The Antitumor Activity of Sodium Selenite Alone and in Combination with Gemcitabine in Pancreatic Cancer: An In Vitro and In Vivo Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Sodium Selenite and Drug Treatments
2.2. Cell Viability Analysis
2.3. Cell Cycle Study
2.4. Mitochondrial Membrane Potential Analysis
2.5. Benzamide Treatment Assays
2.6. Apoptosis-Inducing Factor Immunofluorescence Assay
2.7. Western-Blot Assay of Phosphorylated p38
2.8. Wound Healing Assay
2.9. Multicellular Tumor Spheroid Assays
2.10. Colony Formation Assay
2.11. CSC Spheroids Formation
2.12. In Vivo Tumor Growth Analysis
2.13. Bioimaging
2.14. Histological Analysis
2.15. Statistical Analyses
3. Results
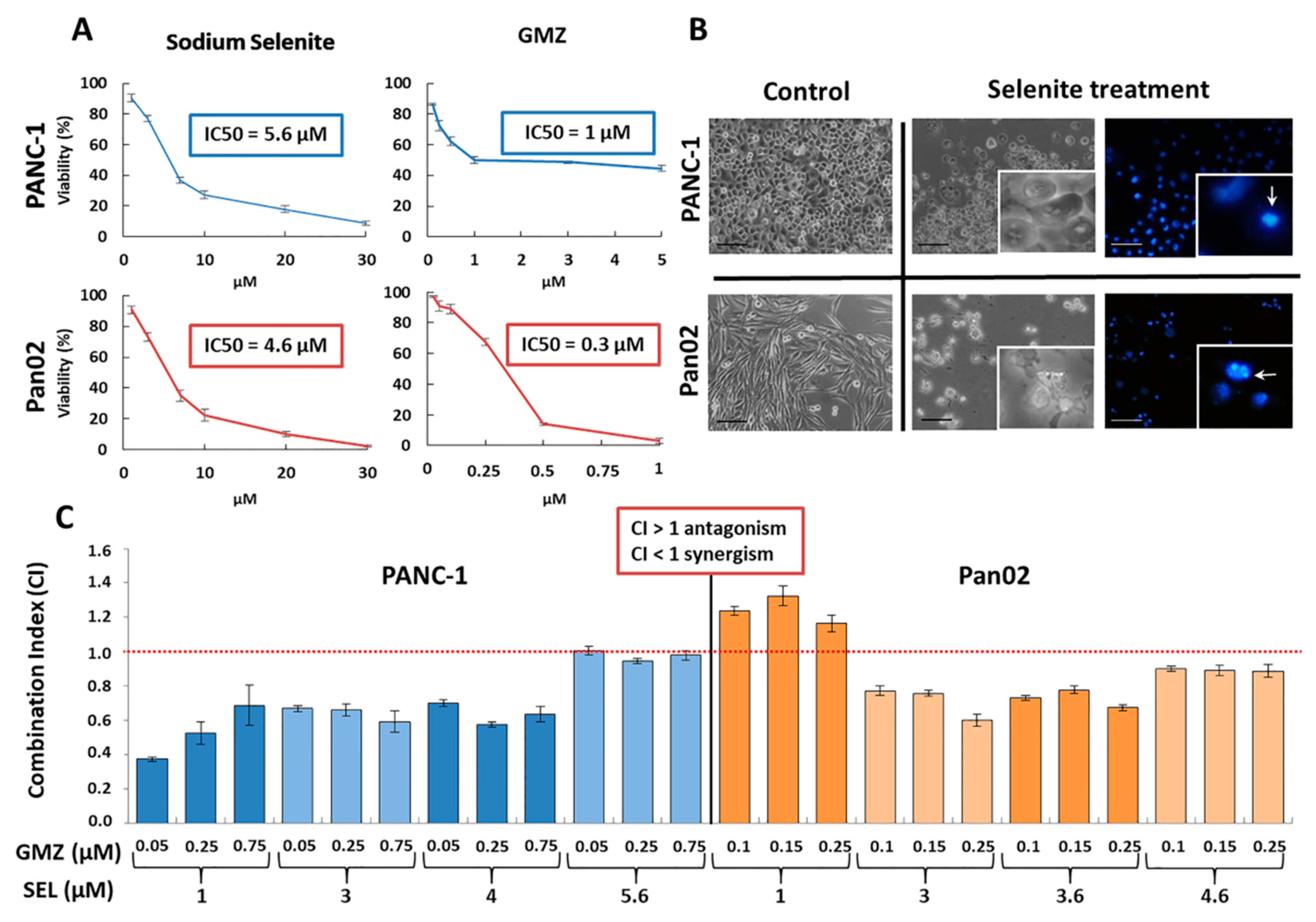
3.1. Sodium Selenite Reduces Cell Viability in Pancreatic Cancer Cells
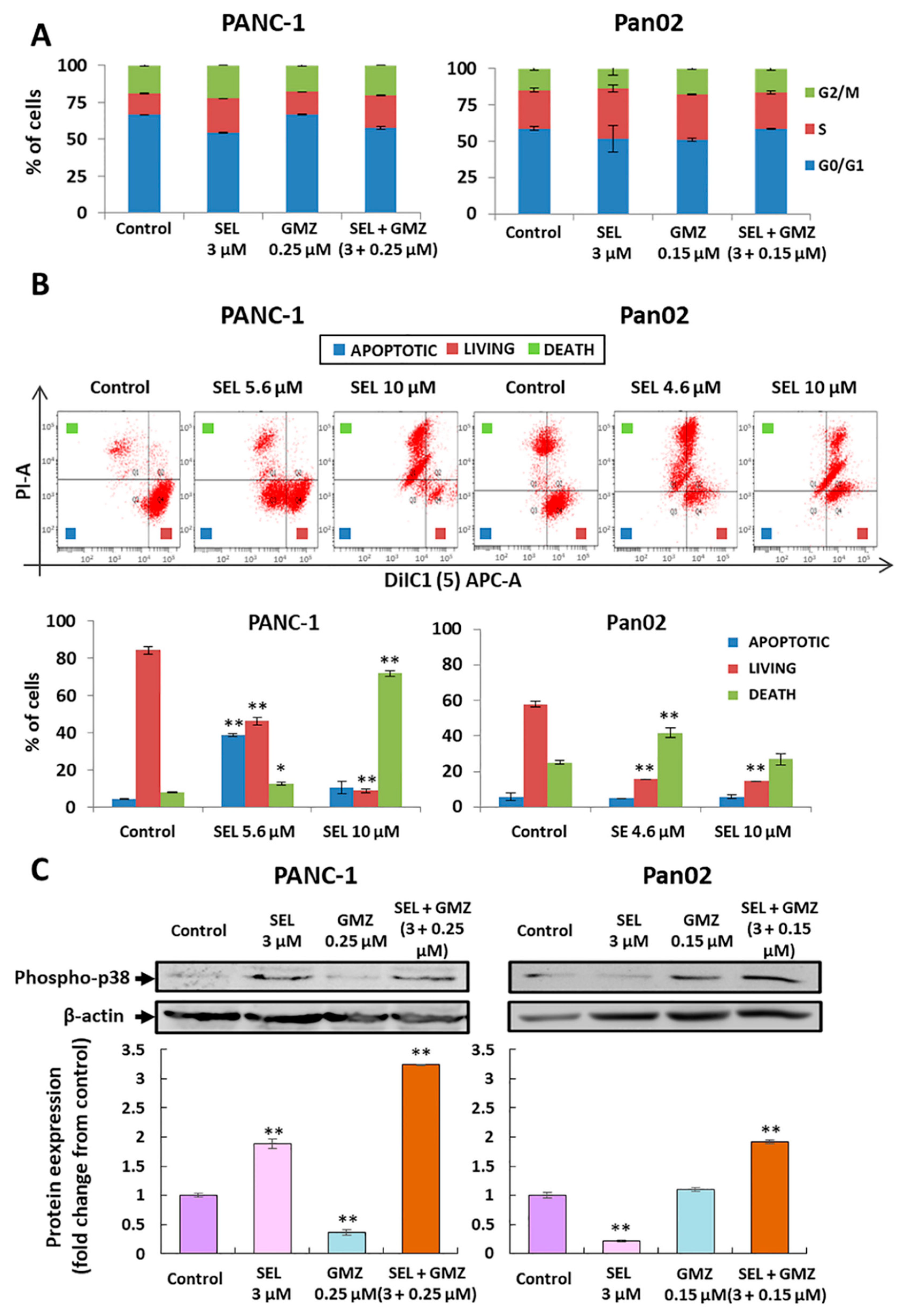
3.2. Modulation of Cell Cycle and Mitochondrial Membrane Potential by Sodium Selenite
