Proposal of a New Prognostic Model for Differentiated Thyroid Cancer with TERT Promoter Mutations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinicopathological Data
2.2. Detection of TERT Promoter Mutations in Thyroid Cancer Samples
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Alternative Grouping According to the AJCC Stage and TERT Promoter Mutation Status
3.3. Comparison of Survival in Patients Staged According to TNM-8 and TNM-8T
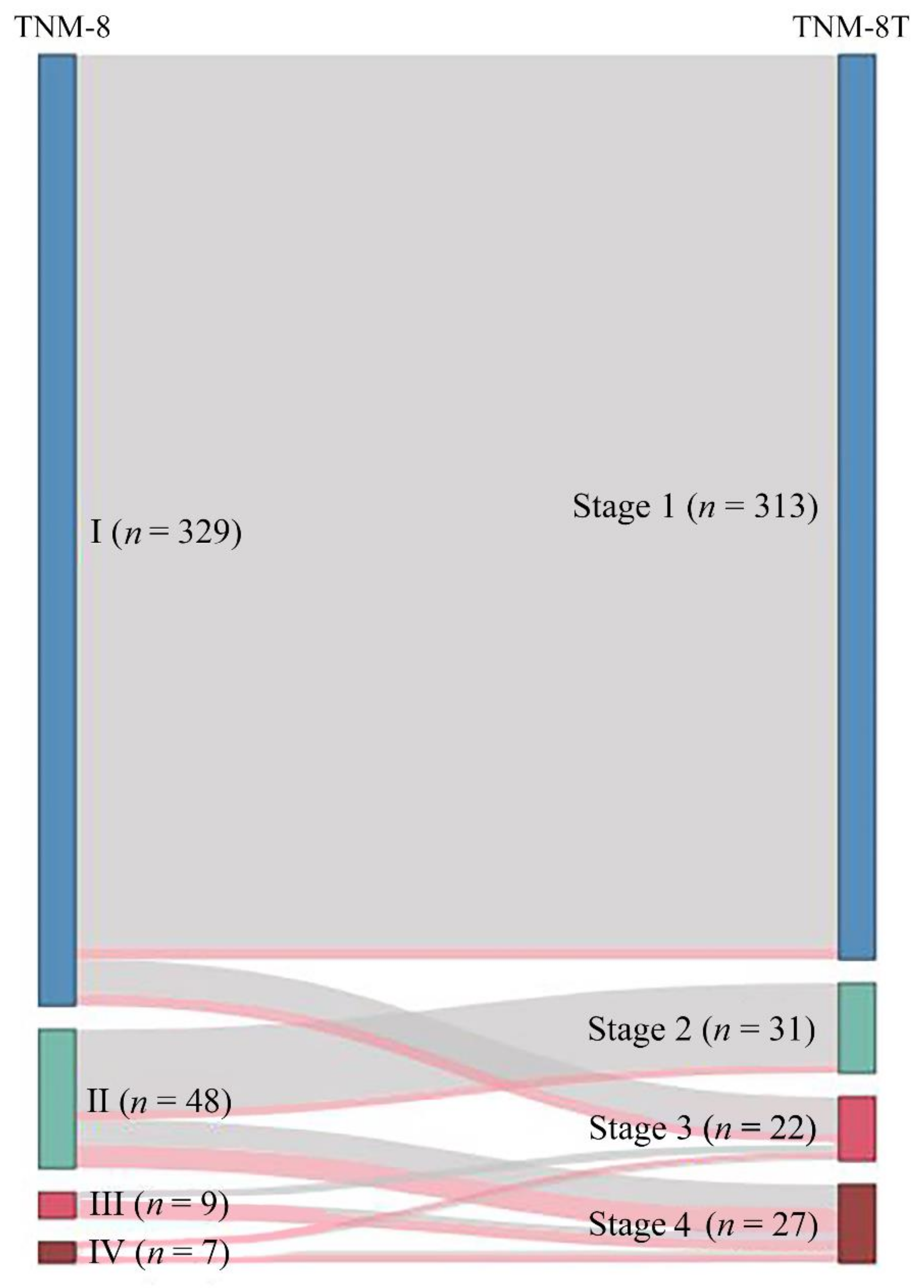
3.4. Comparison of TNM Staging Groups According to TNM-8 vs. TNM-8T
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, Y.E.; Ahn, S.; Kim, J.Y.; Ki, C.S.; Oh, Y.L.; Kim, K.; Yun, J.W.; Park, W.Y.; Choe, J.H.; et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr. Relat. Cancer 2016, 23, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Hwang, T.S.; Choi, Y.L.; Han, H.S.; Kim, W.S.; Jang, M.H.; Kim, S.K.; Yang, J.H. Prognostic Significance of TERT Promoter Mutations in Papillary Thyroid Carcinomas in a BRAF(V600E) Mutation-Prevalent Population. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Nixon, I.J.; Wang, L.Y.; Migliacci, J.C.; Eskander, A.; Campbell, M.J.; Aniss, A.; Morris, L.; Vaisman, F.; Corbo, R.; Momesso, D.; et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 373–380. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.G.; Oh, H.S.; Park, S.; Kwon, H.; Song, D.E.; Kim, T.Y.; Shong, Y.K.; Kim, W.B.; Sung, T.Y.; et al. Comparison of the Seventh and Eighth Editions of the American Joint Committee on Cancer/Union for International Cancer Control Tumor-Node-Metastasis Staging System for Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 1149–1155. [Google Scholar] [CrossRef]
- Shteinshnaider, M.; Kalmovich, L.; Koren, S.; Or, K.; Cantrell, D.; Benbassat, C. Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: A comparative study. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 201–209. [Google Scholar] [CrossRef]
- Shaha, A.R.; Migliacci, J.C.; Nixon, I.J.; Wang, L.Y.; Wong, R.J.; Morris, L.G.T.; Patel, S.G.; Shah, J.P.; Tuttle, R.M.; Ganly, I. Stage migration with the new American Joint Committee on Cancer (AJCC) staging system (8th edition) for differentiated thyroid cancer. Surgery 2019, 165, 6–11. [Google Scholar] [CrossRef]
- Xing, M.; Haugen, B.R.; Schlumberger, M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 2013, 381, 1058–1069. [Google Scholar] [CrossRef]
- McKelvey, B.A.; Umbricht, C.B.; Zeiger, M.A. Telomerase reverse transcriptase (TERT) regulation in thyroid cancer: A review. Front. Endocrinol. 2020, 11, 485. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.I.; Jeon, M.J.; Kim, H.K.; Kim, E.H.; Yi, H.S.; Kim, E.S.; Kim, H.; Kim, B.H.; Kim, T.Y.; et al. Eighth edition of tumor-node-metastasis staging system improve survival predictability for papillary, but not follicular thyroid carcinoma: A multicenter cohort study. Oral Oncol. 2018, 87, 97–103. [Google Scholar] [CrossRef]
