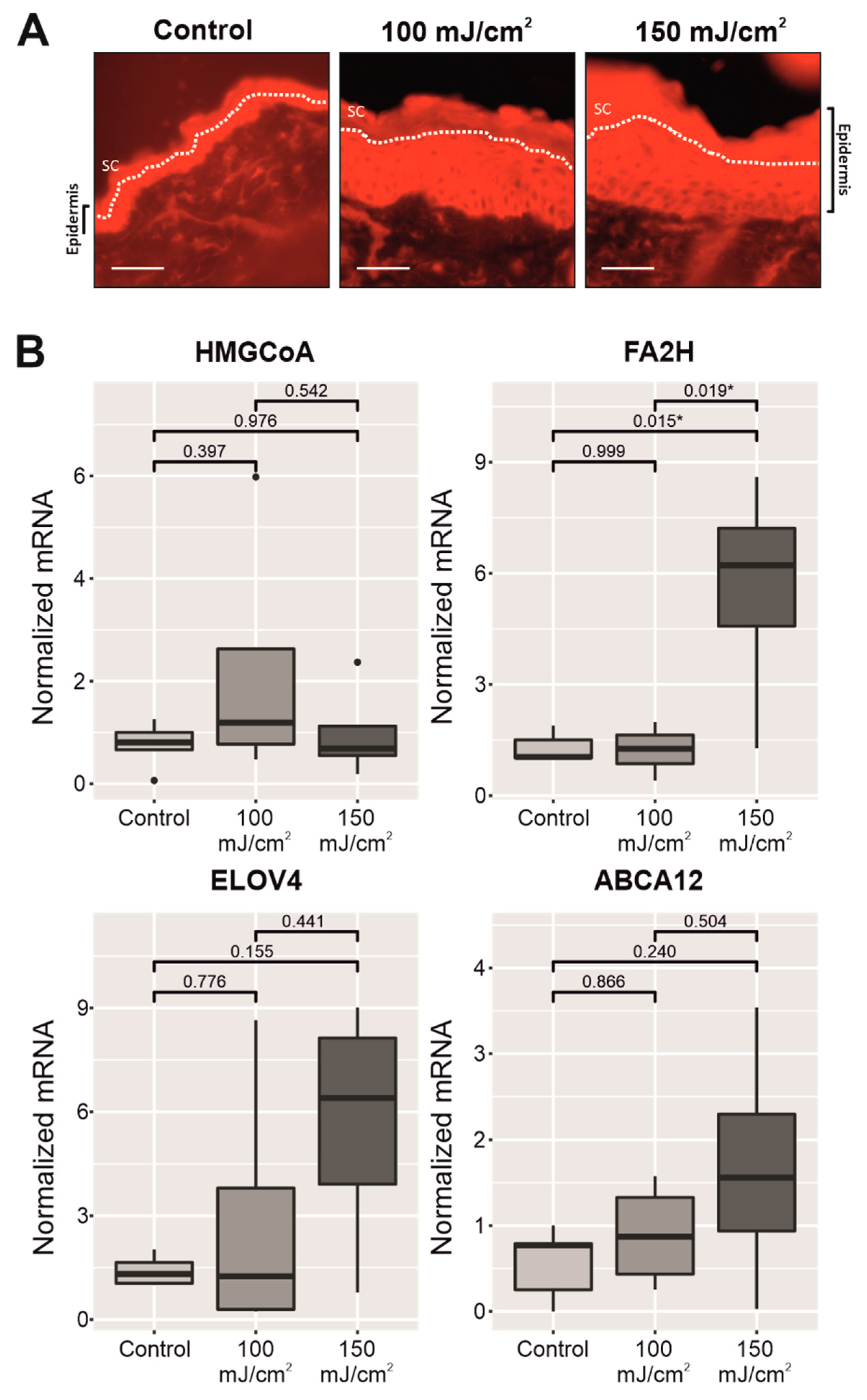
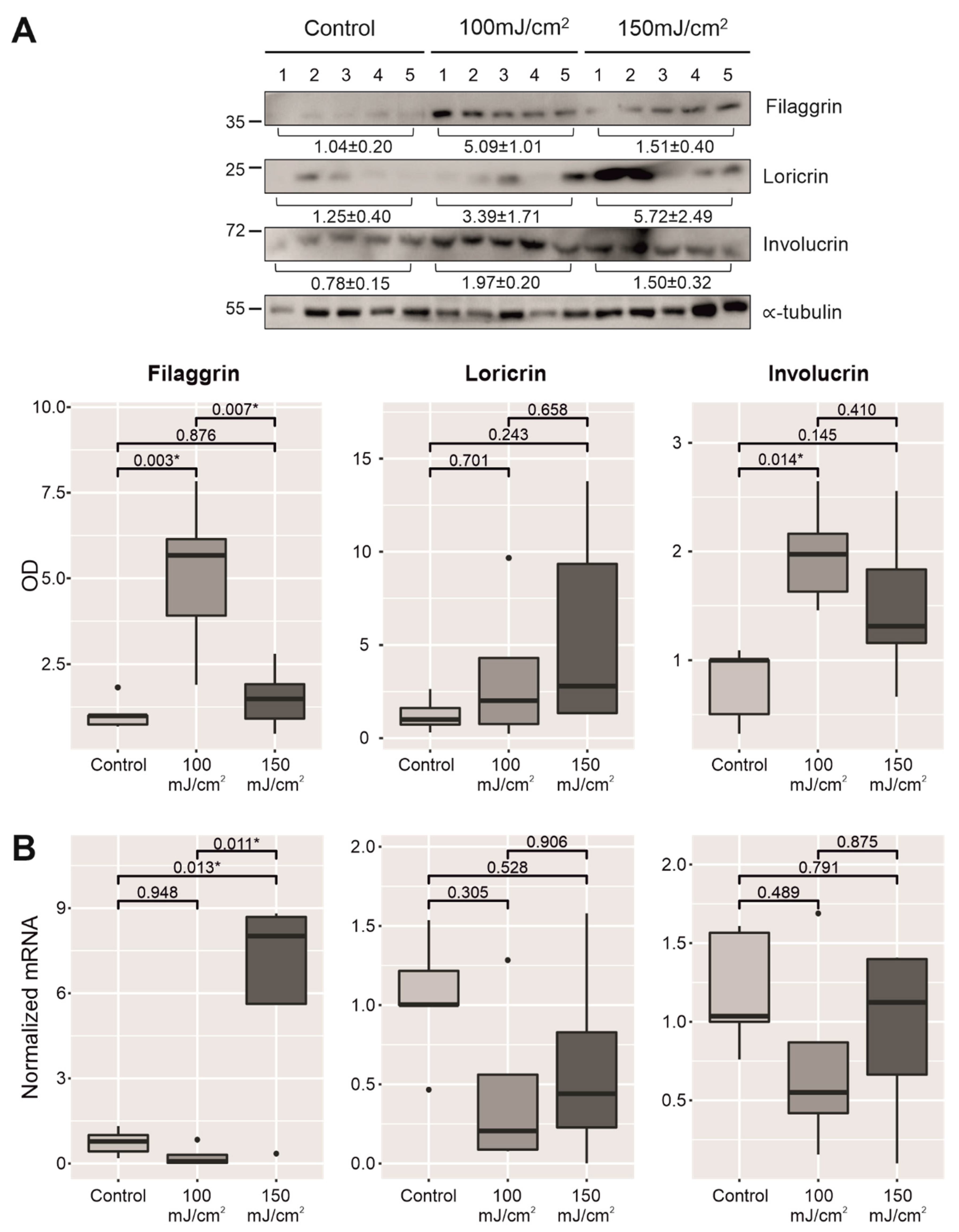
2.3. Chronic UV-B Exposure of Skin with CFC Upregulated the mRNA Expression and Protein Synthesis of Several Biomarkers Linked to the Permeability Barrier
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Assessment of Chronic UV-B Irradiation and Development of CFC
4.3. Measurement of the Skin Barrier Parameters in Skin with CFC
4.4. Histological and Immunohistochemical Analyses
4.5. Western Immunoblotting
4.6. qPCR for mRNA Expression
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA12 | ATP-binding cassette subfamily A member 12 |
| AK | Actinic keratosis |
| CFC | Cutaneous field cancerization |
| DNA | Deoxyribonucleic acid |
| ELOVL4 | Elongation of very long chain fatty acids protein 4 |
| FA2H | Fatty acid 2-hydroxylase |
| HMGCoA reductase | 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase |
| mRNA | Messenger RNA |
| PCNA | Proliferating cell nuclear antigen |
| PI3K | Phosphoinositide 3-kinase |
| PIP | p53 immunopositive patch |
| p53 | Tumor suppressor p53 |
| qPCR | Quantitative polymerase chain reaction |
| SCC | Squamous cell carcinoma |
| SEM | Standard error of the mean |
| TEWL | Transepidermal water loss |
| UV-A light | Ultraviolet A light |
| UV-B light | Ultraviolet B light |
| WB | Western blotting |
Appendix A

| Group | n | Skin Appearance | Number of Macroscopic AKs/SCCs | Dermoscopy | Histopathology (Skin with CFC) |
|---|---|---|---|---|---|
| Control group | 1 | Aged mouse skin: thin pale skin with milia cysts. | None | Thin pale skin. Milia cysts. | Thin (normal) epidermis without keratinocyte atypia. Milia cysts in the dermis. |
| 2 | Aged mouse skin: thin pale skin with milia cysts. | None | Thin pale skin. Milia cysts. | Thin (normal) epidermis without keratinocyte atypia. Milia cysts in the dermis. | |
| 3 | Aged mouse skin: thin pale skin with milia cysts. | None | Thin pale skin. Milia cysts. | Thin (normal) epidermis without keratinocyte atypia. Milia cysts in the dermis. | |
| 4 | Aged mouse skin: thin pale skin with milia cysts. | None | Thin pale skin. Milia cysts. | Thin (normal) epidermis without keratinocyte atypia. Milia cysts in the dermis. | |
| 5 | Aged mouse skin: thin pale skin with milia cysts. | None | Thin pale skin. Milia cysts. | Thin (normal) epidermis without keratinocyte atypia. Milia cysts in the dermis. | |
| UV-B Irradiated Skin 100 mJ/cm2 | 1 | Thicker erythematous scaly skin. Cutaneous dryness. Few hyperkeratotic verrucous lesions. | Nº AKs: 5 Nº SCCs: 0 | AKs: Pink scaly structures without vessels. | Orthokeratotic hyperplasia of the epidermis with few patches of parakeratosis corresponding to microscopic AKs. Hypergranulosis. Keratinocyte atypia. Mast cells in the dermis. Milia cysts in the dermis. |
