Pleuropneumonectomy as Salvage Therapy in Children Suffering from Primary or Metastatic Sarcomas with Pleural Localizations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment
2.3. Indication for PP
- -
- The only alternative to PP was palliative treatment.
- -
- The pleural lesions were limited to one hemithorax, possibly associated with one or more lesions within the ipsilateral lung parenchyma. No other metastatic localizations were found.
- -
- Patients received preoperative chemotherapy with sufficient response.
- -
- The patient must be in a reasonable good general condition, i.e., a Lansky performance scale above 80 (i.e., active but tires more quickly) [25].
2.4. Surgery
2.5. Radiotherapy
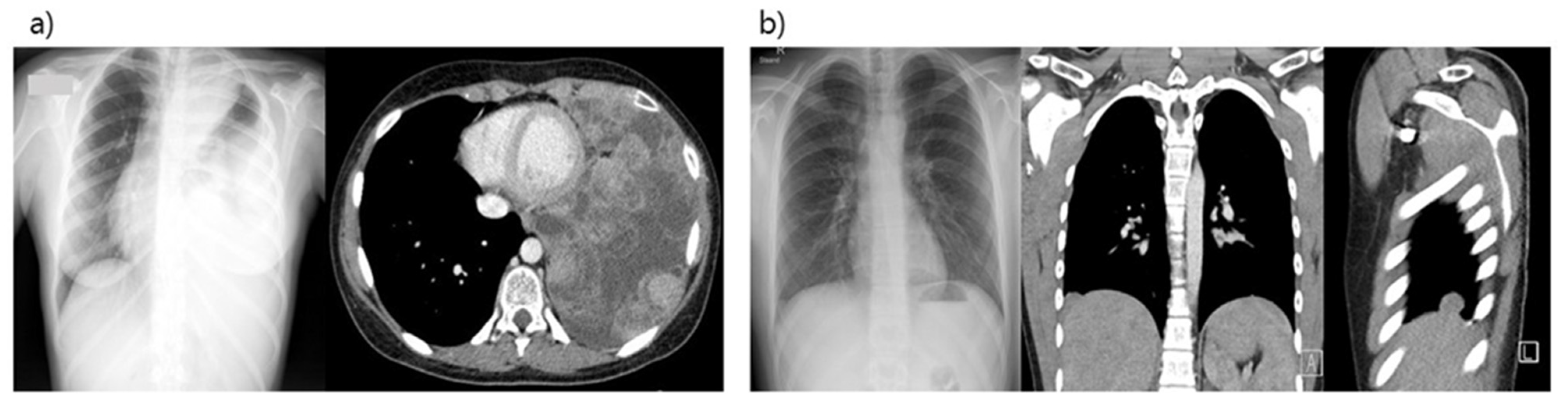
3. Results
3.1. Patient Characteristics
3.2. Pre- and Postoperative Treatment
3.3. Surgical Treatment
3.4. Pulmonary Function Tests
3.5. Survival and Recurrence
3.6. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotterill, S.J.; Ahrens, S.; Paulussen, M.; Jürgens, H.F.; Voûte, P.A.; Gadner, H.; Craft, A.W. Prognostic factors in Ewing’s tumor of bone: Analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J. Clin. Oncol. 2000, 18, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Branscheid, D.; Carrle, D.; Friedel, G.; Helmke, K.; Kevric, M.; Jundt, G.; Kühne, T.; Maas, R.; et al. Second and subsequent recurrences of osteosarcoma: Presentation, treatment and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J. Clin. Oncol. 2009, 27, 557–565. [Google Scholar] [CrossRef]
- Duranti, L.; Pardolesi, A.; Bertolaccini, L.; Tavecchio, L.; Scanagatta, P.; Rolli, L.; Pastorino, U. Extra-pleural pneumonectomy. J. Thorac. Dis. 2019, 11, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Sarot, I.A. Extrapleural pneumonectomy and pleurectomy in pulmonary tuberculosis. Thorax 1949, 4, 173–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butchart, E.G.; Ashcroft, T.; Barnsley, W.C.; Holden, M.P. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976, 31, 15–24. [Google Scholar] [PubMed]
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; et al. MARS trialists. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar]
- Tilleman, T.R.; Richards, W.G.; Zellos, L.; Johnson, B.E.; Jaklitsch, M.T.; Mueller, J.; Yeap, B.Y.; Mujoomdar, A.A.; Ducko, C.T.; Bueno, R.; et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: A phase II prospective study. J. Thorac. Cardiovasc. Surg. 2009, 138, 405–411. [Google Scholar] [CrossRef]
- Wright, C.D. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann. Thorac. Surg. 2006, 82, 1234–1239. [Google Scholar] [CrossRef]
- Huang, T.W.; Cheng, Y.L.; Tzao, C.; Chang, H.; Tsai, W.C.; Lee, S.C. Middle mediastinal thymoma. Respirology 2007, 12, 934–936. [Google Scholar] [CrossRef]
- Jin, W.B.; Liang, C.Y.; Peng, Y.H.; Zhou, N.K. Pleuropneumonectomy for diffuse pleural metastasis in primary lung cancer. J. Cancer Res. Ther. 2013, 9, 92–97. [Google Scholar]
- Bedini, A.V.; Tavecchio, L.; Delledonne, V. Extrapleural pneumonectomy for sarcomas report of two cases. Tumori 2000, 86, 422–423. [Google Scholar] [CrossRef]
- Yamaki, M.; Yonehara, S.; Noriyuki, T. Large primary pleural synovial sarcoma with severe dyspnea: A case report. Surg. Case Rep. 2017, 3, 29. [Google Scholar] [CrossRef][Green Version]
