Utility of CT to Differentiate Pancreatic Parenchymal Metastasis from Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
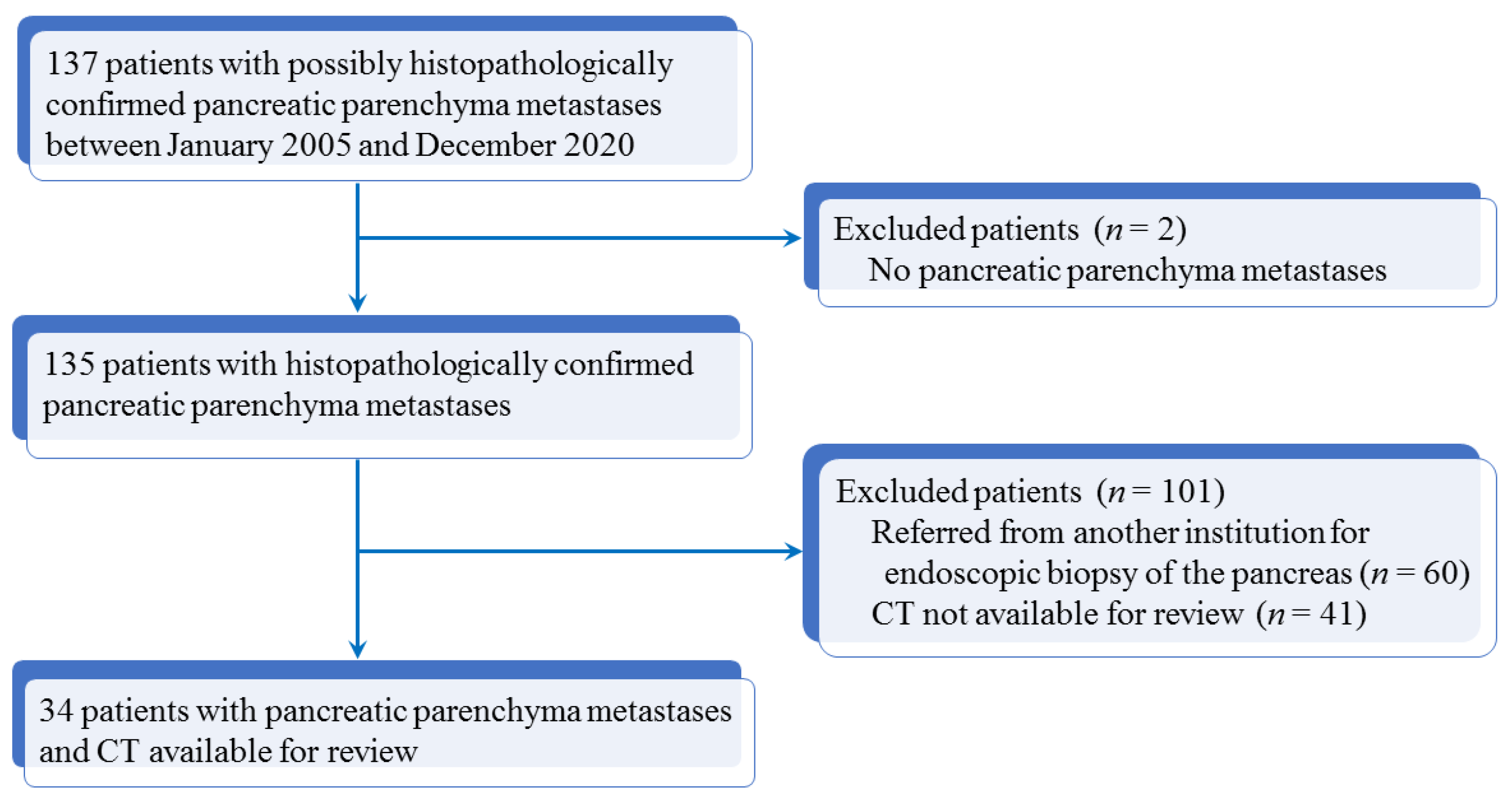
2.1. Patients
2.2. CT Protocol
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Results of Descriptive Statistics
3.2. Results of Univariable Analysis
3.3. Results of Multivariable Analysis and Nomogram
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Tumor Staging in 34 Patients with Pancreatic Ductal Adenocarcinoma
| Primary tumor (T) | |
| T1 | 3 (3/34; 9%) |
| T2 | 8 (8/34; 24%) |
| T3 | 13 (13/34; 38%) |
| T4 | 10 (10/34; 29%) |
| Regional lymph nodes (N) | |
| N0 | 15 (15/34; 44%) |
| N1 | 14 (14/34; 41%) |
| N2 | 5 (5/34; 15%) |
| Distant metastases (M) | |
| M0 | 26 (26/34; 76%) |
| M1 | 8 (8/34; 24%) |
| Stage * | |
| Stage IA | 3 (3/34; 9%) |
| Stage IB | 5 (5/34; 15%) |
| Stage IIA | 3 (3/34; 9%) |
| Stage IIB | 9 (9/34; 26%) |
| Stage III | 6 (6/34; 18%) |
| Stage IV | 8 (8/34; 23%) |
| Note. * Staging was performed according to according to the American Joint Committee on Cancer. | |
Appendix B. Imaging Variables Used for Image Analysis on CT in 34 Patients with Pancreatic Parenchyma Metastasis and 34 Patients with Pancreatic Ductal Adenocarcinoma
| Quantitative Variables | Qualitative Variables |
| Largest tumor diameter (mm) | Tumor location (head, body, tail) Tumor shape (round, oval) Tumor margins (well or ill-defined contours) Tumor capsule (yes, no) Tumor content (purely solid, cystic, mixed) Internal necrosis (yes, no) Tumor enhancement (yes, no) Homogeneous tumor enhancement (yes, no) Tumor enhancement on arterial phase * (>, =, <) Maximal tumor enhancement (arterial, portal) Tumor enhancement on portal phase * (>, =, <) Tumor calcification (yes, no) Wirsung duct enlargement (yes, no) Upstream pancreatic atrophy Vascular involvement (yes, no) Segmental portal hypertension (yes, no) Hepatic metastases (yes, no) Bile duct dilatation (yes, no) Visible lymph nodes (yes, no) Direct adjacent organ involvement (yes, no) Mesenteric panniculitis (yes, no) Ascites (yes, no) |
| Note. Tumor enhancement was compared to that of apparently normal pancreatic parenchyma. | |
Appendix C. Interobserver Agreement for Categorical Data in 34 Patients with Pancreatic Parenchyma Metastases
| Variable | κ Value | 95% CI | Rating of Agreement |
| Tumor oval shape | 0.822 | 0.631–1 | Almost perfect |
| Well-defined tumor margins | 0.717 | 0.458–0.975 | Substantial |
| Tumor capsule | 1 | 1–1 | Perfect |
| Solid content | 0.820 | 0.580–1 | Almost perfect |
| Internal necrosis | 0.883 | 0.726–1 | Almost perfect |
| Tumor enhancement | 100% * | * | * |
| Homogeneous tumor enhancement | 0.822 | 0.631–1 | Almost perfect |
