The Expression of Genes Related to Lipid Metabolism and Metabolic Disorders in Children before and after Hematopoietic Stem Cell Transplantation—A Prospective Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.2.1. Laboratory Testing
2.2.2. Anthropometric Measurements
2.2.3. Molecular Analysis (Microarrays)
2.3. Statistical Analysis
3. Results
3.1. Participants in HSCT and Control Groups
3.2. Metabolic and Anthropometric Characteristics
3.3. Patterns of Gene Expression
3.4. Changes in Gene Expression Related to Lipid-Metabolism Parameters
4. Discussion
4.1. Biochemical Parameters
4.2. Gene Expression
4.2.1. DPP4
4.2.2. PLAG1
4.2.3. SCD
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bis, G.; Szlasa, W.; Sondaj, K.; Zendran, I.; Mielcarek-Siedziuk, M.; Barg, E. Lipid complications after hematopoietic stem cell transplantation (HSCT) in pediatric patients. Nutrients 2020, 12, 2500. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Bregni, M.; Brune, M.; Cornelissen, J.; De Witte, T.; Dini, G.; Einsele, H.; Gaspar, H.B.; Gratwohl, A.; Passweg, J.; et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe 2009. Bone Marrow Transplant. 2009, 45, 219–234. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Bader, P.; Bonini, C.; Cesaro, S.; Dreger, P.; Duarte, R.F.; Dufour, C.; Falkenburg, J.H.F.; Farge-Bancel, D.; et al. Hematopoietic SCT in europe 2013: Recent trends in the use of alternative donors showing more hap-loidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 2015, 50, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Premstaller, M.; Perren, M.; Koçack, K.; Arranto, C.; Favre, G.; Lohri, A.; Gerull, S.; Passweg, J.R.; Halter, J.P.; Leuppi-Taegtmeyer, A.B. Dyslipidemia and lipid-lowering treatment in a hematopoietic stem cell transplant cohort: 25 years of follow-up data. J. Clin. Lipidol. 2018, 12, 464–480.e3. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.L.; Savani, B.N.; Boord, J.B. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: Evaluation and management. Blood 2010, 116, 1197–1204. [Google Scholar] [CrossRef]
- Bhatia, S.; Francisco, L.; Carter, A.; Sun, C.-L.; Baker, K.S.; Gurney, J.G.; McGlave, P.B.; Nademanee, A.; O’Donnell, M.; Ramsay, N.K.C.; et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood 2007, 110, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Hierlmeier, S.; Eyrich, M.; Wölfl, M.; Schlegel, P.-G.; Wiegering, V. Early and late complications following hematopoietic stem cell transplantation in pediatric patients–A retrospective analysis over 11 years. PLoS ONE 2018, 13, e0204914. [Google Scholar] [CrossRef]
