Quercetin Induces Anticancer Activity by Upregulating Pro-NAG-1/GDF15 in Differentiated Thyroid Cancer Cells
Abstract
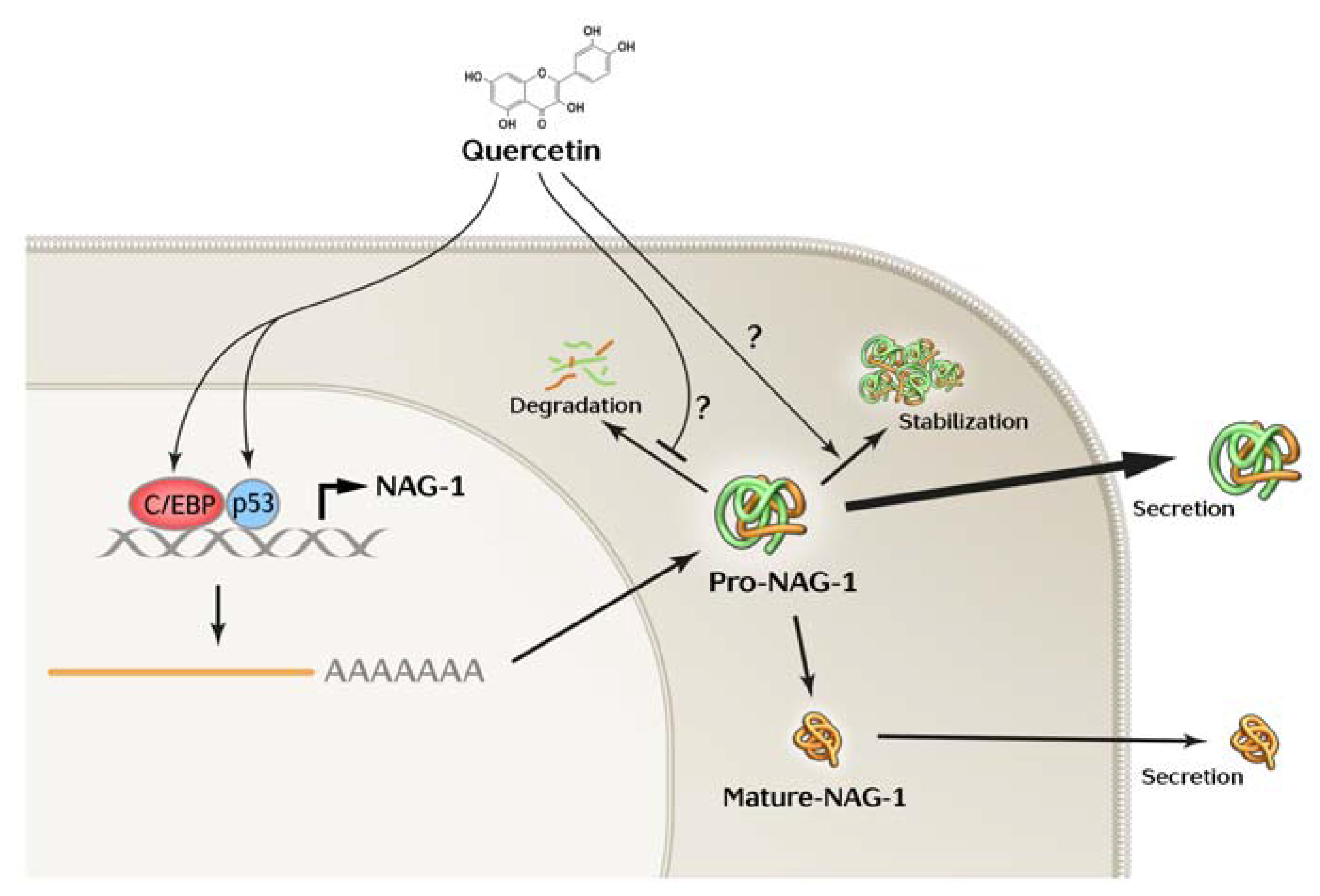
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Tissue Samples
2.3. Antibody Array
2.4. Protien Isolation and Western Blot Analysis
2.5. Reverse Transcriptase Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Dual-Luciferase Assay
2.7. Cytotoxicity Assay Using High-Content Screening
2.8. Apoptosis Analysis by Flow Cytometry
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistical Analysis
3. Results
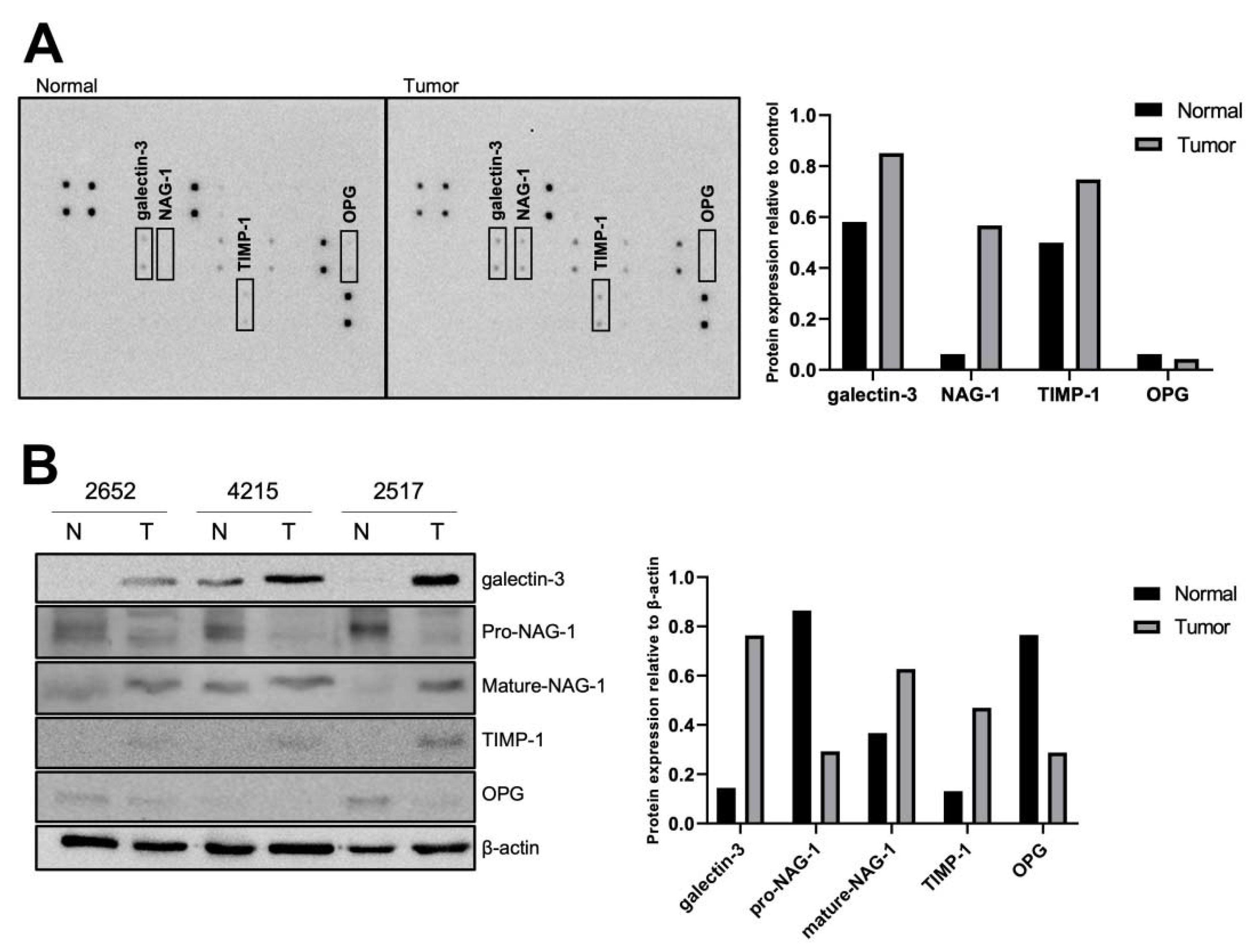
3.1. Identification of Differentially Expressed Proteins in Thyroid Normal and Tumor Tissues
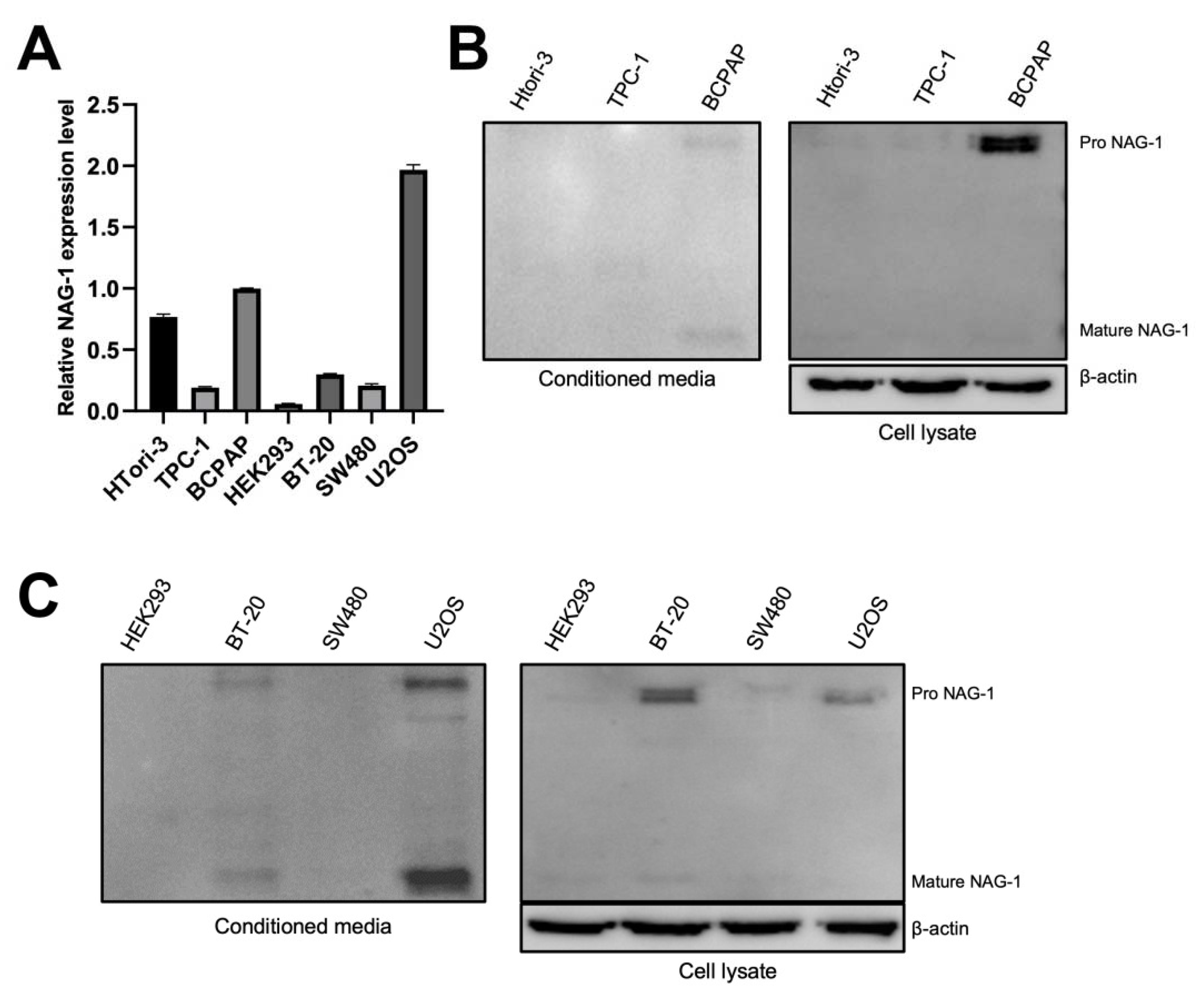
3.2. Mature and Pro-NAG-1 Expression in Various Cancer Cells
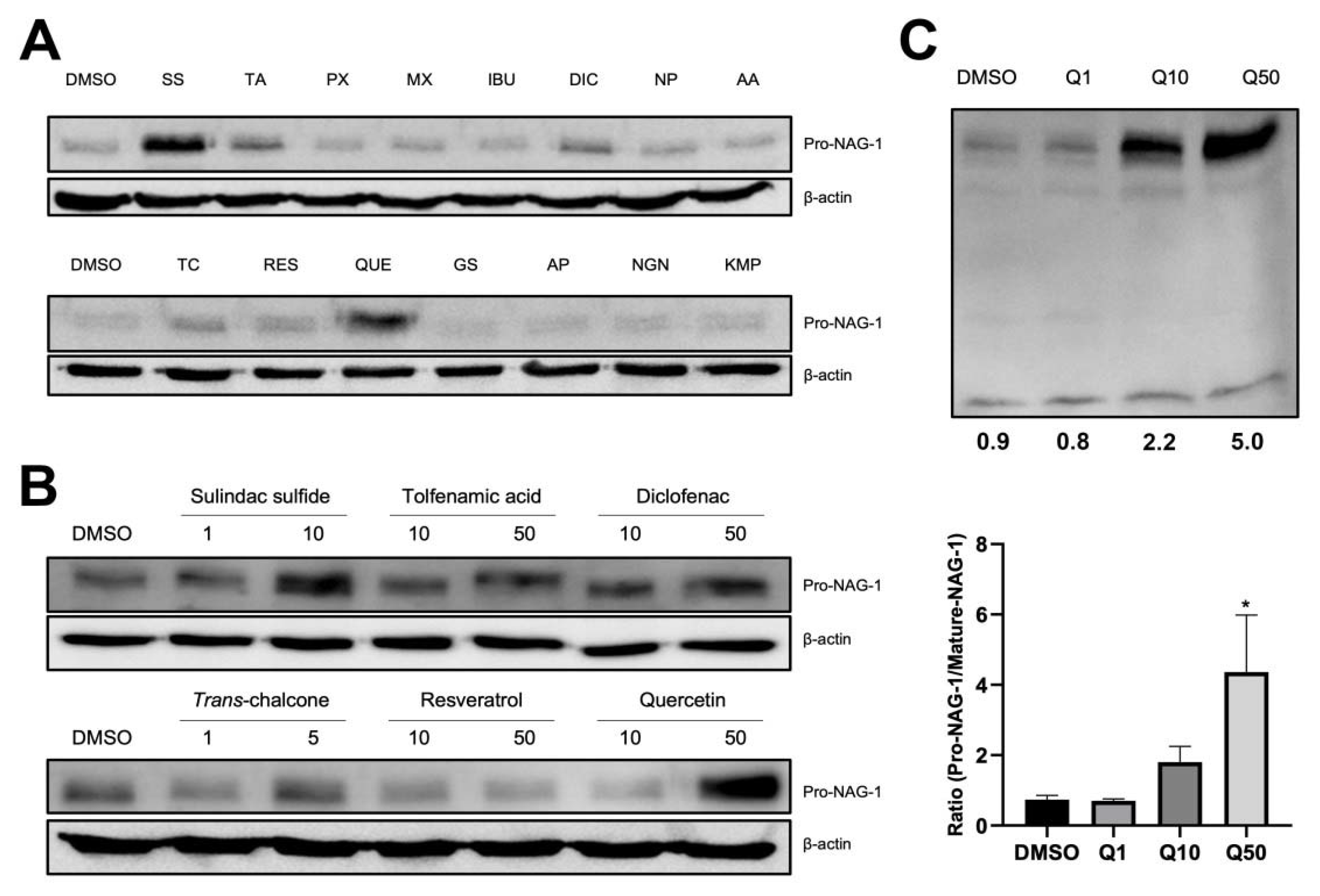
3.3. Quercetin Increases Pro-NAG-1 Levels but Not Mature NAG-1 Levels
3.4. Quercetin Induces Apoptosis and Cell Cycle Arrest
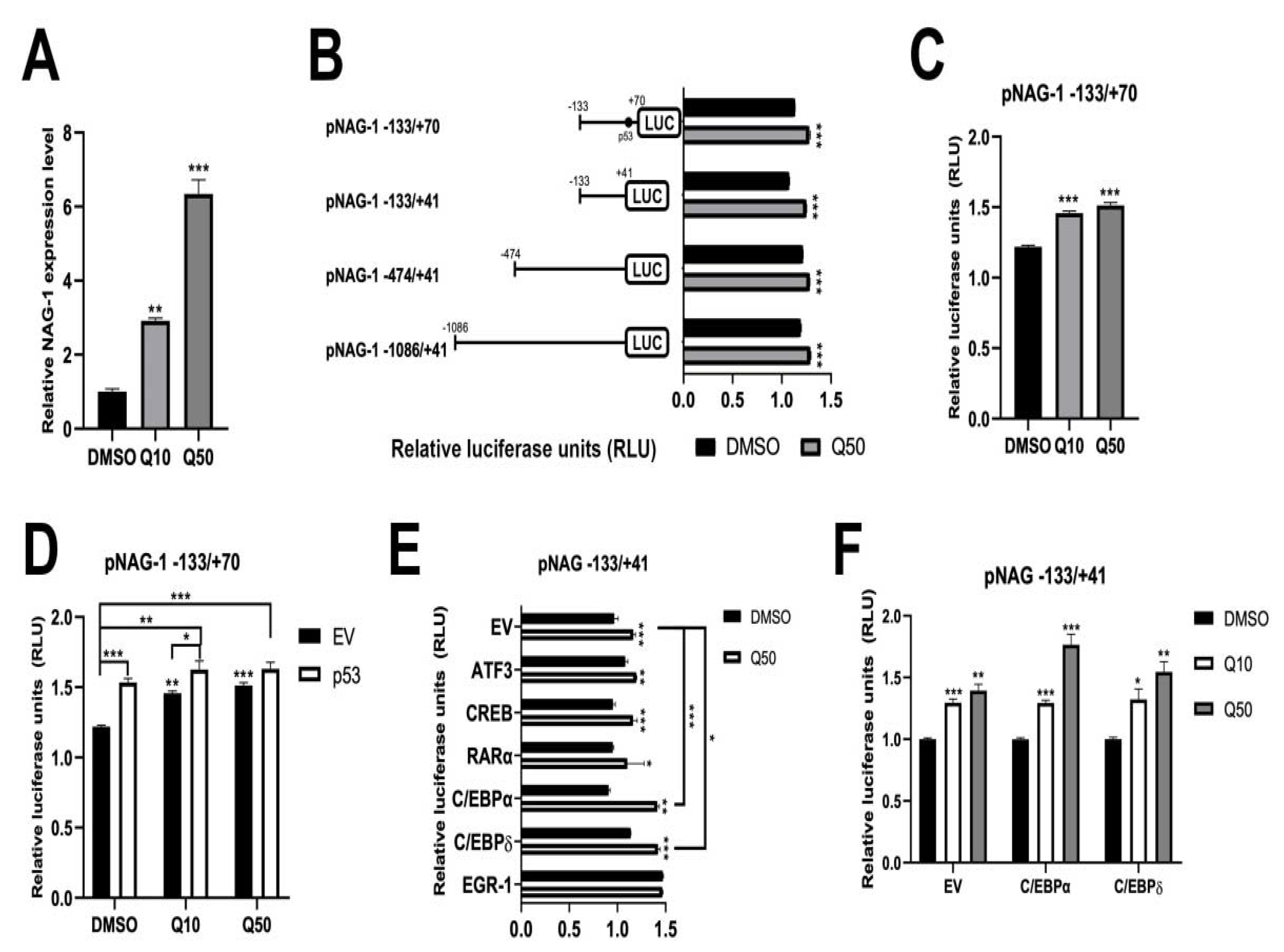
3.5. Quercetin Increases NAG-1 Promoter Activity through p53, C/EBPα, and C/EBPδ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Hong, S.; Won, Y.-J.; Lee, J.J.; Jung, K.-W.; Kong, H.-J.; Im, J.-S.; Seo, H.G. The Community of Population-Based Regional Cancer Registries Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2018. Cancer Res. Treat. 2021, 53, 301–315. [Google Scholar] [CrossRef]
