Impact of Smoking-Related Chronic Obstruction Pulmonary Disease on Mortality of Invasive Ductal Carcinoma Patients Receiving Standard Treatments: Propensity Score-Matched, Nationwide, Population-Based Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.3. Propensity Score Matching and Covariates
2.4. Statistics
3. Results
3.1. Propensity Score Matching and Study Cohort
3.2. Prognostic Factors of All-Cause Mortality after Multivariate Cox Regression Analyses
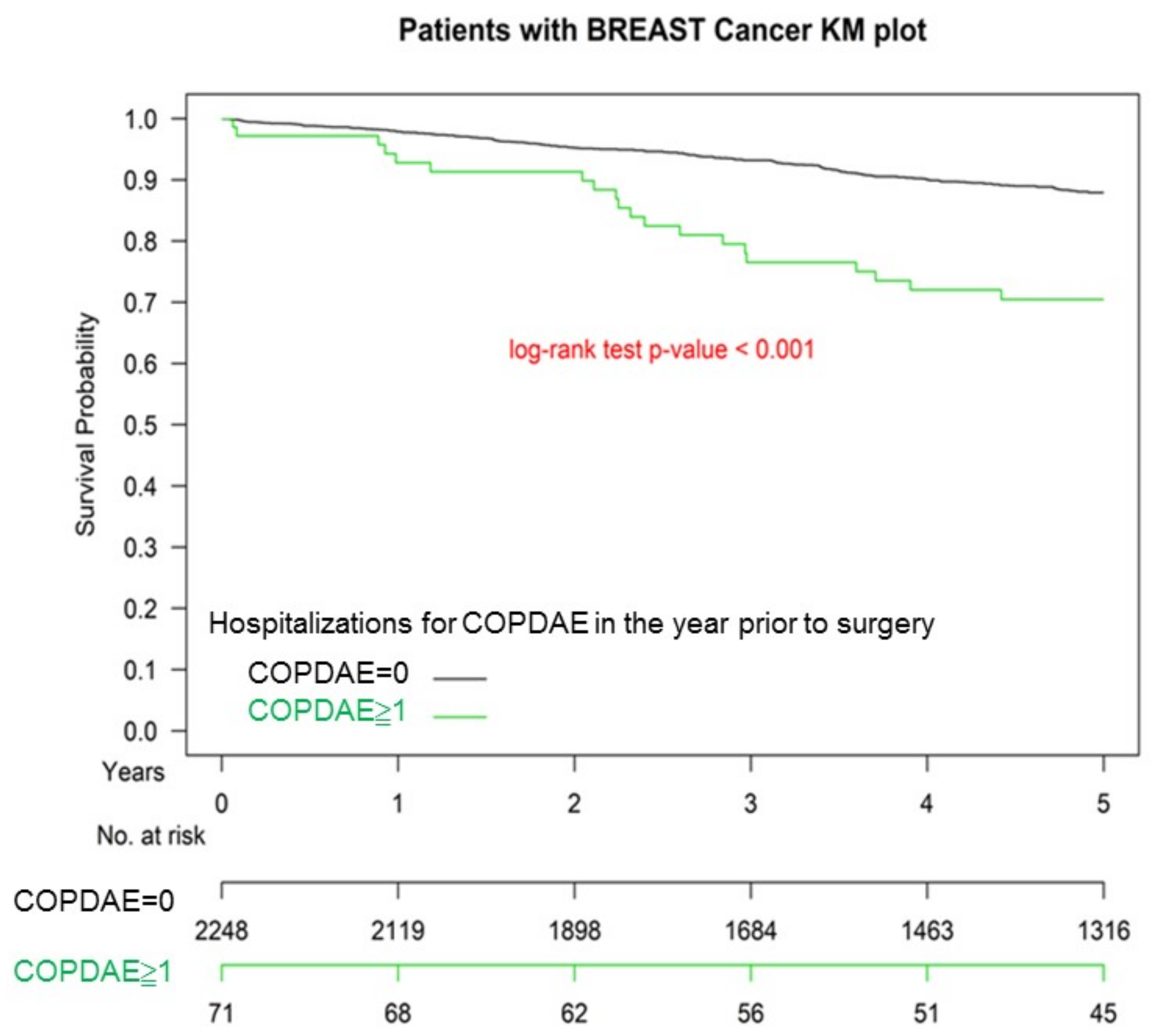
3.3. Kaplan–Meier OS among Non-COPD, COPD, and Hospitalization for COPDAE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | Chronic Obstructive Pulmonary Disease |
| IDC | Invasive Ductal Carcinoma |
| COPDAE | COPD with Acute Exacerbation |
| aHR | Adjusted Hazard Ratio |
| CI | Confidence Interval |
| MACE | Major Adverse Cardiac Events |
| AJCC | American Joint Committee on Cancer |
| RILI | Radiation-Induced Lung Injury |
| RP | Radiation Pneumonitis |
| TCRD | Taiwan Cancer Registry Database |
| PSM | Propensity Score Matching |
| SD | Standard Deviation |
| AJCC | American Joint Committee on Cancer |
| HR | Hormone Receptor |
| Her-2 | Human Epidermal Growth Factor Receptor-2 |
| OS | Overall Survival |
| SLNB | Sentinel Lymph Node Biopsy |
| ALND | Axillary Lymph Node Dissection |
| CKD | Chronic Kidney Disease |
| CCI | Charlson Comorbidity Index |
| ICD-10-CM | International Classification of Diseases, 10th Revision, Clinical Modification |
| NCCN | National Comprehensive Cancer Network |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
References
- Bhatt, S.P.; Dransfield, M.T. Chronic obstructive pulmonary disease and cardiovascular disease. Transl. Res. 2013, 162, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Laratta, C.R.; van Eeden, S. Acute exacerbation of chronic obstructive pulmonary disease: Cardiovascular links. Biomed. Res. Int. 2014, 2014, 528789. [Google Scholar] [CrossRef] [PubMed]