| Hyperattenuating tumor on arterial phase | 0.933 | 0.803–1 | Almost perfect |
| Maximal tumor enhancement on arterial phase | 0.820 | 0.625–1 | Almost perfect |
| Hypo/isoattenuating tumor on portal phase | 100% * | * | * |
| Tumor calcification | 1 | 1–1 | Perfect |
| No Wirsung duct enlargement | 0.766 | 0.455–1 | Substantial |
| No upstream pancreatic atrophy | 0.766 | 0.455–1 | Substantial |
| No vascular involvement | 0.837 | 0.622–1 | Almost perfect |
| Segmental portal hypertension | 1 | 1–1 | Perfect |
| Hepatic metastases | 1 | 1–1 | Perfect |
| No bile duct dilatation | 1 | 1–1 | Perfect |
| No visible lymph nodes | 0.850 | 0.650–1 | Almost perfect |
| Direct adjacent organ involvement | 1 | 1–1 | Perfect |
| Mesenteric panniculitis | 1 | 1–1 | Perfect |
| Ascites | 1 | 1–1 | Perfect |
| Note. CI indicates confidence interval. The Cohen Kappa test was used. The rating was as follows: κ values of 0.00, poor; 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–0.99, almost perfect; 1, perfect. * indicates 100% agreement with no calculation of kappa value. No Wirsung duct enlargement corresponds to Wirsung duct diameter ≤4 mm. | |||
References
- Younan, G. Pancreas solid tumors. Surg Clin N. Am. 2020, 100, 565–580. [Google Scholar] [CrossRef]
- Dioguardi Burgio, M.; Ronot, M.; Vilgrain, V. Rare solid tumor of the exocrine pancreas: A pictorial review. Semin Ultrasound CT MR 2019, 40, 483–499. [Google Scholar] [CrossRef]
- Barral, M.; Faraoun, S.A.; Fishman, E.K.; Dohan, A.; Pozzessere, C.; Berthelin, M.A.; Bazeries, P.; Barat, M.; Hoeffel, C.; Soyer, P. Imaging features of rare pancreatic tumors. Diagn. Interv. Imaging 2016, 97, 1259–1273. [Google Scholar] [CrossRef]
- Chu, L.C.; Singhi, A.D.; Haroun, R.R.; Hruban, R.H.; Fishman, E.K. The many faces of pancreatic serous cystadenoma: Radiologic and pathologic correlation. Diagn. Interv. Imaging 2017, 98, 191–202. [Google Scholar] [CrossRef]
- Bezerra, R.O.F.; Machado, M.C.; Dos Santos Mota, M.M.; Ezzedine, T.A.; Siqueira, L.T.B.; Cerri, G.G. Rare pancreatic masses: A pictorial review of radiological concepts. Clin. Imaging 2018, 50, 314–323. [Google Scholar] [CrossRef]
- Barat, M.; Dohan, A.; Gaujoux, S.; Hoeffel, C.; Jornet, D.; Oudjit, A.; Coriat, R.; Barret, M.; Terris, B.; Soyer, P. Computed tomography features of acinar cell carcinoma of the pancreas. Diagn. Interv. Imaging 2020, 101, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.; Taouli, B.; Guiu, B.; Koh, D.M.; Luciani, A.; Manfredi, R.; Vilgrain, V.; Hoeffel, C.; Kanematsu, M.; Soyer, P. Diffusion-weighted MR imaging of the pancreas: Current status and recommendations. Radiology 2015, 274, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.V.; Tirkes, T. Advanced MR imaging of the pancreas. Magn. Reson. Imaging Clin. N. Am. 2020, 28, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, R.; Spaggiari, M.; Cautero, N.; De Ruvo, N.; Montalti, R.; Longo, C.; Pecchi, A.; Giacobazzi, P.; De Marco, G.; D’Amico, G.; et al. Pancreatic metastases from renal cell carcinoma: The state of the art. World J. Gastroenterol. 2011, 17, 4747–4756. [Google Scholar] [CrossRef]
