The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Innate Immune Cells
2.1. Tumour-Associated Macrophages (TAMs)
2.2. Dendritic Cells
2.3. Natural Killer Cells (NKs)
2.4. Innate Lymphoid Cells (ILCs)
3. Adaptive Immune Cells
3.1. Tumour-Infiltrating Lymphocytes
3.2. Exhausted T-Cells
3.3. Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Lymphocyte -to-Monocyte Ratio (LMR)
4. Immune Checkpoints
4.1. Programmed Cell Death-1 (PD-1) and PD Ligand-1 (PD-L1)
4.2. Expression of Other Immune Checkpoints
5. Novel Insight in the Treatment of Malignant Pleural Mesothelioma
5.1. Immune Checkpoints Inhibitors (ICIs)
Dendritic Cell Immunotherapy
5.2. Adoptive T-Cell Therapy
5.3. Exosome-Based Therapy
5.4. Stimulator of INterferon Genes (STING) Agonists
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wagner, J.C.; Sleggs, C.A.; Marchand, P. Diffuse Pleural Mesothelioma and Asbestos Exposure in the North Western Cape Province. Occup. Environ. Med. 1960, 17, 260–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmes, P.C.; McCaughey, W.T.E.; Wade, O.L. Diffuse Mesothelioma of the Pleura and Asbestos. BMJ 1965, 1, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.-T.; Takahashi, K.; Karjalainen, A.; Hoshuyama, T.; Wilson, D.; Kameda, T.; Chan, C.-C.; Wen, C.-P.; Furuya, S.; Higashi, T.; et al. Ecological association between asbestos-related diseases and historical asbestos consumption: An international analysis. Lancet 2007, 369, 844–849. [Google Scholar] [CrossRef]
- Robinson, B.M. Malignant pleural mesothelioma: An epidemiological perspective. Ann. Cardiothorac. Surg. 2012, 1, 491. [Google Scholar]
- Henley, S.J.; Larson, T.C.; Wu, M.; Antao, V.C.S.; Lewis, M.; Pinheiro, G.A.; Eheman, C. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003–2008. Int. J. Occup. Environ. Heal. 2013, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Peto, J.; Decarli, A.; La Vecchia, C.; Levi, F.; Negri, E. The European mesothelioma epidemic. Br. J. Cancer 1999, 79, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Yap, T.A.; Aerts, J.G.; Popat, S.; Fennell, D.A. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475. [Google Scholar] [CrossRef] [PubMed]
- Galateau-Salle, F.; Churg, A.; Roggli, V.; Travis, W.D.; World Health Organization Committee for Tumors of the Pleura. The 2015 World Health Organization Classification of Tumors of the Pleura: Advances since the 2004 Classification. J. Thorac. Oncol. 2016, 11, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Curran, D.; Sahmoud, T.; Therasse, P.; van Meerbeeck, J.; Postmus, P.E.; Giaccone, G. Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experience. J. Clin. Oncol. 1998, 16, 145–152. [Google Scholar] [CrossRef]
- Habougit, C.; Trombert-Paviot, B.; Karpathiou, G.; Casteillo, F.; Bayle-Bleuez, S.; Fournel, P.; Vergnon, J.-M.; Tiffet, O.; Péoc’h, M.; Forest, F. Histopathologic features predict survival in diffuse pleural malignant mesothelioma on pleural biopsies. Virchows Archiv 2017, 470, 639–646. [Google Scholar] [CrossRef]
- Robinson, B.W.S.; Lake, R.A. Advances in Malignant Mesothelioma. New Engl. J. Med. 2005, 353, 1591–1603. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.; Nowak, A.; Robinson, C.; Creaney, J. Malignant mesothelioma. Lancet 2005, 366, 397–408. [Google Scholar] [CrossRef]
- Sekido, Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis 2013, 34, 1413–1419. [Google Scholar] [CrossRef]
- Karpathiou, G.; Stefanou, D.; Froudarakis, M.E. Pleural neoplastic pathology. Respir. Med. 2015, 109, 931–943. [Google Scholar] [CrossRef] [Green Version]
- Ault, J.G.; Cole, R.W.; Jensen, C.G.; Jensen, L.C.W.; Bachert, L.A.; Rieder, C.L. Behavior of crocidolite asbestos during mitosis in living vertebrate lung epithelial cells. Cancer Res. 1995, 55, 792–798. [Google Scholar]
- Kamp, D.W.; Israbian, V.A.; Preusen, S.E.; Zhang, C.X.; Weitzman, S.A. Asbestos causes DNA strand breaks in cultured pulmonary epithelial cells: Role of iron-catalyzed free radicals. Am. J. Physiol. Lung Cell. Mol. Physiol. 1995, 268, L471–L480. [Google Scholar] [CrossRef] [PubMed]
- Zanella, C.L.; Posada, J.; Tritton, T.R.; Mossman, B.T. Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996, 56, 5334–5338. [Google Scholar] [PubMed]
- Maeda, M.; Chen, Y.; Lee, S.; Kumagai-Takei, N.; Yoshitome, K.; Matsuzaki, H.; Yamamoto, S.; Hatayama, T.; Ikeda, M.; Nishimura, Y.; et al. Induction of IL-17 production from human peripheral blood CD4+ cells by asbestos exposure. Int. J. Oncol. 2017, 50, 2024–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Matsuzaki, H.; Maeda, M.; Yamamoto, S.; Kumagai-Takei, N.; Hatayama, T.; Ikeda, M.; Yoshitome, K.; Nishimura, Y.; Otsuki, T. Accelerated cell cycle progression of human regulatory T cell-like cell line caused by continuous exposure to asbestos fibers. Int. J. Oncol. 2017, 50, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Pairon, J.-C.; Laurent, F.; Rinaldo, M.; Clin, B.; Andujar, P.; Ameille, J.; Brochard, P.; Chammings, S.; Ferretti, G.; Galateau-Sallé, F.; et al. Pleural plaques and the risk of pleural mesothelioma. J. Natl. Cancer Inst. 2013, 105, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011, 43, 1022–1125. [Google Scholar] [CrossRef] [Green Version]
- Nasu, M.; Emi, M.; Pastorino, S.; Tanji, M.; Powers, A.; Luk, H.; Baumann, F.; Zhang, Y.-A.; Gazdar, A.; Kanodia, S.; et al. High Incidence of Somatic BAP1 Alterations in Sporadic Malignant Mesothelioma. J. Thorac. Oncol. 2015, 10, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Illei, P.B.; Rusch, V.W.; Zakowski, M.F.; Ladanyi, M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin. Cancer Res. 2003, 9, 2108–2113. [Google Scholar] [PubMed]
