Control of Skeletal Muscle Atrophy Associated to Cancer or Corticosteroids by Ceramide Kinase
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Biochemicals, Cell Culture Reagents
2.2. Animals and Treatments
2.3. Plasmids and Tissue Electroporation
2.4. Cell Culture and Treatments
2.5. Conditioned Medium (CM) Collection
2.6. Cell Gene Silencing
2.7. Preparation of RNA and Measurement of Gene Expression by Quantitative Real-Time Polymerase Chain Reaction
2.8. Western Blot Analysis
2.9. Cell Cycle Analysis
2.10. Presentation of Data and Statistical Analysis
2.11. Study Approval
3. Results
3.1. CerK Expression Is Reduced in the SkM of the C26 and LLC Hosts
3.2. CerK Expression in C2C12 Cultures
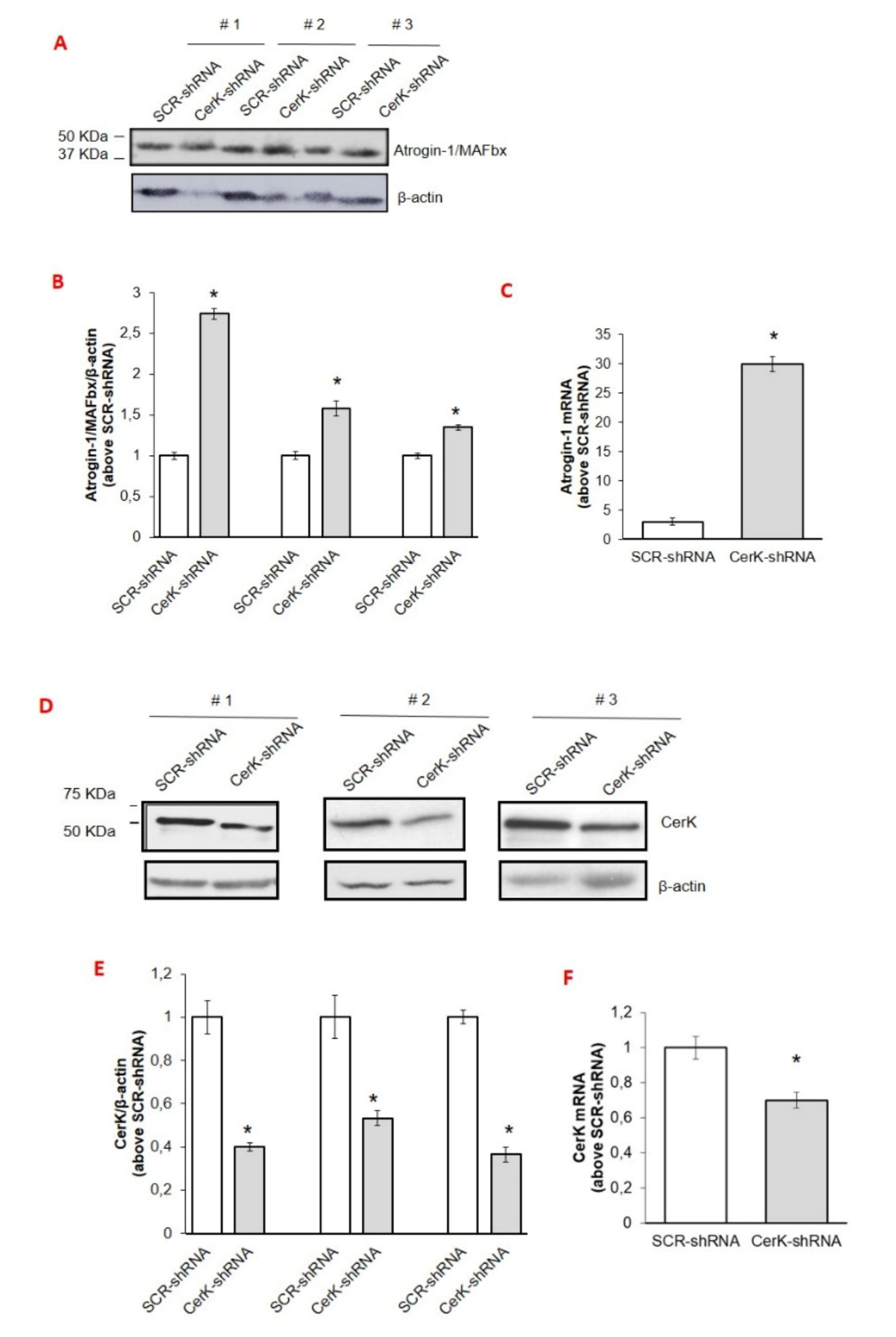
3.3. CerK Silencing Induces Atrogin-1/MAFbx Expression in Both In Vivo and In Vitro Models
3.4. Involvement of the Cer/C1P/SphK1 Axis to Dexa-Induced SKM Atrophy in C2C12 Myotubes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Lòpez-Soriano, F.J. Cancer Cachexia, a clinical challenge. Curr. Opin. Oncol. 2019, 31, 286–290. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Furrer, R.; Handschin, C. Muscle wasting diseases: Novel targets and treatments. Annu. Rev. Pharm. Toxicol. 2019, 59, 315–339. [Google Scholar] [CrossRef]
- Lee, D.; Goldberg, A. Atrogin1/MAFbx: What atrophy, hypertrophy, and cardiac failure have in common. Circ. Res. 2011, 109, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in cancer cachexia. Semin. Cell Dev. Biol. 2016, 54, 11–19. [Google Scholar] [CrossRef]
- Sartori, R.; Schirwis, E.; Blaauw, B.; Bortolanza, S.; Zhao, J.; Enzo, E.; Stantzou, A.; Mouisel, E.; Toniolo, L.; Ferry, A.; et al. BMP signaling controls muscle mass. Nat. Genet. 2013, 45, 1309–1318. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 10, 6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meacci, E.; Nuti, F.; Donati, C.; Cencetti, F.; Farnararo, M.; Bruni, P. Sphingosine kinase activity is required for myogenic differentiation of C2C12 myoblasts. J. Cell. Physiol. 2008, 214, 210–220. [Google Scholar] [CrossRef]
- Olivera, A.; Allende, M.L.; Proia, R.L. Shaping the landscape: Metabolic regulation of S1P gradients. Biochim. Biophys. Acta 2013, 1831, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Nishi, T.; Kobayashi, N.; Hisano, Y.; Kawahara, A.; Yamaguchi, A. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta 2014, 1841, 759–765. [Google Scholar] [CrossRef]
- Pyne, N.J.; El Buri, A.; Adams, D.R.; Pyne, S. Sphingosine 1-phosphate and cancer. Adv. Biol. Regul. 2018, 68, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Pierucci, F.; Frati, A.; Battistini, C.; Matteini, F.; Iachini, M.C.; Vestri, A.; Penna, F.; Costelli, P.; Meacci, E. Involvement of released sphingosine 1-phosphate/sphingosine 1-phosphate receptor axis in skeletal muscle atrophy. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864, 3598–3614. [Google Scholar] [CrossRef]