- Orio, F.; Muscogiuri, G.; Palomba, S.; Serio, B.; Sessa, M.; Giudice, V.; Ferrara, I.; Tauchmanovà, L.; Colao, A.; Selleri, C. Endo-crinopathies after allogeneic and autologous transplantation of hematopoietic stem cells. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Bhatia, S. Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for fol-low-up. Expert Rev. Hematol. 2011, 4, 437–454. [Google Scholar] [CrossRef]
- Cherian, K.E.; Kapoor, N.; Devasia, A.J.; Mathews, V.; Srivastava, A.; Thomas, N.; George, B.; Paul, T.V. Endocrine challeng-es and metabolic profile in recipients of allogeneic haematopoietic stem cell transplant: A cross-sectional study from southern india. Indian J. Hematol. Blood Transfus. 2020, 36, 484–490. [Google Scholar] [CrossRef]
- Ragbourne, S.C.; Crook, M.A. Metabolic syndrome in long-term survivors of hematopoietic stem-cell transplantation. Clin. Lymphoma Myeloma Leuk. 2017, 17, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Mueller, B.A.; Baker, K.S.; Cushing-Haugen, K.L.; Flowers, M.E.D.; Martin, P.J.; Friedman, D.L.; Lee, S.J. Cardio-vascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann. Intern. Med. 2011, 155, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.S.; Ness, K.K.; Steinberger, J.; Carter, A.; Francisco, L.; Burns, L.J.; Sklar, C.; Forman, S.; Weisdorf, D.; Gurney, J.G.; et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: A report from the bone marrow transplantation survivor study. Blood 2006, 109, 1765–1772. [Google Scholar] [CrossRef]
- Rodgers, C.; Gregurich, M.A.; Hockenberry, M. Lipid Profiles of Pediatric Hematopoietic Stem Cell Transplant Survivors. J. Pediatr. Oncol. Nurs. 2012, 29, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Marini, B.L.; Choi, S.W.; Byersdorfer, C.A.; Cronin, S.; Frame, D.G. Treatment of dyslipidemia in allogeneic hematopoietic stem cell transplant patients. Biol. Blood Marrow Transplant. 2015, 21, 809–820. [Google Scholar] [CrossRef]
- Blaser, B.W.; Kim, H.T.; Alyea, E.P.; Ho, V.T.; Cutler, C.; Armand, P.; Koreth, J.; Antin, J.H.; Plutzky, J.; Soiffer, R.J. Hyper-lipidemia and statin use after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2012, 18, 575–583. [Google Scholar] [CrossRef]
- Taskinen, M.; Saarinen-Pihkala, U.M.; Hovi, L.; Lipsanen-Nyman, M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet 2000, 356, 993–997. [Google Scholar] [CrossRef]
- Baig, S.; Rizi, E.P.; Shabeer, M.; Chhay, V.; Mok, S.F.; Loh, T.P.; Magkos, F.; Vidal-Puig, A.; Tai, E.S.; Khoo, C.M.; et al. Meta-bolic gene expression profile in circulating mononuclear cells reflects obesity-associated metabolic inflexibility. Nutr. Metab. 2016, 13, 74. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Yoon, J.M. Dyslipidemia in children and adolescents: When and how to diagnose and treat? Pediatr. Gastroenterol. Hepatol. Nutr. 2014, 17, 85–92. [Google Scholar] [CrossRef]