- Pylkkänen, L.; Sainio, M.; Ollikainen, T.; Mattson, K.; Nordling, S.; Carpen, O.; Linnainmaa, K.; Husgafvel-Pursiainen, K. Concurrent LOH at multiple loci in human malignant mesothelioma with preferential loss of NF2 gene region. Oncol. Rep. 2002, 9, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Fleury-Feith, J.; Boulange-Lecomte, C.; Renier, A.; Matrat, M.; Kheuang, L.; Abramowski, V.; Levy, F.; Janin, A.; Giovannini, M.; Jaurand, M.-C. Hemizygosity of Nf2 is associated with increased susceptibility to asbestos-induced peritoneal tumours. Oncogene 2003, 22, 3799–3805. [Google Scholar] [CrossRef] [Green Version]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407. [Google Scholar] [CrossRef]
- Ceresoli, G.L.; Zucali, P.A.; Favaretto, A.G.; Grossi, F.; Bidoli, P.; Del Conte, G.; Ceribelli, A.; Bearz, A.; Morenghi, E.; Cavina, R.; et al. Phase II Study of Pemetrexed Plus Carboplatin in Malignant Pleural Mesothelioma. J. Clin. Oncol. 2006, 24, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Opitz, I.; Scherpereel, A.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020, 58, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Astoul, P.; Roca, E.; Galateau-Salle, F.; Scherpereel, A. Malignant Pleural Mesothelioma: From the Bench to the Bedside. Respiration 2012, 83, 481–493. [Google Scholar] [CrossRef]
- Yang, H.; Bocchetta, M.; Kroczynska, B.; Elmishad, A.G.; Chen, Y.; Liu, Z.; Bubici, C.; Mossman, B.T.; Pass, H.I.; Testa, J.R.; et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 10397–10402. [Google Scholar] [CrossRef] [Green Version]
- Hegmans, J.P.J.J.; Hemmes, A.; Hammad, H.; Boon, L.; Hoogsteden, H.C.; Lambrecht, B.N. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur. Respir. J. 2006, 27, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.M.; Rodig, S.J.; Tilleman, T.R.; ElBardissi, A.W.; Bueno, R.; Sugarbaker, D.J. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer 2011, 117, 5234–5244. [Google Scholar] [CrossRef]
- Minnema-Luiting, J.; Vroman, H.; Aerts, J.; Cornelissen, R. Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2018, 19, 1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Kopecka, J.; Napoli, F.; Pradotto, M.; Maletta, F.; Costardi, L.; Gagliasso, M.; Milosevic, V.; Ananthanarayanan, P.; Bironzo, P.; et al. Potential Diagnostic and Prognostic Role of Microenvironment in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2019, 14, 1458–1471. [Google Scholar] [CrossRef] [Green Version]
- Ujiie, H.; Kadota, K.; Nitadori, J.-I.; Aerts, J.G.; Woo, K.M.; Sima, C.S.; Travis, W.D.; Jones, D.R.; Krug, L.M.; Adusumilli, P.S. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. OncoImmunology 2015, 4, e1009285. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, R.; Lievense, L.A.; Maat, A.P.; Hendriks, R.W.; Hoogsteden, H.C.; Bogers, A.J.; Hegmans, J.P.; Aerts, J.G. Ratio of Intratumoral Macrophage Phenotypes Is a Prognostic Factor in Epithelioid Malignant Pleural Mesothelioma. PLoS ONE 2014, 9, e106742. [Google Scholar] [CrossRef]
- Marcq, E.; Siozopoulou, V.; De Waele, J.; van Audenaerde, J.; Zwaenepoel, K.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L.J. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. OncoImmunology 2016, 6, e1261241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Cornwall, S.M.J.; Wikstrom, M.; Musk, A.W.; Alvarez, J.; Nowak, A.K.; Nelson, D.J. Human mesothelioma induces defects in dendritic cell numbers and antigen-processing function which predict survival outcomes. OncoImmunology 2015, 5, e1082028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolani, B.; Acevedo, L.A.; Hoang, N.T.; He, B. Heterogeneous Contributing Factors in MPM Disease Development and Progression: Biological Advances and Clinical Implications. Int. J. Mol. Sci. 2018, 19, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegmans, J.P.J.J.; Hemmes, A.; Aerts, J.G.; Hoogsteden, H.C.; Lambrecht, B.N. Immunotherapy of Murine Malignant Mesothelioma Using Tumor Lysate–pulsed Dendritic Cells. Am. J. Respir. Crit. Care Med. 2005, 171, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Marcq, E.; De Waele, J.; Van Audenaerde, J.; Lion, E.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L.J. Abundant expression of TIM-3, LAG-3, PD-1 and PD-L1 as immunotherapy checkpoint targets in effusions of mesothelioma patients. Oncotarget 2017, 8, 89722. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, Y.; Kumagai-Takei, N.; Matsuzaki, H.; Lee, S.; Maeda, M.; Kishimoto, T.; Fukuoka, K.; Nakano, T.; Otsuki, T. Functional Alteration of Natural Killer Cells and Cytotoxic T Lymphocytes upon Asbestos Exposure and in Malignant Mesothelioma Patients. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Moretta, L.; Pietra, G.; Vacca, P.; Pende, D.; Moretta, F.; Bertaina, A.; Mingari, M.C.; Locatelli, F.; Moretta, A. Human NK cells: From surface receptors to clinical applications. Immunol. Lett. 2016, 178, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Nishimura, Y.; Miura, Y.; Maeda, M.; Kumagai, N.; Murakami, S.; Hayashi, H.; Fukuoka, K.; Nakano, T.; Otsuki, T. Impairment in Cytotoxicity and Expression of NK Cell- Activating Receptors on Human NK Cells following Exposure to Asbestos Fibers. Int. J. Immunopathol. Pharmacol. 2009, 22, 579–590. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Yun, Z.; Tagawa, T.; De la Maza, L.; Wu, M.O.; Yu, J.; Zhao, Y.; de Perrot, M. Activation of CD1d-restricted natural killer T cells can inhibit cancer cell proliferation during chemotherapy by promoting the immune responses in murine mesothelioma. Cancer Immunol. Immunother. 2014, 63, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R.; Manning, L.S.; Whitaker, D.; Garlepp, M.J.; Robinson, B.W.S. Establishment of a murine model of malignant mesothelioma. Int. J. Cancer 1992, 52, 881–886. [Google Scholar] [CrossRef]