- Tosson, R.; Krismann, M. Primary myxoid sarcoma of the pleura: 5 years follow-up. Thorac. Cardiovasc. Surg. 2000, 48, 238–240. [Google Scholar] [CrossRef]
- Shapiro, M.; Swanson, S.J.; Wright, C.D.; Chin, C.; Sheng, S.; Wisnivesky, J.; Weiser, T.S. Predictors of major morbidity and mortality after pneumonectomy utilizing the society for thoracic surgeons general thoracic surgery database. Ann. Thorac. Surg. 2010, 90, 927–935. [Google Scholar] [CrossRef]
- Thomas, P.A.; Berbis, J.; Baste, J.M.; Le Pimpec-Barthes, F.; Tronc, F.; Falcoz, P.E.; Dahan, M.; Loundou, A.; EPITHOR Group. Pneumonectomy for lung cancer: Contemporary national early morbidity and mortality outcomes. J. Thorac. Cardiovasc. Surg. 2015, 149, 73–82. [Google Scholar] [CrossRef]
- Giubergia, V.; Alessandrini, F.; Barrias, C.; Giuseppucci, C.; Reusmann, A.; Barrenechea, M.; Castaños, C. Risk factors for morbidities and mortality in children following pneumonectomy. Respirology 2017, 22, 187–191. [Google Scholar] [CrossRef]
- Eren, S.; Eren, M.N.; Balci, A.E. Pneumonectomy in children for destroyed lung and the long-term consequences. J. Thorac. Cardiovasc. Surg. 2003, 126, 574–581. [Google Scholar] [CrossRef]
- Blyth, D.F.; Buckels, N.J.; Sewsunker, R.; Soni, M.A. Pneumonectomy in children. Eur. J. Cardiothorac. Surg. 2002, 22, 587–594. [Google Scholar] [CrossRef]
- Yalcin, S.; Ciftci, A.; Karnak, I.; Ekinci, S.; Tanyel, F.C.; Şenocak, M. Childhood pneumonectomies: Two decades’ experience of a referral center. Eur. J. Pediatr. Surg. 2013, 23, 115–220. [Google Scholar] [PubMed]
- Kosar, A.; Orki, A.; Kiral, H.; Demirhan, R.; Arman, B. Pneumonectomy in children for destroyed lung: Evaluation of 18 cases. Ann. Thorac. Surg. 2010, 89, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.M.; Su, W.; Lal, D.; Rusch, V.W.; La Quaglia, M.P. Extrapleural pneumonectomy in children. J. Pediatr. Surg. 2006, 41, 1738–1742. [Google Scholar] [CrossRef]
- Polites, S.F.; Heaton, T.E.; LaQuaglia, M.P.; Kim, E.S.; Barry, W.E.; Goodhue, C.J.; Murphy, A.J.; Davidoff, A.M.; Langham, M.R.; Meyers, R.L.; et al. Pneumonectomy for Pediatric Tumors-a Pediatric Surgical Oncology Research Collaborative Study. Ann. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sandler, G.; Hayes-Jordan, A. Chest wall reconstruction after tumor resection. Semin. Pediatr. Surg. 2018, 27, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lansky, S.B.; List, M.A.; Lansky, L.L.; Ritter-Sterr, C.; Miller, D.R. The measurement of performance in childhood cancer patients. Cancer 1987, 60, 1651–1656. [Google Scholar] [CrossRef]
- Andreou, D.; Ranft, A.; Gosheger, G.; Timmermann, B.; Ladenstein, R.; Hartmann, W.; Bauer, S.; Baumhoer, D.; van den Berg, H.; Dijkstra, P.D.S.; et al. Which Factors Are Associated with Local Control and Survival of Patients with Localized Pelvic Ewing’s Sarcoma? A Retrospective Analysis of Data from the Euro-EWING99 Trial. Clin. Orthop. Relat. Res. 2020, 478, 290–302. [Google Scholar]
- Haveman, L.M.; Ranft, A.; Vd Berg, H.; Smets, A.; Kruseova, J.; Ladenstein, R.; Brichard, B.; Paulussen, M.; Kuehne, T.; Juergens, H.; et al. The relation of radiological tumor volume response to histological response and outcome in patients with localized Ewing Sarcoma. Cancer Med. 2019, 8, 1086–1094. [Google Scholar] [CrossRef]