- Singh, Y.; Garg, M.K.; Tandon, N.; Marwaha, R.K. A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban indian adolescents. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 245–251. [Google Scholar] [PubMed]
- Madeira, I.R.; Miranda Carvalho, C.N.; Gazolla, F.M.; de Matos, H.J.; Borges, M.A.; Bordallo, M.A.N. Ponto de corte do índice homeostatic model assessment for insulin resistance (HOMA-IR) Avaliado Pela Curva Receiver Operating Characteristic (ROC) Na Detecção de Síndrome Metabólica Em Crianças Pré-Púberes Com Excesso de Peso. Arq. Bras. Endocrinol. Metabol. 2008, 52, 1466–1473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arellano-Ruiz, P.; García-Hermoso, A.; Cavero-Redondo, I.; Pozuelo-Carrascosa, D.; Martínez-Vizcaíno, V.; Solera-Martinez, M. Homeostasis Model Assessment cut-off points related to metabolic syndrome in children and adolescents: A systematic review and meta-analysis. Eur. J. Pediatr. 2019, 178, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- WHO. Anthro Survey Analyser and Other Tools. Available online: https://www.who.int/toolkits/child-growth-standards/software (accessed on 6 April 2021).
- Palczewska, I.; Niedźwiecka, Z. Indices of somatic development of warsaw children and adolescents (wskaźniki rozwoju somatycznego dzieci i młodzieży warszawskiej). Med. Wieku Rozwojowego 2001, 5. Available online: https://www.researchgate.net/publication/284773718_Wskazniki_rozwoju_somatycznego_dzieci_i_mlodziezy_warszawskiej (accessed on 6 April 2021).
- Gołąb, S.; Chrzanowska, M. Dziecko Krakowskie 2000—Poziom Rozwoju Biologicznego Dzieci i Młodzieży Miasta Krakowa, 1st ed.; Studia i Monografie AWF w Krakowie: Krakowa, Poland, 2002; nr 19; p. 98. [Google Scholar]
- Kushner, R.F.; Schoeller, D.A. Estimation of total body water by bioelectrical impedance analysis. Am. J. Clin. Nutr. 1986, 44, 417–424. [Google Scholar] [CrossRef]
- Schübel, R.; Sookthai, D.; Greimel, J.; Johnson, T.S.; Grafetstätter, M.E.; Kirsten, R.; Kratz, M.; Ulrich, C.M.; Kaaks, R.; Kühn, T. Key genes of lipid metabolism and WNT-signaling are downregulated in subcutaneous adipose tissue with moderate weight loss. Nutrients 2019, 11, 639. [Google Scholar] [CrossRef]
- Liu, X.; Bai, C.; Ding, X.; Wei, Z.; Guo, H.; Li, G. Microarray analysis of the gene expression profile and lipid metabolism in fat-1 transgenic cattle. PLoS ONE 2015, 10, e0138874. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The genecards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Dalman, M.R.; Deeter, A.; Nimishakavi, G.; Duan, Z.-H. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinform. 2012, 13, S11. [Google Scholar] [CrossRef]
- Annaloro, C.; Usardi, P.; Airaghi, L.; Giunta, V.; Forti, S.; Orsatti, A.; Baldini, M.; Delle Volpe, A.; Deliliers, G.L. Preva-lence of Metabolic Syndrome in Long-Term Survivors of Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant. 2008, 41, 797–804. [Google Scholar] [CrossRef]