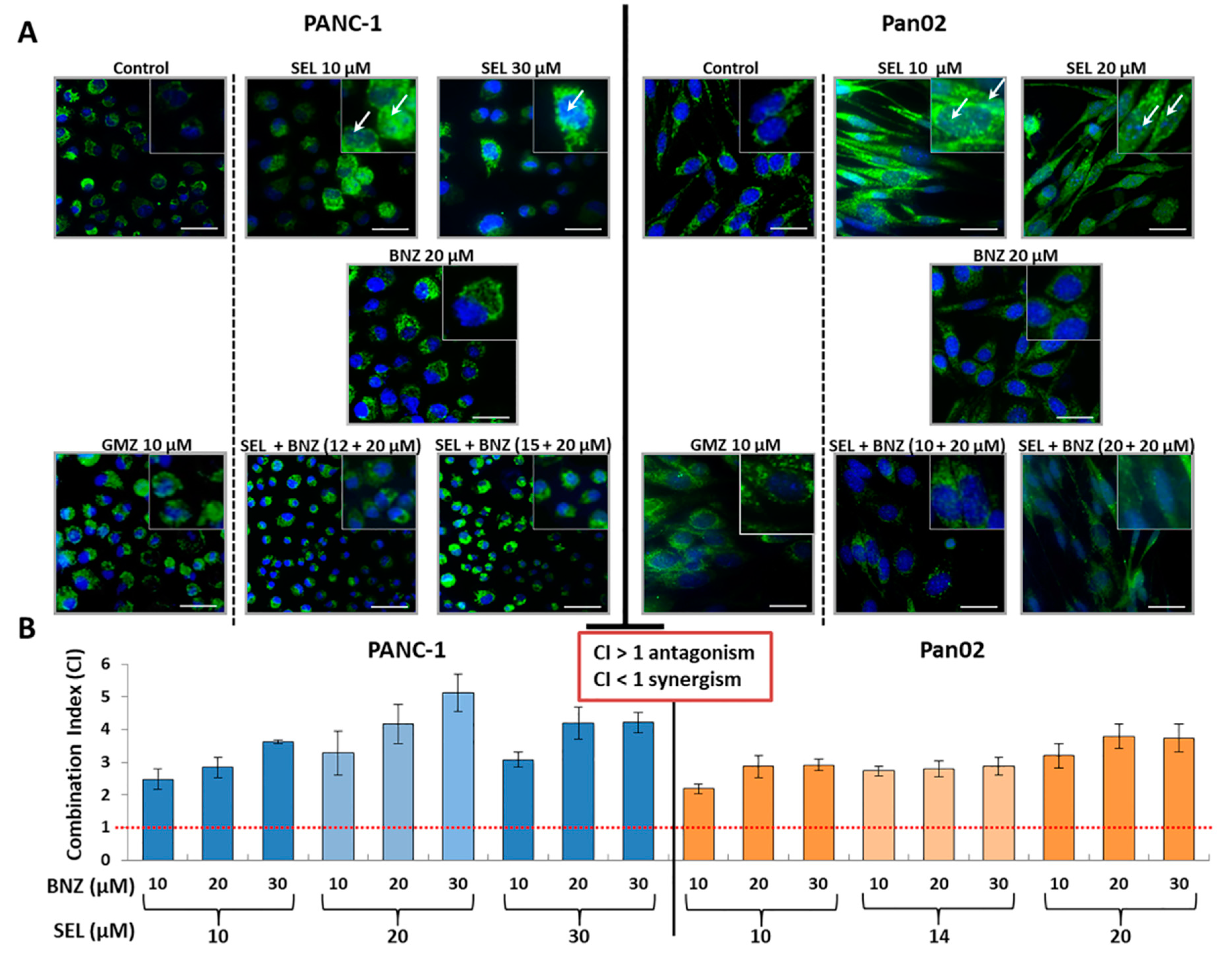
3.3. Sodium Selenite and Its Association with Gemcitabine: Molecular Analysis
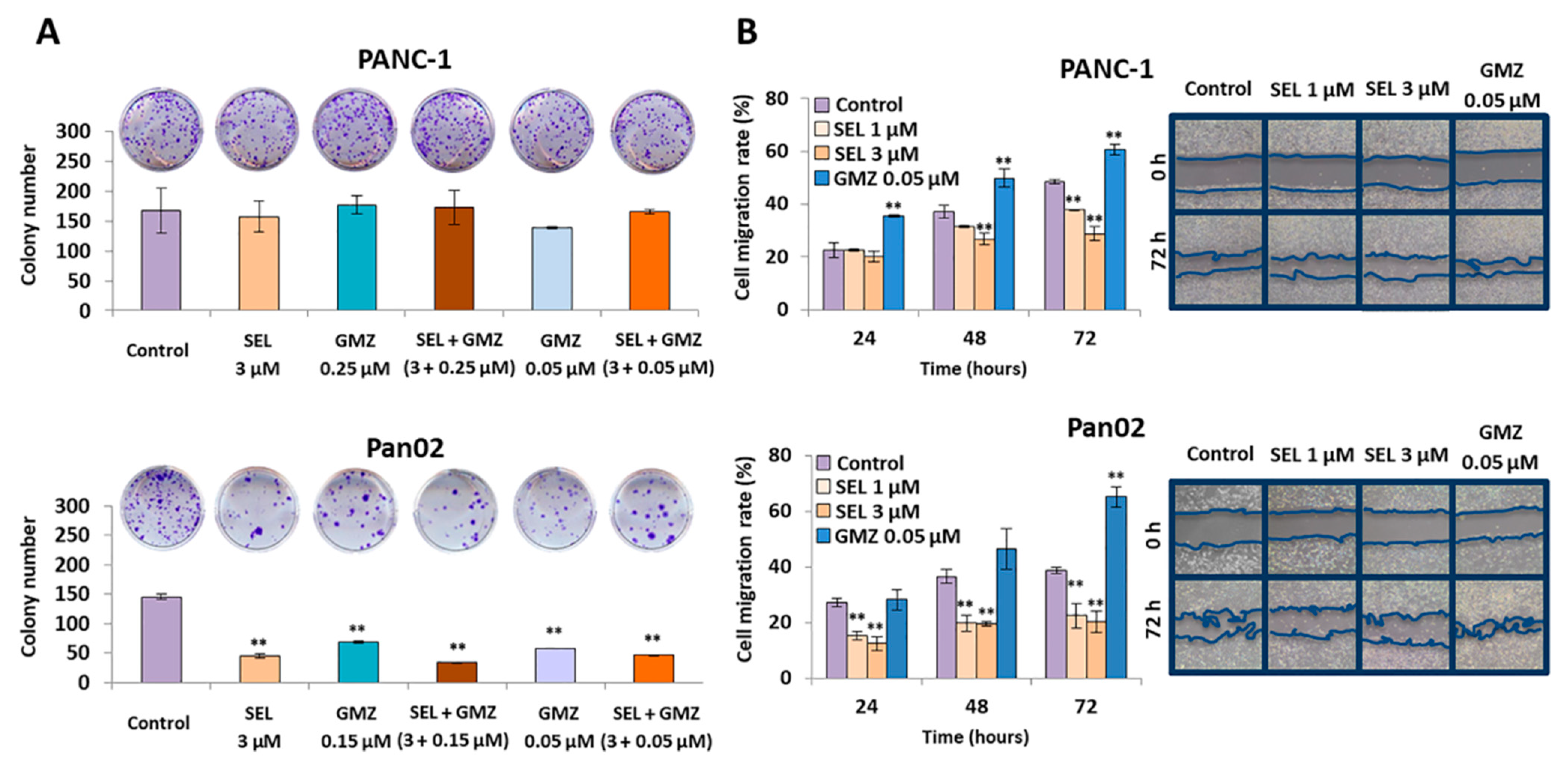
3.4. Sodium Selenite Inhibited Cell Colony Formation and Migration Capacity in Pancreatic Cancer Cells
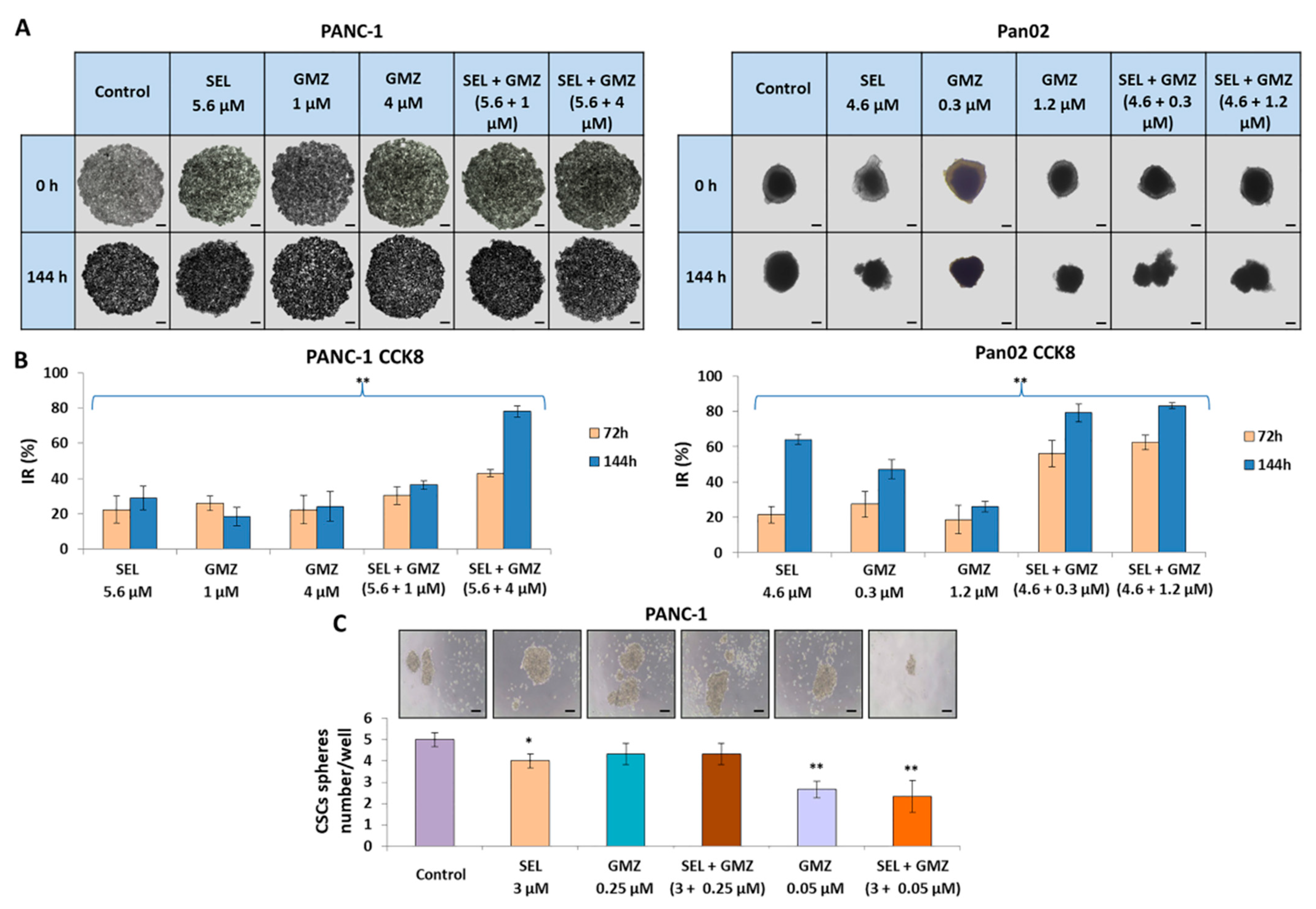
3.5. Sodium Selenite Inhibited Growth of Multicellular Tumor Spheroids from Pancreatic Cancer Cells
3.6. Sodium Selenite Modulated Cancer Stem Cells Sphere Formation
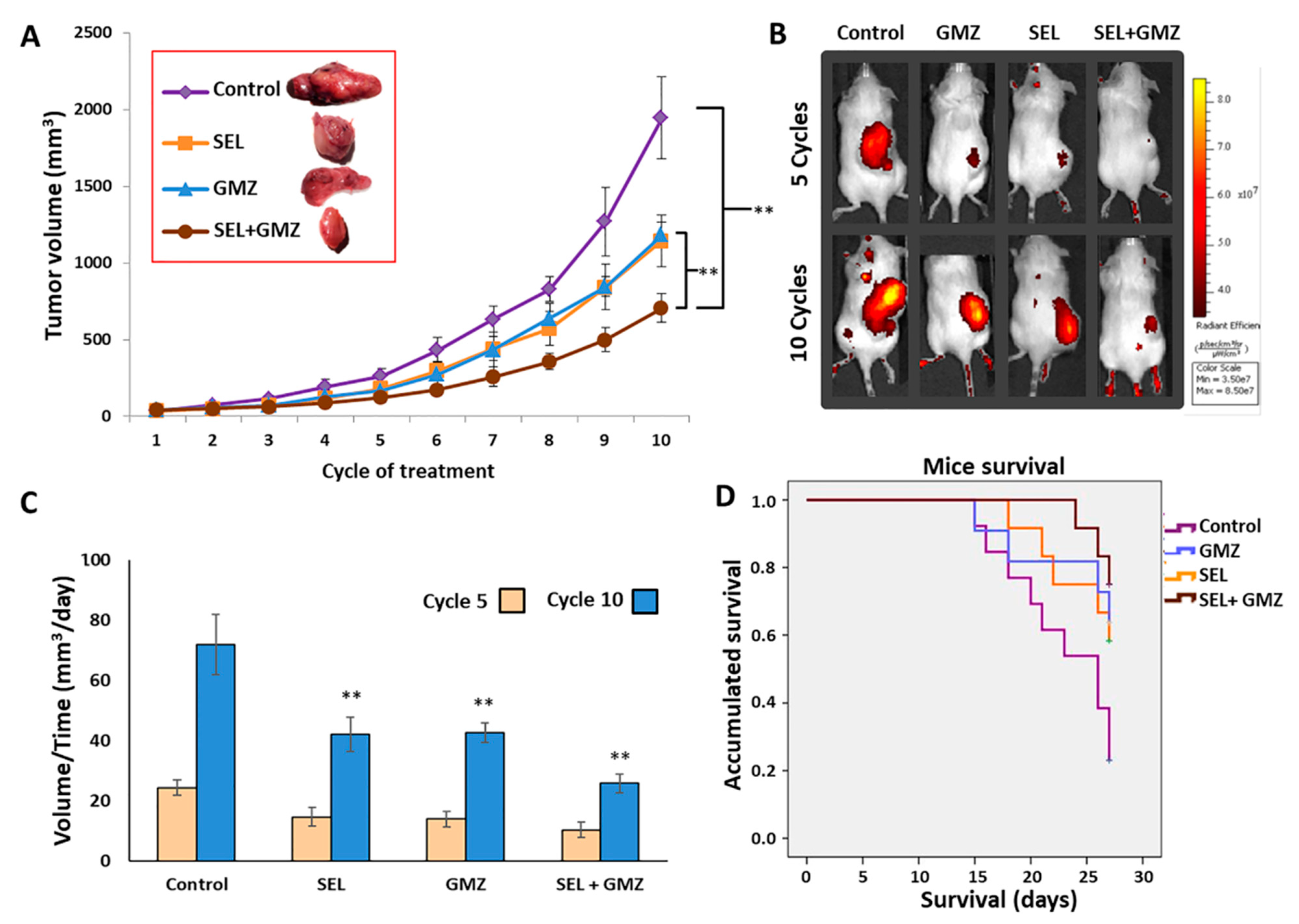
3.7. Sodium Selenite Inhibited Growth of Pancreatic Cancer Tumor and Enhanced Gemcitabine Effect: In Vivo Assays
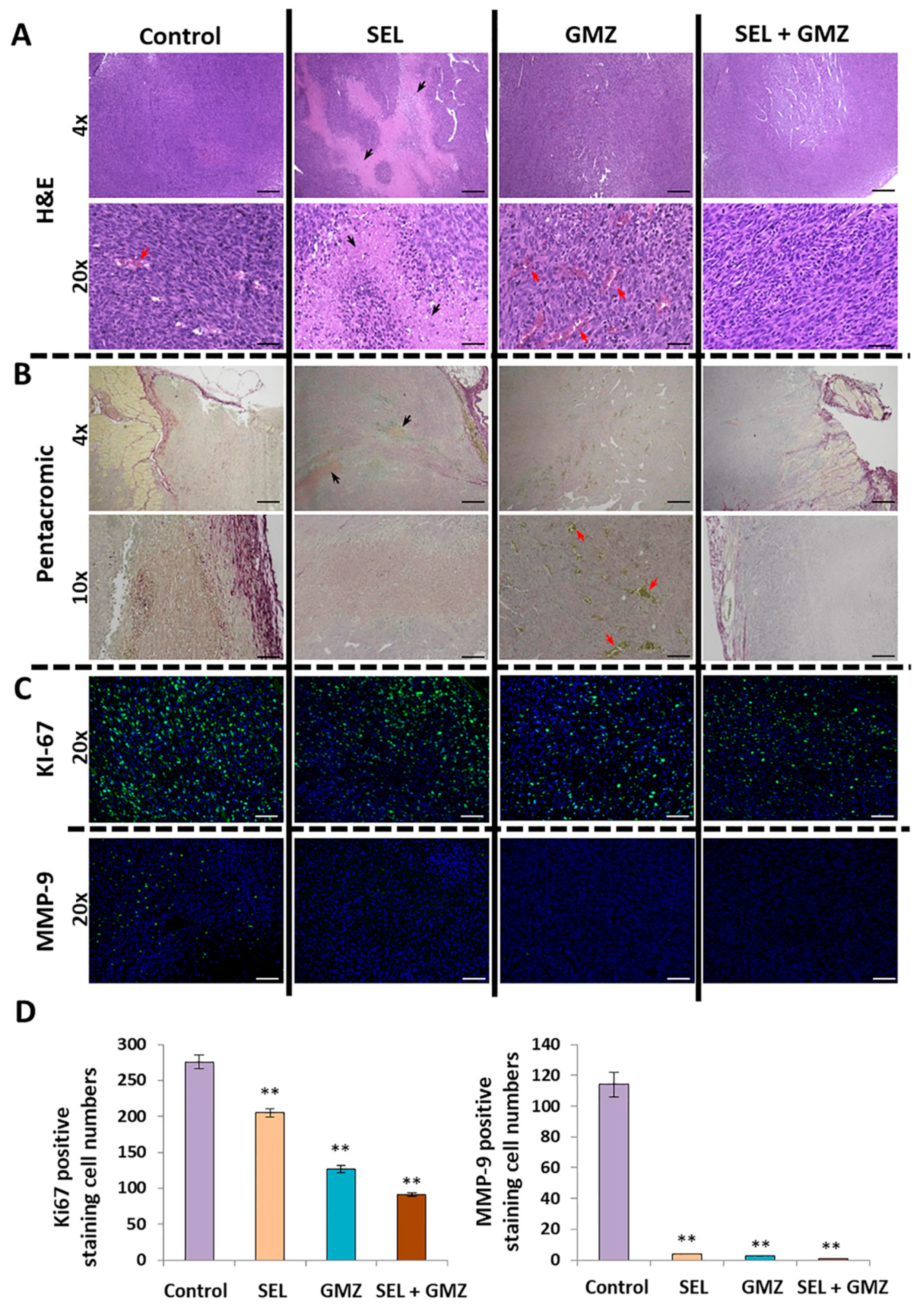
3.8. Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satoh, M.; Naganuma, A.; Imura, N. Effect of Coadministration of Selenite on the Toxicity and Antitumor Activity of Cis-Diamminedichloroplatinum (II) given Repeatedly to Mice. Cancer Chemother. Pharmacol. 