- Kim, T.H.; Ki, C.S.; Kim, H.S.; Kim, K.; Choe, J.H.; Kim, J.H.; Kim, J.S.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; et al. Refining Dynamic Risk Stratification and Prognostic Groups for Differentiated Thyroid Cancer with TERT Promoter Mutations. J. Clin. Endocrinol. Metab. 2017, 102, 1757–1764. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Kim, K.; Park, H.; Ki, C.-S.; Oh, Y.L.; Shin, J.H.; Kim, J.S.; Kim, S.W.; Chung, J.H.; et al. TERT Promoter Mutations and the 8th Edition TNM Classification in Predicting the Survival of Thyroid Cancer Patients. Cancers 2021, 13, 648. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Sherman, S.I.; Tuttle, R.M. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2006, 16, 109–142. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Zheng, Y. Survival model predictive accuracy and ROC curves. Biometrics 2005, 61, 92–105. [Google Scholar] [CrossRef]
- Schemper, M.; Stare, J. Explained variation in survival analysis. Stat. Med. 1996, 15, 1999–2012. [Google Scholar] [CrossRef]
- Brier, G.W. Verification of forecasts expressed in terms of probability. Mon. Weather Rev. 1950, 78, 1–3. [Google Scholar] [CrossRef]
- Xing, M. Genetic-guided risk assessment and management of thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 109–124. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Alsaadi, R.; Murugan, A.K.; Sadiq, B.B. TERT Promoter Mutations in Thyroid Cancer. Horm. Cancer 2016, 7, 165–177. [Google Scholar] [CrossRef]
- Li, F.; Chen, G.; Sheng, C.; Gusdon, A.M.; Huang, Y.; Lv, Z.; Xu, H.; Xing, M.; Qu, S. BRAFV600E mutation in papillary thyroid microcarcinoma: A meta-analysis. Endocr. Relat. Cancer 2015, 22, 159–168. [Google Scholar] [CrossRef]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef]
- Tufano, R.P.; Teixeira, G.V.; Bishop, J.; Carson, K.A.; Xing, M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: A systematic review and meta-analysis. Medicine 2012, 91, 274–286. [Google Scholar] [CrossRef]
- Xing, M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 2007, 28, 742–762. [Google Scholar] [CrossRef]
- Huang, D.-S.; Wang, Z.; He, X.-J.; Diplas, B.H.; Yang, R.; Killela, P.J.; Meng, Q.; Ye, Z.-Y.; Wang, W.; Jiang, X.-T.; et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer 2015, 51, 969–976. [Google Scholar] [CrossRef]
- Liu, X.; Qu, S.; Liu, R.; Sheng, C.; Shi, X.; Zhu, G.; Murugan, A.K.; Guan, H.; Yu, H.; Wang, Y.; et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1130–E1136. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Li, X.; Liang, Z.; Gao, W.; Liang, J.; Cheng, S.; Lin, Y. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J. Nucl. Med. 2017, 58, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Xu, J.; Wang, Y. The role of TERT promoter mutations in postoperative and preoperative diagnosis and prognosis in thyroid cancer. Medicine 2018, 97, e11548. [Google Scholar] [CrossRef]
- Jin, L.; Chen, E.; Dong, S.; Cai, Y.; Zhang, X.; Zhou, Y.; Zeng, R.; Yang, F.; Pan, C.; Liu, Y.; et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: A study of 653 patients. Oncotarget 2016, 7, 18346–18355. [Google Scholar] [CrossRef]
