Predictors of Patient-Reported Incontinence at Adjuvant/Salvage Radiotherapy after Prostatectomy: Impact of Time between Surgery and Radiotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. The IHU-WPRT TOX Study
2.2. The ICIQ-SF Questionnaire—Objective and Subjective UI
2.3. Role of Personality
2.4. Patient Population
2.5. ICIQ Based Incontinence Endpoints
- (a)
- daily frequency: ICIQ3 > 2
- (b)
- amount of urine loss: ICIQ4 > 2
- (c)
- subjective: ICIQ5 > 4
- (d)
- objective: ICIQ3 + ICIQ4 > 5
- (e)
- total: ICIQ3 + ICIQ4 + ICIQ5 > 8
2.6. Statistical Methods
Relationship between ICIQ Scores and Time from Prostatectomy
2.7. Logistic Regression Analysis and Internal Validation
2.8. “Completely Dry” Patients
3. Results
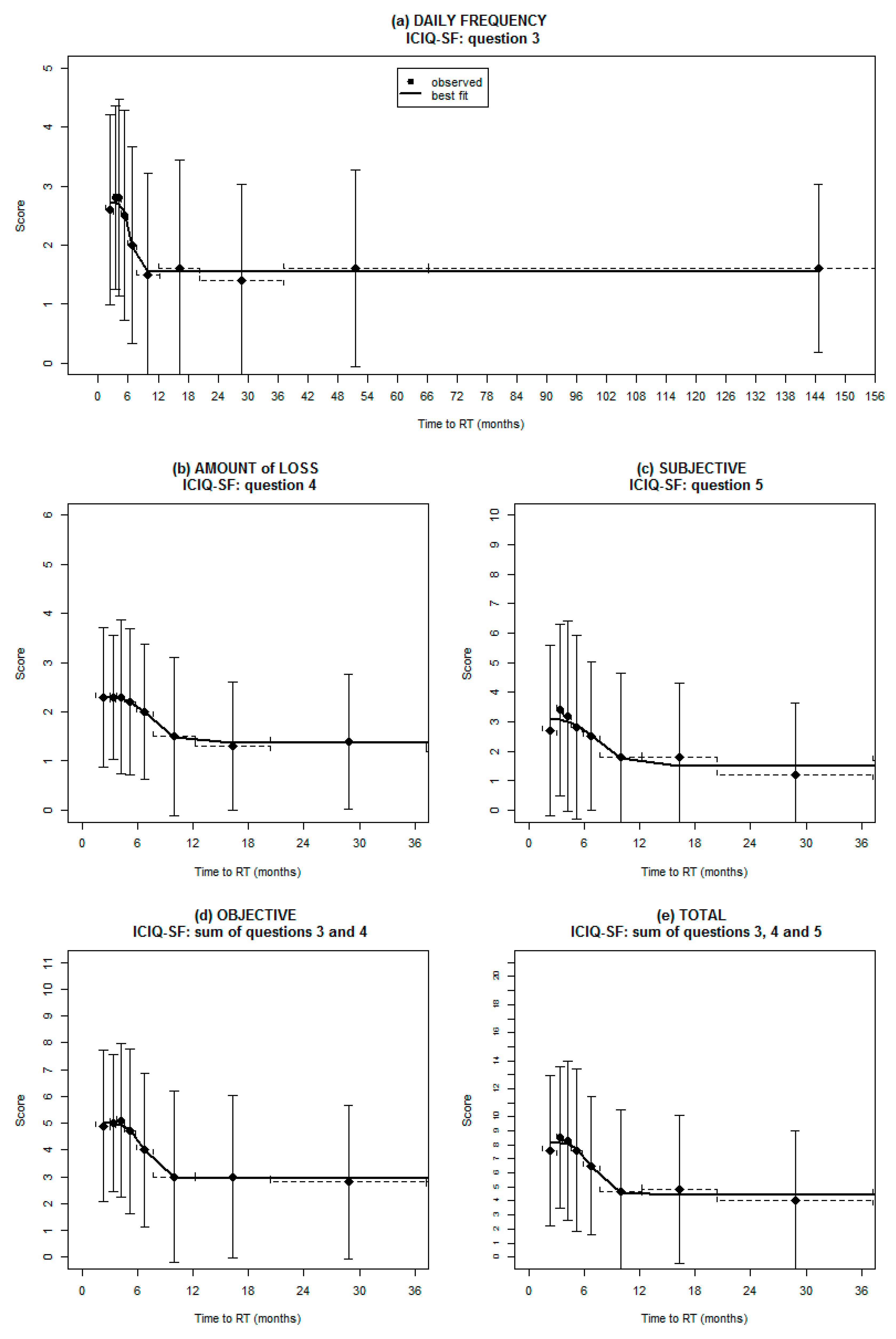
3.1. Relationship between Baseline ICIQ Scores and TTRT
3.2. Predictors of Pre-Radiotherapy UI
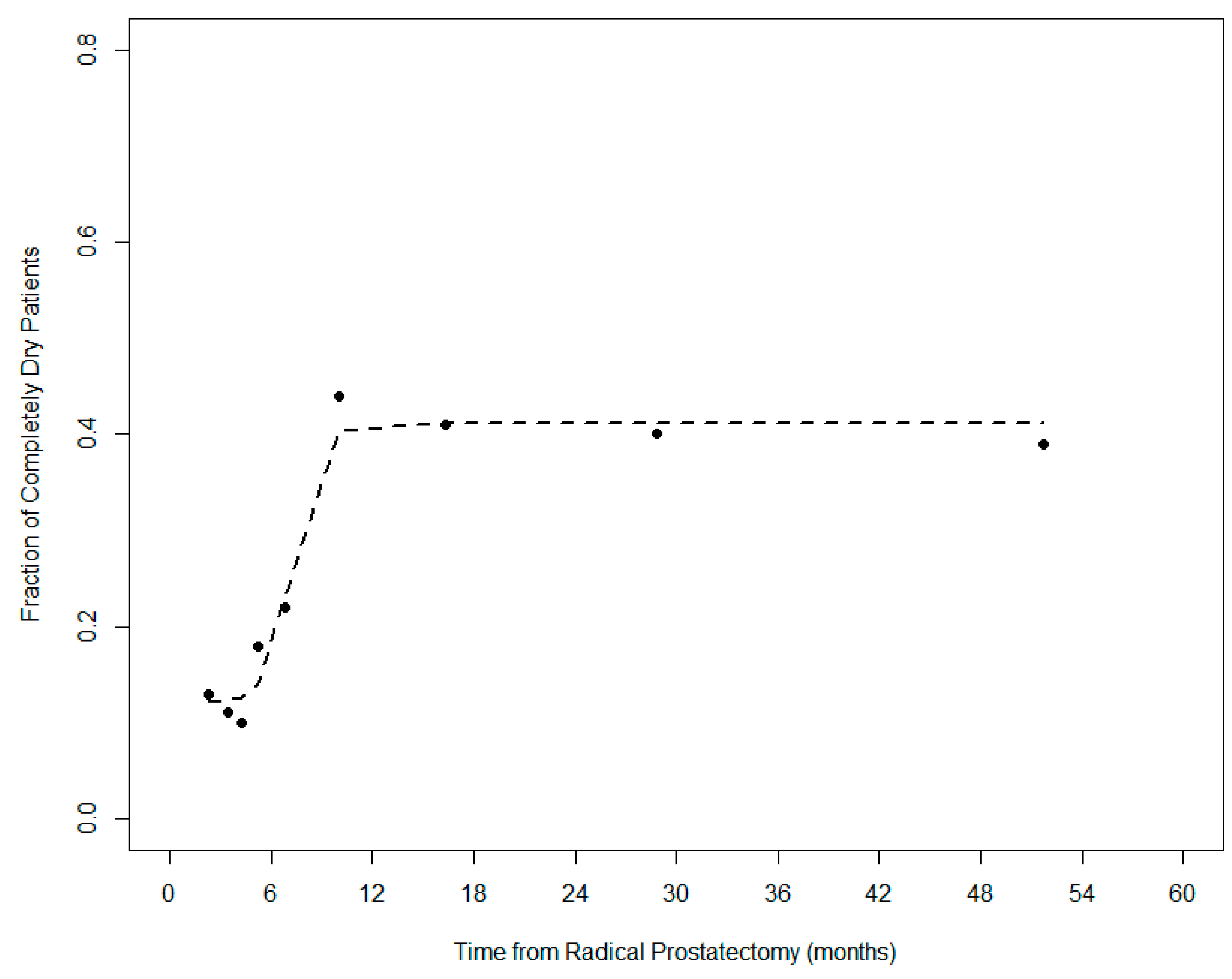
3.3. Predictors of the “Completely-Dry” Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef]
- Parker, C.C.; Clarke, N.W.; Cook, A.D.; Kynaston, H.G.; Petersen, P.M.; Catton, C.; Cross, W.; Logue, J.; Parulekar, W.; Payne, H.; et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomized controlled phase 3 trial. Lancet 2020, 396, 1413–1421. [Google Scholar] [CrossRef]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magné, N.; Benyoucef, A.; Supiot, S.; Pasquier, D.; Abdiche, M.S.; Gilliot, O.; Graff-Cailleaud, P.; et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef]
- Kneebone, A.; Fraser-Browne, C.; Duchesne, G.M.; Fisher, R.; Frydenberg, M.; Herschtal, A.; Williams, S.G.; Brown, C.; Delprado, W.; Haworth, A.; et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 1331–1340. [Google Scholar] [CrossRef]
- Nyarangi-Dix, J.N.; Steimer, J.; Bruckner, T.; Jakobi, H.; Koerber, S.A.; Hadaschik, B.; Debus, J.; Hohenfellner, M. Post-prostatectomy radiotherapy adversely affects urinary continence irrespective of radiotherapy regime. World J. Urol. 2017, 35, 1841–1847. [Google Scholar] [CrossRef]
