Preoperative Detection of Liver Involvement by Right-Sided Adrenocortical Carcinoma Using CT and MRI
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
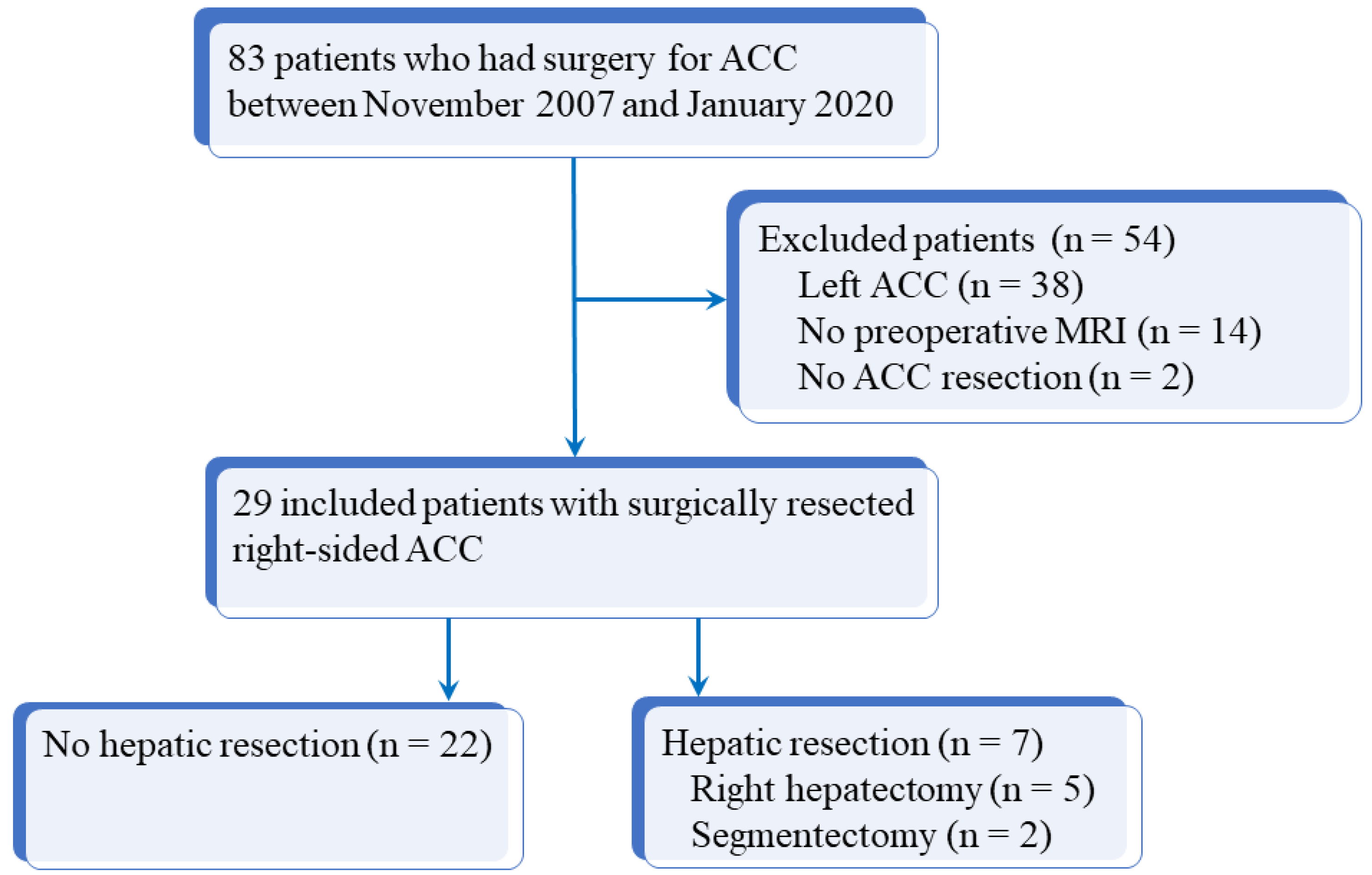
2.1. Patients
2.2. Procedure and Imaging Protocol
2.3. Imaging Analysis
2.4. Standard of Reference
2.5. Statistical Analysis
3. Results
3.1. Correlation of Survival of the Overall Cohort with DLI
3.2. Quantitative Findings
3.3. Qualitative Findings
3.4. Univariable and Multivariable Analysis
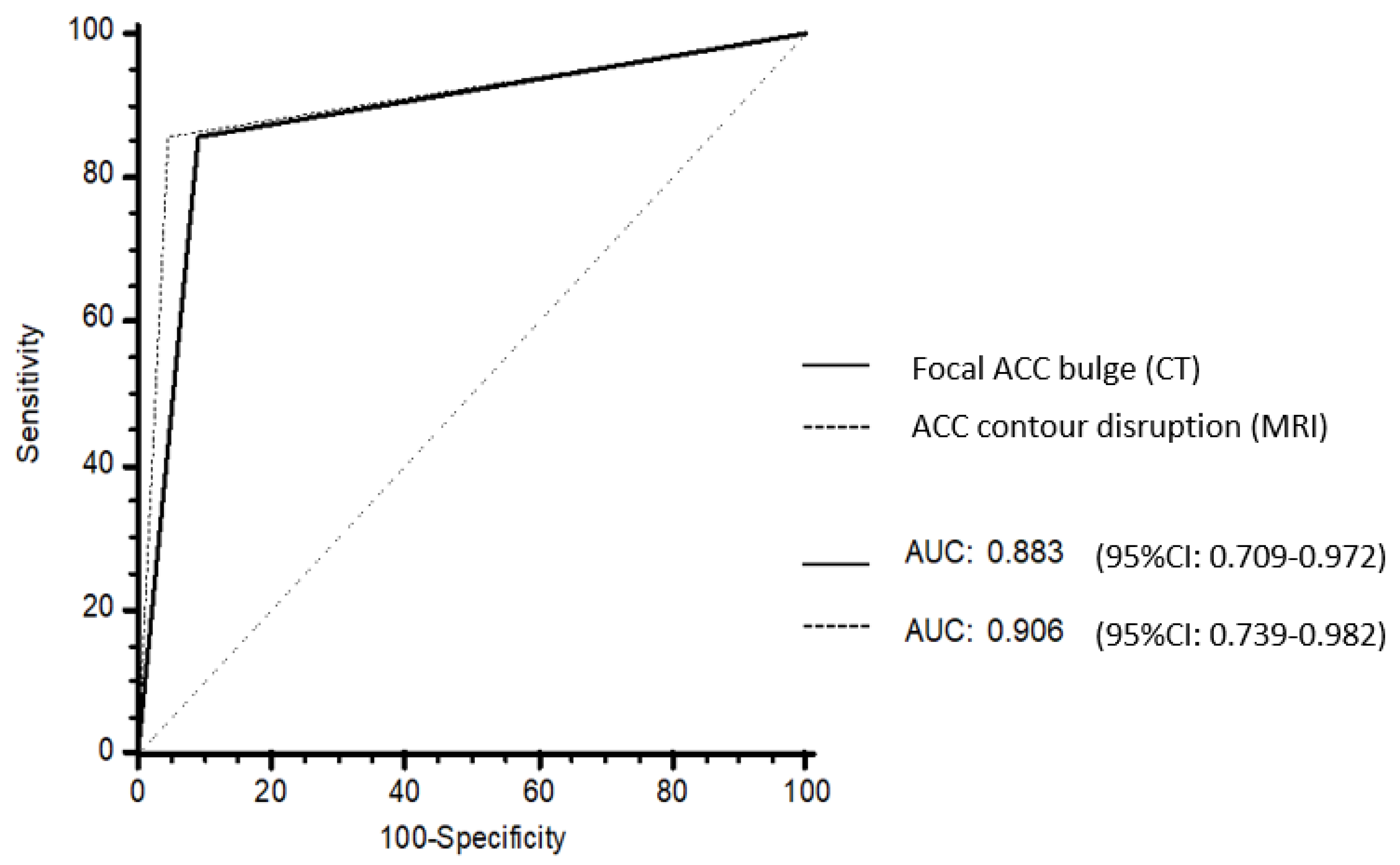
3.5. Added Value of MRI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Souteiro, P.; Donato, S.; Costa, C.; Pereira, C.A.; Simões-Pereira, J.; Oliveira, J.; Belo, S.; Santos, A.P.; Cardoso, H.; Leite, V.; et al. Diagnosis, treatment, and survival analysis of adrenocortical carcinomas: A multicentric study. Hormones 2019, 19, 197–203. [Google Scholar] [CrossRef]
- Abiven, G.; Coste, J.; Groussin, L.; Anract, P.; Tissier, F.; Legmann, P.; Dousset, B.; Bertagna, X.; Bertherat, J. Clinical and biological features in the prognosis of adrenocortical cancer: Poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J. Clin. Endocrinol. Metab. 2006, 91, 2650–2655. [Google Scholar] [CrossRef]
- Grubbs, E.; Lee, J.E. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a revised TNM classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef]
- Ragazzon, B.; Assié, G.; Bertherat, J. Transcriptome analysis of adrenocortical cancers: From molecular classification to the identification of new treatments. Endocr. Relat. Cancer 2011, 18, R15–R27. [Google Scholar] [CrossRef]
- Lughezzani, G.; Sun, M.; Perrotte, P.; Jeldres, C.; Alasker, A.; Isbarn, H.; Budaus, L.; Shariat, S.F.; Guazzoni, G.; Montorsi, F.; et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the International Union Against Cancer-staging system: A North American validation. Eur. J. Cancer 2010, 46, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Brennan, M.F. Recommendation for standardized surgical management of primary adrenocortical carcinoma. Surgery 2012, 152, 123–132. [Google Scholar] [CrossRef]