- Rumancik, W.M.; Megibow, A.J.; Bosniak, M.A.; Hilton, S. Metastatic disease to the pancreas: Evaluation by computed tomography. J. Comput. Assist. Tomogr. 1984, 8, 829–834. [Google Scholar] [CrossRef]
- Merkle, E.M.; Boaz, T.; Kolokythas, O.; Haaga, J.R.; Lewin, J.S.; Brambs, H.-J. Metastases to the pancreas. Br. J. Radiol. 1998, 71, 1208–1214. [Google Scholar] [CrossRef]
- Ferrozzi, F.; Bova, D.; Campodonico, F.; Chiara, F.D.; Passari, A.; Bassi, P. Pancreatic metastases: CT assessment. Eur. Radiol. 1997, 7, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, T.; Teshima, K.; Honda, H.; Nanjo, T.; Hanada, K.; Oshiumi, Y. Computed tomography and histologic appearance of pancreatic metastases from distant sources. Acta Radiol. 1989, 30, 615–619. [Google Scholar] [CrossRef]
- Maeno, T.; Satoh, H.; Ishikawa, H.; Yamashita, Y.T.; Naito, T.; Fujiwara, M.; Kamma, H.; Ohtsuka, M.; Hasegawa, S. Patterns of pancreatic metastasis from lung cancer. Anticancer Res. 1998, 18, 2881–2884. [Google Scholar] [PubMed]
- Roland, C.F.; van Heerden, J.A. Nonpancreatic primary tumors with metastasis to the pancreas. Surg. Gynecol. Obstet. 1989, 168, 345–347. [Google Scholar]
- Sweeney, A.D.; Fisher, W.E.; Wu, M.F.; Hilsenbeck, S.G.; Brunicardi, F.C. Value of pancreatic resection for cancer metastatic to the pancreas. J. Surg. Res. 2010, 160, 268–276. [Google Scholar] [CrossRef]
- Tsitouridis, I.; Diamantopoulou, A.; Michaelides, M.; Arvanity, M.; Papaioannou, S. Pancreatic metastases: CT and MRI findings. Diagn. Interv. Radiol. 2010, 16, 45–51. [Google Scholar] [CrossRef]
- Hiotis, S.P.; Klimstra, D.S.; Conlon, K.C.; Brennan, M.F. Results after pancreatic resection for metastatic lesions. Ann. Surg. Oncol. 2002, 9, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Le Borgne, J.; Partensky, C.; Glemain, P.; Dupas, B.; de Kerviller, B. Pancreaticoduodenectomy for metastatic ampullary and pancreatic tumors. Hepatogastroenterology 2000, 47, 540–544. [Google Scholar] [PubMed]
- Scataridge, J.C.; Horton, K.M.; Sheth, S.; Fishman, E.K. Pancreatic parenchymal metastases: Observations on helical CT. AJR Am. J. Roentgenol. 2001, 176, 695–699. [Google Scholar] [CrossRef]
- Adsay, N.V.; Andea, A.; Basturk, O.; Kilinc, N.; Nassar, H.; Cheng, J.D. Secondary tumors of the pancreas: An analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004, 444, 527–535. [Google Scholar] [CrossRef]