- Sassoli, C.; Pierucci, F.; Zecchi-Orlandini, S.; Meacci, E. Sphingosine 1-phosphate (S1P)/S1P Receptor Signaling and Mechanotransduction: Implications for intrinsic tissue repair/regeneration. Int. J. Mol. Sci. 2019, 20, 5545. [Google Scholar] [CrossRef] [Green Version]
- Bornancin, F. Ceramide kinase: The first decade. Cell. Signal. 2011, 23, 999–1008. [Google Scholar] [CrossRef]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp. Cell Res. 2015, 333, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Muñoz, A.; Presa, N.; Gomez-Larrauri, A.; Rivera, I.G.; Trueba, M.; Ordoñez, M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 2016, 61, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Kono, K.; Liu, H.; Shimizugawa, T.; Minekura, H.; Spiegel, S.; Kohama, T. Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J. Biol. Chem. 2002, 277, 23294–23300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mebarek, S.; Komati, H.; Naro, F.; Zeiller, C.; Alvisi, M.; Lagarde, M.; Prigent, A.F.; Némoz, G. Inhibition of de novo ceramide synthesis upregulates phospholipase D and enhances myogenic differentiation. J. Cell Sci. 2007, 120, 407–416. [Google Scholar] [CrossRef] [Green Version]
- De Larichaudy, J.; Zufferli, A.; Serra, F.; Isidori, A.M.; Naro, F.; Dessalle, K.; Desgeorges, M.; Piraud, M.; Cheillan, H.V.; Vidal, H.; et al. TNF -α- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet. Muscle 2012, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Hoeferlin, L.A.; Wijesinghe, D.S.; Chalfant, C.E. The role of ceramide-1-phosphate in biological functions. In Sphingolipids: Basic Science and Drug Development; Handbook of Experimental Pharmacology; Springer: Vienna, Austria, 2013; Volume 215, pp. 153–166. [Google Scholar]
- Hait, N.C.; Maiti, A. The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediat. Inflamm. 2017. [Google Scholar] [CrossRef]
- Marycz, K.; Śmieszek, A.; Jeleń, M.; Chrząstek, K.; Grzesiak, J.; Meissner, J. The effect of the bioactive sphingolipids S1P and C1P on multipotent stromal cells—New opportunities in regenerative medicine. Cell. Mol. Biol. Lett. 2015, 20, 510–533. [Google Scholar] [CrossRef]
- Bonetto, A.; Penna, F.; Minero, V.G.; Reffo, P.; Bonelli, G.; Baccino, F.M.; Costelli, P. Deacetylase inhibitors modulate the myostatin/follistatin axis without improving cachexia in tumor-bearing mice. Curr. Cancer Drug Targets 2009, 9, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Bonetto, A.; Aversa, Z.; Minero, V.G.; Rossi Fanelli, F.; Costelli, P.; Muscaritoli, M. Effect of the specific proteasome inhibitor bortezomib on cancer-related muscle wasting. J. Cachexia Sarcopenia Muscle 2016, 7, 345–354. [Google Scholar] [CrossRef]