- Gaujoux, S.; Mihai, R. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br. J. Surg. 2017, 104, 358–376. [Google Scholar] [CrossRef]
- Margonis, G.A.; Amini, N.; Kim, Y.; Tran, T.B.; Postlewait, L.M.; Maithel, S.K.; Wang, T.S.; Evans, D.B.; Hatzaras, I.; Shenoy, R.; et al. Incidence of perioperative complications following resection of adrenocortical carcinoma and its association with long-term survival. World J. Surg. 2016, 40, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Sa Cunha, A.; Couderc, P.; Rullier, E.; Saric, J. Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Br. J. Surg. 2003, 90, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Araki, H.; Uchida, T.; Manabe, Y.; Miyazaki, Y.; Itoh, H. Predictive factors and radiological findings of adrenohepatic adhesion during laparoscopic adrenalectomy. Investig. Clin. Urol. 2020, 61, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Park, B.K.; Kim, C.K. Direct and indirect imaging features of adrenohepatic fusion. Abdom. Radiol. 2016, 41, 377–383. [Google Scholar] [CrossRef]
- Libé, R. Clinical and molecular prognostic factors in adrenocortical carcinoma. Minerva Endocrinol. 2019, 44, 58–69. [Google Scholar] [CrossRef]
- Fassnacht, M.; Dekkers, O.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Thomas, A.J.; Ganeshan, D.M.; Blair, K.J.; Lall, C.; Lee, J.T.; Morshid, A.I.; Habra, M.A.; Elsayes, K.M. Adrenal cortical carcinoma: Pathology, genomics, prognosis, imaging features, and mimics with impact on management. Abdom. Radiol. 2020, 45, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, T.K.; Eun, H.W.; Kim, B.S.; Lee, M.-G.; Kim, P.N.; Ha, H.K. Preoperative evaluation of gallbladder carcinoma: Efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J. Magn. Reson. Imaging 2002, 16, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Das, C.J.; Dhamija, E.; Kumar, R.; Gupta, A.K. Adrenal imaging (Part 1): Imaging techniques and primary cortical lesions. Indian J. Endocrinol. Metab. 2015, 19, 8–15. [Google Scholar] [PubMed]