- Ahmed, S.; Johnson, P.T.; Hruban, R.; Fishman, E.K. Metastatic disease to the pancreas: Pathologic spectrum and CT patterns. Abdom. Imaging 2013, 38, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hirashita, T.; Iwashita, Y.; Endo, Y.; Fujinaga, A.; Shin, T.; Mimata, H.; Inomata, M. How should we treat pancreatic metastases from renal cell carcinoma? A meta-analysis. World J. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.F.; DiFronzo, L.A.; Rose, D.M.; Haigh, P.I.; Stern, S.L.; Wanek, L.; Essner, R.; Morton, D.L. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival. Ann. Surg. Oncol. 2001, 8, 658–662. [Google Scholar] [CrossRef]
- Shi, H.Y.; Zhao, X.S.; Miao, F. Metastases to the pancreas: Computed tomography imaging spectrum and clinical features: A retrospective study of 18 patients with 36 metastases. Medicine 2015, 94, e913. [Google Scholar] [CrossRef]
- Benaissa, A.; Fornès, P.; Ladam-Marcus, V.; Grange, F.; Amzallag-Bellenger, E.; Hoeffel, C. Multimodality imaging of melanoma metastases to the abdomen and pelvis. Clin. Imaging 2011, 35, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B. (Ed.) AJCC Cancer Staging Manual. In Pancreatic Adenocarcinoma, 8th ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Protin-Catteau, L.; Thiéfin, G.; Barbe, C.; Jolly, D.; Soyer, P.; Hoeffel, C. Mesenteric panniculitis: Review of consecutive abdominal MDCT examinations with a matched-pair analysis. Acta Radiol. 2016, 57, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Benchoufi, M.; Matzner-Lober, E.; Molinari, N.; Jannot, A.S.; Soyer, P. Interobserver agreement issues in radiology. Diagn. Interv. Imaging 2020, 101, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, T.; Shima, Y.; Okabayashi, T.; Kozuki, A.; Nakamura, T. Comparison of pancreatic acinar cell carcinoma and adenocarcinoma using multidetector-row computed tomography. World J. Gastroenterol. 2013, 19, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- Keogan, M.T.; McDermott, V.G.; Paulson, E.K.; Sheafor, D.H.; Frederick, M.G.; de Long, D.M.; Nelson, R.C. Pancreatic malignancy: Effect of dual-phase helical CT in tumor detection and vascular opacification. Radiology 1997, 205, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Barat, M.; Guegan-Bart, S.; Cottereau, A.S.; Guillo, E.; Hoeffel, C.; Barret, M.; Soyer, P. CT, MRI and PET/CT features of abdominal manifestations of cutaneous melanoma: A review of current concepts in the era of tumor-specific therapies. Abdom. Radiol. 2020. [Google Scholar] [CrossRef]
- Palmowski, M.; Hacke, N.; Satzl, S.; Klauss, M.; Wente, M.N.; Neukamm, M.; Kleeff, J.; Hallscheidt, P. Metastasis to the pancreas: Characterization by morphology and contrast enhancement features on CT and MRI. Pancreatology 2008, 8, 199–203. [Google Scholar] [CrossRef]