| 2 | Thicker erythematous scaly skin. Cutaneous dryness. Few hyperkeratotic verrucous lesions. | Nº AKs: 3 Nº SCCs: 2 | AKs: Pink scaly structures without vessels. SCCs: Scaly crusted lesions with polymorphic vessels. | Orthokeratotic hyperplasia of the epidermis with few patches of microscopic AKs. Hypergranulosis. Keratinocyte atypia. Few inflammatory infiltrates in AKs. Mast cells in the dermis. Milia cysts in the dermis. | |
| 3 | Thicker erythematous scaly skin. Cutaneous dryness. Few hyperkeratotic lesions. | Nº AKs: 5 Nº SCCs: 0 | AKs: Pink scaly structures without vessels. | Orthokeratotic hyperplasia of the epidermis with few patches of microscopic AKs and in situ SCC. Hypergranulosis. Keratinocyte atypia. Few inflammatory infiltrates in AKs or in situ SCC. Mast cells in the dermis. Milia cysts in the dermis. | |
| 4 | Thicker erythematous scaly skin. Cutaneous dryness. | Nº AKs: 2 Nº SCCs: 0 | AKs: Pink scaly structures without vessels. | Orthokeratotic hyperplasia of the epidermis with few patches of microscopic AKs. Hypergranulosis. Keratinocyte atypia. Few inflammatory infiltrates in AKs. Mast cells in the dermis. Ectatic blood vessels in the dermis. Milia cysts in the dermis. | |
| 5 | Thicker erythematous scaly skin. Cutaneous dryness. Few hyperkeratotic verrucous lesions. | Nº AKs: 9 Nº SCCs: 4 | AKs: Pink scaly structures without vessels. SCCs: Whitish structures with polymorphic vessels. | Orthokeratotic hyperplasia of the epidermis with few patches of microscopic AKs. Hypergranulosis. Keratinocyte atypia. Few inflammatory infiltrates in some of the AKs. Ectatic blood vessels in the dermis. Milia cysts in the dermis. | |
| UV-B Irradiated Skin 150 mJ/cm2 | 1 | Thicker scaly erythematous skin. Cutaneous dryness. Hyperkeratotic verrucous lesions. | Nº AKs: 2 Nº SCCs: 6 | AKs: Pink scaly structures without vessels. SCCs: Verrucous hyperkeratotic lesions, central crust, and atypical vessels. Polypoid whitish lesions with polymorphic vessels. | Orthokeratotic hyperplasia of the epidermis. Hypergranulosis. Microscopic AKs (more common). Marked keratinocyte atypia. Few inflammatory infiltrates in some of the AKs. Ectatic blood vessels in the dermis. Mast cells in the dermis. Milia cysts in the dermis. |
| 2 | Thicker erythematous scaly skin. Cutaneous dryness. Few hyperkeratotic verrucous lesions. | Nº AKs: 3 Nº SCCs: 3 | AKs: Pink scaly structures without vessels. SCCs: Crusted and whitish structures with polymorphic vessels. | Orthokeratotic hyperplasia of the epidermis. Hypergranulosis. Microscopic AKs (more common) and in situ SCC. Marked keratinocyte atypia. Few inflammatory infiltrates in some of the AKs. Mast cells in the dermis. Milia cysts in the dermis. | |
| 3 | Thicker erythematous scaly skin. Cutaneous dryness. Hyperkeratotic verrucous lesions. | Nº AKs: 5 Nº SCCs: 6 | AKs: Pink scaly structures without vessels. SCCs: Hyperkeratotic whitish structures, central crust, and looped vessels. Polypoid whitish lesions with polymorphic vessels. | Orthokeratotic hyperplasia of the epidermis. Hypergranulosis. Microscopic AKs. Keratinocyte atypia. Few inflammatory infiltrates in AKs. Mast cells in the dermis. Milia cysts in the dermis. | |
