Stereotactic Ablative Brachytherapy: Recent Advances in Optimization of Radiobiological Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Main Principles of Ablative RT
3. Characteristics of Modern BT
3.1. Utilization of 3D After-Loading Machines
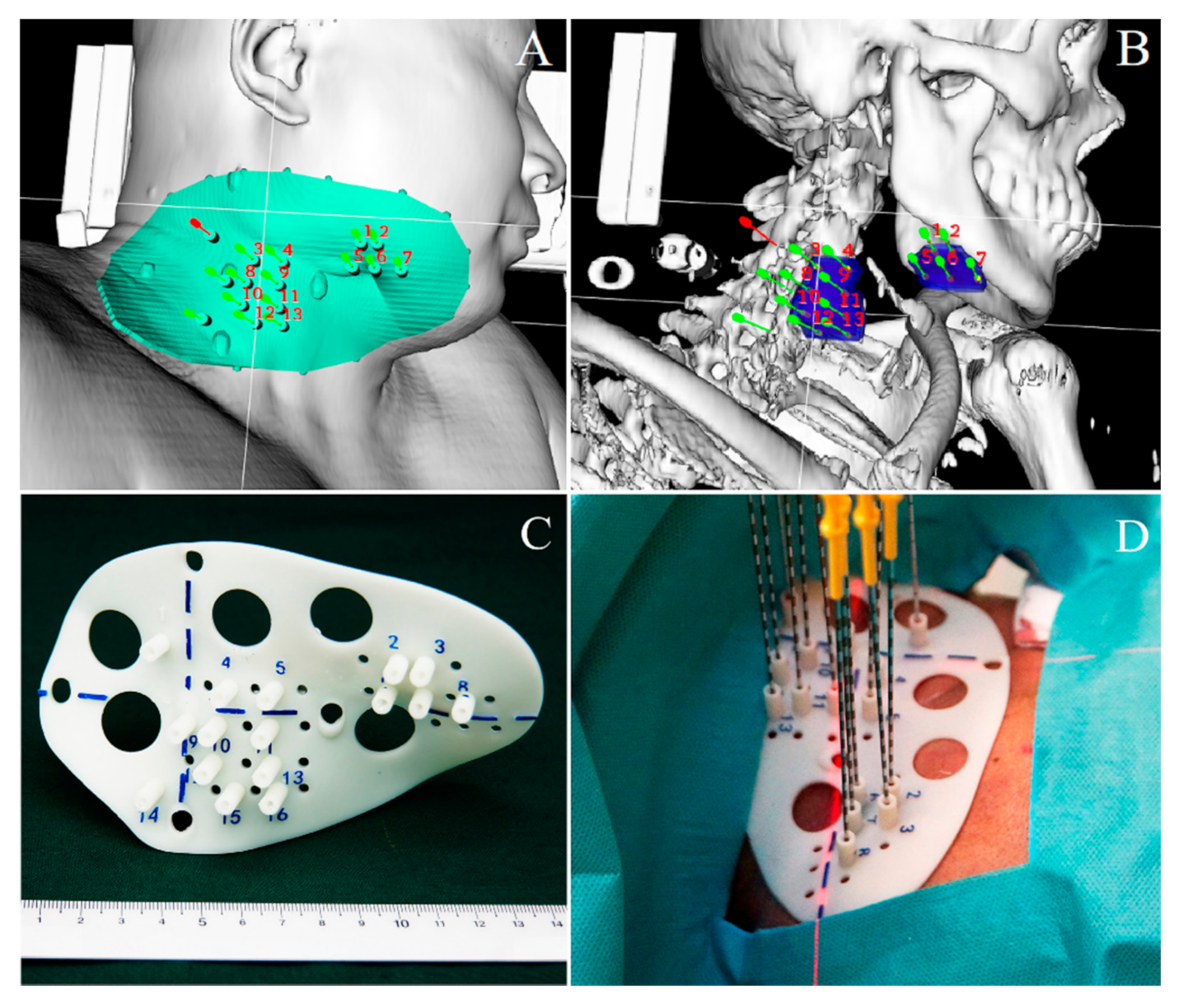
3.2. 3D-PT and Increased Implantation Accuracy
4. SABT Concept and Design
4.1. Image Guidance during Delivery of Treatment
4.2. Precise Calculation of RT Dose/Duration Defines HDR and LDR Effectiveness and Curative Effects
4.3. SABT Allows Shortening the Duration of the RT Exposure
4.4. BT Facilitates Organ Preservation
5. The Practice of SABT: Outcomes, Advances, and Perspectives
5.1. Application of SABT in Prostate Cancer Patients
5.2. Application of SABT in Lung Cancer Patients
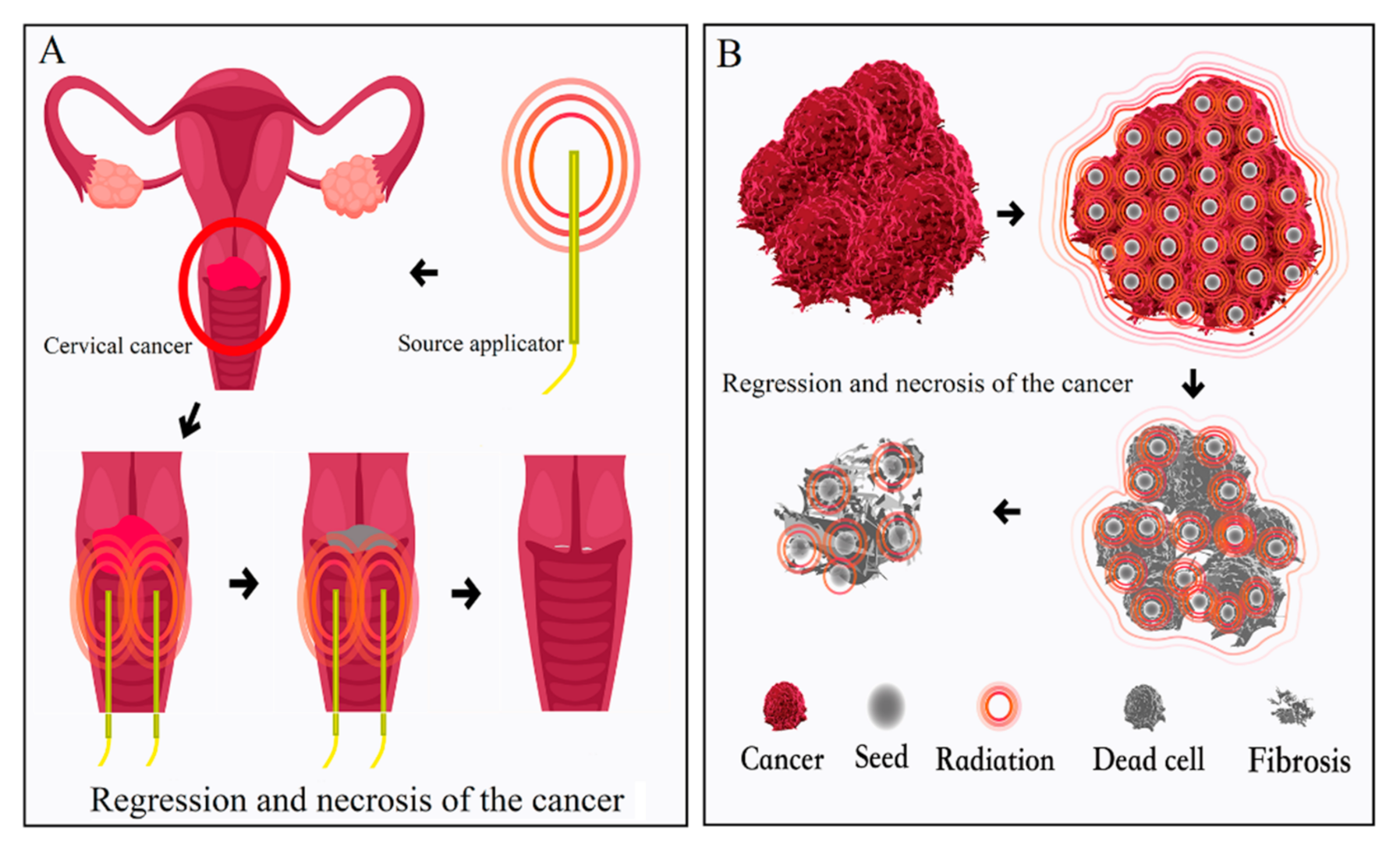
5.3. Application of SABT for Treatment of Gynecological Tumors
5.4. Application of SABT in Liver Cancer Patients
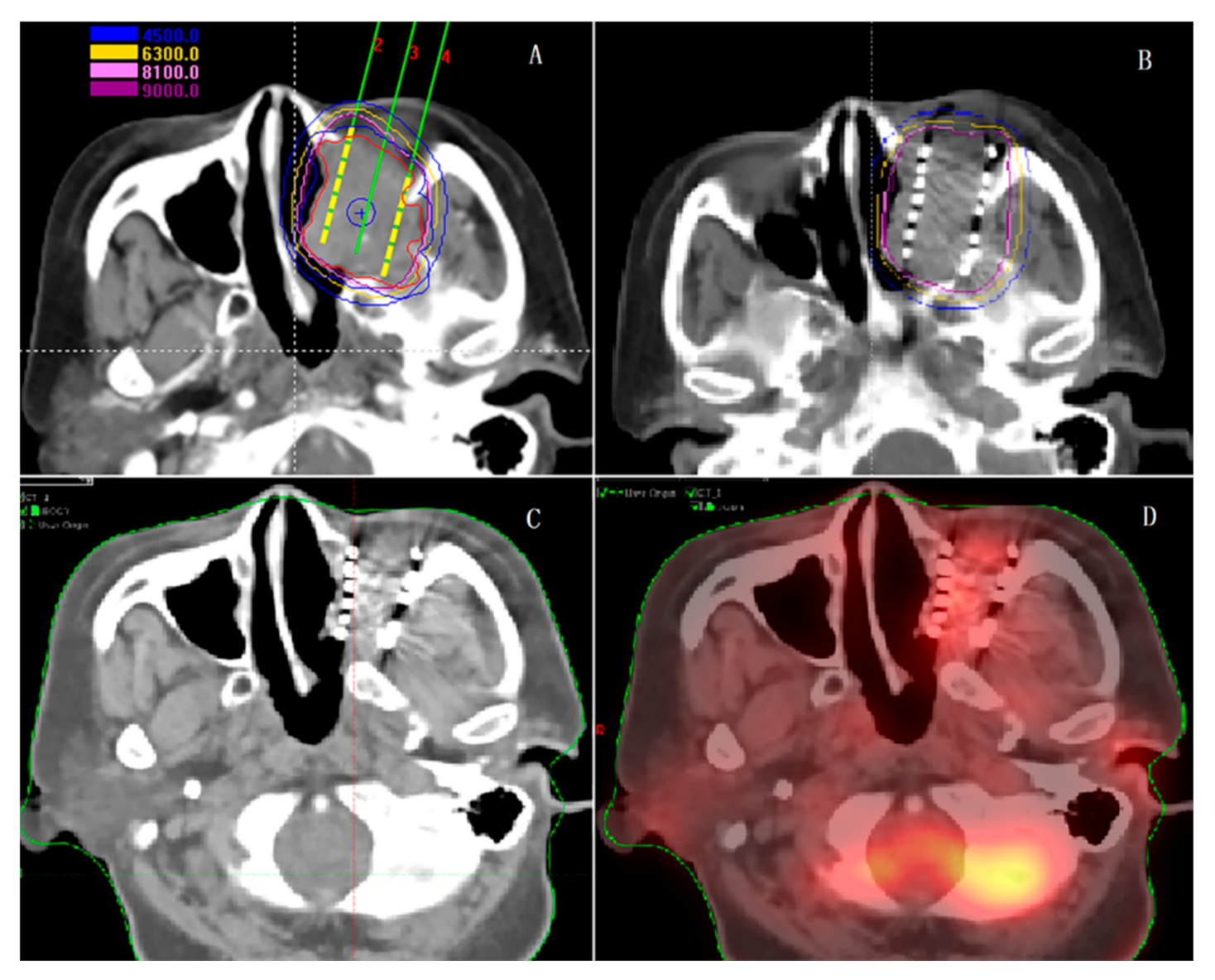
5.5. SABT in Other Cancers
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers. 2019, 5, 13. [Google Scholar] [CrossRef]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.B.; Krishnan, S.; Hodge, J.W.; Chang, J.Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat. Rev. Clin. Oncol. 2016, 13, 516–524. [Google Scholar] [CrossRef]
- Chargari, C.; Deutsch, E.; Blanchard, P.; Gouy, S.; Martelli, H.; Guerin, F.; Dumas, I.; Bossi, A.; Morice, P.; Viswanathan, A.N.; et al. Brachytherapy: An overview for clinicians. CA Cancer J. Clin. 2019, 69, 386–401. [Google Scholar] [CrossRef]
- Pang, H.; Wu, K.; Shi, X.; Tang, T.; Sun, X.; Yang, B.; Wu, J.; Lin, S. Hypofractionated (192)Ir source stereotactic ablative brachytherapy with coplanar template assistance in the primary treatment of peripheral lung cancer. J. Contemp. Brachyther. 2019, 11, 370–378. [Google Scholar] [CrossRef]
- Shi, X.X.; Pang, H.W.; Ren, P.R.; Sun, X.Y.; Wu, J.B.; Lin, S. Template-assisted (192)Ir-based stereotactic ablative brachytherapy as a neoadjuvant treatment for operable peripheral non-small cell lung cancer: A phase I clinical trial. J. Contemp. Brachyther. 2019, 11, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Y.; Ji, Z.; Jiang, P.; Xu, F.; Zhang, Y.; Guo, F.; Peng, R.; Li, X.; Sun, H.; et al. Efficacy and safety of CT-guided (125)I seed implantation as a salvage treatment for locally recurrent head and neck soft tissue sarcoma after surgery and external beam radiotherapy: A 12-year study at a single institution. Brachytherapy 2020, 19, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Aili, A.; Xue, L.; Jiang, P.; Wang, J. Advances in Radiobiology of Stereotactic Ablative Radiotherapy. Front. Oncol. 2020, 10, 1165. [Google Scholar] [CrossRef]
- Sharma, D.N.; Rath, G.K.; Thulkar, S.; Bahl, A.; Pandit, S.; Julka, P.K. Computerized tomography-guided percutaneous high-dose-rate interstitial brachytherapy for malignant lung lesions. J. Cancer Res. Ther. 2011, 7, 174–179. [Google Scholar] [CrossRef]
- Williams, M.; James, N.; Summers, E.; Barrett, A.; Ash, D. National survey of radiotherapy fractionation practice in 2003. Clin. Oncol. 2006, 18, 3–14. [Google Scholar] [CrossRef]
- RodneyWithers, H. The Four R’s of Radiotherapy. Adv. Radiat. Biol. 1975, 5, 241–271. [Google Scholar]
- Leksell, L. The stereotaxic method and radiosurgery of the brain. Acta Chir. Scand. 1951, 102, 316–319. [Google Scholar]
- Sheehan, J.P.; Yen, C.-P.; Lee, C.-C.; Loeffler, J.S. Cranial Stereotactic Radiosurgery: Current Status of the Initial Paradigm Shifter. J. Clin. Oncol. 2014, 32, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.D.; Herman, J.; Cho, L.C. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J. Clin. Oncol. 2014, 32, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.; Papiez, L.; McGarry, R.; Likes, L.; DesRosiers, C.; Frost, S.; Williams, M. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003, 124, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.R.; Stenmark, M.H.; Tao, Y.; Pollom, E.L.; Caoili, E.M.; Lawrence, T.S.; Schipper, M.J.; Feng, M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J. Clin. Oncol. 2016, 34, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Stokes, W.A.; Bronsert, M.R.; Meguid, R.; Blum, M.G.; Jones, B.; Koshy, M.; Sher, D.J.; Louie, A.V.; Palma, D.A.; Senan, S.; et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 642–651. [Google Scholar] [CrossRef]
- Brown, J.M.; Carlson, D.J.; Brenner, D.J. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 254–262. [Google Scholar] [CrossRef]