- Klein, K.A.; Stephens, D.H.; Welch, T.J. CT characteristics of metastatic disease of the pancreas. Radiographics 1998, 18, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Wolske, K.M.; Ponnatapura, J.; Kolokythas, O.; Burke, L.M.B.; Tappouni, R.; Lalwani, N. Chronic pancreatitis or pancreatic tumor? A problem-solving approach. Radiographics 2019, 39, 1965–1982. [Google Scholar] [CrossRef]
- Charnsangavej, C.; Whitley, N.O. Metastases to the pancreas and peripancreatic lymph nodes from carcinoma of the right side of the colon: CT findings in 12 patients. AJR Am. J. Roentgenol. 1993, 160, 49–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.H.; Tamm, E.P.; Marcal, L.; Balachandran, A.; Charnsangavej, C.; Vikram, R.; Bhosale, P. Imaging features of hematogenous metastases to the pancreas: Pictorial essay. Cancer Imaging 2011, 11, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triantopoulou, C.; Kolliakou, E.; Karoumpalis, I.; Yarmenitis, S.; Dervenis, C. Metastatic disease to the pancreas: An imaging challenge. Insights Imaging 2012, 3, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellner, F.; Tykalsky, N.; De Santis, M.; Pont, J.; Klimpfinger, M. Solitary and multiple isolated metastases of clear cell carcinoma to the pancreas: An indication for pancreatic surgery. Ann. Surg. Oncol. 2006, 13, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zerbi, A.; Ortolano, E.; Balzano, G.; Borri, A.; Beneduce, A.A.; Di Carlo, V. Pancreatic metastasis from renal cell carcinoma: Which patients benefit from surgical resection? Ann. Surg. Oncol. 2008, 15, 1161–1168. [Google Scholar] [CrossRef]
- Reddy, S.; Wolfgang, C.L. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009, 10, 287–293. [Google Scholar] [CrossRef]
- Papalampros, A.; Vailas, M.G.; Deladetsima, I.; Moris, D.; Sotiropoulou, M.; Syllaios, A.; Petrou, A.; Felekouras, E. Irreversible electroporation in a case of pancreatic leiomyosarcoma: A novel weapon versus a rare malignancy? World J. Surg. Oncol. 2019, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Tasu, J.P.; Vesselle, G.; Herpe, G.; Richer, J.P.; Boucecbi, S.; Vélasco, S.; Debeane, B.; Carretier, M.; Tougeron, D. Irreversible electroporation for locally advanced pancreatic cancer: Where do we stand in 2017? Pancreas 2017, 46, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Kim, S.H.; Lee, J.; Kim, A.Y.; Jang, K.M.; Choi, D.; Kim, M.J. Differentiation between pancreatic metastases from renal cell carcinoma and hypervascular neuroendocrine tumour: Use of relative percentage washout value and its clinical implication. Eur. J. Radiol. 2015, 84, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, C.B.; Lee, S.; Tsai, S.; Larocque, N.; Alayed, A.; Williams, P.; Schieda, N. Differentiation of pancreatic neuroendocrine tumors from pancreas renal cell carcinoma metastases on CT using qualitative and quantitative features. Abdom. Radiol. 2019, 44, 992–999. [Google Scholar] [CrossRef]