- Trinh, T.; Broxmeyer, H.E. Role for Leptin and Leptin Receptors in Stem Cells During Health and Diseases. Stem Cell Rev. Rep. 2021, 1–12. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Baumeier, C.; Schlüter, L.; Saussenthaler, S.; Laeger, T.; Rödiger, M.; Alaze, S.A.; Fritsche, L.; Häring, H.-U.; Stefan, N.; Fritsche, A.; et al. Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol. Metab. 2017, 6, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, L.; Xing, J.; Li, P.; Sang, H.; Hu, X.; Du, Y.; Zhao, L.; Song, R.; Gu, H. The protective role of DPP4 inhibitors in atherosclerosis. Eur. J. Pharmacol. 2020, 875, 173037. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.A.; Park, Y.M.; Yun, J.S.; Lim, T.S.; Song, K.H.; Yoo, K.D.; Ahn, Y.B.; Ko, S.H. A comparison of effects of DPP-4 inhibitor and SGLT2 inhibitor on lipid profile in patients with type 2 diabetes. Lipids Health Dis. 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Sakai, Y.; Chen, G.; Ni, Y.; Zhuge, F.; Xu, L.; Nagata, N.; Kaneko, S.; Ota, T.; Nagashimada, M. DPP-4 inhibition with anagliptin reduces lipotoxicity-induced insulin resistance and steatohepatitis in male mice. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- Turcot, V.; Bouchard, L.; Faucher, G.; Tchernof, A.; Deshaies, Y.; Perusse, L.; Bélisle, A.; Marceau, S.; Biron, S.; Lescelleur, O.; et al. DPP4 gene DNA methylation in the omentum is associated with its gene expression and plasma lipid profile in severe obesity. Obesity 2011, 19, 388–395. [Google Scholar] [CrossRef]
- Rohmann, N.; Schlicht, K.; Geisler, C.; Hollstein, T.; Knappe, C.; Krause, L.; Hagen, S.; Beckmann, A.; Seoudy, A.K.; Wietzke-Braun, P.; et al. Circulating SDPP-4 is increased in obesity and insulin resistance but is not related to systemic meta-bolic inflammation. J. Clin. Endocrinol. Metab. 2021, 106, 592–601. [Google Scholar] [CrossRef]
- Anoop, S.; Misra, A.; Bhatt, S.P.; Gulati, S.; Pandey, R.M.; Mahajan, H. High circulating plasma dipeptidyl peptidase- 4 levels in non-obese Asian Indians with type 2 diabetes correlate with fasting insulin and LDL-C levels, triceps skinfolds, total intra-abdominal adipose tissue volume and presence of diabetes: A case–control study. BMJ Open Diabetes Res. Care 2017, 5, e000393. [Google Scholar] [CrossRef]
- Dynkevich, Y.; Rother, K.I.; Whitford, I.; Qureshi, S.; Galiveeti, S.; Szulc, A.L.; Danoff, A.; Breen, T.L.; Kaviani, N.; Shanik, M.H.; et al. Tumors, IGF-2, and hypoglycemia: Insights from the clinic, the laboratory, and the historical archive. Endocr. Rev. 2013, 34, 798–826. [Google Scholar] [CrossRef]