- Ghoorah, K.; De Soyza, A.; Kunadian, V. Increased cardiovascular risk in patients with chronic obstructive pulmonary disease and the potential mechanisms linking the two conditions: A review. Cardiol. Rev. 2013, 21, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N. The effect of reducing the number of cigarettes smoked on risk of lung cancer, COPD, cardiovascular disease and FEV(1)—A Review. Regul. Toxicol. Pharmacol. 2013, 67, 372–381. [Google Scholar] [CrossRef]
- Reilev, M.; Pottegard, A.; Lykkegaard, J.; Sondergaard, J.; Ingebrigtsen, T.S.; Hallas, J. Increased risk of major adverse cardiac events following the onset of acute exacerbations of COPD. Respirology 2019, 24, 1183–1190. [Google Scholar] [CrossRef]
- Gram, I.T.; Park, S.Y.; Kolonel, L.N.; Maskarinec, G.; Wilkens, L.R.; Henderson, B.E.; Le Marchand, L. Smoking and Risk of Breast Cancer in a Racially/Ethnically Diverse Population of Mainly Women Who Do Not Drink Alcohol: The MEC Study. Am. J. Epidemiol. 2015, 182, 917–925. [Google Scholar] [CrossRef]
- Gaudet, M.M.; Carter, B.D.; Brinton, L.A.; Falk, R.T.; Gram, I.T.; Luo, J.; Milne, R.L.; Nyante, S.J.; Weiderpass, E.; Beane Freeman, L.E.; et al. Pooled analysis of active cigarette smoking and invasive breast cancer risk in 14 cohort studies. Int. J. Epidemiol. 2017, 46, 881–893. [Google Scholar] [CrossRef]
- Macacu, A.; Autier, P.; Boniol, M.; Boyle, P. Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 154, 213–224. [Google Scholar] [CrossRef]
- Gaudet, M.M.; Gapstur, S.M.; Sun, J.; Diver, W.R.; Hannan, L.M.; Thun, M.J. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J. Natl. Cancer Inst. 2013, 105, 515–525. [Google Scholar] [CrossRef]
- Johnson, K.C.; Miller, A.B.; Collishaw, N.E.; Palmer, J.R.; Hammond, S.K.; Salmon, A.G.; Cantor, K.P.; Miller, M.D.; Boyd, N.F.; Millar, J.; et al. Active smoking and secondhand smoke increase breast cancer risk: The report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009). Tob. Control. 2011, 20, e2. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed on 28 June 2021).