- Ghanim, B.; Klikovits, T.; Hoda, M.A.; Lang, G.; Szirtes, I.; Setinek, U.; Rozsas, A.; Renyi-Vamos, F.; Laszlo, V.; Grusch, M.; et al. Ki67 index is an independent prognostic factor in epithelioid but not in non-epithelioid malignant pleural mesothelioma: A multicenter study. Br. J. Cancer 2015, 112, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Sottile, R.; Tannazi, M.; Johansson, M.H.; Cristiani, C.M.; Calabró, L.; Ventura, V.; Cutaia, O.; Chiarucci, C.; Covre, A.; Garofalo, C.; et al. NK- and T-cell subsets in malignant mesothelioma patients: Baseline pattern and changes in the context of anti-CTLA-4 therapy. Int. J. Cancer 2019, 145, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Tumino, N.; Martini, S.; Munari, E.; Scordamaglia, F.; Besi, F.; Mariotti, F.R.; Bogina, G.; Mingari, M.C.; Vacca, P.; Moretta, L. Presence of innate lymphoid cells in pleural effusions of primary and metastatic tumors: Functional analysis and expression of PD-1 receptor. Int. J. Cancer 2019, 145, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.J.; van Zandwijk, N.; Rasko, J.E.J. The Immune Microenvironment in Mesothelioma: Mechanisms of Resistance to Immunotherapy. Front. Oncol. 2019, 9, 1366. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non–Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311. [Google Scholar] [PubMed]
- Zitvogel, L.; Tesniere, A.; Kroemer, G. Cancer despite immunosurveillance: Immunoselection and immunosubversion. Nat. Rev. Immunol. 2006, 6, 715–727. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Fusco, N.; Vaira, V.; Righi, I.; Sajjadi, E.; Venetis, K.; Lopez, G.; Cattaneo, M.; Castellani, M.; Rosso, L.; Nosotti, M.; et al. Characterization of the immune microenvironment in malignant pleural mesothelioma reveals prognostic subgroups of patients. Lung Cancer 2020, 150, 53–61. [Google Scholar] [CrossRef]
- Shen, M.; Sun, Q.; Wang, J.; Pan, W.; Ren, X. Positive and negative functions of B lymphocytes in tumors. Oncotarget 2016, 7, 55828. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.B.; Sørensen, J.F.; Olsen, B.N.; Pedersen, A.E. Anti-CD40-mediated cancer immunotherapy: An update of recent and ongoing clinical trials. Immunopharmacol. Immunotoxicol. 2014, 36, 96–104. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Lu, Q.; Meng, Y.; Wang, C.; Wang, L.; Wang, H.; Yu, X.; Zhang, Y.; Zhao, L.; et al. Optimization of anti-CD20 humanized antibody hu8E4 by site-directed mutation based on epitope analysis. Biochem. Biophys. Res. Commun. 2015, 459, 617–622. [Google Scholar] [CrossRef]
- von Muenchow, L.; Engdahl, C.; Karjalainen, K.; Rolink, A.G. The selection of mature B cells is critically dependent on the expression level of the co-receptor CD19. Immunol. Lett. 2014, 160, 113–119. [Google Scholar] [CrossRef]
- Gao, Z.-W.; Dong, K.; Zhang, H.-Z. The Roles of CD73 in Cancer. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Santarpia, M.; Karachaliou, N. Tumor immune microenvironment characterization and response to anti-PD-1 therapy. Cancer Biol. Med. 2015, 12, 74. [Google Scholar]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Lemos, L.G.T.; Victorino, V.J.; Herrera, A.C.S.A.; Aranome, A.M.F.; Cecchini, A.L.; Simão, A.N.C.; Panis, C.; Cecchini, R. Trastuzumab-based chemotherapy modulates systemic redox homeostasis in women with HER2-positive breast cancer. Int. Immunopharmacol. 2015, 27, 8–14. [Google Scholar] [CrossRef]
- Harp, C.R.P.; Archambault, A.S.; Sim, J.; Ferris, S.T.; Mikesell, R.J.; Koni, P.A.; Shimodaet, M.; Linington, C.; Russell, J.H.; Wu, G.F. B cell antigen presentation is sufficient to drive neuroinflammation in an animal model of multiple sclerosis. J. Immunol. 2015, 194, 5077–5084. [Google Scholar] [CrossRef] [Green Version]
- Crawford, A.; MacLeod, M.; Schumacher, T.; Corlett, L.; Gray, D.; Primary, T. Cell Expansion and Differentiation In Vivo Requires Antigen Presentation by B Cells. J. Immunol. 2006, 176, 3498–3506. [Google Scholar] [CrossRef] [Green Version]
- Hahne, M.; Renno, T.; Schroeter, M.; Irmler, M.; French, L.; Bornand, T.; Macdonald, H.R.; Tschopp, J. Activated B cells express functional Fas ligand. Eur. J. Immunol. 1996, 26, 721–724. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.-F.; Jiang, D.; Zhao, J.; Ge, J.-F.; Zheng, S.-Y. Significance of Fas and FasL protein expression in cardiac carcinoma and local lymph node tissues. Int. J. Clin. Exp. Pathol. 2015, 8, 11915. [Google Scholar]
- Horikawa, M.; Minard-Colin, V.; Matsushita, T.; Tedder, T.F. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J. Clin. Investig. 2011, 121, 4268–4280. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Min, Z.; Zhang, D.; Wang, W.; Marincola, F.; Wang, X. Enhanced frequency and potential mechanism of B regulatory cells in patients with lung cancer. J. Transl. Med. 2014, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Lo, C.M.; Ling, C.C.; Liu, X.B.; Ng, K.T.-P.; Chu, A.C.Y.; Ma, Y.Y.; Li, C.X.; Fan, S.T.; Man, K. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014, 355, 264–272. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Gawanbacht, A.; Beyer, T.; Lindner, S.; Trzaska, T.; van der Merwe, J.A.; Härter, G.; Grüner, B.; Fabricius, D.; Lotfi, R.; et al. CD4+T Cell–Derived IL-21 and Deprivation of CD40 Signaling Favor the In Vivo Development of Granzyme B–Expressing Regulatory B Cells in HIV Patients. J. Immunol. 2015, 194, 3768–3777. [Google Scholar] [CrossRef] [Green Version]