- Cornud, F.; Rouanne, M.; Beuvon, F.; Eiss, D.; Flam, T.; Liberatore, M.; Zerbib, M.; Delongchamps, N.B. Endorectal 3D T2-weighted 1mm-slice thickness MRI for prostate cancer staging at 1.5Tesla: Should we reconsider the indirects signs of extracapsular extension according to the D’Amico tumor risk criteria? Eur. J. Radiol. 2012, 81, e591–e597. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.-H.; Wan, Y.-L.; Hung, C.-F.; Ng, K.-K.; Pan, K.-T.; Chou, A.S.-B.; Liu, N.J. Diagnosis and staging of gallbladder carcinoma: Evaluation with dynamic MR imaging. Clin. Imaging 2002, 26, 177–182. [Google Scholar] [CrossRef]
- Fuhrman, S.A.; Lasky, L.C.; Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 1982, 6, 655–663. [Google Scholar] [CrossRef]
- Jain, M.; Kapoor, S.; Mishra, A.; Gupta, S.; Agarwal, A. Weiss criteria in large adrenocortical tumors: A validation study. Indian J. Pathol. Microbiol. 2010, 53, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Benchoufi, M.; Matzner-Lober, E.; Molinari, N.; Jannot, A.-S.; Soyer, P. Interobserver agreement issues in radiology. Diagn. Interv. Imaging 2020, 101, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.-Y.; Zou, Z.-M.; Wang, Q.; He, C.-N.; Zou, Q.; Wang, B. MRI manifestations of hepatic perfusion disorders. Exp. Ther. Med. 2018, 15, 5199–5204. [Google Scholar] [CrossRef]
- Lee, M.J.; Saini, S.; Compton, C.C.; Malt, R.A. MR demonstration of edema adjacent to a liver metastasis: Pathologic correlation. AJR Am. J. Roentgenol. 1991, 157, 499–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.J.; Kim, A.Y.; Kim, T.K.; Byun, J.H.; Won, H.J.; Kim, K.W.; Shin, Y.M.; Kim, P.N.; Ha, H.K.; Lee, M.-G. Transient hepatic attenuation differences in focal hepatic lesions: Dynamic CT features. AJR Am. J. Roentgenol. 