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Bronnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Hooning, M.J.; Botma, A.; Aleman, B.M.; Baaijens, M.H.; Bartelink, H.; Klijn, J.G.; Taylor, C.W.; van Leeuwen, F.E. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J. Natl. Cancer Inst. 2007, 99, 365–375. [Google Scholar] [CrossRef]
- Qin, A.; Thompson, C.L.; Silverman, P. Predictors of late-onset heart failure in breast cancer patients treated with doxorubicin. J. Cancer Surviv. 2015, 9, 252–259. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Drafts, B.C.; Twomley, K.M.; D’Agostino, R., Jr.; Lawrence, J.; Avis, N.; Ellis, L.R.; Thohan, V.; Jordan, J.; Melin, S.A.; Torti, F.M.; et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc. Imaging 2013, 6, 877–885. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Doroshow, J.H. Doxorubicin-induced cardiac toxicity. N. Engl. J. Med. 1991, 324, 843–845. [Google Scholar] [CrossRef]
- Rancati, T.; Ceresoli, G.L.; Gagliardi, G.; Schipani, S.; Cattaneo, G.M. Factors predicting radiation pneumonitis in lung cancer patients: A retrospective study. Radiother. Oncol. 2003, 67, 275–283. [Google Scholar] [CrossRef]
- Robnett, T.J.; Machtay, M.; Vines, E.F.; McKenna, M.G.; Algazy, K.M.; McKenna, W.G. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 89–94. [Google Scholar] [CrossRef]
- Yu, J.M.; Hsieh, M.C.; Qin, L.; Zhang, J.; Wu, S.Y. Metformin reduces radiation-induced cardiac toxicity risk in patients having breast cancer. Am. J. Cancer Res. 2019, 9, 1017–1026. [Google Scholar]
- Zhang, J.; Lu, C.Y.; Chen, C.H.; Chen, H.M.; Wu, S.Y. Effect of pathologic stages on postmastectomy radiation therapy in breast cancer receiving neoadjuvant chemotherapy and total mastectomy: A Cancer Database Analysis. Breast 2020, 54, 70–78. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Pathologic response rates for breast cancer stages as a predictor of outcomes in patients receiving neoadjuvant chemotherapy followed by breast-conserving surgery. Surg. Oncol. 2020, 36, 91–98. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, C.Y.; Qin, L.; Chen, H.M.; Wu, S.Y. Breast-conserving surgery with or without irradiation in women with invasive ductal carcinoma of the breast receiving preoperative systemic therapy: A cohort study. Breast 2020, 54, 139–147. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, M.; Chang, E.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Pathologic response as predictor of recurrence, metastasis, and survival in breast cancer patients receiving neoadjuvant chemotherapy and total mastectomy. Am. J. Cancer Res. 2020, 10, 3415–3427. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Lu, C.Y.; Qin, L.; Chen, H.M.; Wu, S.Y. Outcome of post-mastectomy radiotherapy after primary systemic treatment in patients with different clinical tumor and nodal stages of breast cancer: A cohort study. Am. J. Cancer Res. 2020, 10, 2185–2198. [Google Scholar]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy with or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC1+ or 2. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef]