- Fusco, A.; Grieco, M.; Santoro, M.; Berlingieri, M.T.; Pilotti, S.; Pierotti, M.A.; Della Porta, G.; Vecchio, G. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nat. Cell Biol. 1987, 328, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Giordano, T.J.; Kuick, R.; Thomas, D.G.; Misek, D.E.; Vinco, M.; Sanders, D.; Zhu, Z.; Ciampi, R.; Roh, M.; Shedden, K.; et al. Molecular classification of papillary thyroid carcinoma: Distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene 2005, 24, 6646–6656. [Google Scholar] [CrossRef]
- Raman, P.; Koenig, R.J. Pax-8–PPAR-γ fusion protein in thyroid carcinoma. Nat. Rev. Endocrinol. 2014, 10, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.A.; Hay, I.D.; Bartelt, D.H.; Ritland, S.R.; Dahl, R.J.; Grant, C.S.; Jenkins, R.B. Cytogenetic and molecular genetic studies of follicular and papillary thyroid cancers. J. Clin. Investig. 1991, 88, 1596–1604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baek, S.J.; Eling, T. Growth differentiation factor 15 (GDF15): A survival protein with therapeutic potential in metabolic diseases. Pharmacol. Ther. 2019, 198, 46–58. [Google Scholar] [CrossRef]
- Baek, S.J.; Kim, K.S.; Nixon, J.B.; Wilson, L.C.; Eling, T.E. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol. Pharmacol. 2001, 59, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.-N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Bezawada, N.; Wu, K.; Garcia-Albeniz, X.; Morikawa, T.; Fuchs, C.S.; Ogino, S.; Giovannucci, E.L.; Chan, A.T. A Prospective Study of Macrophage Inhibitory Cytokine-1 (MIC-1/GDF15) and Risk of Colorectal Cancer. J. Natl. Cancer Inst. 2014, 106, dju016. [Google Scholar] [CrossRef]
