Mechanical Stimulation Modulates Osteocyte Regulation of Cancer Cell Phenotype
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
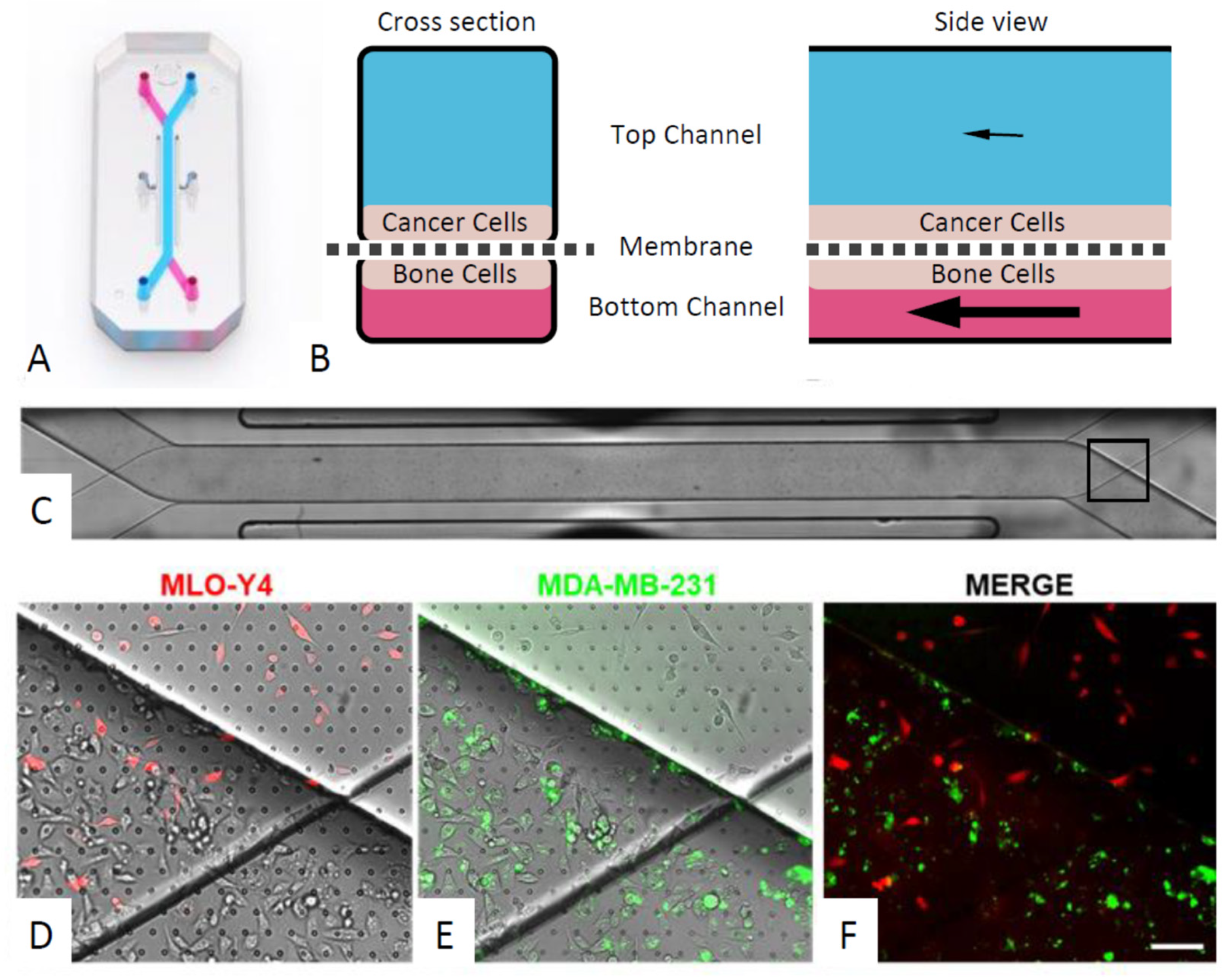
2.2. Microfluidic Organ-Chip Culture Conditions