- Bahreini, F.; Soltanian, A.R.; Mehdipour, P. A meta-analysis on concordance between immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to detect HER2 gene overexpression in breast cancer. Breast Cancer 2015, 22, 615–625. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Chen, J.H.; Yen, Y.C.; Yang, H.C.; Liu, S.H.; Yuan, S.P.; Wu, L.L.; Lee, F.P.; Lin, K.C.; Lai, M.T.; Wu, C.C.; et al. Curative-Intent Aggressive Treatment Improves Survival in Elderly Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma and High Comorbidity Index. Medicine 2016, 95, e3268. [Google Scholar] [CrossRef] [PubMed]
- Bonora, B.M.; Avogaro, A.; Fadini, G.P. Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence. Diabetes Metab. Syndr. Obes. 2020, 13, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Yanoff, L.B.; Andraca-Carrera, E.; Thanh Hai, M. Assessing the Safety of Glucose-Lowering Drugs—A New Focus for the FDA. N. Engl. J. Med. 2020, 383, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; van der Worp, H.B.; van der Graaf, Y.; Visseren, F.L.J.; Westerink, J. Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc. Diabetol. 2017, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Arnott, C.; Li, Q.; Kang, A.; Neuen, B.L.; Bompoint, S.; Lam, C.S.P.; Rodgers, A.; Mahaffey, K.W.; Cannon, C.P.; Perkovic, V.; et al. Sodium-Glucose Cotransporter 2 Inhibition for the Prevention of Cardiovascular Events in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e014908. [Google Scholar] [CrossRef]
- Ramchand, J.; Patel, S.K.; Srivastava, P.M.; Farouque, O.; Burrell, L.M. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS ONE 2018, 13, e0198144. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Austin, P.C. The performance of different propensity score methods for estimating marginal hazard ratios. Stat. Med. 2013, 32, 2837–2849. [Google Scholar] [CrossRef]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef]
- van Gestel, Y.R.; Hoeks, S.E.; Sin, D.D.; Huzeir, V.; Stam, H.; Mertens, F.W.; van Domburg, R.T.; Bax, J.J.; Poldermans, D. COPD and cancer mortality: The influence of statins. Thorax 2009, 64, 963–967. [Google Scholar] [CrossRef][Green Version]
- Janssen-Heijnen, M.L.; Maas, H.A.; Houterman, S.; Lemmens, V.E.; Rutten, H.J.; Coebergh, J.W. Comorbidity in older surgical cancer patients: Influence on patient care and outcome. Eur. J. Cancer 2007, 43, 2179–2193. [Google Scholar] [CrossRef]
- Pierce, J.P.; Patterson, R.E.; Senger, C.M.; Flatt, S.W.; Caan, B.J.; Natarajan, L.; Nechuta, S.J.; Poole, E.M.; Shu, X.O.; Chen, W.Y. Lifetime cigarette smoking and breast cancer prognosis in the after Breast Cancer Pooling Project. J. Natl. Cancer Inst. 2014, 106, djt359. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2018 Report); Global Initiative for Chronic Obstructive Lung Disease, Inc.: Fontana, WI, USA, 2018. [Google Scholar]
- Anand, A.J. Fluorouracil cardiotoxicity. Ann. Pharmacother. 1994, 28, 374–378. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Salim, K.P.; Bano, Z.A. Symptomatic cardiotoxicity with high-dose 5-fluorouracil infusion: A prospective study. Oncology 1993, 50, 441–444. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Colan, S.D.; Gelber, R.D.; Perez-Atayde, A.R.; Sallan, S.E.; Sanders, S.P. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 1991, 324, 808–815. [Google Scholar] [CrossRef]