- Pin, F.; Busquets, S.; Toledo, M.; Camperi, A.; Lòpez-Soriano, F.J.; Costelli, P.; Argilés, J.M.; Penna, F. Combination of exercise training and erythropoietin prevents cancer-induced muscle alterations. Oncotargert 2015, 6, 43202–43215. [Google Scholar] [CrossRef]
- Alamdari, N.; Aversa, Z.; Castillero, E.; Gurav, A.; Petkova, V.; Tizio, S.; Hasselgren, P.O. Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin uigases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochem. Biophys. Res. Commun. 2012, 417, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Castillero, E.; Alamdari, N.; Lecker, S.H.; Hasselgren, P.O. Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes. Metabolism 2013, 62, 1495–1502. [Google Scholar] [CrossRef]
- Brown, J.L.; Lee, D.E.; Rosa-Caldwell, M.E.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Huseman, K.; Sataranatarajan, K.; Van Remmen, H.; Washington, T.A.; et al. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2018, 9, 987–1002. [Google Scholar] [CrossRef]
- Penna, F.; Bonetto, A.; Muscaritoli, A.; Costamagna, D.; Minero, V.G.; Bonelli, G.; Rossi Fanelli, F.; Baccino, F.M.; Costelli, P. Muscle atrophy in experimental cancer cachexia: Is the IGF-1 signaling pathway involved? Int. J. Cancer 2010, 127, 1706–1717. [Google Scholar] [CrossRef]
- Camperi, A.; Pin, F.; Costamagna, D.; Penna, F.; Menduina, M.L.; Aversa, Z.; Zimmers, T.; Verzaro, R.; Fittipaldi, R.; Caretti, G.; et al. Vitamin D and VDR in cancer cachexia and muscle regeneration. Oncotarget 2017, 8, 21778–21793. [Google Scholar] [CrossRef] [Green Version]
- Kumada, H.; Mitsutake, S.; Inagaki, Y.; Mitsunaga, S.; Katsumura, S.; Igarashi, Y. Kinetics of the ceramide kinase inhibitor K1, a suppressor of mast-cell activation. Biosci. Biotechnol. Biochem. 2007, 71, 2581–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squecco, R.; Pierucci, F.; Idrizaj, E.; Frati, A.; Lenci, E.; Vicenti, C.; Iachini, M.C.; Martinesi, M.; Garella, R.; Baccari, M.C.; et al. Ceramide/protein phosphatase 2a axis is engaged in gap junction impairment elicited by PCB153 in liver stem-like progenitor cells. Mol. Cell. Biochem. 2021, 1–16. [Google Scholar] [CrossRef]
- Bedia, C.; Triola, G.; Casas, J.; Llebaria, A.; Fabriàs, G. Analogs of the dihydroceramide desaturase inhibitor GT11 modified at the amide function: Synthesis and biological activities. Org. Biomol. Chem. 2005, 3, 3707–3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, D.; Liefshitz, A. Potential of the proteasome inhibitor MG-132 as an anticancer agent, alone and in combination. Anticancer Res. 2001, 21, 3941–3947. [Google Scholar]
- Murakami, M.; Ito, H.; Hagiwara, K.; Yoshida, K.; Sobue, S.; Ichihara, M.; Takagi, A.; Kojima, T.; Tanaka, K.; Tamiya-Koizumi, K.; et al. ATRA inhibits ceramide kinase transcription in a human neuroblastoma cell line, SH-SY5Y cells: The role of COUP-TFI. J. Neurochem. 2010, 112, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Cencetti, F.; Donati, C.; Nuti, F.; Farnararo, M.; Kohno, T.; Igarashi, Y.; Bruni, P. Down-regulation of EDG5/S1P2 during myogenic differentiation results in the specific uncoupling of sphingosine 1-phosphate signalling to phospholipase D. Biochim. Biophys. Acta 2003, 1633, 133–142. [Google Scholar] [CrossRef]