- Miwa, S.; Takeuchi, A.; Shirai, T.; Taki, J.; Yamamoto, N.; Nishida, H.; Hayashi, K.; Tanzawa, Y.; Kimura, H.; Igarashi, K.; et al. Prognostic value of radiological response to chemotherapy in patients with osteosarcoma. PLoS ONE 2013, 8, e70015. [Google Scholar] [CrossRef][Green Version]
- Bacci, G.; Ferrari, S.; Bertoni, F.; Picci, P.; Bacchini, P.; Longhi, A.; Donati, D.; Forni, C.; Campanacci, L.; Campanacci, M. Histologic response of high-grade nonmetastatic osteosarcoma of the extremity to chemotherapy. Clin. Orthop. Relat. Res. 2001, 386, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Picci, P.; Rougraff, B.T.; Bacci, G.; Neff, J.R.; Sangiorgi, L.; Baldini, A.C.; Ferrari, S.; Mercuri, M.; Ruggieri, P. Prognostic significance of histopathologic response to chemotherapy in nonmetastatic Ewing’s sarcoma of the extremities. J. Clin. Oncol. 1993, 11, 1763–1769. [Google Scholar] [CrossRef]
- Sauer, R.; Jürgens, H.; Burgers, J.M.; Dunst, J.; Hawlicek, R.; Michaelis, J. Prognostic factors in the treatment of Ewing’s sarcoma. The Ewing’s Sarcoma Study Group of the German Society of Paediatric Oncology CESS 81. Radiother. Oncol. 1987, 10, 101–110. [Google Scholar] [CrossRef]
- Harting, M.T.; Blakely, M.L.; Jaffe, N.; Cox, C.S., Jr.; Hayes-Jordan, A.; Benjamin, R.S.; Raymond, A.K.; Andrassy, R.J.; Lally, K.P. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J. Pediatr. Surg. 2006, 41, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; LaQuaglia, M.P.; Cordeiro, P.G. Prevention of postpneumonectomy syndrome in children with prophylactic tissue expander insertion. J. Pediatr. Surg. 2012, 47, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, D.; Krupnick, A.S.; Huddleston, C.B. Outcomes and late complications after pulmonary resections in the pediatric population. Semin. Thorac. Cardiovasc. Surg. 2004, 16, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.J.; Helenowski, I.; Murphy, A.J.; Mansfield, S.A.; LaQuaglia, M.P.; Heaton, T.E.; Cavalli, M.; Murphy, J.T.; Newman, E.; Overmen, R.E.; et al. Implications of Tumor Characteristics and Treatment Modality on Local Recurrence and Functional Outcomes in Children with Chest Wall Sarcoma: A Pediatric Surgical Oncology Research Collaborative Study. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Lopez, C.; Correa, A.; Vaporciyan, A.; Austin, M.; Rice, D.; Hayes-Jordan, A. Outcomes of chest wall resections in pediatric sarcoma patients. J. Pediatr. Surg. 2017, 52, 109–114. [Google Scholar] [CrossRef]
- Barrena, S.; Miguel, M.; Burgos, L.; Fernández, A.; Queizán, A.; Hernández, F.; Lassaletta, L.; Tovar, J.A. Neumonectomía en niños [Pneumonectomy in children]. Cir. Pediatr. 2010, 23, 74–76. [Google Scholar]
| Pat.nr. | Age at Diagnosis/Sex | Initial Diagnosis | First Line Chemotherapy According Treatment Protocol | Time between End of Treatment and Relapse with Pleuropulmonary Lesions | Number of Relapses | 2nd Line Chemo-Therapy | Histological Response after PP | Additional Therapy after PP | EFS (Months/Years) | OS (Months/Years) | Patient Status (Months/Years after PP) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 y/Male | Osteosarcoma of the femur | French OS2006 protocol M-EI courses | 28 months | 1 | IE–APx4 | Poor > 10% viable cells | No | 7.1 years | 7.1 years | Alive |
| 2 | 6 y/Male | Osteosarcoma of the humerus | French OS2006 protocol M-EI courses | 11 months | 1 | APx2 | Poor > 50% vital tumor cells. | RT and HD-CT with Thiotepa followed by aSCT | 11.3 years | 11.3 years | Alive |