- Perez, E.A.; Rodeheffer, R. Clinical cardiac tolerability of trastuzumab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 322–329. [Google Scholar] [CrossRef]
- Keefe, D.L. Trastuzumab-associated cardiotoxicity. Cancer 2002, 95, 1592–1600. [Google Scholar] [CrossRef]
- Roversi, S.; Fabbri, L.M.; Sin, D.D.; Hawkins, N.M.; Agusti, A. Chronic Obstructive Pulmonary Disease and Cardiac Diseases. An Urgent Need for Integrated Care. Am. J. Respir. Crit. Care Med. 2016, 194, 1319–1336. [Google Scholar] [CrossRef]
- Leung, A.A.; McAlister, F.A.; Rogers, S.O., Jr.; Pazo, V.; Wright, A.; Bates, D.W. Preoperative hyponatremia and perioperative complications. Arch. Intern. Med. 2012, 172, 1474–1481. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, H.B.; Han, W.; Noh, D.Y.; Park, S.K.; Kim, W.H.; Kim, J.T. Total Intravenous Anesthesia versus Inhalation Anesthesia for Breast Cancer Surgery: A Retrospective Cohort Study. Anesthesiology 2019, 130, 31–40. [Google Scholar] [CrossRef]
- Oh, T.K.; Kim, H.H.; Jeon, Y.T. Retrospective analysis of 1-year mortality after gastric cancer surgery: Total intravenous anesthesia versus volatile anesthesia. Acta Anaesthesiol. Scand. 2019, 63, 1169–1177. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.H.; Kim, Y.; Kim, H.A.; Kim, B.S. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: A retrospective study. Korean J. Anesthesiol. 2016, 69, 126–132. [Google Scholar] [CrossRef]
- Enlund, M.; Berglund, A.; Ahlstrand, R.; Wallden, J.; Lundberg, J.; Warnberg, F.; Ekman, A.; Sjoblom Widfeldt, N.; Enlund, A.; Bergkvist, L. Survival after primary breast cancer surgery following propofol or sevoflurane general anesthesia-A retrospective, multicenter, database analysis of 6305 Swedish patients. Acta Anaesthesiol. Scand. 2020, 64, 1048–1054. [Google Scholar] [CrossRef]
- Makito, K.; Matsui, H.; Fushimi, K.; Yasunaga, H. Volatile versus Total Intravenous Anesthesia for Cancer Prognosis in Patients Having Digestive Cancer Surgery. Anesthesiology 2020, 133, 764–773. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Neoadjuvant Chemotherapy or Endocrine Therapy for Invasive Ductal Carcinoma of the Breast with High Hormone Receptor Positivity and Human Epidermal Growth Factor Receptor 2 Negativity. JAMA Netw. Open 2021, 4, e211785. [Google Scholar] [CrossRef]
- Zhang, Z. Too much covariates in a multivariable model may cause the problem of overfitting. J. Thorac. Dis. 2014, 6, E196–E197. [Google Scholar] [CrossRef]
- Elston, D.A.; Proe, M.F. Smoothing Regression Coefficients in an Overspecified Regression Model with Interrelated Explanatory Variables. J. R. Stat. Soc. Ser. C (Appl. Stat.) 1995, 44, 395–406. [Google Scholar] [CrossRef]

| Variables | Nonsmokers without COPD | Smokers with COPD | p-Value | ||
|---|---|---|---|---|---|
| N = 1546 | N = 773 | ||||
| N, % | N, % | ||||
| Age (mean ± SD) | (58.37 ± 12.59) | (58.83 ± 12.37) | 0.404 | ||
| Age (years) | 0.467 | ||||
| ≤50 | 396 | 25.61% | 209 | 27.04% | |
| 51–60 | 444 | 28.72% | 212 | 27.43% | |
| 61–70 | 414 | 26.78% | 191 | 24.71% | |
| >70 | 292 | 18.89% | 161 | 20.83% | |
| CCI score | 0.310 | ||||