- Frati, A.; Landi, D.; Marinelli, C.; Gianni, G.; Fontana, L.; Migliorini, M.; Pierucci, F.; Gargia-Gil, M.; Meacci, E. Nutraceutical properties of tuscany chestnut flours: Beneficial effects on skeletal muscle atrophy. Food Funct. 2014, 5, 2870–2882. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, F.; Garcia-Gil, M.; Frati, A.; Bini, F.; Martinesi, M.; Vannini, E.; Mainardi, M.; Luzzati, F.; Peretto, P.; Caleo, M.; et al. Vitamin D3 protects against Aβ peptide cytotoxicity in differentiated human neuroblastoma SH- SY5Y cells: A role for S1P1/p38MAPK/ATF4 axis. Neuropharmacology 2017, 116, 328–342. [Google Scholar] [CrossRef]
- Kisselev, A.F.; Goldberg, A.L. Proteasome Inhibitors: From research tools to drug candidates. Chem. Biol. 2001, 8, 739–758. [Google Scholar] [CrossRef] [Green Version]
- Penna, F.; Baccino, F.M.; Costelli, P. Coming back: Autophagy in cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 241–246. [Google Scholar] [CrossRef]
- Nishino, S.; Yamashita, H.; Tamori, M.; Mashimo, M.; Yamagata, K.; Nakamura, H.; Murayama, T. Translocation and activation of sphingosine kinase 1 by ceramide-1-phosphate. J. Cell. Biochem. 2019, 120, 5396–5408. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef]
- Meacci, E.; Garcia-Gil, M. S1P/S1P receptor signaling in neuromuscolar disorders. Int. J. Mol. Sci. 2019, 20, 6364. [Google Scholar] [CrossRef] [Green Version]
- Sassoli, C.; Frati, A.; Tani, A.; Anderloni, G.; Pierucci, F.; Matteini, F.; Chellini, F.; Zecchi-Orlandini, S.; Formigli, L.; Meacci, E. Mesenchymal stromal cell secreted sphingosine 1-phosphate (S1P) exerts a stimulatory effect on skeletal muscle myoblast proliferation. PLoS ONE 2014, 9, e108662. [Google Scholar] [CrossRef] [Green Version]
- Sassoli, C.; Formigli, L.; Bini, F.; Tani, A.; Squecco, R.; Battistini, C.; Zecchi-Orlandini, S.; Francini, F.; Meacci, E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J. Cell. Mol. Med. 2011, 15, 2498–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squecco, R.; Sassoli, C.; Nuti, F.; Martinesi, M.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L.; Meacci, E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: A role for a gap junction-dependent and -independent function. Mol. Biol. Cell 2006, 17, 4896–4910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formigli, L.; Sassoli, C.; Squecco, R.; Bini, F.; Martinesi, M.; Chellini, F.; Luciani, G.; Sbrana, F.; Zecchi-Orlandini, S.; Francini, F.; et al. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J. Cell Sci. 2009, 122, 1322–1333. [Google Scholar] [CrossRef] [Green Version]