- Breit, S.N.; Johnen, H.; Cook, A.; Tsai, V.W.W.; Mohammad, M.G.; Kuffner, T.; Zhang, H.P.; Marquis, C.; Jiang, L.; Lockwood, G.; et al. The TGF-β superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors 2011, 29, 187–195. [Google Scholar] [CrossRef]
- Min, K.-W.; Liggett, J.L.; Silva, G.; Wu, W.W.; Wang, R.; Shen, R.-F.; Eling, T.; Baek, S.J. NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway. Oncogene 2016, 35, 377–388. [Google Scholar] [CrossRef]
- Lee, J.; Kim, I.; Yoo, E.; Baek, S.J. Competitive inhibition by NAG-1/GDF-15 NLS peptide enhances its anti-cancer activity. Biochem. Biophys. Res. Commun. 2019, 519, 29–34. [Google Scholar] [CrossRef]
- Min, K.-W.; Lee, S.-H.; Baek, S.J. Moonlighting proteins in cancer. Cancer Lett. 2016, 370, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Cekanova, M.; Baek, S.J. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol. Carcinog. 2008, 47, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, J.-S.; Yamaguchi, K.; Eling, T.E.; Baek, S.J. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem. Biophys. Res. Commun. 2005, 328, 63–69. [Google Scholar] [CrossRef]
- Lee, S.-H.; Min, K.-W.; Zhang, X.; Baek, S.J. 3,3′-Diindolylmethane induces activating transcription factor 3 (ATF3) via ATF4 in human colorectal cancer cells. J. Nutr. Biochem. 2013, 24, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of β-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef]