| 0 | 1417 | 91.66% | 694 | 89.78% | |
| 1 | 30 | 1.94% | 20 | 2.59% | |
| ≥2 | 99 | 6.40% | 59 | 7.63% | |
| CCI score (mean ± SD) | (0.16 ± 0.59) | (0.21 ± 0.70) | 0.108 | ||
| Menopausal status | 0.320 | ||||
| Postmenopausal | 996 | 64.42% | 462 | 59.77% | |
| Premenopausal | 550 | 35.58% | 311 | 40.23% | |
| Her2 status | 0.422 | ||||
| Negative | 1259 | 81.44% | 618 | 79.95% | |
| Positive | 287 | 18.56% | 155 | 20.05% | |
| Nodal surgery | 0.891 | ||||
| SLNB | 1082 | 69.99% | 543 | 70.25% | |
| ALND | 464 | 30.01% | 230 | 29.75% | |
| AJCC clinical stage | 0.782 | ||||
| I | 801 | 51.81% | 408 | 52.78% | |
| II | 376 | 24.32% | 193 | 24.97% | |
| III | 369 | 23.87% | 172 | 22.25% | |
| Hormone receptor | 0.792 | ||||
| Negative | 345 | 22.32% | 177 | 22.90% | |
| Positive | 1201 | 77.68% | 596 | 77.10% | |
| Breast surgery | 0.726 | ||||
| Total mastectomy | 228 | 14.75% | 119 | 15.39% | |
| Breast-conserving surgery | 1318 | 85.25% | 654 | 84.61% | |
| Differentiation | 0.692 | ||||
| I | 228 | 14.75% | 119 | 15.39% | |
| II | 731 | 47.28% | 351 | 45.41% | |
| III | 587 | 37.97% | 303 | 39.20% | |
| Adjuvant chemotherapy | 0.177 | ||||
| No | 756 | 48.90% | 403 | 52.13% | |
| Yes | 790 | 51.10% | 370 | 47.87% | |
| Adjuvant radiotherapy | 0.812 | ||||
| No | 228 | 14.75% | 119 | 15.39% | |
| Yes | 1318 | 85.25% | 654 | 84.61% | |
| MACE history | 0.322 | ||||
| No | 1114 | 72.06% | 541 | 69.99% | |
| Yes | 432 | 27.94% | 232 | 30.01% | |
| Hyperlipidemia | 0.566 | ||||
| No | 1138 | 78.16% | 589 | 76.20% | |
| Yes | 318 | 21.84% | 184 | 23.80% | |
| Hypertension | 0.664 | ||||
| No | 965 | 66.28% | 502 | 64.94% | |
| Yes | 491 | 33.72% | 271 | 35.06% | |
| Diabetes | 0.645 | ||||
| No | 1164 | 79.95% | 610 | 78.91% | |
| Yes | 292 | 20.05% | 163 | 21.09% | |
| Chronic kidney disease | 1.000 | ||||
| No | 1441 | 98.97% | 765 | 98.97% | |
| Yes | 15 | 1.03% | 8 | 1.03% | |
| Alcohol use | 0.492 | ||||
| No | 1268 | 82.02% | 618 | 79.94% | |
| Yes | 278 | 17.98% | 155 | 20.06% | |
| Drug abuse | 0.284 | ||||
| No | 1500 | 97.02% | 743 | 96.12% | |
| Yes | 46 | 2.98% | 30 | 3.88% | |
| Frequency of hospitalization for COPDAE within 1 year before breast surgery | <0.001 | ||||
| 0 | 1546 | 100.00% | 702 | 90.82% | |
| 1 | 0 | 0.00% | 39 | 5.05% | |
| ≥2 | 0 | 0.00% | 32 | 4.14% | |
| Follow-up (All-cause mortality) Years, Median (IQR, Q1–Q3) | 7.21 (3.53–12.06) | 5.79 (2.59–9.81) | <0.001 | ||
| Follow-up (Did not die) Years, Median (IQR, Q1–Q3) | 5.41 (3.49–11.93) | 5.15 (2.56–9.71) | 0.788 | ||
| Variables | Crude HR (95% CI) | Adjusted HR * (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| COPD status (ref: non-COPD) | |||||
| COPD | 1.07 | (0.88–1.31) | 1.04 | (0.83–1.22) | 0.782 |
| Frequency of hospitalization for COPDAE within 1 year before breast surgery (ref: 0) | |||||
| ≥1 | 2.86 | (1.78–3.54) | 1.51 | (1.18–2.36) | 0.002 |
| Age (years, ref: ≤50) | |||||
| 51–60 | 1.71 | (1.23–2.31) | 1.54 | (1.12–2.13) | 0.004 |
| 61–70 | 2.51 | (1.85–3.39) | 2.32 | (1.67–3.21) | <0.001 |
| >70 | 4.81 | (3.61–6.48) | 4.92 | (3.50–6.90) | <0.001 |
| CCI score (ref: 0) | |||||
| 1 | 2.87 | (1.81–4.55) | 1.52 | (1.24–2.12) | <0.001 |
| ≥2 | 2.55 | (1.88–3.48) | 1.85 | (1.26–2.70) | <0.001 |