- Anraku, M.; Cunningham, K.S.; Yun, Z.; Tsao, M.; Zhang, L.; Keshavjee, S.; Johnston, M.R.; de Perrot, M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2008, 135, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Pasello, G.; Zago, G.; Lunardi, F.; Urso, L.; Kern, I.; Vlacic, G.; Grosso, F.; Mencoboni, M.; Ceresoli, G.; Schiavon, M.; et al. Malignant pleural mesothelioma immune microenvironment and checkpoint expression: Correlation with clinical–pathological features and intratumor heterogeneity over time. Ann. Oncol. 2018, 29, 1258–1265. [Google Scholar] [CrossRef]
- Leigh, R.A.; Webster, I. Lymphocytic infiltration of pleural mesothelioma and its significance for survival. South. Afr. Med. J. 1982, 61, 1007–1009. [Google Scholar]
- Yamada, N.; Oizumi, S.; Kikuchi, E.; Shinagawa, N.; Konishi-Sakakibara, J.; Ishimine, A.; Aoe, K.; Gemba, K.; Kishimoto, T.; Torigoe, T.; et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol. Immunother. 2010, 59, 1543–1549. [Google Scholar] [CrossRef]
- Losi, L.; Bertolini, F.; Guaitoli, G.; Fabbiani, L.; Banchelli, F.; Ambrosini-Spaltro, A.; Botticelli, L.; Scurani, L.; Baldessari, C.; Barbieri, F.; et al. Role of evaluating tumor-infiltrating lymphocytes, programmed death-1 ligand 1 and mismatch repair proteins expression in malignant mesothelioma. Int. J. Oncol. 2019, 55, 1157–1164. [Google Scholar] [CrossRef]
- Chee, S.J.; Lopez, M.; Mellows, T.; Gankande, S.; Moutasim, K.A.; Harris, S.; Clarke, J.; Vijayanand, P.; Thomas, G.J.; Ottensmeier, C.H. Evaluating the effect of immune cells on the outcome of patients with mesothelioma. Br. J. Cancer 2017, 117, 1341–1348. [Google Scholar] [CrossRef]
- Cho, B.C.; Feld, R.; Leighl, N.B.; Opitz, I.; Anraku, M.; Tsao, M.-S.; Hwang, D.M.; Hope, A.; de Perrot, M. A Feasibility Study Evaluating Surgery for Mesothelioma after Radiation Therapy: The “SMART” Approach for Resectable Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2014, 9, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Kohno, M.; Murakami, J.; Wu, L.; Chan, M.-L.; Yun, Z.; Cho, B.C.J.; de Perrot, M. Foxp3+ Regulatory T Cell Depletion after Nonablative Oligofractionated Irradiation Boosts the Abscopal Effects in Murine Malignant Mesothelioma. J. Immunol. 2020, 205, 2519–2531. [Google Scholar] [CrossRef]
- Kiyotani, K.; Park, J.-H.; Inoue, H.; Husain, A.; Olugbile, S.; Zewde, M.G.; Nakamura, Y.; Vigneswaran, W.T. Integrated analysis of somatic mutations and immune microenvironment in malignant pleural mesothelioma. OncoImmunology 2017, 6, e1278330. [Google Scholar] [CrossRef] [Green Version]
- Lievense, L.A.; Bezemer, K.; Cornelissen, R.; Kaijen-Lambers, M.E.; Hegmans, J.P.J.J.; Aerts, J.G.J.V. Precision immunotherapy; dynamics in the cellular profile of pleural effusions in malignant mesothelioma patients. Lung Cancer 2017, 107, 36–40. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadzada, T.; Lee, K.; Clarke, C.; Cooper, W.A.; Linton, A.; McCaughan, B.; Asher, R.; Clarke, S.; Reid, G.; Kao, S. High BIN1 expression has a favorable prognosis in malignant pleural mesothelioma and is associated with tumor infiltrating lymphocytes. Lung Cancer 2019, 130, 35–41. [Google Scholar] [CrossRef]
- Chen, N.; Liu, S.; Huang, L.; Li, W.; Yang, W.; Cong, T.; Ding, L.; Qiu, M. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with malignant pleural mesothelioma: A meta-analysis. Oncotarget 2017, 8, 57460. [Google Scholar] [CrossRef] [Green Version]
- Linton, A.; van Zandwijk, N.; Reid, G.; Clarke, S.; Cao, C.; Kao, S. Inflammation in malignant mesothelioma-friend or foe? Ann. Cardiothorac. Surg. 2012, 1, 516. [Google Scholar]
- Anevlavis, S.; Kouliatsis, G.; Sotiriou, I.; Koukourakis, M.I.; Archontogeorgis, K.; Karpathiou, G.; Giatromanolaki, A.; Froudarakis, M.E. Prognostic Factors in Patients Presenting with Pleural Effusion Revealing Malignancy. Respiration 2014, 87, 311–316. [Google Scholar] [CrossRef]
- Kao, S.C.H.; Pavlakis, N.; Harvie, R.; Vardy, J.L.; Boyer, M.J.; van Zandwijk, N.; Clarke, S. High Blood Neutrophil-to-Lymphocyte Ratio Is an Indicator of Poor Prognosis in Malignant Mesothelioma Patients Undergoing Systemic Therapy. Clin. Cancer Res. 2010, 16, 5805–5813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, S.C.-H.; Klebe, S.; Henderson, D.W.; Reid, G.; Chatfield, M.; Armstrong, N.J.; Yan, T.D.; Vardy, J.; Clarke, S.; van Zandwijk, N.; et al. Low Calretinin Expression and High Neutrophil-To-Lymphocyte Ratio Are Poor Prognostic Factors in Patients with Malignant Mesothelioma Undergoing Extrapleural Pneumonectomy. J. Thorac. Oncol. 2011, 6, 1923–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagawa, T.; Anraku, M.; Morodomi, Y.; Takenaka, T.; Okamoto, T.; Takenoyama, M.; Ichinose, Y.; Maehara, Y.; Cho, B.C.J.; Feld, R.; et al. Clinical role of a new prognostic score using platelet-to-lymphocyte ratio in patients with malignant pleural mesothelioma undergoing extrapleural pneumonectomy. J. Thorac. Dis. 2015, 7, 1898. [Google Scholar]