- Liu, R.; Bishop, J.; Zhu, G.; Zhang, T.; Ladenson, P.W.; Xing, M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol. 2017, 3, 202–208. [Google Scholar] [CrossRef]
- Xing, M.; Liu, R.; Liu, X.; Murugan, A.K.; Zhu, G.; Zeiger, M.A.; Pai, S.; Bishop, J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2718–2726. [Google Scholar] [CrossRef]
- Hundahl, S.A.; Cady, B.; Cunningham, M.P.; Mazzaferri, E.; McKee, R.F.; Rosai, J.; Shah, J.P.; Fremgen, A.M.; Stewart, A.K.; Hölzer, S. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German thyroid cancer study group. An American college of Surgeons commission on cancer patient care evaluation study. Cancer 2000, 89, 202–217. [Google Scholar] [CrossRef]
- Oh, C.M.; Kong, H.J.; Kim, E.; Kim, H.; Jung, K.W.; Park, S.; Won, Y.J. National epidemiologic survey of thyroid cancer (NEST) in Korea. Epidemiol. Health 2018, 40, e2018052. [Google Scholar] [CrossRef]
- Xing, M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, D.L.; Han, H.S.; Kim, W.S.; Kim, S.J.; Moon, W.J.; Oh, S.Y.; Hwang, T.S. Pyrosequencing analysis for detection of a BRAFV600E mutation in an FNAB specimen of thyroid nodules. Diagn. Mol. Pathol. Am. J. Surg. Pathol. Part B 2008, 17, 118–125. [Google Scholar] [CrossRef]

| Characteristic | Number (%), (Total = 393) |
|---|---|
| Age, years | |
| Median (range) | 42.8 (15.8–81.4) |
| Sex | |
| Male | 329 (83.7) |
| Female | 64 (16.3) |
| Tumor size, cm | |
| Median (range) | 2.7 (0.4–12.0) |
| Histological type | |
| PTC | 327 (83.2) |
| FTC | 66 (16.8) |
| Multifocality | |
| Absent | 286 (72.8) |
| Present | 107 (27.2) |
| Lymph node metastasis | |
| Absent | 199 (50.6) |
| Present | 193 (49.1) |
| Missing | 1 (0.3) |
| Extrathyroidal invasion | |
| Absent | 352 (89.6) |
| Present | 41 (10.4) |
| Distant metastasis | |
| Absent | 370 (94.1) |
| Present | 23 (5.9) |
| Stage at diagnosis | |
| I | 329 (83.7) |
| II | 48 (12.2) |
| III/IV | 16 (4.1) |
| TERT promoter mutations | |
| WT | 350 (89.1) |
| Mutant | 43 (10.9) |
| RAI treatment | |
| Absent | 29 (7.4) |
| Present | 364 (92.6) |
| Incidence related | |
| Incident cases | 27 |
| Persons-year | 6366 |
| Incident rate (1000 persons-year) | 4 |
| Death | |
| Survival | 366 (93.1) |
| Death | 27 (6.9) |
| Combination | Cancer-Related Death | Unadjusted | Adjusted * | ||||
|---|---|---|---|---|---|---|---|
| Events/Total (%) | 10-y CSS | 15-y CSS | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| WT/stage I | 4/313 (1.3) | 98.72 | 98.72 | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| WT/stage II | 3/31 (9.7) | 93.55 | 90.32 | 8.05 (1.80–35.98) | 0.0063 | 10.66 (2.37–47.95) | 0.002 |
| WT/stage III/IV | 1/6 (16.7) | 83.33 | 83.33 | 15.44 (1.72–138.16) | 0.0144 | 17.82 (2.44–130.18) | 0.0045 |
| MUT/stage I | 4/16 (25.0) | 75.00 | 75.00 | 23.66 (5.92–94.63) | <0.001 | 20.63 (5.17–82.40) | <0.001 |
| MUT/stage II | 8/17 (47.1) | 70.59 | 52.29 | 45.46 (13.68–151.13) | <0.001 | 55.37 (16.06–190.87) | <0.001 |