| Menopausal status (ref: Postmenopausal) | |||||
| Premenopausal | 1.38 | (1.08–1.75) | 1.00 | (0.60–1.04) | 0.126 |
| HER2 (ref: Negative) | |||||
| Positive | 1.51 | (1.18–1.93) | 0.89 | (0.66–1.19) | 0.508 |
| Breast surgery (ref: Total mastectomy) | |||||
| Breast-conserving surgery | 1.31 | (0.86–1.68) | 1.11 | (0.88–1.20) | 0.382 |
| Nodal surgery (ref: SLND) | |||||
| ALND | 1.28 | (0.50–1.48) | 1.18 | (0.68–1.87) | 0.492 |
| AJCC clinical stage (ref. stage I) | |||||
| Stage II | 1.81 | (1.23–2.48) | 1.22 | (1.06–1.93) | 0.003 |
| Stage III | 2.13 | (1.60–2.83) | 1.47 | (1.13–1.85) | 0.008 |
| Hormone receptor (ref. Negative) | |||||
| Positive | 0.92 | (0.81–1.40) | 0.90 | (0.87–1.37) | 0.337 |
| Differentiation (ref: Grade I) | |||||
| Grade II | 1.08 | (1.02–1.36) | 1.03 | (1.01–1.47) | 0.044 |
| Grade III | 1.12 | (1.04–1.38) | 1.08 | (1.07–1.35) | 0.013 |
| Adjuvant chemotherapy (ref: No) | |||||
| Yes | 0.73 | (0.43–1.10) | 0.83 | (0.72–1.06) | 0.361 |
| Adjuvant radiotherapy (ref: No) | |||||
| Yes | 0.77 | (0.46–1.13) | 0.70 | (0.52–1.09) | 0.304 |
| MACE history (ref: No) | |||||
| Yes | 1.16 | (1.01–2.57) | 1.31 | (1.14–2.25) | 0.005 |
| Hyperlipidemia (ref: No) | |||||
| Yes | 1.65 | (1.01–2.24) | 0.93 | (0.61–1.51) | 0.798 |
| Hypertension (ref: No) | |||||
| Yes | 1.66 | (1.13–2.45) | 1.13 | (0.71–1.79) | 0.521 |
| Diabetes (ref: No) | |||||
| Yes | 1.90 | (1.35–2.66) | 1.43 | (0.97–2.11) | 0.061 |
| Chronic kidney disease (ref: No) | |||||
| Yes | 1.28 | (0.88–1.84) | 1.01 | (0.48–1.16) | 0.174 |
| Alcohol use (ref: No) | |||||
| Yes | 1.44 | (0.98–2.13) | 0.98 | (0.69–1.56) | 0.452 |
| Drug abuse (ref: No) | |||||
| Yes | 1.39 | (0.71–2.49) | 0.90 | (0.65–1.63) | 0.833 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-Q.; Cheng, T.-M.; Lin, W.-C.; Chiu, K.-C.; Wu, S.-Y. Impact of Smoking-Related Chronic Obstruction Pulmonary Disease on Mortality of Invasive Ductal Carcinoma Patients Receiving Standard Treatments: Propensity Score-Matched, Nationwide, Population-Based Cohort Study. Cancers 2021, 13, 3654. https://doi.org/10.3390/cancers13153654
Zhang J-Q, Cheng T-M, Lin W-C, Chiu K-C, Wu S-Y. Impact of Smoking-Related Chronic Obstruction Pulmonary Disease on Mortality of Invasive Ductal Carcinoma Patients Receiving Standard Treatments: Propensity Score-Matched, Nationwide, Population-Based Cohort Study. Cancers. 2021; 13(15):3654. https://doi.org/10.3390/cancers13153654
Chicago/Turabian StyleZhang, Jia-Qiang, Tsai-Mu Cheng, Wei-Chun Lin, Kuo-Chin Chiu, and Szu-Yuan Wu. 2021. "Impact of Smoking-Related Chronic Obstruction Pulmonary Disease on Mortality of Invasive Ductal Carcinoma Patients Receiving Standard Treatments: Propensity Score-Matched, Nationwide, Population-Based Cohort Study" Cancers 13, no. 15: 3654. https://doi.org/10.3390/cancers13153654
APA StyleZhang, J.-Q., Cheng, T.-M., Lin, W.-C., Chiu, K.-C., & Wu, S.-Y. (2021). Impact of Smoking-Related Chronic Obstruction Pulmonary Disease on Mortality of Invasive Ductal Carcinoma Patients Receiving Standard Treatments: Propensity Score-Matched, Nationwide, Population-Based Cohort Study. Cancers, 13(15), 3654. https://doi.org/10.3390/cancers13153654