- Yoo, E.; Lee, J.; Lertpatipanpong, P.; Ryu, J.; Kim, C.-T.; Park, E.-Y.; Baek, S.J. Anti-proliferative activity of A. Oxyphylla and its bioactive constituent nootkatone in colorectal cancer cells. BMC Cancer 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Gol, R.M.; Kheirouri, S. The Effects of Quercetin on the Apoptosis of Human Breast Cancer Cell Lines MCF-7 and MDA-MB-231: A Systematic Review. Nutr. Cancer 2021, 1–18. [Google Scholar] [CrossRef]
- Lan, C.-Y.; Chen, S.-Y.; Kuo, C.-W.; Lu, C.-C.; Yen, G.-C. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 2019, 27, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hisaka, T.; Sakai, H.; Sato, T.; Goto, Y.; Nomura, Y.; Fukutomi, S.; Fujita, F.; Mizobe, T.; Nakashima, O.; Tanigawa, M.; et al. Quercetin Suppresses Proliferation of Liver Cancer Cell Lines In Vitro. Anticancer. Res. 2020, 40, 4695–4700. [Google Scholar] [CrossRef]
- Altundag, E.M.; Kasacı, T.; Yılmaz, A.M.; Karademir, B.; Koçtürk, S.; Taga, Y.; Yalçın, A.S. Quercetin-Induced Cell Death in Human Papillary Thyroid Cancer (B-CPAP) Cells. J. Thyroid. Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Celano, M.; Maggisano, V.; Bulotta, S.; Allegri, L.; Pecce, V.; Abballe, L.; Damante, G.; Russo, D. Quercetin improves the effects of sorafenib on growth and migration of thyroid cancer cells. Endocr. 2019, 67, 496–498. [Google Scholar] [CrossRef]