2005, 184, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, A.; Frattolillo, G.; Paradiso, G.; De Gori, A.; Scarano Catanazaro, V.; Avantifiori, R.; Fiori, E.; De Toma, G. Current role of open surgery in adrenal tumors. G. Chir. 2020, 41, 79–83. [Google Scholar]
- Kwok, G.T.Y.; Zhao, J.-T.; Glover, A.R.; Ip, J.C.Y.; Sywak, M.; Clifton-Bligh, R.; Clarke, S.; Robinson, B.; Sidhu, S.B. Treatment and management of adrenal cancer in a specialized Australian endocrine surgical unit: Approaches, outcomes and lessons learnt. ANZ J. Surg. 2019, 89, 48–52. [Google Scholar] [CrossRef]
- Zheng, G.-Y.; Li, H.-Z.; Deng, J.-H.; Zhang, X.-B.; Wu, X.-C. Open adrenalectomy versus laparoscopic adrenalectomy for adrenocortical carcinoma: A retrospective comparative study on short-term oncologic prognosis. Onco Targets Ther. 2018, 11, 1625–1632. [Google Scholar] [CrossRef]

| Patients without DLI n = 22 | Patients with DLI n = 7 | p-Value | |
|---|---|---|---|
| Age (years) | 40 (19–73) (q1 = 34; q3 = 58) | 57 (34–76) (q1 = 43; q3 = 60) | 0.33 |
| Sex (M/F) | 6/16 (38%) | 2/5 (40%) | 1.00 |
| Ki 67 | 15/(2–95) (q1 = 5; q3 = 27) | 45 (13–70) (q1 = 26; q3 = 63) | 0.080 |
| Weiss score | 6 (3–9) (q1 = 4; q3 = 8) | 9 (7–9) (q1 = 7; q3 = 9) | 0.035 |
| Overall survival (months) | 110 (86–134) (q1 = 13; q3 = 82) | 25 (7–44) (q1 = 4; q3 = 33) | 0.002 |
| Tumor size | |||
| Length (mm) | 80 (21–170) (q1 = 49; q3 = 103) | 105 (63–166) (q1 = 82; q3 = 132) | 0.15 |
| Width (mm) | 56 (12–151) (q1 = 37; q3 = 67) | 66 (39–119) (q1 = 58; q3 = 99) | 0.19 |
| Height (mm) | 62 (19–235) (q1 = 51; q3 = 109) | 101 (45–179) (q1 = 81; q3 = 131) | 0.15 |
| ENSAT stage (median (range)) | 2 (1–4) (q1 = 2; q3 = 2) | 3 (3–4) (q1 = 3; q3 = 3) | 0.012 |
| I | 3 | 0 | 0.55 |
| II | 16 | 0 | 0.001 |
| III | 0 | 5 | <0.001 |
| IV | 3 | 2 | 0.57 |
| Imaging Sign | Imaging Modality | Analysis Criterion |
|---|---|---|