- Lo, S.S.; Fakiris, A.J.; Chang, E.L.; Mayr, N.A.; Wang, J.Z.; Papiez, L.; Teh, B.S.; McGarry, R.C.; Cardenes, H.R.; Timmerman, R.D. Stereotactic body radiation therapy: A novel treatment modality. Nat. Rev. Clin. Oncol. 2010, 7, 44–54. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Insana, M.F. Stromal responses to fractionated radiotherapy. Int. J. Radiat Biol. 2012, 88, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.A.; Kwak, J.T.; Insana, M.F. Stromal-epithelial responses to fractionated radiotherapy in a breast cancer microenvironment. Cancer Cell Int. 2015, 15, 67. [Google Scholar] [CrossRef]
- Song, C.W.; Lee, Y.-J.; Griffin, R.J.; Park, I.; Koonce, N.A.; Hui, S.; Kim, M.-S.; Dusenbery, K.E.; Sperduto, P.W.; Cho, L.C. Indirect Tumor Cell Death After High-Dose Hypofractionated Irradiation: Implications for Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 166–172. [Google Scholar] [CrossRef]
- Annede, P.; Cosset, J.-M.; Van Limbergen, E.; Deutsch, E.; Haie-Meder, C.; Chargari, C. Radiobiology: Foundation and New Insights in Modeling Brachytherapy Effects. Semin. Radiat. Oncol. 2020, 30, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Van Limbergen, E.; Mahantshetty, U.; Deutsch, É.; Haie-Méder, C. Radiobiology of brachytherapy: The historical view based on linear quadratic model and perspectives for optimization. Cancer/Radiothérapie 2018, 22, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Mazeron, J.J. Radiobiology in brachytherapy. Cancer Radiother. 2013, 17, 81–84. [Google Scholar] [CrossRef]
- Kovács, G. Modern head and neck brachytherapy: From radium towards intensity modulated interventional brachytherapy. J. Contemp. Brachytherapy 2014, 6, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Jiang, P.; Ji, Z.; Huo, X.; Sun, H.; Wang, J. Brachytherapy for lung cancer. Brachytherapy 2021, 20, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Rogers, L.; Demanes, D.J.; Morton, G.; Prestidge, B.R.; Pouliot, J.; Cohen, G.; Zaider, M.; Ghilezan, M.; Hsu, I.-C. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy 2012, 11, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Parashar, B.; Patel, M.; O’Farrell, D.; Biagioli, M.; Devlin, P.; Mutyala, S. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Albuquerque, K.; Hrycushko, B.A.; Harkenrider, M.M.; Mayadev, J.; Klopp, A.; Beriwal, S.; Petereit, D.G.; Scanderbeg, D.J.; Yashar, C. Compendium of fractionation choices for gynecologic HDR brachytherapy-An American Brachytherapy Society Task Group Report. Brachytherapy 2019, 18, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, J. Current status of brachytherapy in cancer treatment—Short overview. J. Contemp. Brachytherapy 2017, 9, 581–589. [Google Scholar] [CrossRef]
- Qiu, B.; Jiang, Y.; Ji, Z.; Sun, H.; Fan, J.; Li, W.; Shao, Y.; Jiang, P.; Wang, J. The Accuracy of Individualized 3D-Printing Template-Assisted I(125) Radioactive Seed Implantation for Recurrent/Metastatic Head and Neck Cancer. Front. Oncol. 2021, 11, 664996. [Google Scholar] [CrossRef]
- Chin, J.; Rumble, R.B.; Kollmeier, M.; Heath, E.; Efstathiou, J.; Dorff, T.; Berman, B.; Feifer, A.; Jacques, A.; Loblaw, D.A. Brachytherapy for Patients With Prostate Cancer: American Society of Clinical Oncology/Cancer Care Ontario Joint Guideline Update. J. Clin. Oncol. 2017, 35, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Asakawa, I.; Hasegawa, M.; Fujimoto, K. Low-dose-rate brachytherapy for prostate cancer: A 15-year experience in Japan. Int. J. Urol. 2020, 27, 17–23. [Google Scholar] [CrossRef]
- Lee, K.K.; Lee, J.Y.; Nam, C.M.; Kim, C.B.; Park, K.R. High-dose-rate vs. low-dose-rate intracavitary brachytherapy for carcinoma of the uterine cervix: Systematic review and meta-analysis. Brachytherapy 2015, 14, 449–457. [Google Scholar] [CrossRef]
- Stewart, A.J.; Viswanathan, A.N. Current controversies in high-dose-rate versus low-dose-rate brachytherapy for cervical cancer. Cancer 2006, 107, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Hattangadi, J.A.; Powell, S.N.; Macdonald, S.M.; Mauceri, T.; Ancukiewicz, M.; Freer, P.; Lawenda, B.; El-Din, M.A.A.; Gadd, M.A.; Smith, B.L.; et al. Accelerated partial breast irradiation with low-dose-rate interstitial implant brachytherapy after wide local excision: 12-year outcomes from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 791–800. [Google Scholar] [CrossRef]