- Baek, S.J.; Horowitz, J.M.; Eling, T.E. Molecular Cloning and Characterization of Human Nonsteroidal Anti-inflammatory Drug-activated Gene Promoter. J. Biol. Chem. 2001, 276, 33384–33392. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Tu, X.; Niu, Y.; Li, X.; Qin, L.; Yang, Z.; Su, Q. Elevated serum growth differentiation factor 15 levels are associated with thyroid nodules in type 2 diabetes aged over 60 years. Oncotarget 2017, 8, 41379–41386. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, J.-W.; Min, D.S.; Chang, J.-S.; Lee, Y.H.; Park, Y.B.; Choi, K.S.; Kwon, T.K.; Lim, J.H.; Park, J.-W.; et al. NAG-1 up-regulation mediated by EGR-1 and p53 is critical for quercetin-induced apoptosis in HCT116 colon carcinoma cells. Apoptosis 2006, 12, 411–421. [Google Scholar] [CrossRef]
- Lee, S.-H.; Bahn, J.H.; Whitlock, N.C.; Baek, S.J. Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene 2010, 29, 5182–5192. [Google Scholar] [CrossRef] [PubMed]
- Nualsanit, T.; Rojanapanthu, P.; Gritsanapan, W.; Lee, S.-H.; Lawson, D.; Baek, S.J. Damnacanthal, a noni component, exhibits antitumorigenic activity in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 915–923. [Google Scholar] [CrossRef]
- Sumana, B.S.; Shashidhar, S.; Shivarudrappa, A.S. Galectin-3 Immunohistochemical Expression in Thyroid Neoplasms. J. Clin. Diagn. Res. 2015, 9, EC07–EC11. [Google Scholar] [CrossRef] [PubMed]
- Hawthorn, L.; Stein, L.; Varma, R.; Wiseman, S.; Loree, T.; Tan, D. TIMP1 and SERPIN-A overexpression and TFF3 and CRABP1 underexpression as biomarkers for papillary thyroid carcinoma. Head Neck 2004, 26, 1069–1083. [Google Scholar] [CrossRef]