| 4 | Thicker erythematous scaly skin. Cutaneous dryness. | Nº AKs: 3 Nº SCCs: 0 | AKs: Pink scaly structures without vessels. | Orthokeratotic hyperplasia of the epidermis. Hypergranulosis. Microscopic AKs. Marked keratinocyte atypia. Few inflammatory infiltrates in AKs. Mast cells in the dermis. Ectatic blood vessels in the dermis. Milia cysts in the dermis. | |
| 5 | Thicker erythematous scaly skin. Cutaneous dryness. | Nº AKs: 2 Nº SCCs: 0 | AKs: Pink scaly structures without vessels. | Orthokeratotic hyperplasia of the epidermis. Hypergranulosis. Microscopic AKs and in situ SCC. Marked keratinocyte atypia. Mast cells in the dermis. Ectatic blood vessels in the dermis. Milia cysts in the dermis. |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| 18S RNA | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGTAGCG |
| mFilaggrin | ATGTCCGCTCTCCTGGAAAG | TGGATTCTTCAAGACTGCCTGTA |
| mLoricrin | GTGGAAAGACCTCTGGTGGA | TGGAACCACCTCCATAGGAA |
| mInvolucrin | AAGGGCTTTCCCAAACATGA | TGCTGGTGCTCACACTTTTGA |
| mHMGCoA reductase | CTTGTGGAATGCCTTGTGAT | CCGAAGCAGCACATGATC |
| mFA2H | CGCTGGCTGGAGCAGTACTAT | TGCAGAGGCTACAGCACCATT |
| mELOVL4 | CGATAAGCATAAGCACGCTCTATC | AACGGCTCGCGGTCTTTC |
| mABCA12 | AGGATGGCTTCCCAGTTTCA | TGGCCATAAGATCAAGACAAGTGT |
References
- Haywood, R.; Rogge, F.; Lee, M. Protein, Lipid, and DNA Radicals to Measure Skin UVA Damage and Modulation by Melanin. Free Radic. Biol. Med. 2008, 44, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Jinnestål, C.L.; Belfrage, E.; Bäck, O.; Schmidtchen, A.; Sonesson, A. Skin Barrier Impairment Correlates with Cutaneous Staphylococcus Aureus Colonization and Sensitization to Skin-Associated Microbial Antigens in Adult Patients with Atopic Dermatitis. Int. J. Dermatol. 2014, 53, 27–33. [Google Scholar] [CrossRef]
- Kim, B.E.; Kim, J.; Goleva, E.; Berdyshev, E.; Lee, J.; Vang, K.A.; Lee, U.H.; Han, S.; Leung, S.; Hall, C.F.; et al. Particulate Matter Causes Skin Barrier Dysfunction. JCI Insight 2021, 6, 145185. [Google Scholar] [CrossRef] [PubMed]
- Jakasa, I.; Thyssen, J.P.; Kezic, S. The Role of Skin Barrier in Occupational Contact Dermatitis. Exp. Dermatol. 2018, 27, 909–914. [Google Scholar] [CrossRef]
- Pigors, M.; Kiritsi, D.; Cobzaru, C.; Schwieger-Briel, A.; Suárez, J.; Faletra, F.; Aho, H.; Mäkelä, L.; Kern, J.S.; Bruckner-Tuderman, L.; et al. TGM5 Mutations Impact Epidermal Differentiation in Acral Peeling Skin Syndrome. J. Investig. Dermatol. 2012, 132, 2422–2429. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Feingold, K.R.; Elias, P.M. Role of Lipids in the Formation and Maintenance of the Cutaneous Permeability Barrier. Biochim. Biophys. Acta 2014, 1841, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Schmuth, M.; Blunder, S.; Dubrac, S.; Gruber, R.; Moosbrugger-Martinz, V. Epidermal Barrier in Hereditary Ichthyoses, Atopic Dermatitis, and Psoriasis. J. Dtsch. Dermatol. Ges. 2015, 13, 1119–1123. [Google Scholar] [CrossRef]