- Ávila, M.; Becerra, V.; Guedea, F.; Suárez, J.F.; Fernandez, P.; Macías, V.; Mariño, A.; Hervás, A.; Herruzo, I.; Ortiz, M.J.; et al. Estimating preferences for treatments in patients with localized prostate cancer. Int. J. Radiat. Oncol. 2015, 91, 277–287. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Lo, M.; Clark, J.A.; Albertsen, P.C.; Barry, M.J.; Goodman, M.; Penson, D.F.; Stanford, J.L.; Stroup, A.M.; Hamilton, A.S. Treatment decision regret among long-term survivors of localized prostate cancer: Results from the prostate cancer outcomes study. J. Clin. Oncol. 2017, 35, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Tendulkar, R.D.; Agrawal, S.; Gao, T.; Efstathiou, J.A.; Pisansky, T.M.; Michalski, J.M.; Koontz, B.F.; Hamstra, D.A.; Feng, F.Y.; Liauw, S.L.; et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J. Clin. Oncol. 2016, 34, 3648–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorino, C.; Broggi, S.; Fossati, N.; Cozzarini, C.; Goldner, G.; Wiegel, T.; Hinkelbein, W.; Karnes, R.J.; Boorjian, S.A.; Haustermans, K.; et al. Predicting the 5-year risk of biochemical relapse after postprostatectomy radiation therapy in ≥ PT2, pN0 patients with a comprehensive tumor control probability model. Int. J. Radiat. Oncol. 2016, 96, 333–340. [Google Scholar] [CrossRef]
- Thompson, I.M.; Valicenti, R.K.; Albertsen, P.C.; Davis, B.; Goldenberg, S.L.; Hahn, C.; Klein, E.A.; Michalski, J.M.; Roach, M.; Sartor, O.; et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J. Urol. 2013, 190, 441–449. [Google Scholar] [CrossRef]
- Suardi, N.; Gallina, A.; Lista, G.; Gandaglia, G.; Abdollah, F.; Capitanio, U.; Dell’Oglio, P.; Nini, A.; Salonia, A.; Montorsi, F.; et al. Impact of adjuvant radiation therapy on urinary continence recovery after radical prostatectomy. Eur. Urol. 2014, 65, 546–551. [Google Scholar] [CrossRef]
- Zaffuto, E.; Gandaglia, G.; Fossati, N.; Dell’Oglio, P.; Moschini, M.; Cucchiara, V.; Suardi, N.; Mirone, V.; Bandini, M.; Shariat, S.F.; et al. Early postoperative radiotherapy is associated with worse functional outcomes in patients with prostate cancer. J. Urol. 2017, 197, 669–675. [Google Scholar] [CrossRef] [PubMed]