- Onur, S.T.; Sokucu, S.N.; Dalar, L.; Iliaz, S.; Kara, K.; Buyukkale, S.; Altin, S. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio reliable parameters as prognostic indicators in malignant mesothelioma? Ther. Clin. Risk Manag. 2016, 12, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagishi, T.; Fujimoto, N.; Nishi, H.; Miyamoto, Y.; Hara, N.; Asano, M.; Fuchimoto, Y.; Wada, S.; Kitamura, K.; Ozaki, S.; et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer 2015, 90, 111–117. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Combaz-Lair, C.; Galateau-Sallé, F.; McLeer-Florin, A.; LE Stang, N.; David-Boudet, L.; Duruisseaux, M.; Ferretti, G.R.; Brambilla, E.; Lebecque, S.; Lantuejoul, S. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum. Pathol. 2016, 52, 9–18. [Google Scholar] [CrossRef]
- Scherpereel, A.; Wallyn, F.; Albelda, S.M.; Munck, C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018, 19, e161–e172. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Roden, A.C.; Peikert, T.; Sheinin, Y.M.; Harrington, S.M.; Krco, C.J.; Dong, H.; Kwon, E.D. B7-H1 Expression in Malignant Pleural Mesothelioma is Associated with Sarcomatoid Histology and Poor Prognosis. J. Thorac. Oncol. 2014, 9, 1036–1040. [Google Scholar] [CrossRef] [Green Version]
- Cedrés, S.; Ponce-Aix, S.; Zugazagoitia, J.; Sansano, I.; Enguita, A.; Navarro-Mendivil, A.; Martinez-Marti, A.; Martinez, P.; Felip, E. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS ONE 2015, 10, e0121071. [Google Scholar] [CrossRef] [PubMed]
- Rrapaj, E.; Giacometti, L.; Spina, P.; Salvo, M.; Baselli, G.A.; Veggiani, C.; Rena, O.; Trisolini, E.; Boldorini, R.L. Programmed cell death 1 ligand 1 (PD-L1) expression is associated with poor prognosis of malignant pleural mesothelioma patients with good performance status. Pathology 2021, 53, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Jones, R.E.; Liu, H.; Lizotte, P.H.; Ivanova, E.V.; Kulkarni, M.; Herter-Sprie, G.S.; Liao, X.; Santos, A.A.; Bittinger, M.A.; et al. Cytotoxic T Cells in PD-L1–Positive Malignant Pleural Mesotheliomas Are Counterbalanced by Distinct Immunosuppressive Factors. Cancer Immunol. Res. 2016, 4, 1038–1048. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.S.; Seidal, T.; Mager, U.; Dobra, K.; Brunnström, H.; Dejmek, A. Higher concordance of PD-L1 expression between biopsies and effusions in epithelioid than in nonepithelioid pleural mesothelioma. Cancer Cytopathol. 2021, 129, 468–478. [Google Scholar] [CrossRef]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of Tissue Inflammation by the Immune Receptor Tim-3 Expressed on Innate Immune Cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef]
- Goldberg, M.V.; Drake, C.G. LAG-3 in Cancer Immunotherapy. Curr. Top. Microbiol. Immunol. 2010, 344, 269–278. [Google Scholar]
- Flem-Karlsen, K.; Fodstad, Ø.; Nunes-Xavier, C.E. B7-H3 Immune Checkpoint Protein in Human Cancer. Curr. Med. Chem. 2020, 27, 4062–4086. [Google Scholar] [CrossRef]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, E.; Kajino, K.; Abe, M.; Ohtsuji, N.; Saeki, H.; Hlaing, M.T.; Hino, O. Expression status of PD-L1 and B7-H3 in mesothelioma. Pathol. Int. 2020, 70, 999–1008. [Google Scholar] [CrossRef]
- Walker, L.S.K.; Sansom, D. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef]
- Engelhardt, J.J.; Sullivan, T.J.; Allison, J.P. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J. Immunol. 2006, 177, 1052–1061. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Manzotti, C.N.; Burke, F.; Dussably, L.; Qureshi, O.; Walker, L.S.K.; Sansom, D.M. Acquisition of suppressive function by activated human CD4+ CD25- T cells is associated with the expression of CTLA-4 not FoxP3. J. Immunol. 2008, 181, 1683–1691. [Google Scholar] [CrossRef] [Green Version]
- Ward, F.J.; Dahal, L.N.; Wijesekera, S.K.; Abdul-Jawad, S.K.; Kaewarpai, T.; Xu, H.; Vickers, M.A.; Barker, R.N. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur. J. Immunol. 2013, 43, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Roncella, S.; Laurent, S.; Fontana, V.; Ferro, P.; Franceschini, M.C.; Salvi, S.; Varesano, S.; Boccardo, S.; Vigani, A.; Morabito, A.; et al. CTLA-4 in mesothelioma patients: Tissue expression, body fluid levels and possible relevance as a prognostic factor. Cancer Immunol. Immunother. 2016, 65, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.C.; Lines, J.L.; Varn, F.S.; Deng, J.; Sarde, A.; Mabaera, R.; Kuta, A.; Le Mercier, I.; Cheng, C.; Noelle, R.J. Immunoregulatory functions of VISTA. Immunol. Rev. 2017, 276, 66–79. [Google Scholar] [CrossRef]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.-F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef]
- Gao, J.; Ward, J.F.; Pettaway, C.A.; Shi, L.Z.; Subudhi, S.K.; Vence, L.M.; Zhao, H.; Chen, J.; Chen, H.; Efstathiou, E.; et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017, 23, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakavand, H.; Jackett, L.A.; Menzies, A.M.; Gide, T.N.; Carlino, M.S.; Saw, R.P.; Thompson, J.F.; Wilmott, J.S.; Long, G.V.; Scolyer, R.A. Negative immune checkpoint regulation by VISTA: A mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod. Pathol. 2017, 30, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, G.; Manick, B.; Hernandez, V.; Renelt, M.; Erickson, C.; Guan, J.; Singh, R.; Rollins, S.; Solorz, A.; et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 2019, 156, 74–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1548–1565. [Google Scholar] [CrossRef] [Green Version]
- Muller, S.; Lai, W.V.; Adusumilli, P.S.; Desmeules, P.; Frosina, D.; Jungbluth, A.; Ni, A.; Eguchi, T.; Travis, W.D.; Ladanyi, M.; et al. V-domain Ig-containing suppressor of T-cell activation (VISTA), a potentially targetable immune checkpoint molecule, is highly expressed in epithelioid malignant pleural mesothelioma. Mod. Pathol. 2019, 33, 303–311. [Google Scholar] [CrossRef]