- Infante, M.; Fabi, A.; Cognetti, F.; Gorini, S.; Caprio, M.; Fabbri, A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Kasimir-Bauer, S.; Göbel, A.; Erdmann, K.; Hoffmann, O.; Browne, A.J.; Wimberger, P.; Rauner, M.; Hofbauer, L.C.; Kimmig, R.; et al. Prognostic Value of RANKL/OPG Serum Levels and Disseminated Tumor Cells in Nonmetastatic Breast Cancer. Clin. Cancer Res. 2019, 25, 1369–1378. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Baek, S.J. Molecular Targets of Dietary Polyphenols with Anti-inflammatory Properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef]
- Baek, S.J.; Wilson, L.C.; Hsi, L.C.; Eling, T.E. Troglitazone, a Peroxisome Proliferator-activated Receptor γ (PPARγ) Ligand, Selectively Induces the Early Growth Response-1 Gene Independently of PPARγ. J. Biol. Chem. 2003, 278, 5845–5853. [Google Scholar] [CrossRef]
- Tsai, V.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef]
- Baek, S.J.; Okazaki, R.; Lee, S.-H.; Martinez, J.; Kim, J.-S.; Yamaguchi, K.; Mishina, Y.; Martin, D.W.; Shoieb, A.; Mcentee, M.F.; et al. Nonsteroidal Anti-Inflammatory Drug-Activated Gene-1 Over Expression in Transgenic Mice Suppresses Intestinal Neoplasia. Gastroenterol. 2006, 131, 1553–1560. [Google Scholar] [CrossRef]