| 3 | 15 y/Female | Ewing sarcoma of the chest wall | Ewing 99 protocol | Pleural involvement at diagnosis | - | no | Good, rare viable cells.in pleura infiltrated | Hemithoracic RT with boost and VAI x7 | 14 years | 14 years | Alive |
| 4 | 10 y/Female | undifferentiated sarcoma of the chest wall | IA 4x + ICE courses | Pleural involvement at diagnosis | - | AP and palliative vinblastin | Poor > 50% viable cells | Hemithoracic RT | 2 months | 3 months | Died |
| 5 | 10 y/Female | Ewing sarcoma of the pelvis with lung mets | Ewing 99 protocol VIDE courses followed by aSCT (BuMel) | 29 months | 2 all in left lung | TemIri | Poor > 50% viable cells | Hemithoracic RT and Tem + CPT11 vinorelbin + C | 7 months | 10 months | Died |
| 6 | 13 y/Female | Osteosarcoma of the femur | Euramos protocol: MAP courses + IE because of radiological progression | 22 months | 3 | GD | Poor vital tumor cells in pleura parietalis | RT and Ca E courses | 10 months | 13 months | Died |
| 7 | 16 y/Male | Ewing sarcoma of the rib | Ewing 2008 protocol | Pleural involvement at diagnosis, early recurrence with pleural lesions after primary surgery | 1 | TemIri | Good, no vital tumor cells, but vital cells in biopsy of diaphragm | GD + RT | 1.5 months | 5 months | Died |
| 8 | 15 y/Male | Osteosarcoma of the femur | Euramos protocol: MAP courses | 31 months | 1 | IE | Good < 5% vital tumor cells, no infiltration in pleura | no | 1.5 years | 1.5 years | Alive |
| 9 | 15 y/Male | Osteosarcoma of the femur | Euramos protocol: MAP courses | 15 months | 1 | IE | Good, 0% tumor cells, no infiltration in pleura | GD after local recurrence | 7 months | 11 months | Alive, with recurrence of disease |
| Pat.nr. | Technique + Resection Margins | Early Complications (<4 Weeks) | Days in Hospital after PP | Pulmonary Function Test | Late Complications (>4 Weeks) | Scores on Quality of Life Questionnaire | |
|---|---|---|---|---|---|---|---|
| Pre- Operative | Post- Operative | ||||||
| 1 | -PP right side -Lateral thoracotomy (7th ICS) -Complete resection of diaphragm -Limits in sano, marginal resection | none | 16 | TLC: 68% FEV1: 80% | 4.5 years after PP TLC: 50% FEV1: 37% | Mild dyspnea on exertion | 5 years after PP Functional Score: 33% Global Health Score: 44.6% Symptom Score: 67% |
| 2 | -PP left side -Lateral thoracotomy (5th and subsequently 9th ICS) -Resection of the medial part of the diaphragm -Limits in sano, marginal resection -No infiltration in pleura | None | 8 | TLC: 104% FEV1: 111% | 3 years after PP TLC: 53% FEV1: 48% 6 years after PP TLC: 38% FEV1: 38% | Severe scoliosis (Cobb angle >25°) Mild dyspnea on exertion | 6 years after PP Functional Score: 98% Global Health Score: 91.6% Symptom Scales: 0% |
| 3 | -PP left side -Lateral thoracotomy (5th then 9th ICS) -Resection part of diaphragm -Partial pericardium resection -Limits not in sano, complete resection | None | 15 | 5.5 years after PP TLC: 66% FEV1: 83.5% 8 years after PP TLC: 81% FEV1: 53% | Hiatal herniation with ulcerated esophagitis Asymmetrical breasts Mild dyspnea on exertion | 9 years after PP Functional Score: 85% Global Health Score: 75% Symptom Score: 20% | |
| 4 | -PP left side -Hemiclamshell -Resection of the medium arch of the 3rd and 4th rib -Positive resection margins | None | 8 | TLC: 71% FEV1: 77% | 1 month after PP TLC: 49% FEV1: 52.5% | ||