- Guedea, F.; Venselaar, J.; Hoskin, P.; Hellebust, T.P.; Peiffert, D.; Londres, B.; Ventura, M.; Mazeron, J.-J.; Van Limbergen, E.; Pötter, R.; et al. Patterns of care for brachytherapy in Europe: Updated results. Radiother. Oncol. 2010, 97, 514–520. [Google Scholar] [CrossRef]
- Ji, Z.; Jiang, Y.; Guo, F.; Sun, H.; Fan, J.; Zhang, L.; Wang, J. Dosimetry verification of radioactive seed implantation for malignant tumors assisted by 3D printing individual templates and CT guidance. Appl. Radiat. Isot. 2017, 124, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Jiang, Y.; Tian, S.; Guo, F.; Peng, R.; Xu, F.; Sun, H.; Fan, J.; Wang, J. The Effectiveness and Prognostic Factors of CT-Guided Radioactive I-125 Seed Implantation for the Treatment of Recurrent Head and Neck Cancer After External Beam Radiation Therapy. Int. J. Radiat. Oncol. 2019, 103, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Sun, H.; Jiang, Y.; Guo, F.; Peng, R.; Fan, J.; Wang, J. Comparative study for CT-guided 125I seed implantation assisted by 3D printing coplanar and non-coplanar template in peripheral lung cancer. J. Contemp. Brachytherapy 2019, 11, 169–173. [Google Scholar] [CrossRef]
- Ji, Z.; Jiang, Y.; Guo, F.; Peng, R.; Sun, H.; Fan, J.; Xu, F.; Wang, J. Safety and efficacy of CT-guided radioactive iodine-125 seed implantation assisted by a 3D printing template for the treatment of thoracic malignancies. J. Cancer Res.Clin. Oncol. 2020, 146, 229–236. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Guo, J.; Chai, S.; Zheng, G.; Zhang, K.; Liao, A.; Jiang, P.; Jiang, Y.; Ji, Z. Expert consensus workshop report: Guideline for three-dimensional printing template-assisted computed tomography-guided (125)I seeds interstitial implantation brachytherapy. J. Cancer Res. Ther. 2017, 13, 607–612. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. The concept of stereotactic ablation brachytherapy and practice. Chin. J. Radiol. Med. Prot. 2020, 40, 173–177. [Google Scholar]
- Wang, J.; Chai, S.; Wang, R.; Zheng, G.; Zhang, K.; Huo, B.; Huo, X.; Jiang, Y.; Ji, Z.; Jiang, P.; et al. Expert consensus on computed tomography-assisted three-dimensional-printed coplanar template guidance for interstitial permanent radioactive 125I seed implantation therapy. J. Cancer Res. Ther. 2019, 15, 1430–1434. [Google Scholar] [CrossRef]
- Davis, B.J.; Horwitz, E.M.; Lee, W.R.; Crook, J.M.; Stock, R.G.; Merrick, G.S.; Butler, W.M.; Grimm, P.D.; Stone, N.N.; Potters, L.; et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012, 11, 6–19. [Google Scholar] [CrossRef]
- Hoskin, P.; Colombo, A.; Henry, A.; Niehoff, P.; Hellebust, T.P.; Siebert, F.-A.; Kovacs, G. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: An update. Radiother. Oncol. 2013, 107, 325–332. [Google Scholar] [CrossRef]
- Cohen, G.N.; Episcopia, K.; Lim, S.B.; LoSasso, T.J.; Rivard, M.J.; Taggar, A.S.; Taunk, N.K.; Wu, A.J.; Damato, A.L. Intraoperative implantation of a mesh of directional palladium sources (CivaSheet): Dosimetry verification, clinical commissioning, dose specification, and preliminary experience. Brachytherapy 2017, 16, 1257–1264. [Google Scholar] [CrossRef]
- Kovács, G.; Martinez-Monge, R.; Budrukkar, A.; Guinot, J.L.; Johansson, B.; Strnad, V.; Skowronek, J.; Rovirosa, A.; Siebert, F.-A. GEC-ESTRO ACROP recommendations for head & neck brachytherapy in squamous cell carcinomas: 1st update—Improvement by cross sectional imaging based treatment planning and stepping source technology. Radiother. Oncol. 2017, 122, 248–254. [Google Scholar]
- Zhang, C.; Hilts, M.; Batchelar, D.; Orlando, N.; Gardi, L.; Fenster, A.; Crook, J. Characterization and registration of 3D ultrasound for use in permanent breast seed implant brachytherapy treatment planning. Brachytherapy 2021, 20, 248–256. [Google Scholar] [CrossRef]
- Doyle, A.J.; King, D.M.; Browne, J.E. A review of the recommendations governing quality assurance of ultrasound systems used for guidance in prostate brachytherapy. Phys. Medica 2017, 44, 51–57. [Google Scholar] [CrossRef]
- Weersink, R.A.; Qiu, J.; Martinez, D.; Rink, A.; Borg, J.; Di Tomasso, A.; Irish, J.C.; Jaffray, D.A. Feasibility study of navigated endoscopy for the placement of high dose rate brachytherapy applicators in the esophagus and lung. Med. Phys. 2019, 47, 917–926. [Google Scholar] [CrossRef]