| Variable | All Patients (n = 68) | PPM Group (n = 34) | PDAC Group (n = 34) | p Value |
|---|---|---|---|---|
| Age (years) | 0.995 * | |||
| Mean ± SD | 63.5 ± 9.5 | 63.3 ± 10.2 | 63.6 ± 8.9 | |
| (median; Q1, Q3) | (64; 57, 70) | (64; 57, 70) | (64.5; 57, 71) | |
| (range) | (40–82) | (40–82) | (40–82) | |
| Gender | 1 ‡ | |||
| Men | 44 (44/68; 65%) | 22 (22/34; 65%) | 22 (22/34; 65%) | |
| Women | 24 (24/68; 35%) | 12 (12/34; 35%) | 12 (12/34; 35%) | |
| Histopathological diagnosis | 0.609 ‡ | |||
| Percutaneous biopsy | 45 (45/68; 66%) | 24 (24/34; 71%) | 21 (21/34; 62%) | |
| Surgical biopsy | 23 (23/68; 34%) | 10 (10/34; 29%) | 13 (13/34; 38%) | |
| Surgical resection | (23/68; 34%) | 10 (11/34; 29%) | 13 (13/34; 38%) | 0.609 ‡ |
| Variable | TP | FP | FN | TN | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| Tumor shape (round) | 15 | 9 | 19 | 25 | 44 (15/34) (27–62) | 74 (25/34) (56–87) | 59 (40/68) (46–71) |
| Well-defined tumor margins | 23 | 7 | 11 | 27 | 68 (23/34) (49–83) | 79 (27/34) (62–91) | 74 (50/68) (61–84) |
| Tumor capsule | 4 | 2 | 30 | 32 | 12 (4/34) (3–27) | 94 (32/34) (80–99) | 53 (36/68) (40–65) |
| Purely solid content | 27 | 24 | 7 | 10 | 79 (27/34) (62–91) | 29 (10/34) (15–47) | 54 (37/68) (42–67) |
| No internal necrosis/hemorrhage | 14 | 17 | 20 | 17 | 41 (14/34) (25–59) | 50 (17/34) (32–68) | 46 (31/68) (33–58) |
| Tumor enhancement | 34 | 34 | 0 | 0 | 100 (34/34) (90–100) | 68 (23/34) (49–83) | 50 (34/68) (38–62) |
| Homogeneous tumor enhancement | 19 | 11 | 15 | 23 | 56 (19/34) (38–73) | 68 (23/34) (49–83) | 62 (42/68) (49–73) |
| Hyperattenuating tumor on arterial phase | 11 | 0 | 23 | 34 | 32 (11/34) (17–51) | 100 (34/34) (90–100) | 66 (45/68) (54–77) |
| Maximal tumor enhancement on arterial phase | 19 | 4 | 15 | 30 | 56 (19/34) (38–73) | 88 (30/34) (73–97) | 72 (49/68) (60–82) |
| Hypo/isoattenuating tumor on portal phase | 34 | 34 | 0 | 0 | 100 (34/34) (90–100) | 0 (0/34) (0–100) | 50 (34/68) (38–62) |
| Tumor calcification | 1 | 1 | 33 | 33 | 3 (1/34) (0–15) | 97 (33/34) (85–100) | 50 (34/68) (38–62) |
| No Wirsung duct enlargement | 29 | 11 | 5 | 23 | 85 (29/34) (69–95) | 68 (23/34) (49–83) | 76 (52/68) (64–86) |
| No upstream pancreatic atrophy | 29 | 15 | 5 | 19 | 85 (29/34) (69–95) | 56 (19/34) (38–73) | 71 (48/68) (58–81) |
| No vascular involvement | 27 | 13 | 7 | 21 | 79 (27/34) (62–91) | 62 (21/34) (44–78) | 71 (48/68) (58–81) |