- Cekanova, M.; Lee, S.-H.; Donnell, R.L.; Sukhthankar, M.; Eling, T.E.; Fischer, S.M.; Baek, S.J. Nonsteroidal Anti-inflammatory Drug-Activated Gene-1 Expression Inhibits Urethane-Induced Pulmonary Tumorigenesis in Transgenic Mice. Cancer Prev. Res. 2009, 2, 450–458. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Wang, J.; Kong, J.; Tang, J.; Wu, Y.; Xu, E.; Zhang, H.; Lai, M. GDF15 promotes EMT and metastasis in colorectal cancer. Oncotarget 2015, 7, 860–872. [Google Scholar] [CrossRef]
- Kang, Y.E.; Kim, J.M.; Lim, M.A.; Lee, S.E.; Yi, S.; Kim, J.T.; Oh, C.; Liu, M.L.; Jin, Y.; Jung, S.-N.; et al. Growth Differentiation Factor 15 is a Cancer Cell-Induced Mitokine That Primes Thyroid Cancer Cells for Invasiveness. Thyroid. 2021, 31, 772–786. [Google Scholar] [CrossRef]
- Baek, S.J.; Wilson, L.C.; Lee, C.-H.; Eling, T.E. Dual Function of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Inhibition of Cyclooxygenase and Induction of NSAID-Activated Gene. J. Pharmacol. Exp. Ther. 2002, 301, 1126–1131. [Google Scholar] [CrossRef]
- Yang, M.H.; Kim, J.; Khan, I.A.; Walker, L.A.; Khan, S.I. Nonsteroidal anti-inflammatory drug activated gene-1 (NAG-1) modulators from natural products as anti-cancer agents. Life Sci. 2014, 100, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Krisanapun, C.; Baek, S.J. NSAID-activated gene-1 as a molecular target for capsaicin-induced apoptosis through a novel molecular mechanism involving GSK3 C/EBP and ATF3. Carcinog. 2010, 31, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Galleggiante, V.; De Santis, S.; Liso, M.; Verna, G.; Sommella, E.; Mastronardi, M.; Campiglia, P.; Chieppa, M.; Serino, G. Quercetin-Induced miR-369-3p Suppresses Chronic Inflammatory Response Targeting C/EBP-β. Mol. Nutr. Food Res. 2019, 63, e1801390. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | Gender | Age at Time of Surgery | Type of Thyroid Cancer |