- Schmidt, M.; Payne, G.S. Radiotherapy planning using MRI. Phys. Med. Biol. 2015, 60, R323–R361. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Erickson, B.; Gaffney, D.K.; Beriwal, S.; Bhatia, S.K.; Lee Burnett, O., 3rd; D’Souza, D.P.; Patil, N.; Haddock, M.G.; Jhingran, A.; et al. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Major, T.; Frohlich, G.; Lovey, K.; Fodor, J.; Polgar, C. Dosimetric experience with accelerated partial breast irradiation using image-guided interstitial brachytherapy. Radiother. Oncol. 2009, 90, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, M.; Li, N.; Miao, M.; Yang, Y.; Hsu, H.-C.; Chen, H.-M.; Wu, S.-Y. Contemporary external beam radiotherapy boost or high dose-rate brachytherapy boost for cervical cancer: A propensity-score-matched, nationwide, population-based cohort study. Am. J. Cancer Res. 2021, 11, 1719–1732. [Google Scholar] [PubMed]
- Zaorsky, N.G.; Davis, B.J.; Nguyen, P.L.; Showalter, T.; Hoskin, P.; Yoshioka, Y.; Morton, G.C.; Horwitz, N.G.Z.E.M. The evolution of brachytherapy for prostate cancer. Nat. Rev. Urol. 2017, 14, 415–439. [Google Scholar] [CrossRef]
- Yamazaki, H.; Masui, K.; Suzuki, G.; Aibe, N.; Shimizu, D.; Kimoto, T.; Yamada, K.; Ueno, A.; Matsugasumi, T.; Yamada, Y.; et al. High-dose-rate brachytherapy with external beam radiotherapy versus low-dose-rate brachytherapy with or without external beam radiotherapy for clinically localized prostate cancer. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Takenaka, Y.; Noda, Y.; Kawai, N.; Yoshikawa, T.; Wakamiya, T.; Hara, I.; Sonomura, T. Reduction of toxicity in brachytherapy using a new technique. Brachytherapy 2021, 20, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, Y.; Miyakawa, A.; Otsuka, S.; Iwata, H. Radiobiology of hypofractionated stereotactic radiotherapy: What are the optimal fractionation schedules? J. Radiat. Res. 2016, 57 (Suppl. S1), i76–i82. [Google Scholar] [CrossRef] [PubMed]
- Tanderup, K.; Ménard, C.; Polgar, C.; Lindegaard, J.C.; Kirisits, C.; Pötter, R. Advancements in brachytherapy. Adv. Drug Deliv. Rev. 2017, 109, 15–25. [Google Scholar] [CrossRef]
- Chen, C.; Wang, W.; Yu, Z.; Tian, S.; Li, Y.; Wang, Y. Combination of computed tomography-guided iodine-125 brachytherapy and bronchial arterial chemoembolization for locally advanced stage III non-small cell lung cancer after failure of concurrent chemoradiotherapy. Lung Cancer 2020, 146, 290–296. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, B.; Li, Z.; Wang, W. 125I Brachytherapy Seeds Implantation for Inoperable Low-Grade Leiomyosarcoma of Inferior Vena Cava. Korean, J. Radiol. 2013, 14, 278–282. [Google Scholar] [CrossRef][Green Version]
- Yang, B.; Guo, W.-H.; Lan, T.; Yuan, F.; Liu, G.-J.; Zan, R.-Y.; You, X.; Tan, Q.-Y.; Liao, Z.-Y. CT-guided 125I seed implantation for inoperable retroperitoneal sarcoma: A technique for delivery of local tumor brachytherapy. Exp. Ther. Med. 2016, 12, 3843–3850. [Google Scholar] [CrossRef][Green Version]
- Voskuilen, C.S.; Bosschieter, J.; van Werkhoven, E.; Hendricksen, K.; Vis, A.N.; Witteveen, T.; Pieters, B.R.; Burger, M.; Bex, A.; van der Poel, H.G.; et al. Long-term survival and complications following bladder-preserving brachytherapy in patients with cT1-T2 bladder cancer. Radiother. Oncol. 2019, 141, 130–136. [Google Scholar] [CrossRef]
- Gérard, J.-P.; Barbet, N.; Gal, J.; Dejean, C.; Evesque, L.; Doyen, J.; Coquard, R.; Gugenheim, J.; Benizri, E.; Schiappa, R.; et al. Planned organ preservation for early T2-3 rectal adenocarcinoma: A French, multicentre study. Eur. J. Cancer 2019, 108, 1–16. [Google Scholar] [CrossRef]
- Blasko, J.C.; Grimm, P.D.; Ragde, H. Brachytherapy and Organ Preservation in the Management of Carcinoma of the Prostate. Semin. Radiat. Oncol. 1993, 3, 240–249. [Google Scholar] [CrossRef]
- Loblaw, A.; Pickles, T.; Crook, J.; Martin, A.-G.; Vigneault, E.; Souhami, L.; Cury, F.; Morris, J.; Catton, C.; Lukka, H.; et al. Stereotactic Ablative Radiotherapy Versus Low Dose Rate Brachytherapy or External Beam Radiotherapy: Propensity Score Matched Analyses of Canadian Data. Clin. Oncol. 2017, 29, 161–170. [Google Scholar] [CrossRef]
- Taussky, D.; Lambert, C.; Meissner, N.; Bahary, J.-P.; Delouya, G. Risk factors for biochemical recurrence after a tissue-ablative prostate-specific antigen <0.2 ng/mL. Brachytherapy 2018, 17, 794–798. [Google Scholar] [CrossRef]
- Nag, S.; DeHaan, M.; Scruggs, G.; Mayr, N.; Martin, E.W. Long-term follow-up of patients of intrahepatic malignancies treated with iodine-125 brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 736–744. [Google Scholar] [CrossRef]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Ramsay, C.R.; Adewuyi, T.E.; Gray, J.; Hislop, J.; Shirley, M.; Jayakody, S.; MacLennan, G.; Fraser, C.; MacLennan, S.; Brazzelli, M.; et al. Ablative therapy for people with localised prostate cancer: A systematic review and economic evaluation. Health Technol. Assess. 2015, 19, 1–490. [Google Scholar] [CrossRef]