- Bondì, M.; Germinario, E.; Pirazzini, M.; Zanetti, G.; Cencetti, F.; Donati, C.; Gorza, L.; Betto, R.; Bruni, P.; Danieli-Betto, D. Ablation of S1P3 receptor protects mouse soleus from age-related drop in muscle mass, force, and regenerative capacity. Am. J. Physiol. Cell. Physiol. 2017, 313, C54–C67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajjalieh, S.M.; Martin, T.F.; Floor, E. Synaptic vesicle ceramide kinase. A calcium-stimulated lipid kinase that co-purifies with brain synaptic vescicles. J. Biol. Chem. 1989, 264, 14354–14360. [Google Scholar] [CrossRef]
- Presa, N.; Gomez-Larrauri, A.; Rivera, I.G.; Ordoñez, M.; Trueba, M.; Gomez-Muñoz, A. Regulation of cell migration and inflammation by ceramide 1-phosphate. Biochim. Biophys. Acta 2016, 1861, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Muñoz, A. The role of ceramide 1-phosphate in tumor cell survival and dissemination. Adv. Cancer Res. 2018, 140, 217–234. [Google Scholar]
- Payne, A.W.; Pant, D.K.; Pan, T.C.; Chodosh, L.A. Ceramide kinase promotes tumor cell survival and mammary tumor recurrence. Cancer Res. 2014, 74, 6352–6363. [Google Scholar] [CrossRef] [Green Version]
- Rivera, I.G.; Ordonez, M.; Presa, N.; Gangoiti, P.; Gomez-Larrauri, A.; Trueba, M.; Fox, T.; Kester, M.; Gomez-Muñoz, A. Ceramide 1-phosphate regulates cell migration and invasion of human pancreatic cancer cells. Biochem. Pharmacol. 2016, 102, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Salaun, E.; Lefeuvre-Orfila, L.; Cavey, T.; Martin, B.; Turlin, B.; Ropert, M.; Loreal, O.; Derbré, F. Myriocin prevents muscle ceramide accumulation but not muscle fiber atrophy during short-term mechanical unloading. J. Appl. Physiol. 2016, 120, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Kukreti, H.; Amuthavalli, K. MicroRNA-34a causes ceramide accumulation and effects insulin signaling pathway by targeting ceramide kinase (CERK) in aging skeletal muscle. J. Cell. Biochem. 2020, 121, 3070–3089. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Fretts, A.M.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma ceramides and sphingomyelins in relation to heart failure risk: The cardiovascular health study. Circ. Heart Fail. 2019, 12, e005708. [Google Scholar] [CrossRef]
- Bilodeau, A.P.; Coyne, E.S.; Wing, S.S. The Ubiquitin proteasome system in atrophying skeletal muscle: Roles and regulation. Am. J. Physiol. Cell Physiol. 2016, 311, 392–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bini, F.; Frati, A.; Garcia-Gil, M.; Battistini, C.; Granado, M.; Martinesi, M.; Mainardi, M.; Vannini, E.; Luzzati, F.; Caleo, M.; et al. New signaling pathway involved in the anti-proliferative action of vitamin D3 and its analogues in human neuroblastoma cells: A role for ceramide kinase. Neuropharmacology 2012, 63, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Caron, A.Z.; Haroun, S.; Leblanc, E.; Trensz, F.; Guindi, C.; Amrani, A.; Grenier, G. The proteasome inhibitor MG132 reduces immobilization-induced skeletal muscle atrophy in mice. BMC Musculoskelet. Disord. 