- Kessler, S.M.; Laggai, S.; van Wonterg, E.; Gemperlein, K.; Müller, R.; Haybaeck, J.; Vandenbroucke, R.E.; Ogris, M.; Libert, C.; Kiemer, A.K. Transient hepatic overexpression of insulin-like growth factor 2 induces free cholesterol and lipid droplet formation. Front. Physiol. 2016, 7, 147. [Google Scholar]
- Kadakia, R.; Zheng, Y.; Zhang, Z.; Zhang, W.; Josefson, J.; Hou, L. Association of cord blood methylation with neonatal leptin: An epigenome wide association study. PLoS ONE 2019, 14, 0226555. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. Role of stearoyl-CoA desaturase in human metabolic disease. Future Lipidol. 2008, 3, 163–173. [Google Scholar] [CrossRef]
- Morcillo, S.; Martín-Núñez, G.M.; Garcia-Serrano, S.; Gutierrez-Repiso, C.; Rodriguez-Pacheco, F.; Valdes, S.; Gonzalo, M.; Rojo-Martínez, G.; Moreno-Ruiz, F.J.; Rodríguez-Cañete, A.; et al. Changes in SCD gene DNA methylation after bariatric surgery in morbidly obese patients are associated with free fatty acids. Sci. Rep. 2017, 7, 46292. [Google Scholar] [CrossRef]
- Oshima, M.; Pechberty, S.; Bellini, L.; Göpel, S.O.; Campana, M.; Rouch, C.; Dairou, J.; Cosentino, C.; Fantuzzi, F.; Toivonen, S.; et al. Stearoyl CoA desaturase is a gatekeeper that protects human beta cells against lipotoxicity and maintains their identity. Diabetologia 2019, 63, 395–409. [Google Scholar] [CrossRef]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a therapeutic target for the treatment of cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef] [PubMed]
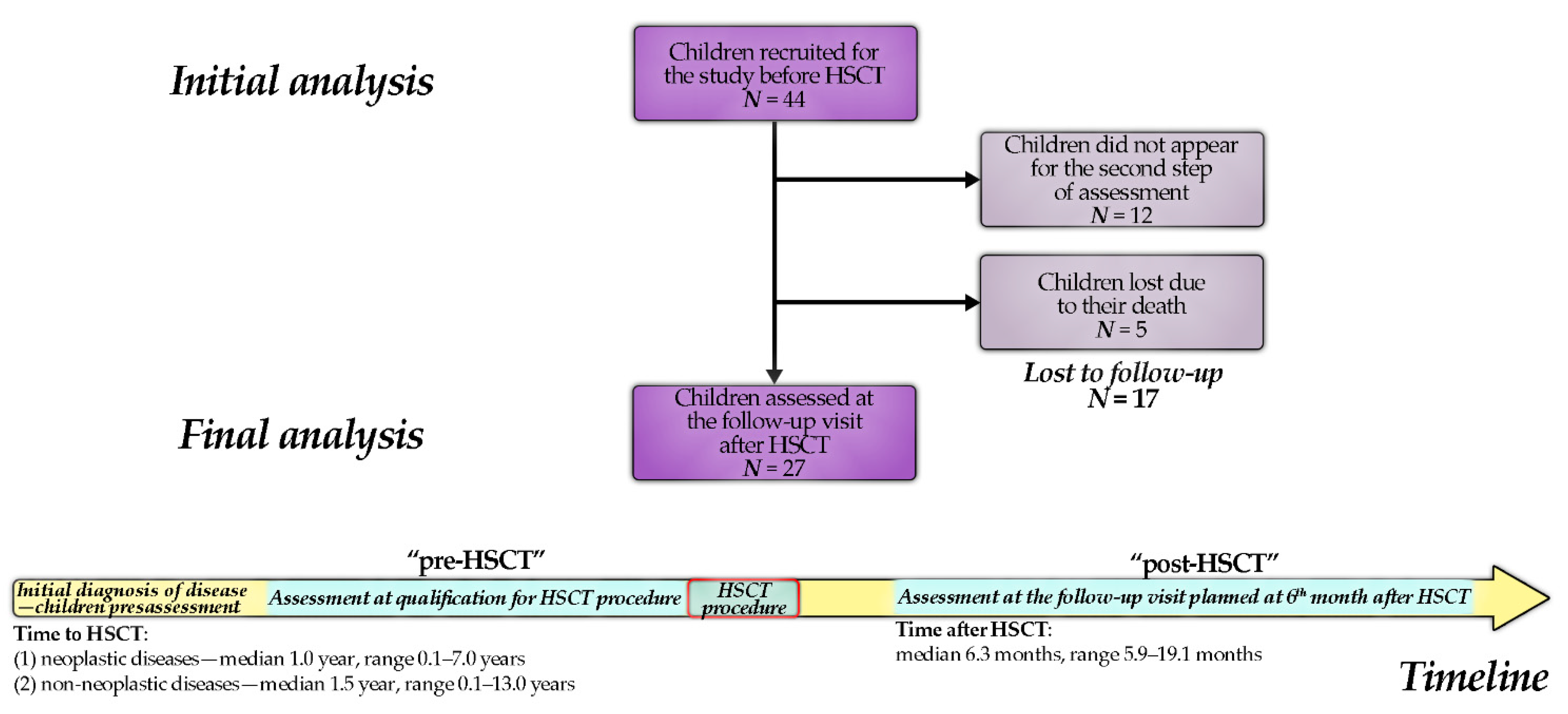

| Diagnosis | Number (%), N = 27 |
|---|---|
| Neoplastic diseases | 18 (67) |
| Acute lymphoblastic leukemia | 11 (41) |
| Acute myeloblastic leukemia | 4 (15) |
| Chronic myelocytic leukemia | 1 (4) |
| Myelodysplastic syndrome | 1 (4) |
| Juvenile myelomonocytic leukemia and acute myeloblastic leukemia | 1 (4) |
| Non-Neoplastic diseases | 9 (33) |
| Severe aplastic anemia | 4 (15) |
| Chronic granulomatous disease | 3 (11) |
| Autoimmune lymphoproliferative syndrome | 1 (4) |
| Hyper IgM syndrome | 1 (4) |
| Conditioning Type | Regimen | Number (%), N = 27 |
|---|---|---|
| Non-myeloablative | CyATG | 3 (11) |
| FluCyATG | 1 (4) | |
| Myeloablative | Bu or Bux-based | 14 (52) |
| Treo-based | 2 (7) | |
| TBI-VP | 7 (26) |
| Treatment | Number of Patients, N = 27 | |
|---|---|---|
| Time since diagnosis (years) | Neoplastic diseases | median: 1.0, mean: 2.0, range: 0.1–7.0 |
| Non-neoplastic diseases | median: 1.5, mean: 3.8, range: 0.1–13.0 | |
| Chemotherapy before HSCT (N, %) | 17 (63) | |
| Local radiotherapy (N, %) | 5 (19): CNS-4 (15), testes-1 (4) | |
| Total body irradiation-12 Gy/6 fractions (N, %) | 7 (27) | |
| Conditioning regimen based on busulfan or treosulfan (N, %) | 16 (59) | |
| GvHD prophylaxis (N, %) | CsA | 4 (15) |