- Nemes, Z.; Steinert, P.M. Bricks and Mortar of the Epidermal Barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-H.; Man, M.-Q.; Xu, P.; Xin, S.; Liu, Z.; Crumrine, D.A.; Jiang, Y.J.; Fluhr, J.W.; Feingold, K.R.; Elias, P.M.; et al. Stratum Corneum Acidification Is Impaired in Moderately Aged Human and Murine Skin. J. Investig. Dermatol. 2007, 127, 2847–2856. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-Induced Skin Damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Ratushny, V.; Gober, M.D.; Hick, R.; Ridky, T.W.; Seykora, J.T. From Keratinocyte to Cancer: The Pathogenesis and Modeling of Cutaneous Squamous Cell Carcinoma. J. Clin. Investig. 2012, 122, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Ramos-e-Silva, M.; Boza, J.C.; Cestari, T.F. Effects of Age (Neonates and Elderly) on Skin Barrier Function. Clin. Dermatol. 2012, 30, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV Radiation Reduces the Barrier Function of Human Skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef] [PubMed]
- Permatasari, F.; Zhou, B.; Luo, D. Epidermal Barrier: Adverse and Beneficial Changes Induced by Ultraviolet B Irradiation Depending on the Exposure Dose and Time (Review). Exp. Ther. Med. 2013, 6, 287–292. [Google Scholar] [CrossRef]
- Fernandez Figueras, M.T. From Actinic Keratosis to Squamous Cell Carcinoma: Pathophysiology Revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. 2), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Oster-Schmidt, C.; Stockfleth, E. Nonmelanoma Skin Cancer—From Actinic Keratosis to Cutaneous Squamous Cell Carcinoma. J. Dtsch. Dermatol. Ges. 2018, 16, 1002–1013. [Google Scholar] [CrossRef]
- Ulrich, M.; Maltusch, A.; Röwert-Huber, J.; González, S.; Sterry, W.; Stockfleth, E.; Astner, S. Actinic Keratoses: Non-Invasive Diagnosis for Field Cancerisation. Br. J. Dermatol. 2007, 156 (Suppl. 3), 13–17. [Google Scholar] [CrossRef]
- Friis, K.B.E.; Themstrup, L.; Jemec, G.B.E. Optical Coherence Tomography in the Diagnosis of Actinic Keratosis-A Systematic Review. Photodiagnosis Photodyn. Ther. 2017, 18, 98–104. [Google Scholar] [CrossRef]
- Tyrrell, J.; Campbell, S.M.; Curnow, A. Monitoring the Accumulation and Dissipation of the Photosensitizer Protoporphyrin IX during Standard Dermatological Methyl-Aminolevulinate Photodynamic Therapy Utilizing Non-Invasive Fluorescence Imaging and Quantification. Photodiagnosis Photodyn. Ther. 2011, 8, 30–38. [Google Scholar] [CrossRef]
- Jonason, A.S.; Kunala, S.; Price, G.J.; Restifo, R.J.; Spinelli, H.M.; Persing, J.A.; Leffell, D.J.; Tarone, R.E.; Brash, D.E. Frequent Clones of P53-Mutated Keratinocytes in Normal Human Skin. Proc. Natl. Acad. Sci. USA 1996, 93, 14025–14029. [Google Scholar] [CrossRef]
- Kramata, P.; Lu, Y.-P.; Lou, Y.-R.; Singh, R.N.; Kwon, S.M.; Conney, A.H. Patches of Mutant P53-Immunoreactive Epidermal Cells Induced by Chronic UVB Irradiation Harbor the Same P53 Mutations as Squamous Cell Carcinomas in the Skin of Hairless SKH-1 Mice. Cancer Res. 2005, 65, 3577–3585. [Google Scholar] [CrossRef]