- Kee, D.L.C.; Gal, J.; Falk, A.T.; Schiappa, R.; Chand, M.-E.; Gautier, M.; Doyen, J.; Hannoun-Levi, J.-M. Brachytherapy versus external beam radiotherapy boost for prostate cancer: Systematic review with meta-analysis of randomized trials. Cancer Treat. Rev. 2018, 70, 265–271. [Google Scholar] [CrossRef]
- Ruge, M.I.; Suchorska, B.; Maarouf, M.; Runge, M.; Treuer, H.; Voges, J.J.; Sturm, V. Stereotactic 125Iodine Brachytherapy for the Treatment of Singular Brain Metastases: Closing a Gap? Neurosurg. 2011, 68, 1209–1219. [Google Scholar] [CrossRef]
- Ruge, M.M.I.; Kocher, M.; Maarouf, M.; Hamisch, C.; Treuer, H.; Voges, J.; Sturm, V. Comparison of Stereotactic Brachytherapy (125Iodine Seeds) with Stereotactic Radiosurgery (LINAC) for the Treatment of Singular Cerebral Metastases. Strahlenther. Onkol. 2010, 187, 7–14. [Google Scholar] [CrossRef]
- Tselis, N.; Ferentinos, K.; Kolotas, C.; Schirren, J.; Baltas, D.; Antonakakis, A.; Ackermann, H.; Zamboglou, N. Computed tomography-guided interstitial high-dose-rate brachytherapy in the local treatment of primary and secondary intrathoracic malignancies. J. Thorac. Oncol. 2011, 6, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.; Rojas, A.; Ostler, P.; Hughes, R.; Alonzi, R.; Lowe, G. Single-dose high-dose-rate brachytherapy compared to two and three fractions for locally advanced prostate cancer. Radiother. Oncol. 2017, 124, 56–60. [Google Scholar] [CrossRef]
- Mulherkar, R.; Hasan, S.; Wegner, R.E.; Verma, V.; Glaser, S.M.; Kalash, R.; Beriwal, S.; Horne, Z.D. National patterns of care for early-stage penile cancers in the United States: How is radiation and brachytherapy utilized? Brachytherapy 2019, 18, 503–509. [Google Scholar] [CrossRef]
- Damm, R.; Streitparth, T.; Hass, P.; Seidensticker, M.; Heinze, C.; Powerski, M.; Wendler, J.J.; Liehr, U.B.; Mohnike, K.; Pech, M.; et al. Prospective evaluation of CT-guided HDR brachytherapy as a local ablative treatment for renal masses: A single-arm pilot trial. Strahlenther. und Onkol. 2019, 195, 982–990. [Google Scholar] [CrossRef]
- Tharmalingam, H.; Tsang, Y.; Ostler, P.; Wylie, J.; Bahl, A.; Lydon, A.; Ahmed, I.; Elwell, C.; Nikapota, A.R.; Hoskin, P.J.; et al. Single dose high-dose rate (HDR) brachytherapy (BT) as monotherapy for localised prostate cancer: Early results of a UK national cohort study. Radiother. Oncol. 2020, 143, 95–100. [Google Scholar] [CrossRef]
- Langley, S.; Uribe, J.; Uribe-Lewis, S.; Franklin, A.; Perna, C.; Horton, A.; Cunningham, M.; Higgins, D.; Deering, C.; Khaksar, S.; et al. Hemi-ablative low-dose-rate prostate brachytherapy for unilateral localised prostate cancer. BJU Int. 2019, 125, 383–390. [Google Scholar] [CrossRef]
- Hosni, A.; Carlone, M.; Rink, A.; Menard, C.; Chung, P.; Berlin, A. Dosimetric feasibility of ablative dose escalated focal monotherapy with MRI-guided high-dose-rate (HDR) brachytherapy for prostate cancer. Radiother. Oncol. 2017, 122, 103–108. [Google Scholar] [CrossRef]
- Kovacs, G.; Muller, K.; Soror, T.; Melchert, C.; Guo, X.; Jocham, D.; Merseburger, A. Results of multiparametric transrectal ultrasound-based focal high-dose-rate dose escalation combined with supplementary external beam irradiation in intermediate- and high-risk localized prostate cancer patients. Brachytherapy 2017, 16, 277–281. [Google Scholar] [CrossRef]
- Wolff, R.F.; Ryder, S.; Bossi, A.; Briganti, A.; Crook, J.; Henry, A.; Karnes, J.; Potters, L.; De Reijke, T.; Stone, N.; et al. A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. Eur. J. Cancer 2015, 51, 2345–2367. [Google Scholar] [CrossRef]
- Wang, P.; Shen, L.Q.; Zhang, H.; Zhang, M.; Ji, Z.; Jiang, Y.; Li, B. Quality of life after I-125 seed implantation using computed tomography and three-dimensional-printed template guidance in patients with advanced malignant tumor. J. Cancer Res. Ther. 2018, 14, 1492–1496. [Google Scholar]