| Mtx + CsA | 23 (85) | |
| ATG | 20 (74) | |
| Mucositis (N, %) | 22 (81) | |
| Grade (N) | I-7, II-8, III-6, IV-1 | |
| Intravenous alimentation due to mucositis (%) | 13 (48) | |
| aGvHD (N, %) | 11 (41) | |
| Localization (%) | Gut-9, liver-27, skin-91 | |
| Grade (N) | IA-1, IB-4, IIB-1, IIC-3, IIIC-2 | |
| Systemic glucocorticoid treatment | N, % | 19 (70) |
| days | median: 3.5, mean: 3.6, range: 0.1–11.0 | |
| Time from HSCT to the second assessment (months) | median: 6.3, range: 5.9–19.1 | |
| Time from discontinuation of systemic glucocorticoids to the second assessment (months) | median: 3.6, mean: 4.5, range: 0.5–14.0 | |
| Time from discontinuation of immunosuppressive treatment to the second assessment (months) | median: 1.6, range: 0.0–9.0 | |
| Hematopoietic stem cells donor (N, %) | MUD: 16 (59), MSD: 9 (33), MFD: 2 (7) | |
| Characteristic | Pre-HSCT N = 27 | Post-HSCT N = 27 |
|---|---|---|
| Boys/girls (N, %) | 20(74)/7(26) | |
| Age (years) | 9.7 ± 5.2 | 10.4 ± 5.0 |
| Height (cm) | 134.7 ± 29.8 | 137.7 ± 27.2 |
| Body mass (kg) | 37.4 ± 18.5 | 37.2 ± 17.4 |
| Waist circumference (cm) | 66.9 ± 12.4 | 66.1 ± 12.4 |
| Parameter | Pre-HSCT N = 27 | Post-HSCT N = 27 | p/pBH-Value Pre-HSCT vs. Post-HSCT |
|---|---|---|---|
| Glc(T0) (mmol/L) | 4.4 ± 0.6 | 4.5 ± 0.6 | 0.7/- |
| Glc(T60) (mmol/L) | 6.1 ± 1.0 | 5.9 ±1.7 | 0.4/- |
| Glc(T120) (mmol/L) | 5.5 ± 1.6 | 5.4 ± 1.1 | 0.7/- |
| AUC glc (mmol/L/h) | 11.0 ± 1.4 | 10.9 ± 1.3 | 0.6/- |
| TC (mmol/L) | 3.3 ± 1.0 | 3.9 ± 0.9 | 0.002/0.04 |
| HDL-C (mmol/L) | 1.0 ± 0.4 | 1.3 ± 0.5 | 0.02/0.4 |
| LDL-C (mmol/L) | 1.4 ± 0.9 | 1.9 ± 0.8 | 0.05/- |
| HDL-C/TC | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4/- |
| TG (mmol/L) | 1.8 ± 0.7 | 1.5 ± 0.6 | 0.3/- |
| Insulin(T0) (mIU/L) | 13.7 ± 13.5 | 11.3 ± 9.5 | 0.3/- |
| Insulin(T60) (mIU/L) | 56.7 ± 56.2 | 38.1 ± 46.7 | 0.05/0.9 |
| Insulin(T120) (mIU/L) | 43.9 ± 56.2 | 28.6 ± 32.5 | 0.3/- |
| AUC insulin (mIU/L/h) | 89.6 ± 98.0 | 60.5 ± 66.4 | 0.1/- |
| Leptin(T0) (µg/L) | 13.9 ± 20.6 | 11.6 ± 21.8 | 0.04/0.7 |
| Leptin(T60) (µg/L) | 16.2 ± 24.4 | 8.0 ± 15.7 | 0.0007/0.02 |
| Leptin(T120) (µg/L) | 16.8 ± 24.4 | 8.8 ± 19.0 | 0.001/0.02 |
| AUC leptin (µg/L/h) | 30.1 ± 46.2 | 18.1 ± 35.5 | 0.001/0.02 |
| Leptin receptor(T0) (µg/L) | 27.9 ± 27.4 | 29.3 ± 24.2 | 0.4/- |
| Leptin receptor(T60) (µg/L) | 28.7 ± 29.5 | 30.4 ± 21.2 | 0.1/- |
| Leptin receptor(T120) (µg/L) | 28.2 ± 31.5 | 30.7 ± 22.8 | 0.3/- |
| AUC leptin receptor (µg/L/h) | 57.4 ± 59.2 | 61.2 ± 44.4 | 0.1/- |
| HOMA-IR | 2.8 ± 2.8 | 2.2 ± 2.0 | 0.4/- |
| hsCRP (mg/L) | 7.7 ± 10.4 | 7.0 ± 11.2 | 0.8/- |
| Insulin resistance (N, %) | 9 (41) | 6 (26) | 0.3/- |
| Dyslipidemia: Abnormal TC (N, %) | 1 (4) | 2(7) | - |
| Dyslipidemia: Abnormal TG (N, %) | 17 (63) | 14 (52) | 0.6/- |