- Robinson, S.; Dixon, S.; August, S.; Diffey, B.; Wakamatsu, K.; Ito, S.; Friedmann, P.S.; Healy, E. Protection against UVR Involves MC1R-Mediated Non-Pigmentary and Pigmentary Mechanisms in Vivo. J. Investig. Dermatol. 2010, 130, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Rebel, H.; Kram, N.; Westerman, A.; Banus, S.; van Kranen, H.J.; de Gruijl, F.R. Relationship between UV-Induced Mutant P53 Patches and Skin Tumours, Analysed by Mutation Spectra and by Induction Kinetics in Various DNA-Repair-Deficient Mice. Carcinogenesis 2005, 26, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Holleran, W.M.; Uchida, Y.; Halkier-Sorensen, L.; Haratake, A.; Hara, M.; Epstein, J.H.; Elias, P.M. Structural and Biochemical Basis for the UVB-Induced Alterations in Epidermal Barrier Function. Photodermatol. Photoimmunol. Photomed. 1997, 13, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Meguro, S.; Arai, Y.; Masukawa, Y.; Uie, K.; Tokimitsu, I. Relationship between Covalently Bound Ceramides and Transepidermal Water Loss (TEWL). Arch. Dermatol. Res. 2000, 292, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nakagawa, H.; Kondo, H.; Takema, Y.; Imokawa, G. Decreased Levels of Covalently Bound Ceramide Are Associated with Ultraviolet B-Induced Perturbation of the Skin Barrier. J. Investig. Dermatol. 2004, 123, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J.; Chu, A.W.; Lu, Z.F.; Pan, M.H.; Che, D.F.; Zhou, X.J. Ultraviolet B-Induced Alterations of the Skin Barrier and Epidermal Calcium Gradient. Exp. Dermatol. 2007, 16, 985–992. [Google Scholar] [CrossRef]
- Schaffer, B.S.; Grayson, M.H.; Wortham, J.M.; Kubicek, C.B.; McCleish, A.T.; Prajapati, S.I.; Nelon, L.D.; Brady, M.M.; Jung, I.; Hosoyama, T.; et al. Immune Competency of a Hairless Mouse Strain for Improved Preclinical Studies in Genetically Engineered Mice. Mol. Cancer Ther. 2010, 9, 2354–2364. [Google Scholar] [CrossRef]
- Pillon, A.; Gomes, B.; Vandenberghe, I.; Cartron, V.; Cèbe, P.; Blanchet, J.-C.; Sibaud, V.; Guilbaud, N.; Audoly, L.; Lamant, L.; et al. Actinic Keratosis Modelling in Mice: A Translational Study. PLoS ONE 2017, 12, e0179991. [Google Scholar] [CrossRef]
- Cozzi, S.-J.; Ogbourne, S.M.; James, C.; Rebel, H.G.; de Gruijl, F.R.; Ferguson, B.; Gardner, J.; Lee, T.T.; Larcher, T.; Suhrbier, A. Ingenol Mebutate Field-Directed Treatment of UVB-Damaged Skin Reduces Lesion Formation and Removes Mutant P53 Patches. J. Investig. Dermatol. 2012, 132, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.-O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P.; et al. Blackberry Extract Inhibits UVB-Induced Oxidative Damage and Inflammation through MAP Kinases and NF-ΚB Signaling Pathways in SKH-1 Mice Skin. Toxicol. Appl. Pharmacol. 2015, 284, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.M.; Liang, S.; Yeung, S.; Oiyemhonlan, E.; Cleveland, K.H.; Parsa, C.; Orlando, R.; Meyskens, F.L.; Andresen, B.T.; Huang, Y. Topically Applied Carvedilol Attenuates Solar Ultraviolet Radiation Induced Skin Carcinogenesis. Cancer Prev. Res. 2017, 10, 598–606. [Google Scholar] [CrossRef]