- van Stam, M.A.; Aaronson, N.K.; Pos, F.J.; Bosch, J.R.; Kieffer, J.M.; Tillier, C.N.; van der Poel, H.G. The effect of salvage radiotherapy and its timing on the health-related quality of life of prostate cancer patients. Eur. Urol. 2016, 70, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Tennstedt, P.; Lanwehr, D.; Tilki, D.; Steuber, T.; Beyer, B.; Thederan, I.; Heinzer, H.; Haese, A.; Salomon, G.; et al. Functional outcomes and quality of life after radical prostatectomy only versus a combination of prostatectomy with radiation and hormonal therapy. Eur. Urol. 2017, 71, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M.; Fischedick, K.; Asadpour, B.; Gagel, B.D.; Piroth, M.D.; Holy, R.; Krenkel, B.; Eble, M.J. Health-related quality of life after adjuvant and salvage postoperative radiotherapy for prostate cancer—A prospective analysis. Radiother. Oncol. 2008, 88, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Sowerby, R.J.; Gani, Y.; Yim, H.; Radomsky, S.B.; Catton, C. Long-term complications in men who have early or late radiotherapy after radical prostatectomy. Can. Urol. Assoc. J. 2014, 8, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, M.M.E.; Kessel, K.A.; Gschwend, J.E.; Weichert, W.; Wilkens, J.J.; Combs, S.E. Early and late toxicity profiles of patients receiving immediate postoperative radiotherapy versus salvage radiotherapy for prostate cancer after prostatectomy. Strahlenther. Onkol. 2018, 195, 131–144. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budäus, L.; Bolla, M.; Bossi, A.; Cozzarini, C.; Crook, J.; Widmark, A.; Wiegel, T. Functional outcomes and complications following radiation therapy for prostate cancer: A critical analysis of the literature. Eur. Urol. 2012, 61, 112–127. [Google Scholar] [CrossRef]
- Sini, C.; Chiorda, B.N.; Gabriele, P.; Sanguineti, G.; Morlino, S.; Badenchini, F.; Cante, D.; Carillo, V.; Gaetano, M.; Giandini, T.; et al. Patient-reported intestinal toxicity from whole pelvis intensity-modulated radiotherapy: First quantification of bowel dose-volume effects. Radiother. Oncol. 2017, 124, 296–301. [Google Scholar] [CrossRef]
- Sini, C.; Fiorino, C.; Perna, L.; Chiorda, B.N.; Deantoni, C.L.; Bianchi, M.; Sacco, V.; Briganti, A.; Montorsi, F.; Calandrino, R.; et al. Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother. Oncol. 2016, 118, 79–84. [Google Scholar] [CrossRef]