| Segmental portal hypertension | 4 | 9 | 30 | 25 | 12 (4/34) (3–27) | 74 (25/34) (56–87) | 43 (29/68) (31–55) |
| Hepatic metastases | 2 | 8 | 32 | 26 | 6 (2/34) (1–20) | 76 (26/34) (59–89) | 41 (28/68) (29–54) |
| No bile duct dilatation | 30 | 15 | 4 | 19 | 88 (30/34) (73–97) | 56 (19/34) (38–73) | 72 (49/68) (60–82) |
| No visible lymph nodes | 24 | 12 | 10 | 22 | 71 (24/34) (52–85) | 65 (22/34) (46–80) | 68 (46/68) (55–78) |
| Adjacent organ involvement | 2 | 3 | 32 | 31 | 6 (2/34) (1–20) | 91 (31/34) (76–98) | 49 (33/68) (36–61) |
| Mesenteric panniculitis | 3 | 5 | 31 | 29 | 9 (3/34) (2–24) | 85 (29/34) (69–95) | 47 (32/68) (35–60) |
| Ascites | 2 | 5 | 32 | 29 | 6 (2/34) (1–20) | 85 (29/34) (69–95) | 46 (31/68) (33–58) |
| Variable | PPM (n = 34) | PDAC (n = 34) | p Value |
|---|---|---|---|
| Quantitative variables | |||
| Largest tumor diameter (mm) | 35.0 ± 21.1 (13–110) (27.5; 21, 39) | 32.1 ± 9.2 (16–59) (32.5; 26, 38) | 0.725 * |
| Visible lymph node size (mm) | 20.5 ± 15.3 (9–60) (15; 11, 20) | 10.3 ± 3.3 (4–18) (10; 7, 13) | 0.014 * |
| Qualitative variables | |||
| Tumor location Head Body Tail | 18 (18/34; 53%) 12 (12/34; 35%) 4 (4/34; 12%) | 18 (18/34; 53%) 12 (12/34; 35%) 4 (4/34; 12%) | >0.999 † |
| Tumor shape Oval Round | 19 (19/34; 56%) 15 (15/34; 44%) | 25 (25/34; 74%) 9 (9/34; 26%) | 0.204 ‡ |
| Well-defined tumor margins | 23 (23/34; 68%) | 7 (7/34; 21%) | <0.001 |
| Tumor capsule | 4 (4/34; 12%) | 2 (2/34; 6%) | 0.673 ‡ |
| Purely solid content | 27 (27/34; 79%) | 24 (24/34; 71%) | 0.576 ‡ |
| Internal necrosis | 14 (14/34; 41%) | 17 (17/34; 50%) | 0.627 ‡ |
| Tumor enhancement | 34 (34/34; 100%) | 34 (34/34; 100%) | >0.999 ‡ |
| Homogeneous tumor enhancement | 19 (19/34; 56%) | 11 (11/34; 32%) | 0.087 ‡ |
| Hyperattenuating tumor on arterial phase | 11 (11/34; 32%) | 0 (0/34; 0%) | <0.001 ‡ |
| Maximal tumor enhancement on arterial phase | 19 (19/34; 56%) | 4 (4/34; 12%) | <0.001 ‡ |
| Hypo/isoattenuating tumor on portal phase | 34 (34/34; 100%) | 34 (34/34; 100%) | >0.999 ‡ |
| Tumor calcification | 1 (1/34; 3%) | 1 (1/34; 3%) | >0.999 ‡ |
| No Wirsung duct enlargement | 29 (29/34; 85%) | 11 (11/34; 32%) | <0.001 ‡ |
| No upstream pancreatic atrophy | 29 (29/34; 85%) | 15 (15/34; 44%) | 0.001 ‡ |
| No vascular involvement | 27 (27/34; 79%) | 13 (13/34; 38%) | 0.001 ‡ |
| Segmental portal hypertension | 4 (4/34; 12%) | 9 (9/34; 26%) | 0.217 ‡ |
| Hepatic metastases | 2 (2/34; 6%) | 8 (8/34; 24%) | 0.083 |
| No bile duct dilatation | 30 (30/34; 88%) | 15 (15/34; 44%) | <0.001 ‡ |
| No visible lymph nodes | 24 (24/34; 71%) | 12 (12/34; 35%) | 0.002 ‡ |
| Direct adjacent organ involvement | 2 (2/34; 6%) | 3 (3/34; 9%) | >0.999 ‡ |