- Sharma, D.N.; Rath, G.K. Brachytherapy for medically inoperable lung cancer. Lancet Oncol. 2009, 10, 1141–1142. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Li, R.; Zhang, Y.; Han, M.; Ma, W. Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non–small cell lung cancer—A meta-analysis. Brachytherapy 2018, 17, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Soror, T.; Kovacs, G.; Furschke, V.; Ismail, M.; Badakhshi, H. Salvage treatment with sole high-dose-rate endobronchial interventional radiotherapy (brachytherapy) for isolated endobronchial tumor recurrence in non-small-cell lung cancer patients: A 20-year experience. Brachytherapy 2019, 18, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Aumont-le Guilcher, M.; Prevost, B.; Sunyach, M.P.; Peiffert, D.; Maingon, P.; Thomas, L.; Williaume, D.; Begue, M.; Lerouge, D.; Campion, L.; et al. High-dose-rate brachytherapy for non-small-cell lung carcinoma: A retrospective study of 226 patients. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1112–1116. [Google Scholar] [CrossRef]
- Knox, M.C.; Bece, A.; Bucci, J.; Moses, J.; Graham, P.H. Endobronchial brachytherapy in the management of lung malignancies: 20 years of experience in an Australian center. Brachytherapy 2018, 17, 973–980. [Google Scholar] [CrossRef]
- Skowronek, J.; Piorunek, T.; Kanikowski, M.; Chicheł, A.; Bielęda, G. Definitive high-dose-rate endobronchial brachytherapy of bronchial stump for lung cancer after surgery. Brachytherapy 2013, 12, 560–566. [Google Scholar] [CrossRef]
- Lewis, J.W.; Ajlouni, M.; Kvale, P.A.; Groux, N.; Bae, Y.; Horowitz, B.S.; Magilligan, D.J. Role of brachytherapy in the management of pulmonary and mediastinal malignancies. Ann. Thorac. Surg. 1990, 49, 728–733. [Google Scholar] [CrossRef]
- Martínez-Monge, R.; Pagola, M.; Vivas, I.; López-Picazo, J.M. CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC). Lung Cancer 2008, 61, 209–213. [Google Scholar] [CrossRef]
- Trombetta, M.G.; Colonias, A.; Makishi, D.; Keenan, R.; Werts, E.D.; Landreneau, R.; Parda, D.S. Tolerance of the aorta using intraoperative iodine-125 interstitial brachytherapy in cancer of the lung. Brachytherapy 2008, 7, 50–54. [Google Scholar] [CrossRef]
- Wei, W.; Shen, X.H.; Sun, H.H.; Lu, W.L.; Chai, S.D.; Yang, J.K. The short term therapeutic effects of radioactive (125)I seeds implantation for treatment of non-small-cell lung cancer. Zhonghua Nei Ke Za Zhi. 2012, 51, 978–981. [Google Scholar]
- Xu, W.; Jiang, G.; Li, Z.; Ding, A.; Zhou, F.; Jiao, W.; Tang, D.; Qiu, W.; Yue, L. Computed tomography-guided iodine-125 interstitial implantation as an alternative treatment option for lung cancer. Indian J. Cancer 2014, 51, 9–12. [Google Scholar] [CrossRef]
- Du, P.; Xiao, Y.; Lu, W. Modified Fan-Shaped Distribution Technology for Computed Tomography (CT)-Guided Radioactive Seed Implantation in Lung Cancer Patients with Lung Dysfunction. Med. Sci. Monit. 2017, 23, 4366–4375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiao, D.; Ren, K.; Li, Z.; Shui, S.; Han, X. Clinical role of guidance by C-arm CT for 125I brachytherapy on pulmonary tumors. La Radiol. Med. 2017, 122, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Doggett, S.W.; Chino, S.; Lempert, T. A novel approach for salvage treatment of non-small-cell lung cancer: Percutaneous CT fluoroscopy-guided permanent seed brachytherapy for salvage treatment of lung cancer: Long-term results of a case series. J. Contemp. Brachytherapy 2019, 11, 174–179. [Google Scholar] [CrossRef]
- Li, W.; Guan, J.; Yang, L.; Zheng, X.; Yu, Y.; Jiang, J. Iodine-125 brachytherapy improved overall survival of patients with inoperable stage III/IV non-small cell lung cancer versus the conventional radiotherapy. Med. Oncol. 2014, 32, 395. [Google Scholar] [CrossRef] [PubMed]
- Fernando, H.C.; Landreneau, R.J.; Mandrekar, S.J.; Nichols, F.C.; Hillman, S.L.; Heron, D.E.; Meyers, B.F.; DiPetrillo, T.A.; Jones, D.R.; Starnes, S.L.; et al. Impact of brachytherapy on local recurrence rates after sublobar resection: Results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J. Clin. Oncol. 2014, 32, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, J.; Zhong, X.; He, J. Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer 2015, 15, 1–6. [Google Scholar] [CrossRef]
- Petereit, D.G.; Frank, S.J.; Viswanathan, A.N.; Erickson, B.; Eifel, P.J.; Nguyen, P.L.; Wazer, D.E. Brachytherapy: Where Has It Gone? J. Clin. Oncol. 2015, 33, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, G.; Geszti, G.; Vízkeleti, J.; Ágoston, P.; Polgár, C.; Major, T. Dosimetric comparison of inverse optimisation methods versus forward optimisation in HDR brachytherapy of breast, cervical and prostate cancer. Strahlenther. und Onkol. 2019, 195, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, K.; Tumati, V.; Lea, J.; Ahn, C.; Richardson, D.; Miller, D.; Timmerman, R. A Phase II Trial of Stereotactic Ablative Radiation Therapy as a Boost for Locally Advanced Cervical Cancer. Int. J. Radiat. Oncol. 2020, 106, 464–471. [Google Scholar] [CrossRef]