| Dyslipidemia: Abnormal HDL-C (N, %) | 18 (64) | 10 (36) | 1.0/- |
| Dyslipidemia: Abnormal LDL-C (N, %) | 1 (4) | 0 (0) | - |
| Dyslipidemia: Any abnormality (N, %) | 24 (86) | 19 (68) | 0.001/0.006 |
| Gene Symbol | Locus and Affimetrix Code | Pre-HSCT N = 27 | Post-HSCT N = 27 | Pre-HSCT vs. Post-HSCT | |
|---|---|---|---|---|---|
| FC | p/pBH-Value | ||||
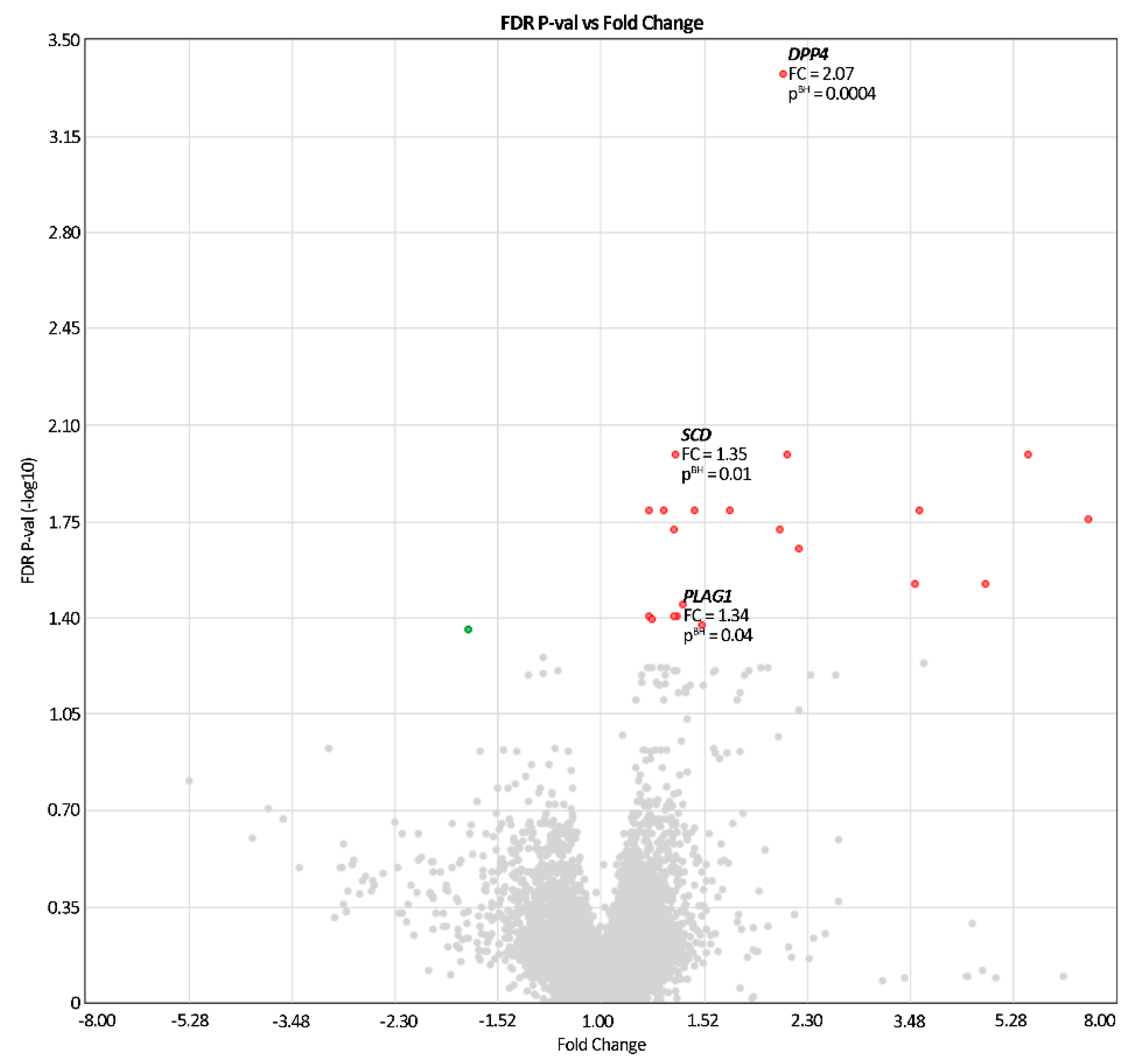
| DPP4 | 2q24.2 8056222 | 481.0 ± 1.5 | 230.7 ± 1.6 | 2.07 | 10−8/0.0004 |
| PLAG1 | 8q12.1 8150881 | 68.1 ± 1.4 | 50.6 ± 1.3 | 1.34 | 10−5/0.04 |
| SCD | 10q24.31 7929816 | 125.4 ± 1.4 | 93.1 ± 1.3 | 1.35 | 10−6/0.01 |
| Lipid Metabolism Parameter | Gene | Pre-HSCT Gene Expression and Pre-HSCT Lipid Metabolism Parameters | Post-HSCT Gene Expression and Post-HSCT Lipid Metabolism Parameters | Pre-HSCT Gene Expression and Post-HSCT Lipid Metabolism Parameters | Gene Expression Change (Δmean) and Post-HSCT Lipid Metabolism Parameters | Gene Expression Relative Change (Δmean(rel)) and Post-HSCT Lipid Metabolism Parameters (%) |
|---|---|---|---|---|---|---|
| Spearman’s Correlation Coefficient r and p/pBH-Value | ||||||
| TC | DPP4 | 0.48, 0.02/0.06 | −0.42, 0.03/0.1 | −0.02, 0.9/- | 0.02, 0.9/- | −0.42, 0.03/0.1 |
| PLAG1 | 0.33, 0.1/0.2 | 0.30, 0.1/0.2 | 0.30, 0.1/0.3 | 0.22, 0.3/0.9 | 0.23, 0.3/0.6 | |
| SCD | −0.13, 0.6/0.6 | 0.05, 0.8/0.8 | 0.09, 0.7/- | −0.14, 0.5/- | −0.08, 0.7/0.7 | |
| HDL-C | DPP4 | 0.23, 0.3/0.9 | 0.04, 0.9/- | −0.31, 0.1/0.3 | −0.18, 0.4/- | −0.15, 0.5/- |
| PLAG1 | 0.01, 1.0/- | 0.23, 0.3/0.9 | 0.01, 1.0/- | 0.05, 0.8/- | 0.03, 0.9/- | |
| SCD | −0.9, 0.7/- | −0.07, 0.7/- | −0.04, 0.9/- | −0.08, 0.7/- | −0.07, 0.7/- | |
| LDL-C | DPP4 | 0.51, 0.01/0.03 | −0.46, 0.02/0.06 | 0.12, 0.6/- | 0.08, 0.7/- | 0.11, 0.6/- |
| PLAG1 | 0.45, 0.03/0.06 | −0.37, 0.06/0.1 | 0.13, 0.5/- | 0.14, 0.5/- | 0.15, 0.5/- | |
| SCD | −0.10, 0.7/0.7 | 0.14, 0.5/0.5 | 0.18, 0.4/- | −0.20, 0.3/0.9 | −0.14, 0.5/- | |
| Gene | Any Feature of Dyslipidemia after the HSCT Procedure, Yes (N = 19) vs. No (N = 8) (Mean ± SD) | p/pBH-Value | |
|---|---|---|---|
| Gene expression before HSCT | |||
| DPP4 | 509.8 ± 170.2 | 348.1 ± 115.9 | 0.02/0.06 |
| PLAG1 | 71.4 ± 22.6 | 69.8 ± 25.1 | 0.5/0.5 |
| SCD | 111.3 ± 44.4 | 142.8 ± 73.6 | 0.1/0.2 |
| Gene expression after HSCT | |||
| DPP4 | 220.9 ± 111.6 | 254.9 ± 85.3 | 0.3/0.6 |
| PLAG1 | 46.9 ± 6.1 | 57.3 ± 19.5 | 0.2/0.6 |
| SCD | 78.4 ± 23.9 | 76.1 ± 13.3 | 0.9/0.9 |
| Difference in gene expression (Δmean) between pre-HSCT and post-HSCT status | |||
| DPP4 | −328.2 ± 204.0 | −46.0 ± 166.6 | 0.002/0.006 |
| PLAG1 | −31.2 ± 12.6 | −12.6 ± 28.1 | 0.3/0.3 |
| SCD | −32.9 ± 38.7 | −66.7 ± 72.7 | 0.1/0.2 |
| Difference in relative gene expression (Δmean(rel)) between pre-HSCT and post-HSCT status (%) | |||