1992, 30, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Noori, S.; Mahboob, T. Sodium Selenite Attenuated Cisplatin-Induced Toxicity in Rats: Role of Electrolytes Homeostasis. Indian J. Exp. Biol. 2011, 49, 791–794. [Google Scholar] [PubMed]
- Dennert, G.; Horneber, M. Selenium for Alleviating the Side Effects of Chemotherapy, Radiotherapy and Surgery in Cancer Patients. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; p. CD005037. [Google Scholar] [CrossRef]
- Spyrou, G.; Björnstedt, M.; Skog, S.; Holmgren, A. Selenite and Selenate Inhibit Human Lymphocyte Growth via Different Mechanisms. Cancer Res. 1996, 56, 4407–4412. [Google Scholar] [PubMed]
- Olm, E.; Jönsson-Videsäter, K.; Ribera-Cortada, I.; Fernandes, A.P.; Eriksson, L.C.; Lehmann, S.; Rundlöf, A.K.; Paul, C.; Björnstedt, M. Selenite Is a Potent Cytotoxic Agent for Human Primary AML Cells. Cancer Lett. 2009, 282, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, J.; Xu, T.J.; Qin, X.Y.; Zhou, X.D. Sodium Selenite Induces Apoptosis in Colon Cancer Cells via Bax-Dependent Mitochondrial Pathway. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2166–2171. [Google Scholar]
- Olm, E. Cytotoxic Mechanisms of Selenium in Cancer; Institutionen för Laboratoriemedicin: Huddinge, Sweden; Department of Laboratory Medicine: Huddinge, Sweden, 2009; ISBN 978-91-7409-732-0. [Google Scholar]
- Wallenberg, M.; Misra, S.; Björnstedt, M. Selenium Cytotoxicity in Cancer. Basic Clin. Pharmacol. Toxicol. 2014, 114, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.; Mo, J.; Zhang, Z.; Huang, C.; Zou, Y.; Sun, J. Sodium Selenite Accentuates the Therapeutic Effect of Adriamycin Prodrug (PADM) against Gastric Cancer. Biomed. Res. Int. 2019, 2019, 2035682. [Google Scholar] [CrossRef]
- Caffrey, P.B.; Frenkel, G.D. Selenite Enhances and Prolongs the Efficacy of Cisplatin Treatment of Human Ovarian Tumor Xenografts. In Vivo 2012, 26, 549–552. [Google Scholar]