- Cozzarini, C.; Chiorda, B.N.; Sini, C.; Fiorino, C.; Briganti, A.; Montorsi, F.; Di Muzio, N. Hematologic toxicity in patients treated with postprostatectomy whole-pelvis irradiation with different intensity modulated radiation therapy techniques is not negligible and is prolonged: Preliminary results of a longitudinal, observational study. Int. J. Radiat. Oncol. 2016, 95, 690–695. [Google Scholar] [CrossRef]
- Avery, K.; Donovan, J.; Peters, T.J.; Shaw, C.; Gotoh, M.; Abrams, P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol. Urodyn. 2004, 23, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.J.; Brown, L.B.; Philipchalk, R. The development of an abbreviated form of the revised Eysenck personality questionnaire (EPQR-A): Its use among students in England, Canada, the U.S.A. and Australia. Pers. Individ. Differ. 1992, 13, 443–449. [Google Scholar] [CrossRef]
- Landoni, V.; Fiorino, C.; Cozzarini, C.; Sanguineti, G.; Valdagni, R.; Rancati, T. Predicting toxicity in radiotherapy for prostate cancer. Phys. Med. 2016, 32, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Cozzarini, C.; Rancati, T.; Palorini, F.; Avuzzi, B.; Garibaldi, E.; Balestrini, D.; Cante, D.; Munoz, F.; Franco, P.; Girelli, G.; et al. Patient-reported urinary incontinence after radiotherapy for prostate cancer: Quantifying the dose-effect. Radiother. Oncol. 2017, 125, 101–106. [Google Scholar] [CrossRef]
- Barry, M.J.; Gallagher, P.M.; Skinner, J.S.; Fowler, F.J. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of medicare-age men. J. Clin. Oncol. 2012, 30, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyberga, M.; Hugossonc, J.; Wiklund, P.; Sjoberg, D.; Wilderäng, U.; Carlsson, S.V.; Carlsson, S.; Stranne, J.; Steineck, G.; Haglind, E.; et al. Functional and oncologic outcomes between open and robotic radical prostatectomy at 24-month follow-up in the Swedish LAPPRO trial. Eur. Urol. Oncol. 2018, 1, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ávila, M.; Patel, L.; López, S.; Cortes-Sanabría, L.; Garín, O.; Pont, À.; Ferrer, F.; Boladeras, A.; Zamora, V.; Fosså, S.; et al. Patient-reported outcomes after treatment for clinically localized prostate cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 66, 23–44. [Google Scholar] [CrossRef]