| DPP4 | −57.1 ± 36.0 | −3.8 ± 51.0 | 0.002/0.006 |
| PLAG1 | −35.8 ± 24.6 | −11.1 ± 36.7 | 0.052/0.1 |
| SCD | −24.5 ± 19.7 | −37.7 ± 22.9 | 0.1/0.1 |
| Variable | Non-Standardized Regression Coefficient ± SEM | p-Value |
|---|---|---|
| Sex, boys vs. girls | 0.24 ± 0.15 | 0.1 |
| BMI WHO, per 1 unit | 0.05 ± 0.04 | 0.2 |
| DPP4 expression after HSCT, per 50 units | −0.12 ± 0.07 | 0.1 |
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p/pBH-Value | OR (95% CI) | p/pBH-Value | OR (95% CI) | p/pBH-Value | |
| Sex, boys vs. girls | 5.46 (0.36–84.10) | 0.2/0.6 | 6.60 (0.27–159.74) | 0.2/0.6 | 8.09 (0.36–182.76) | 0.2/0.6 |
| Age after HSCT, per 1 year | 0.92 (0.70–1.21) | 0.6/- | 0.94 (0.69–1.27) | 0.7/- | 0.97 (0.73–1.30) | 0.9/- |
| Indication for HSCT, non-malignant vs. malignant | 0.29 (0.03–3.28) | 0.3/0.9 | 0.34 (0.03–4.63) | 0.4/0.9 | 0.27 (0.02–3.53) | 0.3/0.9 |
| BMI WHO, per 1 unit | 0.83 (0.55–1.23) | 0.4/0.9 | 0.80 (0.50–1.29) | 0.4/0.9 | 0.77 (0.48–1.25) | 0.3/0.9 |
| DPP4 expression † | 1.51 (1.01–2.25) | 0.04/0.04 | 1.45 (1.08–1.95) | 0.01/0.03 | 1.35 (1.05–1.73) | 0.02/0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czogała, W.; Czogała, M.; Kwiecińska, K.; Bik-Multanowski, M.; Tomasik, P.; Hałubiec, P.; Łazarczyk, A.; Miklusiak, K.; Skoczeń, S. The Expression of Genes Related to Lipid Metabolism and Metabolic Disorders in Children before and after Hematopoietic Stem Cell Transplantation—A Prospective Observational Study. Cancers 2021, 13, 3614. https://doi.org/10.3390/cancers13143614
Czogała W, Czogała M, Kwiecińska K, Bik-Multanowski M, Tomasik P, Hałubiec P, Łazarczyk A, Miklusiak K, Skoczeń S. The Expression of Genes Related to Lipid Metabolism and Metabolic Disorders in Children before and after Hematopoietic Stem Cell Transplantation—A Prospective Observational Study. Cancers. 2021; 13(14):3614. https://doi.org/10.3390/cancers13143614
Chicago/Turabian StyleCzogała, Wojciech, Małgorzata Czogała, Kinga Kwiecińska, Mirosław Bik-Multanowski, Przemysław Tomasik, Przemysław Hałubiec, Agnieszka Łazarczyk, Karol Miklusiak, and Szymon Skoczeń. 2021. "The Expression of Genes Related to Lipid Metabolism and Metabolic Disorders in Children before and after Hematopoietic Stem Cell Transplantation—A Prospective Observational Study" Cancers 13, no. 14: 3614. https://doi.org/10.3390/cancers13143614
APA StyleCzogała, W., Czogała, M., Kwiecińska, K., Bik-Multanowski, M., Tomasik, P., Hałubiec, P., Łazarczyk, A., Miklusiak, K., & Skoczeń, S. (2021). The Expression of Genes Related to Lipid Metabolism and Metabolic Disorders in Children before and after Hematopoietic Stem Cell Transplantation—A Prospective Observational Study. Cancers, 13(14), 3614. https://doi.org/10.3390/cancers13143614