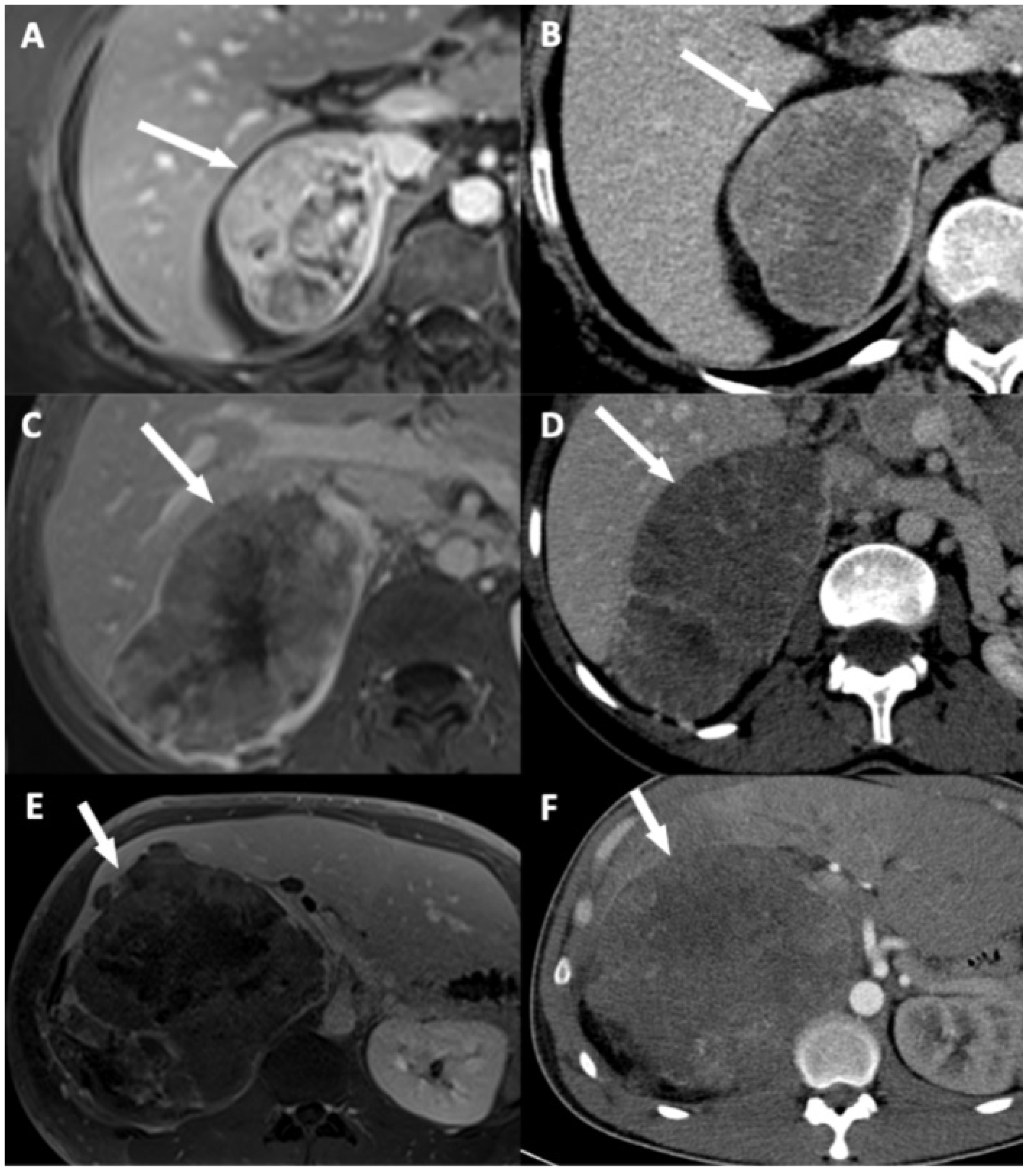
| Disappearance of fat border between ACC and liver | Portal phase CT T2 HASTE MRI (**) | Fat border between ACC and liver non-measurable (<1 mm) |
| Periadrenal fat densification | Portal phase CT T2 FSE MRI | ΔHU between periadrenal fat and normal retroperitoneal fat >10 HU Hyperintense areas in the periadrenal fat |
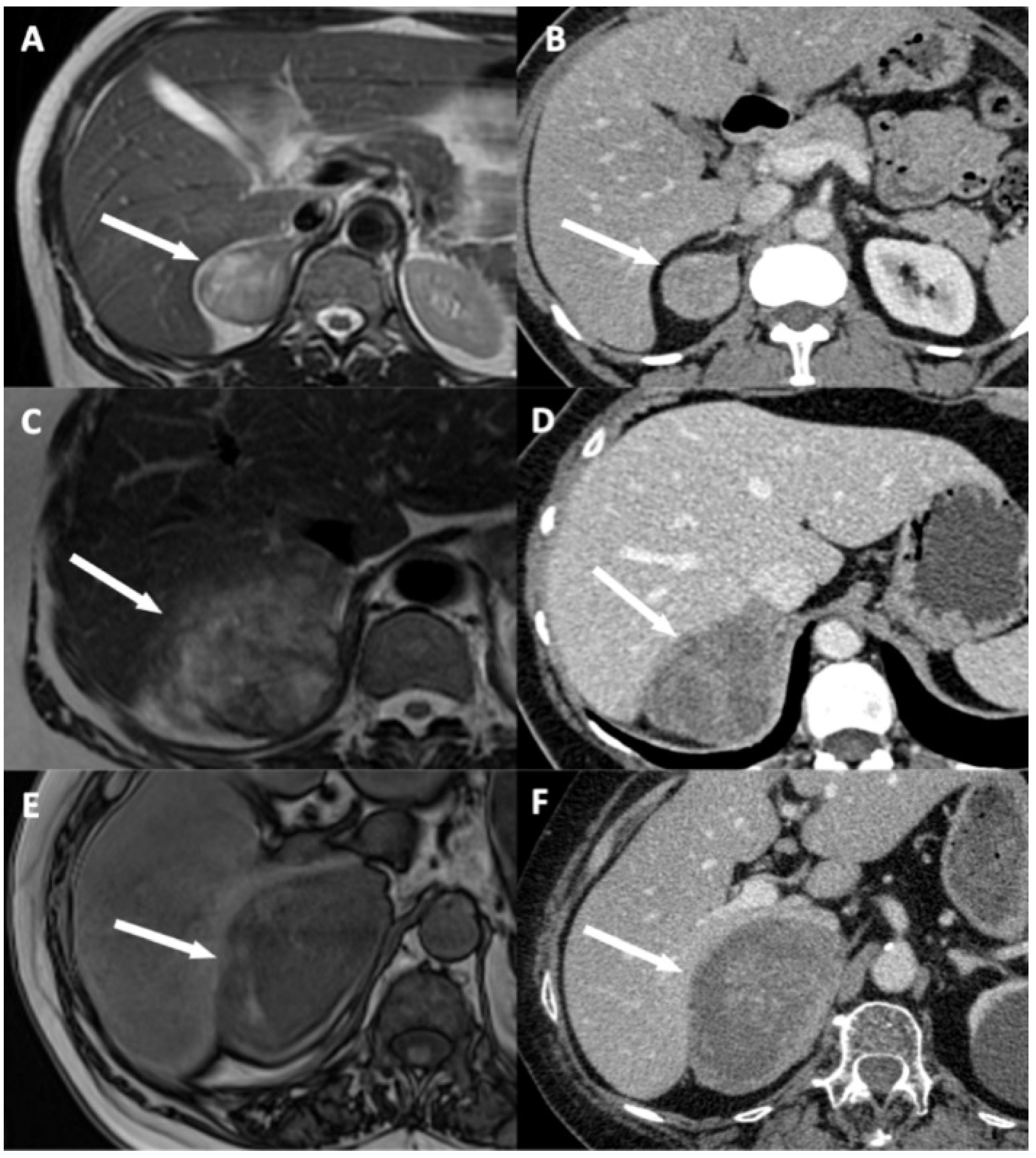
| ACC contour disruption | Portal phase CT Portal phase MRI | Measurable adrenal capsular defect without any enhancement |
| Macroscopic mass effect on inferior vena cava | Portal phase CT Portal phase MRI | Intrahepatic displacement of the vessel and direct contact with the tumor ± changes of its caliber |
| Macroscopic mass effect on right hepatic vein | Portal phase CT Portal phase MRI | Intrahepatic displacement of the vessel and direct contact with the tumor ± changes of its caliber |
| Focal ACC bulge | Portal phase CT Portal phase MRI | Focal and abrupt irregularity of ACC shape |
| Periadrenal hepatic parenchyma enhancement | Portal phase CT | ΔHU > 20 HU between periadrenal liver parenchyma and normal adjacent parenchyma |
| ACC inclusion by hepatic parenchyma > 180° | Portal phase CT Portal phase MRI | ACC surrounded by the liver parenchyma over its half-circumference |
| Categorical Data | CT | MRI | ||||
|---|---|---|---|---|---|---|
| κ Value | 95% CI | p Value | κ Value | 95% CI | p Value | |
| Disappearance of fat border between ACC and liver | 0.94 | 0.87–1.00 | <0.001 | 0.85 | 0.75–0.95 | 0.001 |
| Periadrenal fat densification | 0.37 | 0.20–0.53 | 0.021 | 0.37 | 0.20–0.53 | 0.02 |
| ACC contour disruption | 1.00 | 0.95–1.00 | <0.001 | 0.91 | 0.82–1.00 | <0.001 |
| Macroscopic mass effect on inferior vena cava | 0.88 | 0.80–0.96 | <0.001 | 0.81 | 0.68–0.94 | <0.001 |
| Macroscopic mass effect on right hepatic vein | 0.68 | 0.53–0.83 | <0.001 | 0.72 | 0.58–0.87 | <0.001 |
| Focal ACC bulge | 0.85 | 0.75–0.95 | <0.001 | 1.00 | 0.92–1.00 | <0.001 |