- Yang, Y.; Luo, H.; Hui, K.; Ci, Y.; Shi, K.; Chen, G.; Shi, L.; Xu, C. Selenite-Induced Autophagy Antagonizes Apoptosis in Colorectal Cancer Cells in Vitro and in Vivo. Oncol. Rep. 2016, 35, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Thamilselvan, V.; Menon, M.; Thamilselvan, S. Combination of Carmustine and Selenite Effectively Inhibits Tumor Growth by Targeting Androgen Receptor, Androgen Receptor-Variants, and Akt in Preclinical Models: New Hope for Patients with Catration Resistant Prostate Cancer. Int. J. Cancer 2016, 139, 1632–1647. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising Selenium for Modulation of Cancer Treatments. Anticancer Res. 2017, 37, 6497–6509. [Google Scholar] [CrossRef] [Green Version]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [Green Version]
- Ganther, H.E. Selenium Metabolism, Selenoproteins and Mechanisms of Cancer Prevention: Complexities with Thioredoxin Reductase. Carcinogenesis 1999, 20, 1657–1666. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Schoene, N.W.; Lartey, F.M.; Cheng, W.H. Selenium Compounds Activate ATM-Dependent DNA Damage Response via the Mismatch Repair Protein HMLH1 in Colorectal Cancer Cells. J. Biol. Chem. 2010, 285, 33010–33017. [Google Scholar] [CrossRef] [Green Version]
- Rudolf, E.; Rudolf, K.; Cervinka, M. Selenium Activates P53 and P38 Pathways and Induces Caspase-Independent Cell Death in Cervical Cancer Cells. Cell. Biol. Toxicol. 2008, 24, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Olm, E.; Fernandes, A.P.; Hebert, C.; Rundlof, A.K.; Larsen, E.H.; Danielsson, O.; Bjornstedt, M. Extracellular Thiol-Assisted Selenium Uptake Dependent on the x(c)(-) Cystine Transporter Explains the Cancer-Specific Cytotoxicity of Selenite. Proc. Natl. Acad. Sci. USA 2009, 106, 11400–11405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, C.; Zhang, T.; Zhu, X.; Qiu, J.; Jiang, K.; Zhao, G.; Wu, H.; Deng, G. Methylseleninic Acid Suppresses Breast Cancer Growth via the JAK2/STAT3 Pathway. Reprod. Sci. 2019, 26, 829–838. [Google Scholar] [CrossRef]
- Goel, A.; Fuerst, F.; Hotchkiss, E.; Boland, C.R. Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol. Ther. 2006, 5, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, A.R.; Jo, M.J.; Jung, M.J.; Kim, H.S.; Yoon, S. Selenate specifically sensitizes drug-resistant cancer cells by increasing apoptosis via G2 phase cell cycle arrest without P-GP inhibition. Eur. J. Pharmacol. 2015, 764, 63–69. [Google Scholar] [CrossRef]
- National Cancer Institute. Available online: https://www.cancer.gov/cancertopics/types/pancreatic (accessed on 5 March 2021).
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 12 December 2020).