- Leufgens, F.; Berneking, V.; Vögeli, T.-A.; Kirschner-Hermanns, R.; Eble, M.J.; Pinkawa, M. Quality of life changes >10 years after postoperative radiation therapy after radical prostatectomy for prostate cancer. Int. J. Radiat. Oncol. 2019, 105, 382–388. [Google Scholar] [CrossRef]
| Age (years) | 67 | (62–71) |
| BMI (kg/m2) | 26 | (24–28) |
| Time to RT (mo) | 7.7 | (4.1–26.4) |
| PSA (ng/mL) | ||
| pre-RP | 8.58 | (5.83–13.98) |
| post-RP | 0.04 | (0.01–0.15) |
| pre-RT | 0.22 | (0.05–0.48) |
| Number of removed lymph nodes | 13 | (7–21) |
| Hypertension | 178 | (44%) |
| Smoke | 69 | (18%) |
| Diabetes | 27 | (7%) |
| ADT | ||
| No ADT | 213 | (58%) |
| Bicalutamide monotherapy | 33 | (9%) |
| LH-RH | 89 | (24%) |
| CAB | 30 | (8%) |
| Surgical margins | ||
| Negative | 237 | (58%) |
| Positive | 171 | (42%) |
| Surgery | ||
| Open | 233 | (59%) |
| Robotic | 120 | (30%) |
| Laparoscopic | 43 | (11%) |
| Gleason score | ||
| ISUP Groups 1–3 | 102 | (27%) |
| ISUP Groups 4–5 | 274 | (73%) |
| Stage T | ||
| pT2 | 121 | (30%) |
| pT3a | 143 | (35%) |
| pT3b and pT4 | 139 | (34%) |
| Parameter | Daily Frequency | Amount of Urine Loss | Subjective | Objective | Total | |
|---|---|---|---|---|---|---|
| Age (yr) | 1.03 (1.00–1.06) 0.062 | 1.00 (0.96–1.05) 0.812 | 1.01 (0.97–1.04) 0.697 | 1.03 (1.00–1.06) 0.080 | 1.02 (0.99–1.05) 0.189 | |
| BMI (kg/m2) | 1.05 (0.99–1.11) 0.091 | 1.05 (0.97–1.13) 0.218 | 1.06 (0.99–1.13) 0.112 | 1.04 (0.98–1.10) 0.230 | 1.06 (0.99–1.12) 0.082 | |
| PSA (ng/mL) | pre-RP | 1.02 (1.00–1.04) 0.024 | 1.01 (0.99–1.03) 0.134 | 1.01 (1.00–1.03) 0.104 | 1.01 (1.00–1.03) 0.032 | 1.01 (1.00–1.03) 0.063 |
| post-RP | 0.97 (0.77–1.19) 0.775 | 1.14 (0.88–1.41) 0.250 | 1.00 (0.73–1.25) 0.981 | 1.00 (0.78–1.22) 0.971 | 0.99 (0.76–1.22) 0.922 | |
| pre-RT | 0.98 (0.90–1.05) 0.587 | 1.04 (0.96–1.11) 0.303 | 1.02 (0.94–1.09) 0.518 | 1.00 (0.93–1.07) 0.977 | 0.99 (0.91–1.06) 0.846 | |
| Time to RT (mo) | ||||||
| ≥cut-off time | Ref. | Ref. | Ref. | Ref. | Ref. | |