|---|---|---|---|
| 2652 | M | 35 | Papillary carcinoma |
| 4215 | F | 56 | Papillary microcarcinoma |
| 2517 | M | 34 | Papillary carcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Lee, J.; Moon, H.; Ryu, C.H.; Seok, J.; Jung, Y.-S.; Ryu, J.; Baek, S.J. Quercetin Induces Anticancer Activity by Upregulating Pro-NAG-1/GDF15 in Differentiated Thyroid Cancer Cells. Cancers 2021, 13, 3022. https://doi.org/10.3390/cancers13123022
Hong Y, Lee J, Moon H, Ryu CH, Seok J, Jung Y-S, Ryu J, Baek SJ. Quercetin Induces Anticancer Activity by Upregulating Pro-NAG-1/GDF15 in Differentiated Thyroid Cancer Cells. Cancers. 2021; 13(12):3022. https://doi.org/10.3390/cancers13123022
Chicago/Turabian StyleHong, Yukyung, Jaehak Lee, Hyunjin Moon, Chang H. Ryu, Jungirl Seok, Yuh-Seog Jung, Junsun Ryu, and Seung J. Baek. 2021. "Quercetin Induces Anticancer Activity by Upregulating Pro-NAG-1/GDF15 in Differentiated Thyroid Cancer Cells" Cancers 13, no. 12: 3022. https://doi.org/10.3390/cancers13123022
APA StyleHong, Y., Lee, J., Moon, H., Ryu, C. H., Seok, J., Jung, Y.-S., Ryu, J., & Baek, S. J. (2021). Quercetin Induces Anticancer Activity by Upregulating Pro-NAG-1/GDF15 in Differentiated Thyroid Cancer Cells. Cancers, 13(12), 3022. https://doi.org/10.3390/cancers13123022