- Tan, J.M.; Lambie, D.; Sinnya, S.; Sahebian, A.; Soyer, H.P.; Prow, T.W.; Ardigò, M. Histopathology and Reflectance Confocal Microscopy Features of Photodamaged Skin and Actinic Keratosis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Persechino, F.; Ranieri, D.; Guttieri, L.; Nanni, M.; Torrisi, M.R.; Belleudi, F. Expression Profile of Fibroblast Growth Factor Receptors, Keratinocyte Differentiation Markers, and Epithelial Mesenchymal Transition-Related Genes in Actinic Keratosis: A Possible Predictive Factor for Malignant Progression? Biology 2021, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Bak, H.; Hong, S.-P.; Jeong, S.-K.; Choi, E.-H.; Lee, S.E.; Lee, S.-H.; Ahn, S.-K. Altered Epidermal Lipid Layers Induced by Long-Term Exposure to Suberythemal-Dose Ultraviolet. Int. J. Dermatol. 2011, 50, 832–837. [Google Scholar] [CrossRef]
- Del Bino, S.; Vioux, C.; Rossio-Pasquier, P.; Jomard, A.; Demarchez, M.; Asselineau, D.; Bernerd, F. Ultraviolet B Induces Hyperproliferation and Modification of Epidermal Differentiation in Normal Human Skin Grafted on to Nude Mice. Br. J. Dermatol. 2004, 150, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Kim, M.J.; Jung, M.-Y.; Jeon, H.; Goo, J.; Ahn, S.K.; Lee, S.H.; Elias, P.M.; Choi, E.H. Biopositive Effects of Low-Dose UVB on Epidermis: Coordinate Upregulation of Antimicrobial Peptides and Permeability Barrier Reinforcement. J. Investig. Dermatol. 2008, 128, 2880–2887. [Google Scholar] [CrossRef]
- Lee, J.H.; An, H.T.; Chung, J.H.; Kim, K.H.; Eun, H.C.; Cho, K.H. Acute Effects of UVB Radiation on the Proliferation and Differentiation of Keratinocytes. Photodermatol. Photoimmunol. Photomed. 2002, 18, 253–261. [Google Scholar] [CrossRef]
- Kambayashi, H.; Yamashita, M.; Odake, Y.; Takada, K.; Funasaka, Y.; Ichihashi, M. Epidermal Changes Caused by Chronic Low-Dose UV Irradiation Induce Wrinkle Formation in Hairless Mouse. J. Dermatol. Sci. 2001, 27 (Suppl. 1), S19–S25. [Google Scholar] [CrossRef]
- Kambayashi, H.; Odake, Y.; Takada, K.; Funasaka, Y.; Ichihashi, M. Involvement of Changes in Stratum Corneum Keratin in Wrinkle Formation by Chronic Ultraviolet Irradiation in Hairless Mice. Exp. Dermatol. 2003, 12 (Suppl. 2), 22–27. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Phenotypic and Lipidomic Characterization of Primary Human Epidermal Keratinocytes Exposed to Simulated Solar UV Radiation. J. Dermatol. Sci. 2018, 92, 97–105. [Google Scholar] [CrossRef]
- Löwenau, L.J.; Zoschke, C.; Brodwolf, R.; Volz, P.; Hausmann, C.; Wattanapitayakul, S.; Boreham, A.; Alexiev, U.; Schäfer-Korting, M. Increased Permeability of Reconstructed Human Epidermis from UVB-Irradiated Keratinocytes. Eur. J. Pharm. Biopharm. 2017, 116, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic Dermatitis: The Skin Barrier and Beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef]