| Periadrenal hepatic parenchyma enhancement | 0.62 | 0.42–0.82 | <0.001 | 0.37 | 0.18–0.57 | 0.04 |
| ACC inclusion by hepatic parenchyma >180° | 0.91 | 0.81–1.00 | <0.001 | 0.81 | 0.68–0.94 | <0.001 |
| Patients without DLI n = 22 | Patients with DLI n = 7 | p Value | |||||
|---|---|---|---|---|---|---|---|
| Pr | % | 95% CI | Pr | % | 95% CI | ||
| Disappearance of fat border between ACC and liver | 3/22 | 14 | 5–33 | 7/7 | 100 | 65–100 | <0.001 |
| Periadrenal fat densification | 1/22 | 5 | 1–22 | 5/7 | 71 | 36–92 | <0.001 |
| ACC contour disruption | 1/22 | 5 | 1–22 | 7/7 | 100 | 65–100 | <0.001 |
| Macroscopic mass effect on inferior vena cava | 12/22 | 55 | 35–73 | 6/7 | 88 | 49–97 | 0.20 |
| Macroscopic mass effect on right hepatic vein | 4/22 | 18 | 7–39 | 4/7 | 57 | 25–84 | 0.07 |
| Focal ACC bulge | 2/22 | 9 | 3–28 | 6/7 | 86 | 49–97 | <0.001 |
| Periadrenal hepatic parenchyma enhancement | 2/22 | 9 | 3–28 | 1/7 | 14 | 3–51 | >0.99 |
| ACC inclusion by hepatic parenchyma >180° | 5/22 | 23 | 10–43 | 1/7 | 14 | 3–51 | >0.99 |
| Patients without DLI n = 22 | Patients with DLI n = 7 | p Value | |||||
|---|---|---|---|---|---|---|---|
| Pr | % | 95% CI | Pr | % | 95% CI | ||
| Disappearance of fat border between ACC and liver | 3/22 | 14 | 5–33 | 7/7 | 100 | 65–100 | <0.001 |
| Periadrenal fat densification | 0/22 | 0 | 0–15 | 5/7 | 71 | 36–92 | <0.001 |
| ACC contour disruption | 1/22 | 5 | 1–22 | 6/7 | 86 | 49–97 | <0.001 |
| Macroscopic mass effect on inferior vena cava | 13/22 | 59 | 39–77 | 6/7 | 86 | 49–97 | 0.37 |
| Macroscopic mass effect on right hepatic vein | 4/22 | 18 | 7–39 | 4/7 | 57 | 25–84 | 0.07 |
| Focal ACC bulge | 3/22 | 14 | 5–33 | 7/7 | 100 | 65–100 | <0.001 |
| Periadrenal hepatic parenchyma enhancement | 3/22 | 14 | 5–33 | 1/7 | 14 | 3–51 | >0.99 |
| ACC inclusion by hepatic parenchyma >180° | 6/22 | 27 | 13–48 | 1/7 | 14 | 3–51 | 0.65 |
| CT | OR (95% CI) | p Value |
|---|---|---|