| <cut-off time | 3.30 (2.20–5.00) <0.0001 | 3.55 (1.94–6.81) <0.0001 | 2.31 (1.41–3.86) 0.001 | 2.94 (1.92–4.57) <0.0001 | 3.16 (2.02–5.01) <0.0001 | |
| N° removed lymph nodes | 1.01 (0.99–1.03) 0.368 | 1.02 (0.99–1.04) 0.113 | 1.01 (0.99–1.03) 0.275 | 1.01 (0.99–1.03) 0.549 | 1.01 (0.99–1.03) 0.424 | |
| Hypertension | No | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes | 1.16 (0.78–1.73) 0.460 | 1.00 (0.57–1.74) 1.000 | 1.09 (0.67–1.77) 0.722 | 1.16 (0.76–1.76) 0.495 | 0.99 (0.64–1.51) 0.945 | |
| Smoke | No | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes | 1.05 (0.62–1.77) 0.864 | 1.60 (0.80–3.07) 0.165 | 1.30 (0.69–2.37) 0.399 | 1.33 (0.77–2.28) 0.300 | 1.29 (0.73–2.22) 0.372 | |
| Diabetes | No | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes | 2.46 (1.12–5.71) 0.029 | 1.36 (0.44–3.48) 0.553 | 0.46 (0.11–1.37) 0.219 | 1.51 (0.66–3.32) 0.313 | 1.41 (0.61–3.13) 0.407 | |
| ADT | No | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes | 1.54 (1.04–2.31) 0.033 | 1.84 (1.05–3.25) 0.034 | 1.31 (0.81–2.13) 0.274 | 1.52 (1.00–2.32) 0.052 | 1.67 (1.09–2.58) 0.019 | |
| Surgery | Open | Ref. | Ref. | Ref. | Ref. | Ref. |
| Robotic | 0.87 (0.55–1.36) 0.547 | 1.18 (0.63–2.17) 0.595 | 0.94 (0.54–1.61) 0.821 | 0.92 (0.57–1.48) 0.737 | 0.83 (0.50–1.35) 0.456 | |
| Laparoscopic | 1.07 (0.55–2.06) 0.836 | 1.44 (0.58–3.25) 0.406 | 0.99 (0.42–2.13) 0.988 | 1.27 (0.64–2.48) 0.483 | 1.22 (0.60–2.40) 0.566 | |
| Surgical margins | ||||||
| Negative | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Positive | 1.23 (0.82–1.86) 0.313 | 1.12 (0.64–1.99) 0.696 | 1.06 (0.65–1.75) 0.803 | 1.12 (0.73–1.72) 0.609 | 1.03 (0.67–1.59) 0.903 | |
| Gleason score | ||||||
| ISUP Groups 1–3 | Ref. | Ref. | Ref. | Ref. | Ref. | |
| ISUP Groups 4–5 | 1.49 (0.94–2.41) 0.095 | 1.67 (0.86–3.52) 0.153 | 115 (0.66–2.06) 0.631 | 1.5 (0.91–2.51) 0.117 | 1.34 (0.81–2.25) 0.263 | |
| Stage T | pT2 | Ref. | Ref. | Ref. | Ref. | Ref. |
| pT3a | 1.50 (0.90–2.52) 0.117 | 1.53 (0.71–3.46) 0.287 | 1.00 (0.53–1.89) 1.000 | 2.06 (1.19–3.65) 0.011 | 1.4 (0.8–2.47) 0.240 | |
| pT3b and pT4 | 2.58 (1.56–4.34) <0.001 | 2.64 (1.29–5.76) 0.011 | 1.51 (0.84–2.79) 0.175 | 2.44 (1.41–4.31) 0.002 | 2.15 (1.25–3.75) 0.006 | |
| EPQ-R | ||||||