- Alcala, N.; Mangiante, L.; Le-Stang, N.; Gustafson, C.E.; Boyault, S.; Damiola, F.; Alcala, K.; Brevet, M.; Thivolet-Bejui, F.; Blanc-Fournier, C.; et al. Redefining malignant pleural mesothelioma types as a continuum uncovers immune-vascular interactions. EBioMedicine 2019, 48, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Thomas, A.; Abate-Daga, D.; Zhang, J.; Morrow, B.; Steinberg, S.M.; Orlandi, A.; Ferroni, P.; Schlom, J.; Guadagni, F.; et al. Malignant Mesothelioma Effusions Are Infiltrated by CD3 + T Cells Highly Expressing PD-L1 and the PD-L1 + Tumor Cells within These Effusions Are Susceptible to ADCC by the Anti–PD-L1 Antibody Avelumab. J. Thorac. Oncol. 2016, 11, 1993–2005. [Google Scholar] [CrossRef] [Green Version]
- Scherpereel, A.; Mazieres, J.; Greillier, L.; Lantuejoul, S.; Dô, P.; Bylicki, O.; Monnet, I.; Corre, R.; Audigier-Valette, C.; Locatelli-Sanchez, M.; et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): A multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019, 20, 239–253. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.; Fujimoto, N.; Peters, S.; Tsao, A.; Mansfield, A.; Popat, S.; Jahan, T.; Antonia, S.; et al. ID:2908 First-Line Nivolumab + Ipilimumab vs Chemotherapy in Unresectable Malignant Pleural Mesothelioma: CheckMate 743. J. Thorac. Oncol. 2020, 15, e42. [Google Scholar] [CrossRef]
- Marcq, E.; Van Audenaerde, J.R.M.; De Waele, J.; Jacobs, J.; Van Loenhout, J.; Cavents, G.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L.J. Building a Bridge between Chemotherapy and Immunotherapy in Malignant Pleural Mesothelioma: Investigating the Effect of Chemotherapy on Immune Checkpoint Expression. Int. J. Mol. Sci. 2019, 20, 4182. [Google Scholar] [CrossRef] [Green Version]
- de Gooijer, C.J.; Borm, F.J.; Scherpereel, A.; Baas, P. Immunotherapy in Malignant Pleural Mesothelioma. Front. Oncol. 2020, 10, 187. [Google Scholar] [CrossRef] [Green Version]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. New Engl. J. Med. 2017, 377, 2500. [Google Scholar] [CrossRef]
- Shrestha, R.; Nabavi, N.; Lin, Y.-Y.; Mo, F.; Anderson, S.; Volik, S.; Adomat, H.H.; Lin, D.; Xue, H.; Dong, X.; et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med. 2019, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ladanyi, M.; Vega, F.S.; Zauderer, M. Loss of BAP1 as a candidate predictive biomarker for immunotherapy of mesothelioma. Genome Med. 2019, 11, 1–3. [Google Scholar] [CrossRef]
- Cornelissen, R.; Hegmans, J.P.J.J.; Maat, A.P.W.M.; Kaijen-Lambers, M.E.H.; Bezemer, K.; Hendriks, R.W.; Hoogsteden, H.C.; Aerts, J.G.J.V. Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am. J. Respir. Crit. Care Med. 2016, 193, 1023–1031. [Google Scholar] [CrossRef]
- Veltman, J.D.; Lambers, M.E.H.; Van Nimwegen, M.; De Jong, S.; Hendriks, R.W.; Hoogsteden, H.C.; Aerts, J.G.J.V.; Hegmans, J.P.J.J. Low-Dose Cyclophosphamide Synergizes with Dendritic Cell-Based Immunotherapy in Antitumor Activity. J. Biomed. Biotechnol. 2010, 2010, 798467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, J.G.; de Goeje, P.; Cornelissen, R.; Kaijen-Lambers, M.E.; Bezemer, K.; Van Der Leest, C.H.; Mahaweni, N.M.; Kunert, A.; Eskens, F.A.; Waasdorp, C.; et al. Autologous Dendritic Cells Pulsed with Allogeneic Tumor Cell Lysate in Mesothelioma: From Mouse to Human. Clin. Cancer Res. 2017, 24, 766–776. [Google Scholar] [CrossRef] [Green Version]
- Belderbos, R.A.; Baas, P.; Berardi, R.; Cornelissen, R.; Fennell, D.A.; Van Meerbeeck, J.P.; Scherpereel, A.; Vroman, H.; Aerts, J.G.J.V.; on behalf of the DENIM team*. A multicenter, randomized, phase II/III study of dendritic cells loaded with allogeneic tumor cell lysate (MesoPher) in subjects with mesothelioma as maintenance therapy after chemotherapy: DENdritic cell Immunotherapy for Mesothelioma (DENIM) trial. Transl. Lung Cancer Res. 2019, 8, 280. [Google Scholar] [CrossRef]
- Castelletti, L.; Yeo, D.; van Zandwijk, N.; Rasko, J.E.J. Anti-Mesothelin CAR T cell therapy for malignant mesothelioma. Biomark. Res. 2021, 9, 1–3. [Google Scholar] [CrossRef]
- Morello, A.; Sadelain, M.; Adusumilli, P.S. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov. 2016, 6, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Burgio, S.; Noori, L.; Gammazza, A.M.; Campanella, C.; Logozzi, M.; Fais, S.; Bucchieri, F.; Cappello, F.; Bavisotto, C.C. Extracellular Vesicles-Based Drug Delivery Systems: A New Challenge and the Exemplum of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2020, 21, 5432. [Google Scholar] [CrossRef]
- Kara-Terki, L.; Treps, L.; Blanquart, C.; Fradin, D. Critical Roles of Tumor Extracellular Vesicles in the Microenvironment of Thoracic Cancers. Int. J. Mol. Sci. 2020, 21, 6024. [Google Scholar] [CrossRef]
- Ahmadzada, T.; Kao, S.; Reid, G.; Clarke, S.; Grau, G.E.; Hosseini-Beheshti, E. Extracellular vesicles as biomarkers in malignant pleural mesothelioma: A review. Crit. Rev. Oncol. Hematol. 2020, 150, 102949. [Google Scholar] [CrossRef]
- Greening, D.W.; Ji, H.; Chen, M.; Robinson, B.W.S.; Dick, I.M.; Creaney, J.; Simpson, R.J. Secreted primary human malignant mesothelioma exosome signature reflects oncogenic cargo. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.-A.A.; Van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic Analysis of Exosomes Isolated from Human Malignant Pleural Effusions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 114–121. [Google Scholar] [CrossRef]