2011, 12, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, S.; Kato, H.; Takahashi, S.; Johno, H.; Kitamura, M. Inhibition of NF-κB by MG132 through ER stress-mediated induction of LAP and LIP. FEBS Lett. 2011, 585, 2249–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, I.G.; Ordoñez, M.; Presa, N.; Gomez-Larrauri, A.; Simón, J.; Trueba, M.; Gomez-Muñoz, A. Sphingomyelinase D/ceramide 1-phosphate in cell survival and inflammation. Toxins 2015, 7, 1457–1466. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Tanaka, A.; Nakamura, H.; Murayama, T. Knockout of ceramide kinase aggravates pathological and lethal responses in mice with experimental colitis. Biol. Pharm. Bull. 2018, 41, 797–805. [Google Scholar] [CrossRef] [Green Version]










| Parameter | C | C26 Hosts |
|---|---|---|
| Initial body weight (g) | 19.16 ± 0.50 | 18.86 ± 0.97 |
| Final body weight (g) | 21.83 ± 0.8 | 17.17 ± 1.2 *** |
| Gastrocnemius (mg/100g i.b.w.) left | 556 ± 18 | 415 ± 47 ** |
| Gastrocnemius right (mg/100g i.b.w.) | 563 ± 30 | 431 ± 51 *** |
| Liver (mg/100g i.b.w.) | 5932 ± 418 | 5378 ± 472 * |
| Spleen (mg/100g i.b.w.) | 420 ± 32 | 1220 ± 296 *** |
| Heart (mg/100g i.b.w.) | 546 ± 32 | 517 ± 57 |
| Tumor mass (mg) | - | 351 ± 56 |
| Tibialis anterior (mg/100g i.b.w.) | ||
| SCR-shRNA | 228 ± 29 | 134 ± 32 *** |
| CerK-shRNA | 170 ± 16 *** | 119 ± 28 |
| CerK mRNA (Above Vehicle ± S.E.M.) | Atrogin-1 mRNA (Above Vehicle ± S.E.M.) | ||
|---|---|---|---|
| Vehicle | 1 ± 0.04 | Vehicle | 1 ± 0.2 |
| Dexa | 1.2 ± 0.12 | Dexa | 6 ± 0.8 ** |
| DMEM 1:4 | 1 ± 0.1 | DMEM 1:4 | 1 ± 0.1 |
| CM-LLC 1:4 | 0.55 ± 0.16 * | CM-LLC 1:4 | 5.2 ± 0.51 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierucci, F.; Frati, A.; Battistini, C.; Penna, F.; Costelli, P.; Meacci, E. Control of Skeletal Muscle Atrophy Associated to Cancer or Corticosteroids by Ceramide Kinase. Cancers 2021, 13, 3285. https://doi.org/10.3390/cancers13133285
Pierucci F, Frati A, Battistini C, Penna F, Costelli P, Meacci E. Control of Skeletal Muscle Atrophy Associated to Cancer or Corticosteroids by Ceramide Kinase. Cancers. 2021; 13(13):3285. https://doi.org/10.3390/cancers13133285
Chicago/Turabian StylePierucci, Federica, Alessia Frati, Chiara Battistini, Fabio Penna, Paola Costelli, and Elisabetta Meacci. 2021. "Control of Skeletal Muscle Atrophy Associated to Cancer or Corticosteroids by Ceramide Kinase" Cancers 13, no. 13: 3285. https://doi.org/10.3390/cancers13133285
APA StylePierucci, F., Frati, A., Battistini, C., Penna, F., Costelli, P., & Meacci, E. (2021). Control of Skeletal Muscle Atrophy Associated to Cancer or Corticosteroids by Ceramide Kinase. Cancers, 13(13), 3285. https://doi.org/10.3390/cancers13133285