- Lin, T.-K.; Crumrine, D.; Ackerman, L.D.; Santiago, J.-L.; Roelandt, T.; Uchida, Y.; Hupe, M.; Fabriàs, G.; Abad, J.L.; Rice, R.H.; et al. Cellular Changes That Accompany Shedding of Human Corneocytes. J. Investig. Dermatol. 2012, 132, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Mauro, T.; Holleran, W.M.; Grayson, S.; Gao, W.N.; Man, M.Q.; Kriehuber, E.; Behne, M.; Feingold, K.R.; Elias, P.M. Barrier Recovery Is Impeded at Neutral PH, Independent of Ionic Effects: Implications for Extracellular Lipid Processing. Arch. Dermatol. Res. 1998, 290, 215–222. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Roelandt, T.; Schürer, N.; Pu, X.; Fluhr, J.; Giddelo, C.; Man, M.-Q.; Crumrine, D.; Roseeuw, D.; Feingold, K.R.; et al. Acute Acidification of Stratum Corneum Membrane Domains Using Polyhydroxyl Acids Improves Lipid Processing and Inhibits Degradation of Corneodesmosomes. J. Investig. Dermatol. 2010, 130, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Hachem, J.-P.; Crumrine, D.; Fluhr, J.; Brown, B.E.; Feingold, K.R.; Elias, P.M. PH Directly Regulates Epidermal Permeability Barrier Homeostasis, and Stratum Corneum Integrity/Cohesion. J. Investig. Dermatol. 2003, 121, 345–353. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kurasawa, M.; Hattori, T.; Maeda, T.; Nakano, H.; Sasaki, H. Relationship between Expression of Tight Junction-Related Molecules and Perturbed Epidermal Barrier Function in UVB-Irradiated Hairless Mice. Arch. Dermatol. Res. 2008, 300, 61–68. [Google Scholar] [CrossRef]
- Kligman, L.H.; Murphy, G.F. Ultraviolet B Radiation Increases Hairless Mouse Mast Cells in a Dose-Dependent Manner and Alters Distribution of UV-Induced Mast Cell Growth Factor. Photochem. Photobiol. 1996, 63, 123–127. [Google Scholar] [CrossRef]
- Bosset, S.; Bonnet-Duquennoy, M.; Barré, P.; Chalon, A.; Kurfurst, R.; Bonté, F.; Schnébert, S.; Le Varlet, B.; Nicolas, J.F. Photoageing Shows Histological Features of Chronic Skin Inflammation without Clinical and Molecular Abnormalities. Br. J. Dermatol. 2003, 149, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory Mast Cells Up-Regulate Angiogenesis during Squamous Epithelial Carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef]
- Andreu, P.; Johansson, M.; Affara, N.I.; Pucci, F.; Tan, T.; Junankar, S.; Korets, L.; Lam, J.; Tawfik, D.; DeNardo, D.G.; et al. FcRgamma Activation Regulates Inflammation-Associated Squamous Carcinogenesis. Cancer Cell 2010, 17, 121–134. [Google Scholar] [CrossRef]
- Chacón-Salinas, R.; Limón-Flores, A.Y.; Chávez-Blanco, A.D.; Gonzalez-Estrada, A.; Ullrich, S.E. Mast Cell-Derived IL-10 Suppresses Germinal Center Formation by Affecting T Follicular Helper Cell Function. J. Immunol. 2011, 186, 25–31. [Google Scholar] [CrossRef]
- Ashida, Y.; Denda, M.; Hirao, T. Histamine H1 and H2 Receptor Antagonists Accelerate Skin Barrier Repair and Prevent Epidermal Hyperplasia Induced by Barrier Disruption in a Dry Environment. J. Investig. Dermatol. 2001, 116, 261–265. [Google Scholar] [CrossRef]