- Clayton, A.; Tabi, Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol. Dis. 2005, 34, 206–213. [Google Scholar] [CrossRef]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef]
- Han, L.; Lam, E.W.-F.; Sun, Y. Extracellular vesicles in the tumor microenvironment: Old stories, but new tales. Mol. Cancer 2019, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Munson, P.B.; Hall, E.M.; Farina, N.H.; Pass, H.; Shukla, A. Exosomal miR-16-5p as a target for malignant mesothelioma. Sci. Rep. 2019, 9, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Mahaweni, N.M.; Kaijen-Lambers, M.E.H.; Dekkers, J.; Aerts, J.G.J.V.; Hegmans, J.P.J.J. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J. Extracell. Vesicles 2013, 2, 22492. [Google Scholar] [CrossRef]
- Flood, B.A.; Higgs, E.F.; Li, S.; Luke, J.J.; Gajewski, T.F. STING pathway agonism as a cancer therapeutic. Immunol. Rev. 2019, 290, 24–38. [Google Scholar] [CrossRef]
- Woo, S.-R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.; et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1130. [Google Scholar] [CrossRef] [Green Version]
- Berry, S.; Giraldo, N.; Nguyen, P.; Green, B.; Xu, H.; Ogurtsova, A.; Soni, A.; Succaria, F.; Wang, D.; Roberts, C.; et al. 33rd Annual Meeting & Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2018), Washington, D.C., USA, 7–11 November 2018. J. Immunother. Cancer 2018, 6 (suppl. S1), 115. [Google Scholar]
- An, X.; Zhu, Y.; Zheng, T.; Wang, G.; Zhang, M.; Li, J.; Ji, H.; Li, S.; Yang, S.; Xu, D.; et al. An Analysis of the Expression and Association with Immune Cell Infiltration of the cGAS/STING Pathway in Pan-Cancer. Mol. Ther. Nucleic Acids 2019, 14, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lu, X.; Qin, N.; Qiao, Y.; Xing, S.; Liu, W.; Feng, F.; Liu, Z.; Sun, H. STING, a promising target for small molecular immune modulator: A review. Eur. J. Med. Chem. 2021, 211, 113113. [Google Scholar] [CrossRef]
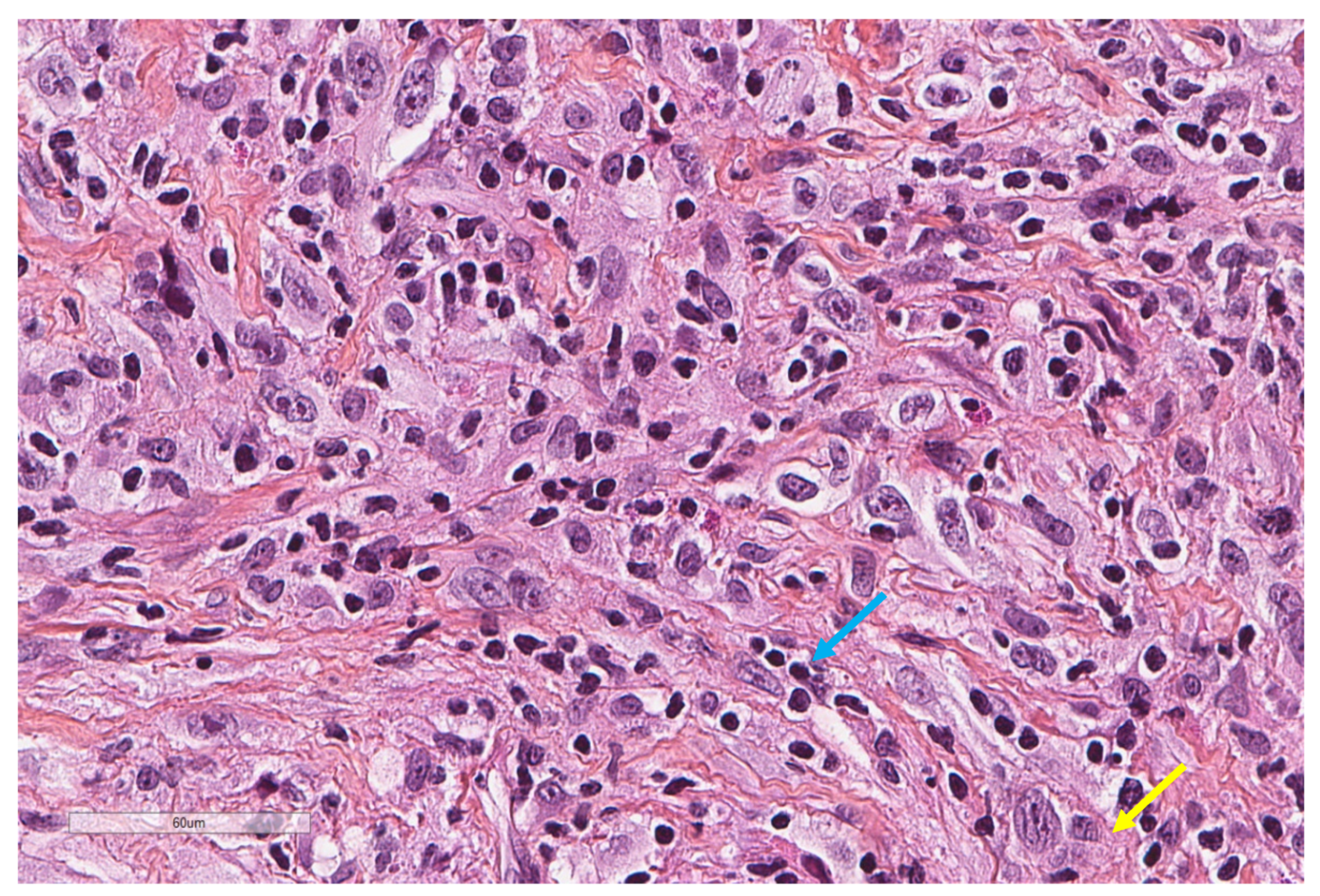
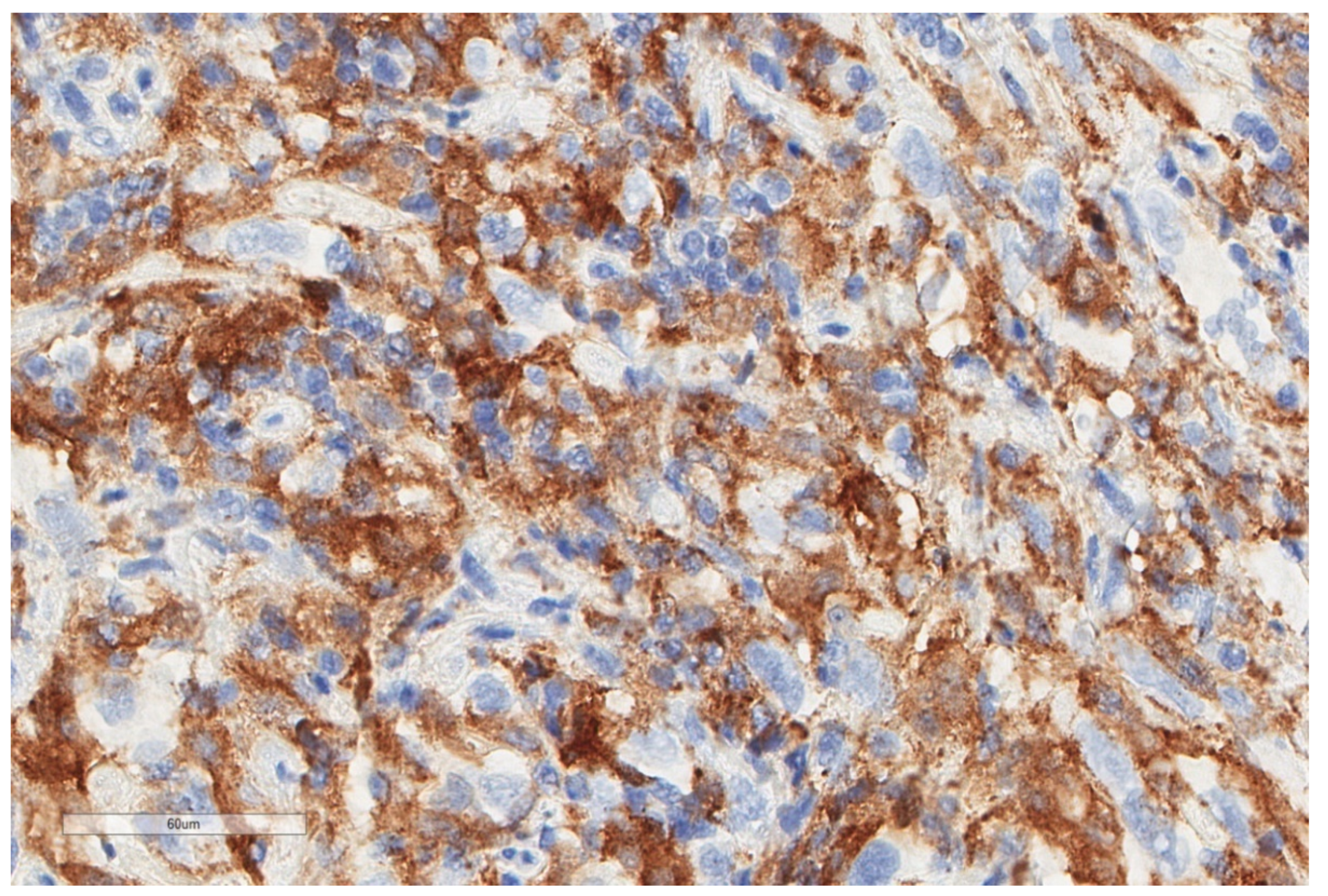
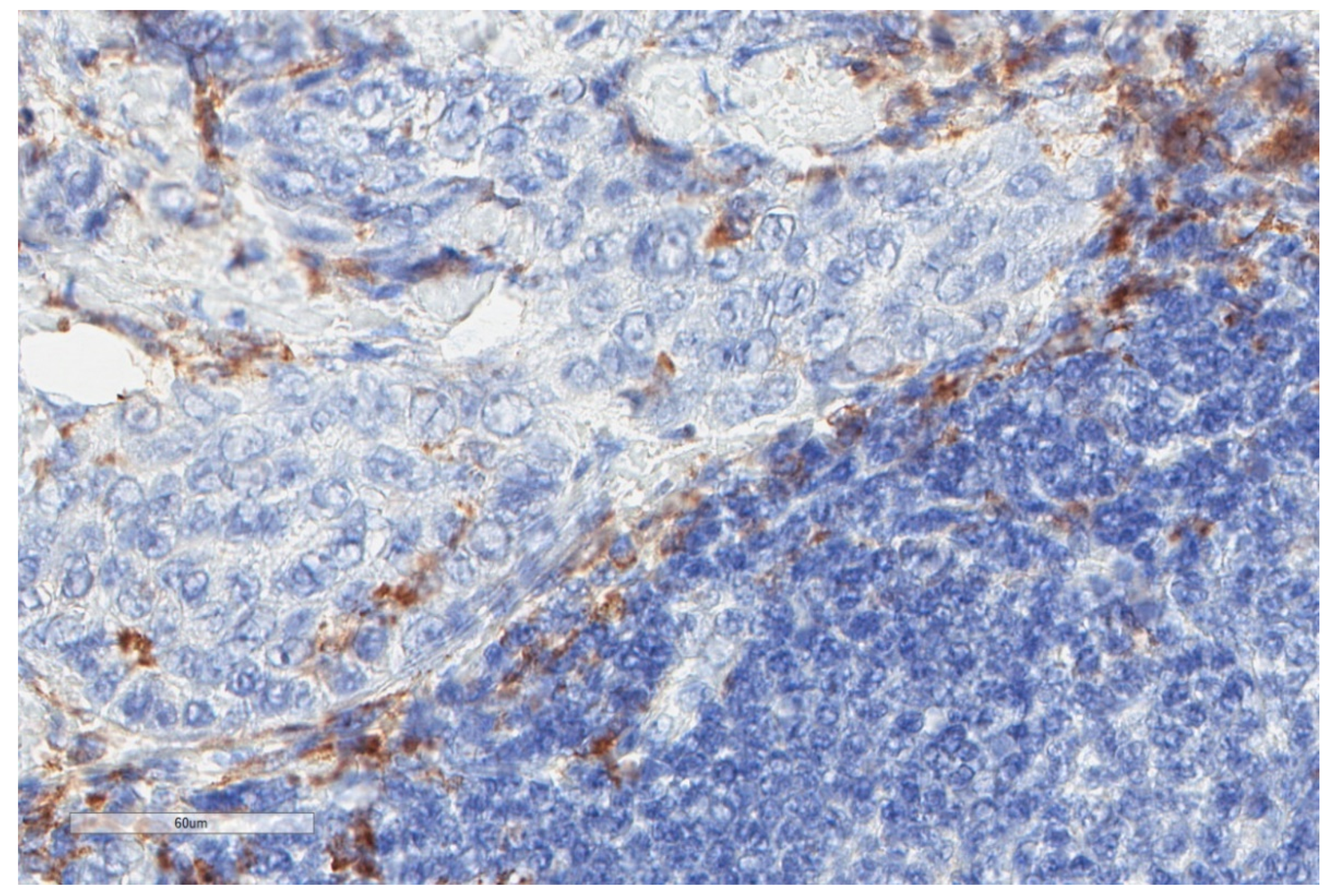
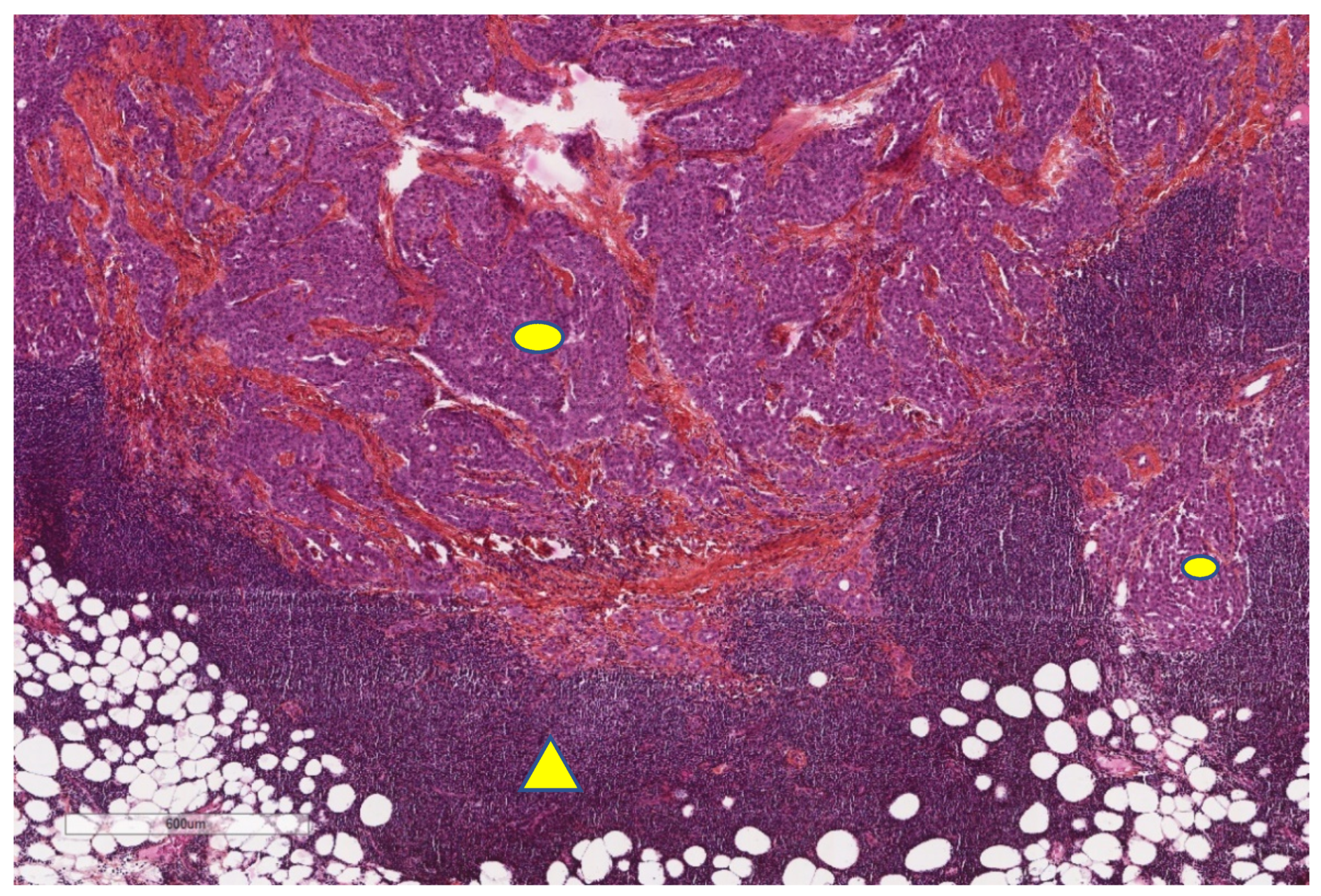
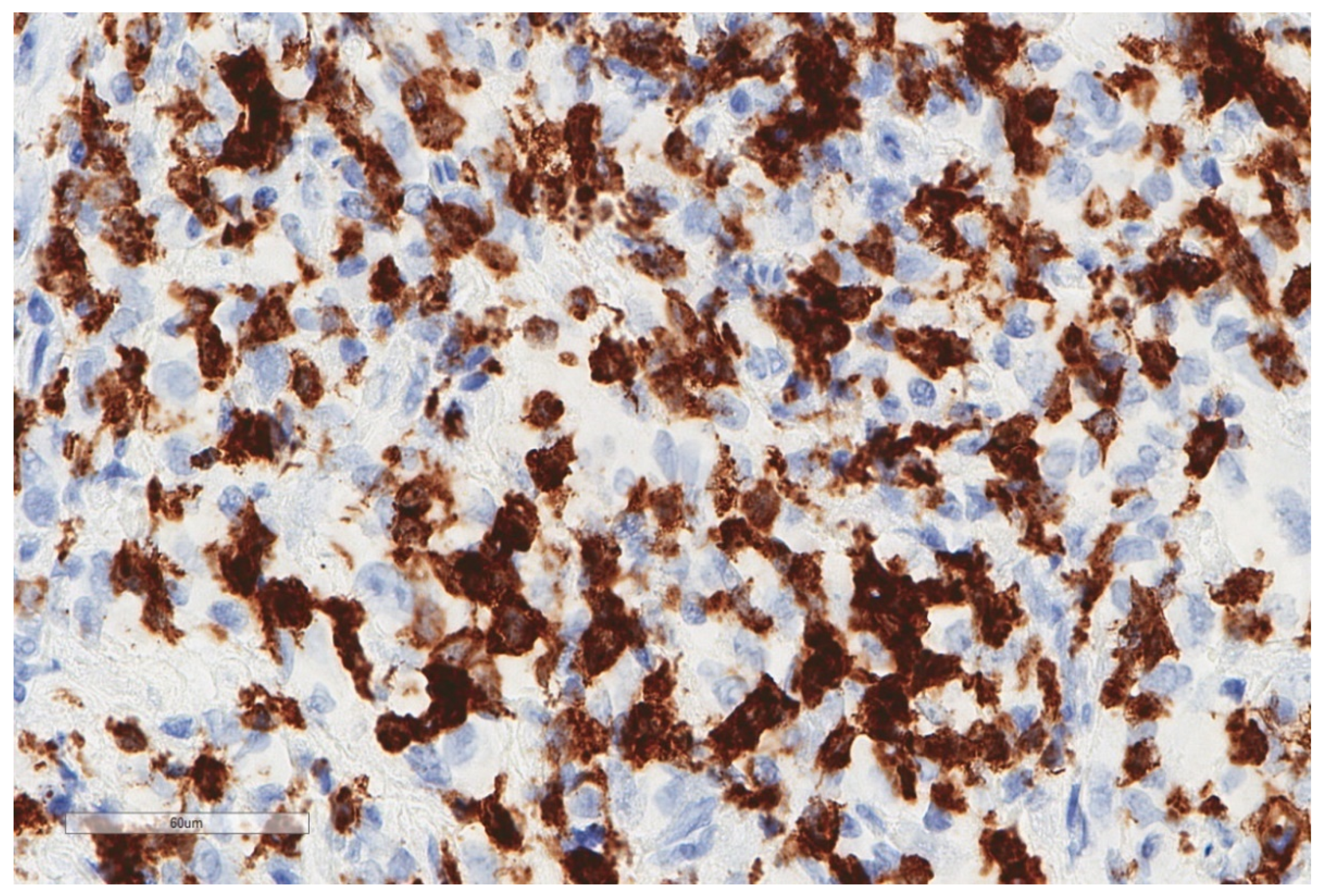
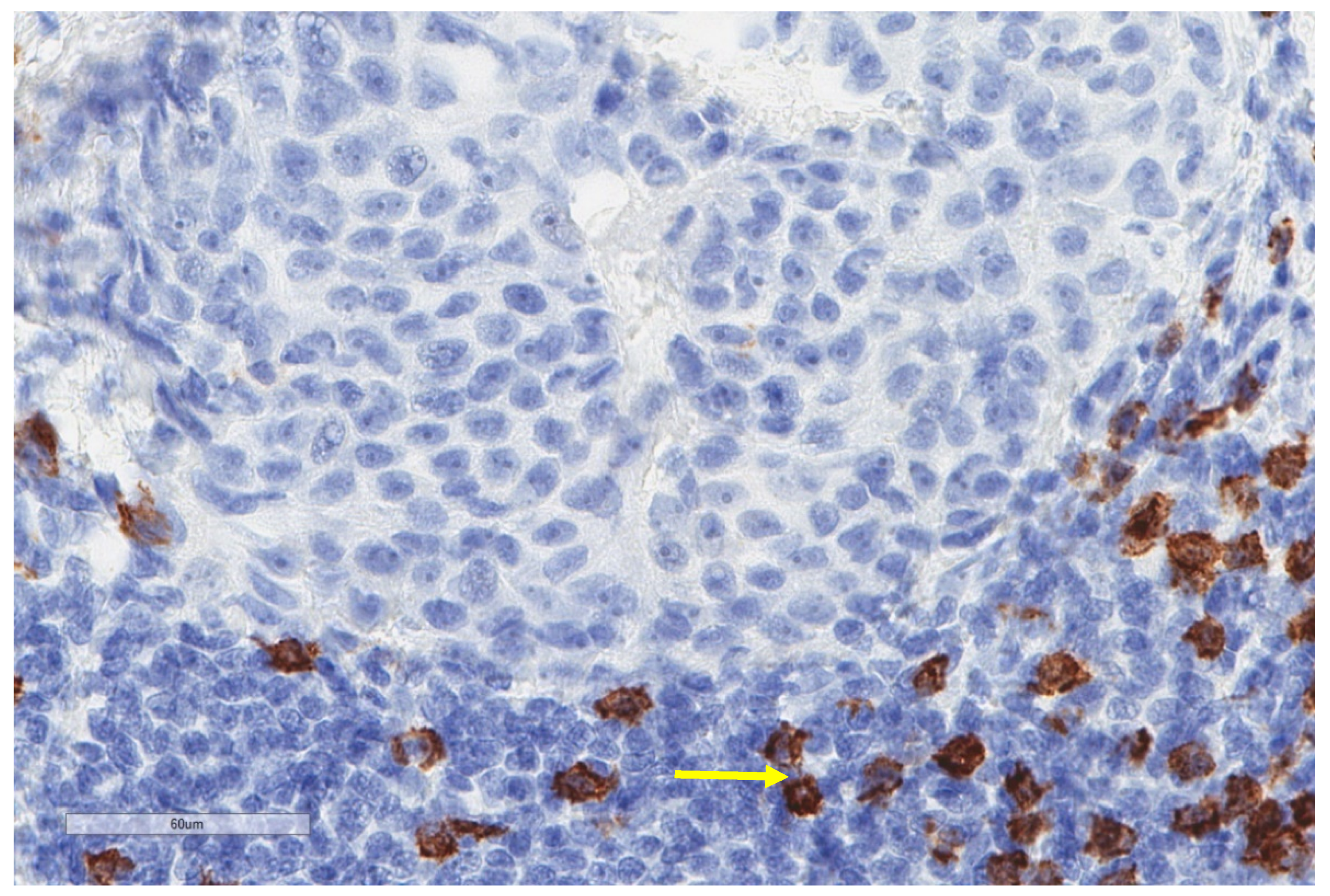
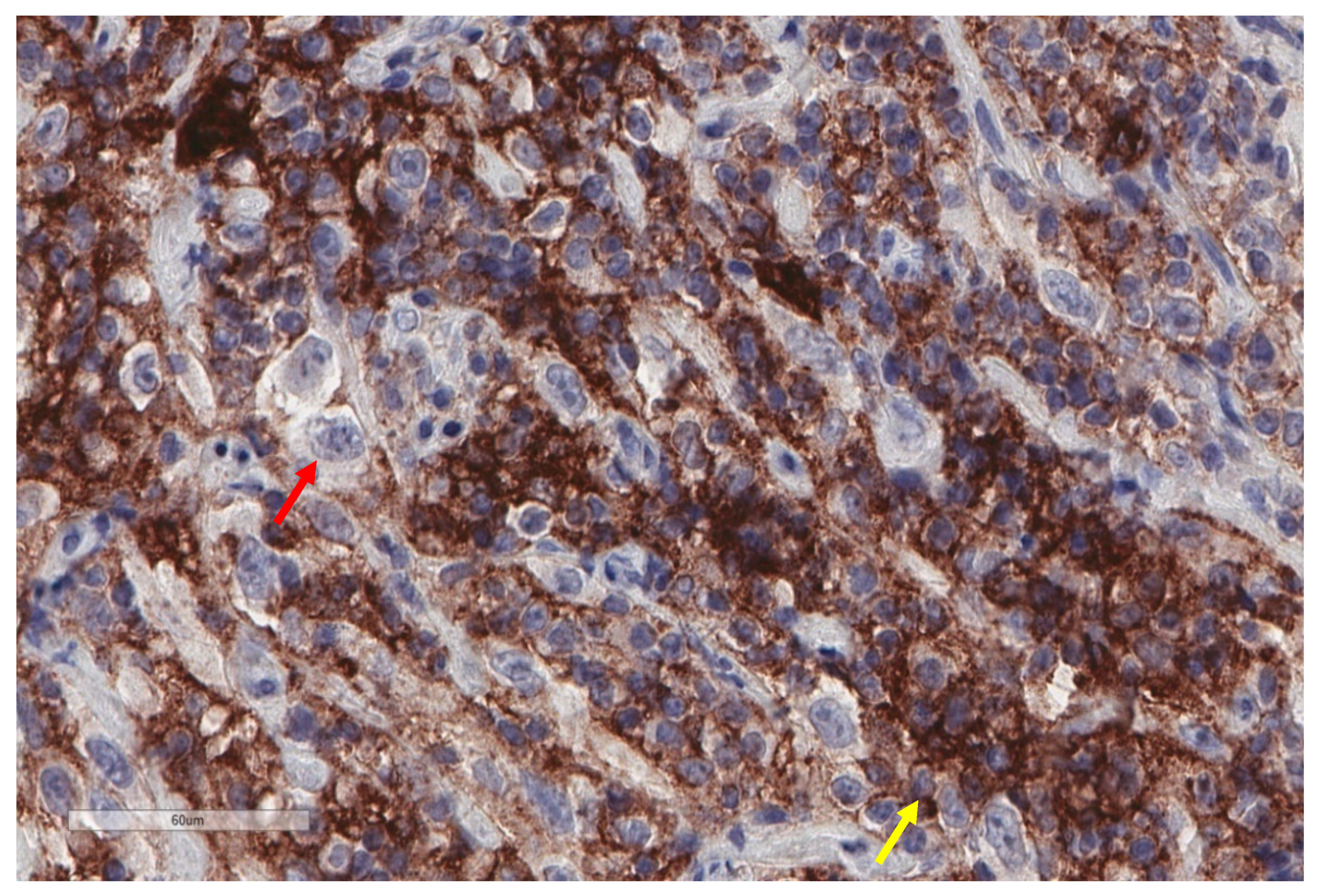
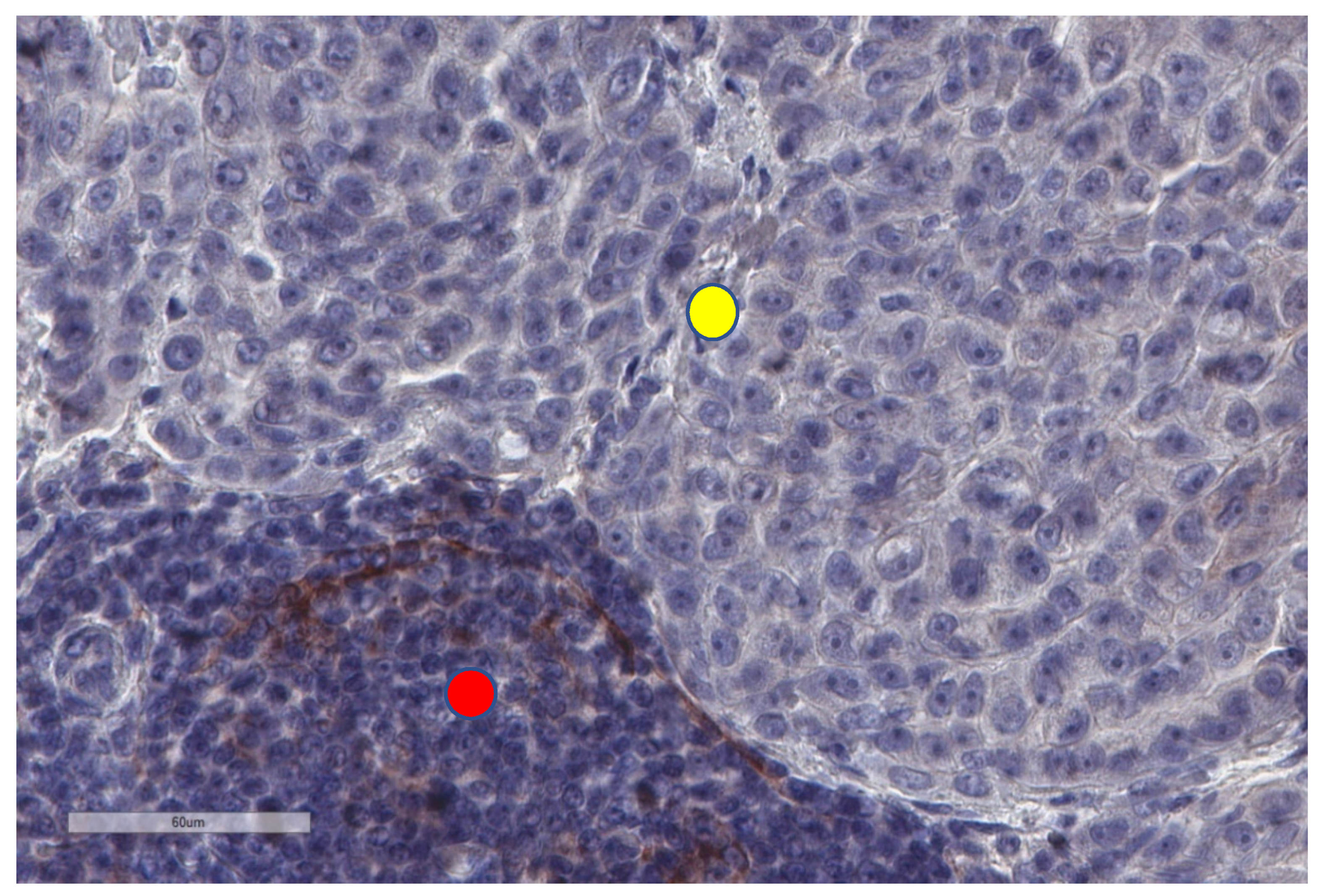
| Study | n | Study Aim | Method | Results |
|---|---|---|---|---|
| Burt., 2011 [34] | 52 | Circulating monocytes, tumour-infiltrating macrophages and OS | Blood and surgical specimen for tissue microarray | Higher number of monocytes was correlated with poor survival in all patients, and high density of macrophages correlated with poor survival in non-epithelial tumours |
| Salaroglio., 2019 [37] | 275 | Intrapleural and Intratumor T-regs, M2, MDSC, TILs and OS, PFS | Pleural fluid and pleural biopsies by flow cytometry | MDSC related to anergy of TILs. High intratumor T-regs, MDSC correlated with poor OS/PFS, not observed in pleural fluid |
| Marcq., 2017 [40] | 54 | Macrophages (CD68), PD-L1, TIM-3, CD4, CD8, CD45RO | Immunohistochemistry Tissue biopsies | Expression of CD68+ macrophages in the stroma correlates with the number of stromal Tregs |
| Cornelissen., 2014 [39] | 16 | Intratumor CD8, total macrophages, M2 and OS | Immunohistochemistry Comparison CT induction + surgery vs. CT alone | CD8+, total and M2 macrophages: no relation to OS; Rate M2/TAMs correlates with poor OS (p < 0.05) |
| Cornwall., 2016 [42] | 48 | Dendritic cells (DC) MPM vs. controls | Whole blood analysis by flow cytometry | Decreased numbers of DC Reduced ability to process antigen and reduced expression of costimulatory molecules inducing anergised/tolerized T cells. |
| Sottile., 2019 [54] | 27 | Circulating NK and T cells before and after treatment with tremelimumab (CTL4) | RNA expression from MPM biopsies | MM patients have perturbed NK cells before CTL4, while, after treatment, NK shift to normal levels |
| Tumino., 2019 [57] | 33 | NK cells Innate lymphoid cells (ILC) 1,2,3 | Pleural fluid Flow cytometry | NK highly expressed ILC3 more highly expressed than ILC2 and ILC1 |
| Study | n | Study Aim | Method | Results |
|---|---|---|---|---|
| Marcq., 2017 [40] | 54 | CD68, PD-L1, TIM-3, CD4, CD8, CD45RO | Immunohistochemistry tissue biopsies | High CD4+ is associated with better survival Increase in the CD45RO expression on stromal lymphocytes was significantly associated with a lower likelihood of partial or complete response to chemotherapy |
| Fusco., 2020 [62] | 88 | CD4, CD8, PD-L1 and survival in patients treated with chemotherapy | Tissue biopsies for microarray | Stromal low CD4+ and high CD8+ were correlated with poor survival In PD-L1 CPS > 1, stromal high CD8+ was a poor prognostic factor CD4/CD8 > 1 associated with a longer survival independently of the histologic sub-type |
| Pasello, 2018 [80] | 130 | CD4, CD8, CD68, Ki-67, PD-L1 and survival/before and after chemotherapy | Tissue biopsies Immunohistochemistry | CD3+ T-cells and CD8+ TILs significantly increased after chemotherapy High CD8+ were correlated with high macrophages, PD-L1 expression and aggressiveness High CD8 were correlated with poor survival and low response to chemotherapy |
| Losi., 2019 [83] | 55 | Tumor CD4, CD8, PD-L1, and survival | Immunohistochemisty Tissue biopsies | Low lymphocytic TILs expression and high CD8+ TILs at baseline associated with shorter survival |
| Anraku., 2008 [79] | 32 | CD4, CD8, CD25, FOXP3, CD45RO and OS, PFS | Immunohistochemistry in MPM from extrapleural pneumonectomy | High level of CD8+ TILs expression is associated with a better OS/PFS for patients with extrapleural pneumonectomy |