| 5 | -PP left side -Lateral thoracotomy (5th then 9th ICS) -Resection of major medial part of diaphragm -Positive resection margins | None | 10 | TLC: 67% FEV1: - | Not performed due to early progression | Feeding difficulties resolved after gastrostomy | |
| 6. | -PP left side -Posterolateral thoracotomy (6th ICS, subsequently 1st ICS) -Complete resection hemidiaphragm -Part of pericard: reconstruction with patch -positive resection margins | Hematothorax due to re-bleeding → re-surgery 4 days after PP | 17 | TLC: 67% FEV1: 62% (after earlier lobectomy) | 9 months after PP TLC: 44% FEV1: 41.5% | Mild dyspnea complaints in case of moderate physical activity | |
| 7. | -PP right side -Posterolateral thoracotomy -Complete resection hemidiaphragm -Pericard patch -positive resection margins | None | 17 | TLC: 71% FEV1: 78% | Not performed due to early progression | ||
| 8. | -PP left side -Posterolateral thoracotomy (4th ICS) -Complete resection hemi -diaphragm -complete resection | Infection (day 7), requiring long term antibiotics | 16 | TLC: 85% FEV1:78% | 8 months after PP TLC: 50% FEV1: 42% | Mild dyspnea complaints in case of moderate physical activity | 18 months after PP Functional Score: 90% Global Health Score: 67% Symptom Score: 6% |
| 9. | -PP right side -Posterolateral thoracotomy -Complete resection hemidiaphragm -Resection of small part pericardium -complete resection | Pneumonia (day 3) pneumopericardium (day 3) -cardiac tamponade → re-surgery: pericardial window (day 7) | 24 | TLC: 72% FEV1: 67% (earlier wedge excision) | 9 months after PP TLC: 38% FEV1: 41% | Slightly reduced cardiac function 5 months after PP → ACE-inhibitor (same range as function before PP) Dyspnea in case of physical activity | 9 months after PP Functional Score: 77% Global Health Score: 75% Symptom Score: 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameury, F.; Marec-Berard, P.; Eymery, M.; Wijnen, M.H.W.; van der Kaaij, N.; Mure, P.-Y.; Tronc, F.; Chotel, F.; Libbrecht, C.; van Boven, W.J.P.; et al. Pleuropneumonectomy as Salvage Therapy in Children Suffering from Primary or Metastatic Sarcomas with Pleural Localizations. Cancers 2021, 13, 3655. https://doi.org/10.3390/cancers13153655
Hameury F, Marec-Berard P, Eymery M, Wijnen MHW, van der Kaaij N, Mure P-Y, Tronc F, Chotel F, Libbrecht C, van Boven WJP, et al. Pleuropneumonectomy as Salvage Therapy in Children Suffering from Primary or Metastatic Sarcomas with Pleural Localizations. Cancers. 2021; 13(15):3655. https://doi.org/10.3390/cancers13153655
Chicago/Turabian StyleHameury, Frédéric, Perrine Marec-Berard, Mathilde Eymery, Marc H. W. Wijnen, Niels van der Kaaij, Pierre-Yves Mure, François Tronc, Franck Chotel, Clara Libbrecht, Wim Jan P. van Boven, and et al. 2021. "Pleuropneumonectomy as Salvage Therapy in Children Suffering from Primary or Metastatic Sarcomas with Pleural Localizations" Cancers 13, no. 15: 3655. https://doi.org/10.3390/cancers13153655
APA StyleHameury, F., Marec-Berard, P., Eymery, M., Wijnen, M. H. W., van der Kaaij, N., Mure, P.-Y., Tronc, F., Chotel, F., Libbrecht, C., van Boven, W. J. P., & Haveman, L. M. (2021). Pleuropneumonectomy as Salvage Therapy in Children Suffering from Primary or Metastatic Sarcomas with Pleural Localizations. Cancers, 13(15), 3655. https://doi.org/10.3390/cancers13153655