| MUT/stage III/IV | 7/10 (70.0) | 50.00 | 30.00 | 78.45 (22.82–269.76) | <0.001 | 78.37 (22.44–273.67) | <0.001 |
| Alternative Grouping | Definition |
|---|---|
| TNM-8T stage 1 | Patients with TNM-8 stage I and wild-type TERT |
| TNM-8T stage 2 | Patients with TNM-8 stage II and wild-type TERT |
| TNM-8T stage 3 | Patients with TNM-8 stage III/IV and wild-type TERT or with TNM-8 stage I and mutant TERT |
| TNM-8T stage 4 | Patients with TNM-8 stage II and mutant TERT or with TNM-8 stage III/IV and mutant TERT |
| Alternative Grouping | TNM-8T | ||||||
|---|---|---|---|---|---|---|---|
| Cancer-Related Death | Unadjusted | Adjusted * | |||||
| Events/Total (%) | 10-y CSS | 15-y CSS | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| TNM-8T stage 1 | 4/313 (1.3) | 98.7 | 98.7 | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| TNM-8T stage 2 | 3/31 (9.7) | 93.5 | 90.3 | 8.05 (1.80–35.97) | 0.006 | 10.62 (2.41–46.71) | 0.002 |
| TNM-8T stage 3 | 5/22 (22.7) | 77.3 | 77.3 | 21.37 (5.74–79.59) | <0.001 | 18.57 (5.02–68.70) | <0.001 |
| TNM-8T stage 4 | 15/27 (55.6) | 63.0 | 44.1 | 56.40 (18.67–170.36) | <0.001 | 62.87 (20.66–191.29) | <0.001 |
| AJCC Stage | TNM-8 | ||||||
| Cancer-Related Death | Unadjusted | Adjusted * | |||||
| Events/Total (%) | 10-y CSS | 15-y CSS | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| TNM-8 stage I | 8/329 (2.4) | 97.6 | 97.6 | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| TNM-8 stage II | 11/48 (22.9) | 85.4 | 76.3 | 10.43 (4.19–25.93) | <0.001 | 13.20 (5.08–34.30) | <0.001 |
| TNM-8 stage III | 4/9 (44.4) | 77.8 | 55.6 | 20.68 (6.21–68.84) | <0.001 | 26.99 (8.06–90.42) | <0.001 |
| TNM-8 stage IV | 4/7 (57.1) | 42.9 | 42.9 | 38.24 (11.44–127.75) | <0.001 | 26.39 (7.84–88.79) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, S.; Park, J.; Park, H.; Ki, C.-S.; Oh, Y.-L.; Shin, J.-H.; Kim, J.-S.; Kim, S.-W.; Chung, J.-H.; et al. Proposal of a New Prognostic Model for Differentiated Thyroid Cancer with TERT Promoter Mutations. Cancers 2021, 13, 2943. https://doi.org/10.3390/cancers13122943
Park J, Lee S, Park J, Park H, Ki C-S, Oh Y-L, Shin J-H, Kim J-S, Kim S-W, Chung J-H, et al. Proposal of a New Prognostic Model for Differentiated Thyroid Cancer with TERT Promoter Mutations. Cancers. 2021; 13(12):2943. https://doi.org/10.3390/cancers13122943
Chicago/Turabian StylePark, Jun, Sungjoo Lee, Jiyun Park, Hyunju Park, Chang-Seok Ki, Young-Lyun Oh, Jung-Hee Shin, Jee-Soo Kim, Sun-Wook Kim, Jae-Hoon Chung, and et al. 2021. "Proposal of a New Prognostic Model for Differentiated Thyroid Cancer with TERT Promoter Mutations" Cancers 13, no. 12: 2943. https://doi.org/10.3390/cancers13122943
APA StylePark, J., Lee, S., Park, J., Park, H., Ki, C.-S., Oh, Y.-L., Shin, J.-H., Kim, J.-S., Kim, S.-W., Chung, J.-H., Kim, K., & Kim, T.-H. (2021). Proposal of a New Prognostic Model for Differentiated Thyroid Cancer with TERT Promoter Mutations. Cancers, 13(12), 2943. https://doi.org/10.3390/cancers13122943