| Yamada., 2010 [82] | 27 | CD4, CD8, NK cells, HLA-1 and OS | Immunohistochemistry in MPM from extrapleural pneumonectomy | High density of CD8+ TILs is independently associated with a significantly better OS in this patient population |
| Chee., 2017 [84] | 302 | CD4, CD8, CD25, CD2O, FOXP3, CD45RO, neutrophils (NP57+), natural killer cells (CD56+) macrophages (CD68+) and OS, PFS | Tissue microarray | High CD4+, CD20+ and low FOXP3 expression were associated with better OS in epithelial tumours Low CD8+ and low FOXP3 expression were associated with better OS in non-epithelial tumours CD4/CD8 > 1 associated with a longer survival in epithelioid tumours |
| Ujiie., 2015 [38] | 230 | TAMs, TILs and survival | Immunohistochemistry. Tissue microarray | High CD163+ TAMs and low CD8+ TILs associated with worse prognosis |
| Study | n | Study Aim | Method | Results |
|---|---|---|---|---|
| Chen, 2017 [91] | 1533 | NLR and overall survival (OS) | Meta-analysis of 11 studies | NLR significantly higher in non-epithelioid group Elevated NLR was associated with a poor OS |
| Kao, 2010 [94] | 173 | NLR and OS | Chemotherapy and chemotherapy naïve patients | NLR < 5 associated with better OS in chemotherapy group and chemotherapy-naïve group Normalization of NLR ratio after one cycle of chemotherapy associated with a better OS |
| Kao, 2011 [95] | 85 | NLR and OS | Patients with extrapleural pneumonectomy | NLR ≥ 3 was associated with poor OS in patients with extrapleural pneumonectomy |
| Tagawa, 2015 [96] | 65 | NLR, PLR and OS | Patients with extrapleural pneumonectomy | NLR ≥ 3.5 was associated with poor OS in univariate analysis PLR ≤ 215 was associated with better OS in both univariate and multivariate analysis |
| Yamagishi, 2015 [98] | 150 | NLR, PLR, LMR and OS | Blood samples from patients with MPM at diagnosis | At univariate analysis, NLR > 5, PLR > 150 and LMR < 2.74 were associated with poor OS. Only LMR was independent predictor of survival in multivariate analysis. |
| Study | n | Study Aim | Method | Results |
|---|---|---|---|---|
| Mansfield, 2014 [102] | 106 | PD-L1 and overall survival (OS) | Tissue biopsy immunohistochemistry PD-L1 > 5% | PD-L1 highly expressed in MPM cells associated with poor OS Patients with PD-L1 highly expressed were less likely to be offered surgery PD-L1 expression associated with poor OS in sarcomatoid MPM |
| Cedrés, 2015 [103] | 77 | PD-L1 and overall survival (OS) | Tissue biopsy immunohistochemistry PD-L1 > 1% | PD-L1 positive cases were associated with poor OS |
| Rrapaj, 2021 [104] | 198 | PD-L1 and overall survival (OS) | Tissue biopsy Immunohistochemistry PD-L1 > 5% | PD-L1 positive cases were associated with poor OS |
| Awad, 2016 [105] | 43 | PD-L1, CD4+, CD8+, TIMP3, CD45+ and overall survival (OS) | Surgically resected MPM Next generation sequencing Flow cytometry | PD-L1 positive cases were associated with CD4+, CD8+, TIM3, CD45+ positive cases No difference in survival according to PD-L1 status |
| Mansour, 2021 [106] | 61 | PD-L1 from biopsies vs. pleural effusions | Immunohistochemistry PD-L1 ≥ 1% vs. 5% vs. 10% vs. 50% | PD-L1 is less expressed in pleural effusions compared to pleural tissues Higher concordance for PD-L1 at ≥ 1% cut-off in epithelioid MPM for histologic and cytologic samples |
| Marcq, 2017 [40] | 54 | CD68, PD-L1, TIM-3, CD4, CD8, CD45RO | Immunohistochemistry Tissue biopsies | TIM-3 expression is an independent prognostic factor of better survival |
| Marcq, 2017 [45] | 6 | PD-1, PD-L1, TIM-3, LAG-3, CD4, CD8, NK | Pleural effusions Flow cytometry | LAG-3 and TIM-3 expressed in pleural effusion on CD4+, CD8+ and NKs |
| Salaroglio, 2019 [37] | 275 | Intrapleural and Intratumor T-regs, M2, MDSC, TILs, TIM-3, LAG-3 and OS, PFS | Pleural fluid and pleural biopsies by flow cytometry | LAG-3 expressed in both pleural effusions and pleural tissue Low PD-1+/LAG-3+/TIM-3+ CD4+ TILS were related to better survival in pleural tissue |
| Matsumura, 2020 [111] | 31 | PD-L1, B7 homolog 3 (B7-H3) | Immunohistochemistry Tissue biopsies Confirmation of B7-H3 by flow cytometry | B7-H3 highly expressed in chemotherapy-pretreated patients, in both epithelioid and non-epithelioid sub-types B7-H3 significantly more expressed compared to PD-L1 in epithelioid MPM No significant difference in the expression levels of PD-L1 and B7-H3 |
| Roncella, 2016 [116] | 45 | CTLA-4 | Tissue biopsies, blood samples and pleural effusions from patients with MPM | Variable expression (56% cases) in biopsies Higher levels in blood samples compared to pleural effusions Higher levels in tissue for epithelioid tumours Higher levels in blood samples for sarcomatoid tumours High CTLA-4 expression associated with a better survival |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Désage, A.-L.; Karpathiou, G.; Peoc’h, M.; Froudarakis, M.E. The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review. Cancers 2021, 13, 3205. https://doi.org/10.3390/cancers13133205
Désage A-L, Karpathiou G, Peoc’h M, Froudarakis ME. The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review. Cancers. 2021; 13(13):3205. https://doi.org/10.3390/cancers13133205
Chicago/Turabian StyleDésage, Anne-Laure, Georgia Karpathiou, Michel Peoc’h, and Marios E. Froudarakis. 2021. "The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review" Cancers 13, no. 13: 3205. https://doi.org/10.3390/cancers13133205
APA StyleDésage, A.-L., Karpathiou, G., Peoc’h, M., & Froudarakis, M. E. (2021). The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review. Cancers, 13(13), 3205. https://doi.org/10.3390/cancers13133205