| Disappearance of fat border between ACC and liver | +∞ (5.16–+∞) | <0.001 |
| Periadrenal fat densification | 39,8 (2.88–2532,19) | <0.001 |
| ACC contour disruption | +∞ (9.96–+∞) | <0.001 |
| Macroscopic mass effect on inferior vena cava | 4.76 (0.45–252.19) | 0.20 |
| Macroscopic mass effect on right hepatic vein | 5.55 (0.66–55.78) | 0.07 |
| Focal ACC bulge | 45.26 (3.47–2813.61) | <0.001 |
| Periadrenal hepatic parenchyma enhancement | 1.63 (0.02–36.93) | >0.99 |
| ACC inclusion by hepatic parenchyma >180° | 0.58 (0.01–6.96) | >0.99 |
| MRI | ||
| Disappearance of fat border between ACC and liver | +∞ (5.16–+∞) | <0.001 |
| Periadrenal fat densification | +∞ (5.02–+∞) | <0.001 |
| ACC contour disruption | 79.65 (5.01–5668.77) | <0.001 |
| Macroscopic mass effect on inferior vena cava | 3.98 (0.38–211.67) | 0.37 |
| Macroscopic mass effect on right hepatic vein | 5.55 (0.66–55.78) | 0.07 |
| Focal ACC bulge | +∞ (5.16–+∞) | <0.001 |
| Periadrenal hepatic parenchyma enhancement | 1.05 (0.02–16.34) | >0.99 |
| ACC inclusion by hepatic parenchyma >180° | 0.46 (0.01–5.21) | 0.65 |
| Imaging Finding | CT | MRI | ||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity 95% CI | Specificity 95% CI | Accuracy 95% CI | p Value | Sensitivity 95% CI | Specificity 95% CI | Accuracy 95% CI | p Value | |
| Disappearance of fat border between ACC and liver | 100 65–100 | 86 67–95 | 90 53–99 | <0.001 | 100 65–100 | 86 67–95 | 90 53–99 | <0.001 |
| Periadrenal fat densification | 71 36–92 | 96 78–99 | 90 53–99 | <0.001 | 71 36–92 | 100 85–100 | 93 56–99 | <0.001 |
| ACC contour disruption | 100 65–100 | 96 78–99 | 97 60–100 | <0.001 | 86 49–97 | 96 78–99 | 93 56–99 | <0.001 |
| Macroscopic mass effect on inferior vena cava | 86 49–97 | 46 27–65 | 55 24–83 | 0.20 | 86 49–97 | 41 23–61 | 52 21–81 | 0.37 |
| Macroscopic mass effect on right hepatic vein | 57 25–84 | 82 62–93 | 76 40–93 | 0.07 | 57 25–84 | 82 62–93 | 76 40–93 | 0.07 |