| Extroversion | 0.97 (0.85–1.10) 0.617 | 0.90 (0.75–1.06) 0.202 | 0.96 (0.82–1.12) 0.555 | 0.93 (0.81–1.06) 0.270 | 0.90 (0.79–1.03) 0.134 | |
| Neuroticism | 1.18 (1.04–1.35) 0.012 | 1.16 (0.97–1.38) 0.098 | 1.22 (1.04–1.42) 0.012 | 1.15 (1.00–1.32) 0.049 | 1.19 (1.04–1.37) 0.014 | |
| Psychoticism | 0.94 (0.77–1.14) 0.518 | 1.07 (0.82–1.38) 0.596 | 1.04 (0.82–1.30) 0.754 | 1.00 (0.81–1.22) 0.992 | 0.97 (0.78–1.18) 0.741 | |
| Lie | 1.00 (0.85–1.19) 0.981 | 0.93 (0.75–1.17) 0.511 | 1.07 (0.87–1.33) 0.557 | 1.06 (0.89–1.28) 0.533 | 1.06 (0.89–1.28) 0.523 | |
| End-Po Int | Coeff. ± St. dev. | Odds Radio (95% CI) | p-Value |
|---|---|---|---|
| (a) Daily Frequency endpoint: ICIQ3 > 2 (n = 152/372, 41%) | |||
| TTRT (cut-off: 6.7 mo) | 1.272 ± 0.223 | 3.57 (2.31–5.56) | <0.0001 |
| Neuroticism | 0.167 ± 0.070 | 1.18 (1.03–1.36) | 0.018 |
| Constant: | −1.266 | ||
| H&L p-value = 0.693; Brier score = 0.216 (corrected for optimism: 0.219); calibration slope = 0.975; R2 = 0.840 | |||
| (b) Amount of Loss endpoint: ICIQ4 > 2 (n = 59/408, 14%) | |||
| TTRT (cut-off: 7.2 mo) | 1.266 ± 0.318 | 3.55 (1.94–6.81) | 0.0001 |
| Constant: | −2.544 | ||
| H&L p-value = 1.000; Brier score = 0.118 (corrected for optimism: 0.120); calibration slope = 1.000; R2 = 1.000 | |||
| (c) Subjective endpoint: ICIQ5 > 4 (n = 75/372, 20%) | |||
| TTRT (cut-off: 7.1 mo) | 0.935 ± 0.272 | 2.55 (1.51–4.39) | 0.001 |
| Neuroticism | 0.190 ± 0.080 | 1.21 (1.03–1.42) | 0.018 |
| Constant: | −2.241 | ||
| H&L p-value = 0.458; Brier score = 0.152 (corrected for optimism: 0.155); calibration slope = 0.952; R2 = 0.794 | |||
| (d) Objective endpoint: ICIQ3+ICIQ4 > 5 (n = 129/408, 32%) | |||
| TTRT (cut-off: 6.7 mo) | 1.080 ± 0.221 | 2.94 (1.92–4.57) | <0.001 |
| Constant: | −1.325 | ||
| H&L p-value = 1.000; Brier score = 0.203 (corrected for optimism: 0.205); calibration slope = 1.000; R2 = 1.000 | |||
| (e) Total endpoint: ICIQ3+ICIQ4+ICIQ5 > 8 (n = 108/365, 30%) | |||
| TTRT (cut-off: 7.8 mo) | 1.206 ± 0.246 | 3.34 (2.08–5.46) | <0.0001 |
| Neuroticism | 0.155 ± 0.075 | 1.17 (1.01–1.35) | 0.039 |
| BMI | 0.064 ± 0.034 | 1.07 (1.00–1.14) | 0.058 |
| Constant: | −2.569 | ||