- Albibas, A.A.; Rose-Zerilli, M.J.J.; Lai, C.; Pengelly, R.J.; Lockett, G.A.; Theaker, J.; Ennis, S.; Holloway, J.W.; Healy, E. Subclonal Evolution of Cancer-Related Gene Mutations in P53 Immunopositive Patches in Human Skin. J. Investig. Dermatol. 2018, 138, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Haratake, A.; Uchida, Y.; Schmuth, M.; Tanno, O.; Yasuda, R.; Epstein, J.H.; Elias, P.M.; Holleran, W.M. UVB-Induced Alterations in Permeability Barrier Function: Roles for Epidermal Hyperproliferation and Thymocyte-Mediated Response. J. Investig. Dermatol. 1997, 108, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.; Elias, P.M.; Grunfeld, C.; Feingold, K.R.; Wood, L.C. Amphiregulin and Nerve Growth Factor Expression Are Regulated by Barrier Status in Murine Epidermis. J. Investig. Dermatol. 1997, 108, 73–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darido, C.; Georgy, S.R.; Jane, S.M. The Role of Barrier Genes in Epidermal Malignancy. Oncogene 2016, 35, 5705–5712. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, J.L.; Muñoz-Rodriguez, J.R.; Cruz-Morcillo, M.A.d.l.; Villar-Rodriguez, C.; Gonzalez-Lopez, L.; Aguado, C.; Nuncia-Cantarero, M.; Redondo-Calvo, F.J.; Perez-Ortiz, J.M.; Galan-Moya, E.M. Characterization of Permeability Barrier Dysfunction in a Murine Model of Cutaneous Field Cancerization Following Chronic UV-B Irradiation: Implications for the Pathogenesis of Skin Cancer. Cancers 2021, 13, 3935. https://doi.org/10.3390/cancers13163935
Santiago JL, Muñoz-Rodriguez JR, Cruz-Morcillo MAdl, Villar-Rodriguez C, Gonzalez-Lopez L, Aguado C, Nuncia-Cantarero M, Redondo-Calvo FJ, Perez-Ortiz JM, Galan-Moya EM. Characterization of Permeability Barrier Dysfunction in a Murine Model of Cutaneous Field Cancerization Following Chronic UV-B Irradiation: Implications for the Pathogenesis of Skin Cancer. Cancers. 2021; 13(16):3935. https://doi.org/10.3390/cancers13163935
Chicago/Turabian StyleSantiago, Juan Luis, Jose Ramon Muñoz-Rodriguez, Miguel Angel de la Cruz-Morcillo, Clara Villar-Rodriguez, Lucia Gonzalez-Lopez, Carolina Aguado, Miriam Nuncia-Cantarero, Francisco Javier Redondo-Calvo, Jose Manuel Perez-Ortiz, and Eva Maria Galan-Moya. 2021. "Characterization of Permeability Barrier Dysfunction in a Murine Model of Cutaneous Field Cancerization Following Chronic UV-B Irradiation: Implications for the Pathogenesis of Skin Cancer" Cancers 13, no. 16: 3935. https://doi.org/10.3390/cancers13163935
APA StyleSantiago, J. L., Muñoz-Rodriguez, J. R., Cruz-Morcillo, M. A. d. l., Villar-Rodriguez, C., Gonzalez-Lopez, L., Aguado, C., Nuncia-Cantarero, M., Redondo-Calvo, F. J., Perez-Ortiz, J. M., & Galan-Moya, E. M. (2021). Characterization of Permeability Barrier Dysfunction in a Murine Model of Cutaneous Field Cancerization Following Chronic UV-B Irradiation: Implications for the Pathogenesis of Skin Cancer. Cancers, 13(16), 3935. https://doi.org/10.3390/cancers13163935