| Focal ACC bulge | 86 49–97 | 91 72–98 | 90 53–99 | <0.001 | 100 65–100 | 86 67–95 | 90 53–99 | <0.001 |
| Periadrenal hepatic parenchyma enhancement | 14 3–51 | 91 72–98 | 72 37–92 | >0.99 | 14 3–51 | 86 67–95 | 69 34–90 | >0.99 |
| ACC inclusion by hepatic parenchyma > 180° | 14 3–51 | 77 57–90 | 62 29–87 | >0.99 | 14 3–51 | 73 52–87 | 59 26–85 | 0.65 |
| Focal ACC bulge or ACC contour disruption | 100 65–100 | 91 72–97 | 93 56–99 | <0.001 | 100 65–100 | 86 67–95 | 90 53–99 | <0.001 |
| Focal ACC bulge and ACC contour disruption | 86 49–97 | 95 78–99 | 93 56–99 | <0.001 | 86 49–97 | 95 78–99 | 93 56–99 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kedra, A.; Dohan, A.; Gaujoux, S.; Sibony, M.; Jouinot, A.; Assié, G.; Groussin Rouiller, L.; Libé, R.; Bertherat, J.; Soyer, P.; et al. Preoperative Detection of Liver Involvement by Right-Sided Adrenocortical Carcinoma Using CT and MRI. Cancers 2021, 13, 1603. https://doi.org/10.3390/cancers13071603
Kedra A, Dohan A, Gaujoux S, Sibony M, Jouinot A, Assié G, Groussin Rouiller L, Libé R, Bertherat J, Soyer P, et al. Preoperative Detection of Liver Involvement by Right-Sided Adrenocortical Carcinoma Using CT and MRI. Cancers. 2021; 13(7):1603. https://doi.org/10.3390/cancers13071603
Chicago/Turabian StyleKedra, Alice, Anthony Dohan, Sébastien Gaujoux, Mathilde Sibony, Anne Jouinot, Guillaume Assié, Lionel Groussin Rouiller, Rossella Libé, Jérôme Bertherat, Philippe Soyer, and et al. 2021. "Preoperative Detection of Liver Involvement by Right-Sided Adrenocortical Carcinoma Using CT and MRI" Cancers 13, no. 7: 1603. https://doi.org/10.3390/cancers13071603
APA StyleKedra, A., Dohan, A., Gaujoux, S., Sibony, M., Jouinot, A., Assié, G., Groussin Rouiller, L., Libé, R., Bertherat, J., Soyer, P., & Barat, M. (2021). Preoperative Detection of Liver Involvement by Right-Sided Adrenocortical Carcinoma Using CT and MRI. Cancers, 13(7), 1603. https://doi.org/10.3390/cancers13071603