- Gill, B.S.; Lin, J.F.; Krivak, T.C.; Sukumvanich, P.; Laskey, R.A.; Ross, M.S.; Lesnock, J.L.; Beriwal, S. National Cancer Data Base Analysis of Radiation Therapy Consolidation Modality for Cervical Cancer: The Impact of New Technological Advancements. Int. J. Radiat. Oncol. 2014, 90, 1083–1090. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Den, R.B.; Doyle, L.A.; Dicker, A.P.; Hurwitz, M.D. Combining theoretical potential and advanced technology in high-dose rate brachytherapy boost therapy for prostate cancer. Expert Rev. Med. Devices 2013, 10, 751–763. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Doyle, L.A.; Yamoah, K.; Andrel, J.A.; Trabulsi, E.J.; Hurwitz, M.D.; Dicker, A.P.; Den, R.B. High dose rate brachytherapy boost for prostate cancer: A systematic review. Cancer Treat. Rev. 2014, 40, 414–425. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Liu, P.; Guo, Z.; Ni, H. Computed tomography-guided 125I seed interstitial implantation in the treatment of recurrent ovarian cancer. Int. J. Gynecol. Cancer 2014, 24, 1414–1419. [Google Scholar] [CrossRef]
- Crane, C.H.; Koay, E.J. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer 2016, 122, 1974–1986. [Google Scholar] [CrossRef]
- Kovács, A.; Iezzi, R.; Cellini, F.; Lancellotta, V.; Bischoff, P.; Carchesio, F.; Tagliaferri, L.; Kovács, G.; Gambacorta, M. Critical review of multidisciplinary non-surgical local interventional ablation techniques in primary or secondary liver malignancies. J. Contemp. Brachytherapy 2019, 11, 589–600. [Google Scholar] [CrossRef]
- Pennington, J.D.; Park, S.J.; Abgaryan, N.; Banerjee, R.; Lee, P.P.; Loh, C.; Lee, E.; Demanes, D.J.; Kamrava, M. Dosimetric comparison of brachyablation and stereotactic ablative body radiotherapy in the treatment of liver metastasis. Brachytherapy 2015, 14, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Heinze, C.; Omari, J.; Damm, R.; Hass, P.; Brunner, T.; Surov, A.; Seidesticker, R.; Seidensticker, M.; Ricke, J.; Powerski, M.; et al. Interstitial Brachytherapy for Limited (<4 cm) and Large (>/=4 cm) Hepatic Metastases from Rare and Less Common Cancers. Anticancer Res. 2020, 40, 4281–4289. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Akinwande, O.; Bodei, L.; Chamarthy, M.R.; Devlin, P.M.; Elman, S.; Ganguli, S.; Kennedy, A.S.; Koo, S.J.; Ouhib, Z.; et al. ACR-ABS-ACNM-ASTRO-SIR-SNMMI practice parameter for selective internal radiation therapy or radioembolization for treatment of liver malignancies. Brachytherapy 2021, 20, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Rivard, M.J. Dosimetric Evaluation of the 103Pd Civastring for Permanent Breast Brachytherapy. Brachytherapy 2015, 14 (Suppl. S1), S65–S66. [Google Scholar] [CrossRef]
- Charaghvandi, R.K.; den Hartogh, M.D.; van Ommen, A.M.; de Vries, W.J.; Scholten, V.; Moerland, M.A.; Philippens, M.E.; Schokker, R.I.; van Vulpen, M.; van Asselen, B.; et al. DHMRI-guided single fraction ablative radiotherapy for early-stage breast cancer: A brachytherapy versus volumetric modulated arc therapy dosimetry study. Radiother. Oncol. 2015, 117, 477–482. [Google Scholar] [CrossRef]
- Faaborg, P.M.; Haas, S.; Liao, D.; Ploen, J.; Jakobsen, A.; Rahr, H.B.; Laurberg, S.; Gregersen, H.; Lundby, L.; Christensen, P.; et al. Long-term anorectal function in rectal cancer patients treated with chemoradiotherapy and endorectal brachytherapy. Color. Dis. 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Liang, Y.; Chen, E.; Zhang, H.; Gao, Z.; Wang, J. The effectiveness and prognostic factors of radioactive iodine-125 seed implantation for the treatment of cervical lymph node recurrence of esophageal squamous cell carcinoma after external beam radiation therapy. J. Contemp. Brachytherapy 2020, 12, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Qu, A.; Jiang, P.; Wei, S.; Jiang, Y.; Ji, Z.; Sun, H.; Li, W.; Shao, Y.; Fan, J.; Wang, J. Accuracy and dosimetric parameters comparison of 3D-printed non-coplanar template-assisted computed tomography-guided iodine-125 seed ablative brachytherapy in pelvic lateral recurrence of gynecological carcinomas. J. Contemp. Brachytherapy 2021, 13, 39–45. [Google Scholar] [CrossRef]
- Patel, R.B.; Baniel, C.C.; Sriramaneni, R.N.; Bradley, K.; Markovina, S.; Morris, Z.S. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities. Brachytherapy 2018, 17, 995–1003. [Google Scholar] [CrossRef]