| H&L p-value = 0.454; Brier score = 0.188 (corrected for optimism: 0.193); calibration slope = 1.112; R2 = 0.633 | |||
| Coeff. ± St. dev. | Odds radio (95% CI) | p-value | |
| Completely Dry endpoint: ICIQ3+ICIQ4 = 0 (n = 108/408, 26%) | |||
| TTRT (cut-off: 6.9 mo) | −1.429 ± 0.257 | 0.24 (0.14–0.40) | <0.0001 |
| Age | −0.059 ± 0.017 | 0.94 (0.90–0.98) | 0.001 |
| Constant: | 3.445 | ||
| H&L p-value = 0.185; Brier score = 0.176 (corrected for optimism: 0.178); calibration slope = 0.956; R2 = 0.837 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munoz, F.; Sanguineti, G.; Bresolin, A.; Cante, D.; Vavassori, V.; Waskiewicz, J.M.; Girelli, G.; Avuzzi, B.; Garibaldi, E.; Faiella, A.; et al. Predictors of Patient-Reported Incontinence at Adjuvant/Salvage Radiotherapy after Prostatectomy: Impact of Time between Surgery and Radiotherapy. Cancers 2021, 13, 3243. https://doi.org/10.3390/cancers13133243
Munoz F, Sanguineti G, Bresolin A, Cante D, Vavassori V, Waskiewicz JM, Girelli G, Avuzzi B, Garibaldi E, Faiella A, et al. Predictors of Patient-Reported Incontinence at Adjuvant/Salvage Radiotherapy after Prostatectomy: Impact of Time between Surgery and Radiotherapy. Cancers. 2021; 13(13):3243. https://doi.org/10.3390/cancers13133243
Chicago/Turabian StyleMunoz, Fernando, Giuseppe Sanguineti, Andrea Bresolin, Domenico Cante, Vittorio Vavassori, Justina Magdalena Waskiewicz, Giuseppe Girelli, Barbara Avuzzi, Elisabetta Garibaldi, Adriana Faiella, and et al. 2021. "Predictors of Patient-Reported Incontinence at Adjuvant/Salvage Radiotherapy after Prostatectomy: Impact of Time between Surgery and Radiotherapy" Cancers 13, no. 13: 3243. https://doi.org/10.3390/cancers13133243
APA StyleMunoz, F., Sanguineti, G., Bresolin, A., Cante, D., Vavassori, V., Waskiewicz, J. M., Girelli, G., Avuzzi, B., Garibaldi, E., Faiella, A., Villa, E., Magli, A., Noris Chiorda, B., Gatti, M., Rancati, T., Valdagni, R., Di Muzio, N. G., Fiorino, C., & Cozzarini, C. (2021). Predictors of Patient-Reported Incontinence at Adjuvant/Salvage Radiotherapy after Prostatectomy: Impact of Time between Surgery and Radiotherapy. Cancers, 13(13), 3243. https://doi.org/10.3390/cancers13133243