- O’Driscoll, L.; Walsh, N.; Larkin, A.; Ballot, J.; Ooi, W.S.; Gullo, G.; O’Connor, R.; Clynes, M.; Crown, J.; Kennedy, S. MDR1/P-Glycoprotein and MRP-1 Drug Efflux Pumps in Pancreatic Carcinoma. Anticancer Res. 2007, 27, 2115–2120. [Google Scholar] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the Pancreas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar] [CrossRef]
- Chou, T.; Martin, N. CompuSyn for Drug Combinations. In Pc Software and User’s Guide; ComboSyn Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Invest. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [Green Version]
- Prados, J.; Melguizo, C.; Rama, A.R.; Ortiz, R.; Segura, A.; Boulaiz, H.; Vélez, C.; Caba, O.; Ramos, J.L.; Aránega, A. Gef Gene Therapy Enhances the Therapeutic Efficacy of Doxorubicin to Combat Growth of MCF-7 Breast Cancer Cells. Cancer Chemother. Pharmacol. 2010, 66, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Ware, M.J.; Colbert, K.; Keshishian, V.; Ho, J.; Corr, S.J.; Curley, S.A.; Godin, B. Generation of Homogenous Three-Dimensional Pancreatic Cancer Cell Spheroids Using an Improved Hanging Drop Technique. Tissue Eng. Part C Methods 2016, 22, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doello, K. A New Pentachrome Method for the Simultaneous Staining of Collagen and Sulfated Mucopolysaccharides. Yale J. Biol. Med. 2014, 87, 341–347. [Google Scholar] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.W.; Batrakova, E.V.; Kabanov, A.V. Inhibition of Multidrug Resistance-Associated Protein (MRP) Functional Activity with Pluronic Block Copolymers. Pharm. Res. 1999, 16, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Gock, M.; Linnebacher, M.; Nauheimer, D.; Schmidt, J.; Klar, E.; Eisold, S. Influence of Chemotherapeutic Treatment on Expression of Human Multidrug Resistance Protein (ABCC, ABCB) Family Members in Pancreatic Cancer Cell Lines. J. Gastroenterol. Hepatol. Res. 2013, 2, 719–725. [Google Scholar]
- Soukupová, K.; Rudolf, E. Suppression of Proliferation and Activation of Cell Death by Sodium Selenite Involves Mitochondria and Lysosomes in Chemoresistant Bladder Cancer Cells. J. Trace Elem. Med. Biol. 2019, 52, 58–67. [Google Scholar] [CrossRef]
- David, K.K.; Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Parthanatos, a Messenger of Death. Front. Biosci. 2009, 14, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.-C.; Papaconstantinou, J. Thioredoxin-ASK1 Complex Levels Regulate ROS-Mediated P38 MAPK Pathway Activity in Livers of Aged and Long-Lived Snell Dwarf Mice. FASEB J. 2006, 20, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Habiro, A.; Tanno, S.; Koizumi, K.; Izawa, T.; Nakano, Y.; Osanai, M.; Mizukami, Y.; Okumura, T.; Kohgo, Y. Involvement of P38 Mitogen-Activated Protein Kinase in Gemcitabine-Induced Apoptosis in Human Pancreatic Cancer Cells. Biochem. Biophys. Res. Commun. 2004, 316, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.Q.; Fu, Z.G.; Meng, Y.; Wu, X.Q.; Wu, B.; Xu, L.; Jiang, J.L.; Li, L.; Chen, Z.N. Gemcitabine Enhances Cell Invasion via Activating HAb18G/CD147-EGFR-PSTAT3 Signaling. Oncotarget 2016, 7, 62177–62193. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Yang, J.; Ni, X.; Liu, S.; Li, Z.; Hodges, S.E.; Fisher, W.E.; Brunicardi, F.C.; Gibbs, R.A.; et al. Genomic sequencing of key genes in mouse pancreatic cancer cells. Curr. Mol. Med. 2012, 12, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Deer, E.L.; González-Hernández, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and genotype of pancreatic cancer cell lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, Z. Increased Oxidative Stress as a Selective Anticancer Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 294303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espey, M.G.; Chen, P.; Chalmers, B.; Drisko, J.; Sun, A.Y.; Levine, M.; Chen, Q. Pharmacologic Ascorbate Synergizes with Gemcitabine in Preclinical Models of Pancreatic Cancer. Free Radic. Biol. Med. 2011, 50, 1610–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed Ali, M.S.; Mohamed Hussein, R.; Ahmed Kandeil, M. The Pro-Oxidant, Apoptotic and Anti-Angiogenic Effects of Selenium Supplementation on Colorectal Tumors Induced by 1,2-Dimethylhydrazine in BALB/C Mice. Rep. Biochem. Mol. Biol. 2019, 8, 216–226. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doello, K.; Mesas, C.; Quiñonero, F.; Perazzoli, G.; Cabeza, L.; Prados, J.; Melguizo, C.; Ortiz, R. The Antitumor Activity of Sodium Selenite Alone and in Combination with Gemcitabine in Pancreatic Cancer: An In Vitro and In Vivo Study. Cancers 2021, 13, 3169. https://doi.org/10.3390/cancers13133169
Doello K, Mesas C, Quiñonero F, Perazzoli G, Cabeza L, Prados J, Melguizo C, Ortiz R. The Antitumor Activity of Sodium Selenite Alone and in Combination with Gemcitabine in Pancreatic Cancer: An In Vitro and In Vivo Study. Cancers. 2021; 13(13):3169. https://doi.org/10.3390/cancers13133169
Chicago/Turabian StyleDoello, Kevin, Cristina Mesas, Francisco Quiñonero, Gloria Perazzoli, Laura Cabeza, Jose Prados, Consolacion Melguizo, and Raul Ortiz. 2021. "The Antitumor Activity of Sodium Selenite Alone and in Combination with Gemcitabine in Pancreatic Cancer: An In Vitro and In Vivo Study" Cancers 13, no. 13: 3169. https://doi.org/10.3390/cancers13133169
APA StyleDoello, K., Mesas, C., Quiñonero, F., Perazzoli, G., Cabeza, L., Prados, J., Melguizo, C., & Ortiz, R. (2021). The Antitumor Activity of Sodium Selenite Alone and in Combination with Gemcitabine in Pancreatic Cancer: An In Vitro and In Vivo Study. Cancers, 13(13), 3169. https://doi.org/10.3390/cancers13133169