- Walle, T.; Monge, R.M.; Cerwenka, A.; Ajona, D.; Melero, I.; Lecanda, F. Radiation effects on antitumor immune responses: Current perspectives and challenges. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef]
- Mayadev, J.; Nunes, A.T.; Li, M.; Marcovitz, M.; Lanasa, M.C.; Monk, B.J. CALLA: Efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy versus chemoradiotherapy alone in women with locally advanced cervical cancer: A phase III, randomized, double-blind, multicenter study. Int. J. Gynecol. Cancer 2020, 30, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, A.; Takekuma, M.; Mori, K.; Usami, T.; Kondo, E.; Nishio, S.; Nishino, K.; Miyamoto, Y.; Yoshimura, R.; Watanabe, M.; et al. A randomized phase III trial of adjuvant chemotherapy versus concurrent chemoradiotherapy for postoperative cervical cancer: Japanese Gynecologic Oncology Group study (JGOG1082). Int. J. Gynecol. Cancer. 2021, 31, 623–626. [Google Scholar] [CrossRef] [PubMed]
| Author(s) (Reference) | Design | Year | Cases | Mean/Median Age (y) | Male (%) | Cancer | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Nag et al. [71] | Retrospective study | 2006 | 64 | 57.4 | 31 | Intrahepatic malignancies | 160-Gy permanent I brachytherapy | 1-y, 3-ys, 5-ys LCR 44%, 22%, and 22%; 1-y, 3-ys, 5-ys OS rate 73%, 23%, and 5% |
| Ruge et al. [75] | Retrospective study | 2011 | 90 | 59 | 48 | Brain Metastases | SBT | 1-y local cerebral relapse 5.4% |
| Ruge et al. [76] | Retrospective study | 2011 | 142SRS/77SBT | 58/58 | 82/35 | Cerebral Metastases | SRS vs. SBT | 1-y LCR SRS/SBT 93.6%vs.96.7% |
| Pötter et al. [72] | Prospective study | 2011 | 156 | 58 | 0 | Cervix cancer (FIGO stages IB–IVA) | EBRT ± chemotherapy + HDR brachytherapy | Complete remission 97%; 3-ys LCR 95%; 3-ys survival 68% |
| Tselis et al. [77] | Retrospective study | 2011 | 55 | 64 | 37 | Metastatic/primary intrathoracic malignancies | HDR brachytherapy | 1-y, 2-ys, 3-ys LCR 93%, 82% and 82% for metastatic/86%, 79%, and 73% for primary cancer |
| Hoskin et al. [78] | Phase II study | 2017 | 293 | 69 | 293 | Prostate cancer | HDR brachytherapy | 4-ys bPFS 91%-94% |
| Loblaw et al. [69] | Propensity score matching | 2017 | 71SABR/213LDR | 64.93 | 284 | Low risk localised prostate cancer | SABR/LDR | 6-ys biochemical failure-free survival SABR 97.1% versus LDR 93.4% |
| Taussky et al. [70] | Retrospective study | 2018 | 454 | 66 | 454 | Low- or intermediate-risk prostate cancer | LDR prostate brachytherapy | 7-ys recurrence-free survival 96% |
| Mulherkar et al. [79] | Propensity-matched study | 2019 | 52Radiation/419surgery | 69 | 471 | Early-stage penile cancer | Brachytherapy/EBRT/surgery | 5-ys OS: definitive radiation vs. surgery 61.6% vs. 62.2% |
| Damm et al. [80] | Prospective study | 2019 | 16 | 76 | 11 | Renal masses | HDR brachytherapy | LCR 95% (median follow-up 22.5 months) |
| Pang et al. [5] | Clinical trial | 2019 | 33 | 55 | 13 | Peripheral lung cancer (stage I 4, II 14, and III 15) | Ir source stereotactic ablative brachytherapy | CR plus PR at 6-month 100% |
| Tharmalingam et al. [81] | Multicenter prospective | 2020 | 441 | 73 | 441 | Prostate cancer | HDR brachytherapy | 2-ys bPFS 94% and 3-ys bPFS 88% |
| Langley et al. [82] | Phase II prospective trial | 2020 | 30 | 65.6 | 30 | Low or intermediate-risk unilateral localised prostate cancer | Hemi-Ablative LDR brachytherapy | PSA was reduced at 24 months by 78% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Qiu, B.; Wang, H.; Jiang, P.; Sukocheva, O.; Fan, R.; Xue, L.; Wang, J. Stereotactic Ablative Brachytherapy: Recent Advances in Optimization of Radiobiological Cancer Therapy. Cancers 2021, 13, 3493. https://doi.org/10.3390/cancers13143493
Xue H, Qiu B, Wang H, Jiang P, Sukocheva O, Fan R, Xue L, Wang J. Stereotactic Ablative Brachytherapy: Recent Advances in Optimization of Radiobiological Cancer Therapy. Cancers. 2021; 13(14):3493. https://doi.org/10.3390/cancers13143493
Chicago/Turabian StyleXue, Hui, Bin Qiu, Hao Wang, Ping Jiang, Olga Sukocheva, Ruitai Fan, Lixiang Xue, and Junjie Wang. 2021. "Stereotactic Ablative Brachytherapy: Recent Advances in Optimization of Radiobiological Cancer Therapy" Cancers 13, no. 14: 3493. https://doi.org/10.3390/cancers13143493
APA StyleXue, H., Qiu, B., Wang, H., Jiang, P., Sukocheva, O., Fan, R., Xue, L., & Wang, J. (2021). Stereotactic Ablative Brachytherapy: Recent Advances in Optimization of Radiobiological Cancer Therapy. Cancers, 13(14), 3493. https://doi.org/10.3390/cancers13143493