| Mesenteric panniculitis | 3 (3/34; 9%) | 5 (5/34; 15%) | 0.709 ‡ |
| Ascites | 2 (2/34; 6%) | 5 (5/34; 15%) | 0.427 ‡ |
| Effect * | Results OR [95% CI] | p Value |
|---|---|---|
| Round shape | 0.46 (0.17–1.26) | 0.102 |
| Well-defined tumor margins | 8.07 (2.69–24.20) | < 0.001 |
| Tumor capsule | 2.13 (0.36–12.51) | 0.336 |
| Purely solid content | 1.61 (0.53–4.88) | 0.288 |
| Internal necrosis | 0.70 (0.27–1.83) | 0.313 |
| Tumor enhancement | 100 (34/34) vs. 100 (34/34) † | > 0.999 ‡ |
| Homogeneous tumor enhancement | 2.65 (0.99–7.11] | 0.043 |
| Hyperattenuating tumor on arterial phase | 32 (11/34) vs. 0 (0/34) † | < 0.001 ‡ |
| Maximal tumor enhancement on arterial phase | 9.50 (2.74–32.95) | < 0.001 |
| Hypo/isoattenuating tumor on portal phase | 100 (34/34) vs. 100 (34/34) † | > 0.999 ‡ |
| Tumor calcification | 1.00 (0.06–16.67) | 0.754 |
| No Wirsung duct duct enlargement | 12.13 (3.69–39.88) | < 0.001 |
| No upstream pancreatic atrophy | 7.35 (2.29–23.57) | < 0.001 |
| No vascular involvement | 6.23 (2.11–18.37) | 0.001 |
| Segmental portal hypertension | 0.37 (0.10–1.35) | 0.108 |
| Hepatic metastases | 0.20 (0.04–1.04) | 0.042 |
| No bile duct dilatation | 9.50 (2.739–32.95) | < 0.001 |
| No visible lymph nodes | 4.80 (1.75–13.21) | 0.002 |
| Direct adjacent organ involvement | 0.65 (0.10–4.13) | 0.500 |
| Mesenteric panniculitis | 0.56 (0.12–2.56) | 0.355 |
| Ascites | 0.36 (0.07–2.01) | 0.214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barat, M.; Aldhaheri, R.; Dohan, A.; Fuks, D.; Kedra, A.; Hoeffel, C.; Oudjit, A.; Coriat, R.; Barret, M.; Terris, B.; et al. Utility of CT to Differentiate Pancreatic Parenchymal Metastasis from Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 3103. https://doi.org/10.3390/cancers13133103
Barat M, Aldhaheri R, Dohan A, Fuks D, Kedra A, Hoeffel C, Oudjit A, Coriat R, Barret M, Terris B, et al. Utility of CT to Differentiate Pancreatic Parenchymal Metastasis from Pancreatic Ductal Adenocarcinoma. Cancers. 2021; 13(13):3103. https://doi.org/10.3390/cancers13133103
Chicago/Turabian StyleBarat, Maxime, Rauda Aldhaheri, Anthony Dohan, David Fuks, Alice Kedra, Christine Hoeffel, Ammar Oudjit, Romain Coriat, Maximilien Barret, Benoit Terris, and et al. 2021. "Utility of CT to Differentiate Pancreatic Parenchymal Metastasis from Pancreatic Ductal Adenocarcinoma" Cancers 13, no. 13: 3103. https://doi.org/10.3390/cancers13133103
APA StyleBarat, M., Aldhaheri, R., Dohan, A., Fuks, D., Kedra, A., Hoeffel, C., Oudjit, A., Coriat, R., Barret, M., Terris, B., Marchese, U., & Soyer, P. (2021). Utility of CT to Differentiate Pancreatic Parenchymal Metastasis from Pancreatic Ductal Adenocarcinoma. Cancers, 13(13), 3103. https://doi.org/10.3390/cancers13133103