2.3. Proliferation Assay
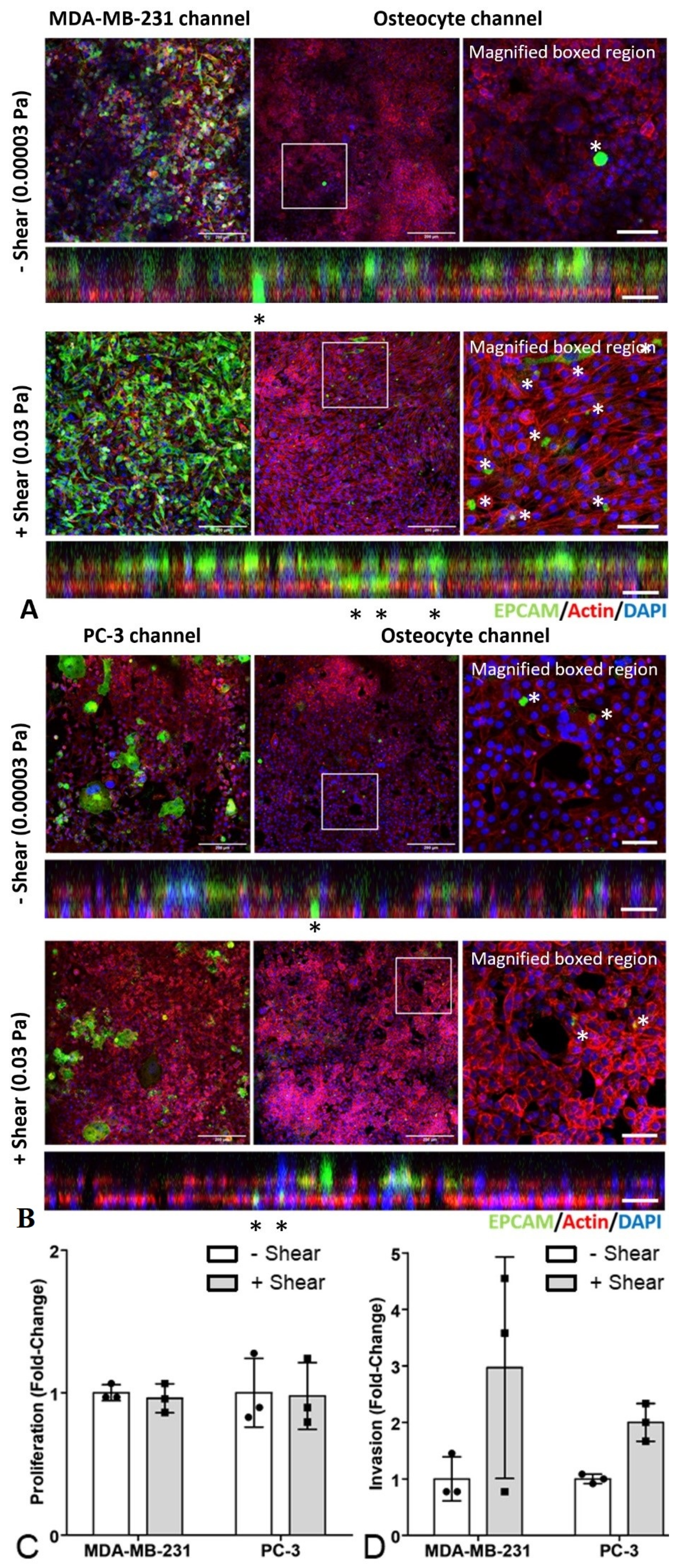
2.4. Invasion Assays
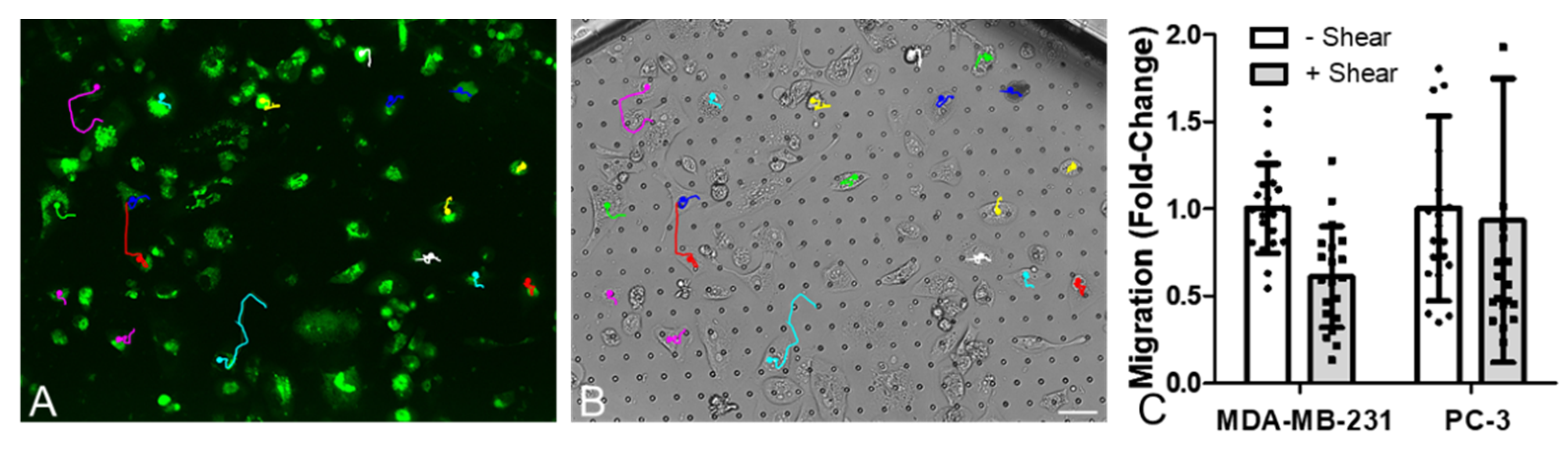
2.5. Migration Assays
2.6. Immunocytochemistry and Microscopy
2.7. Statistical Analysis
3. Results
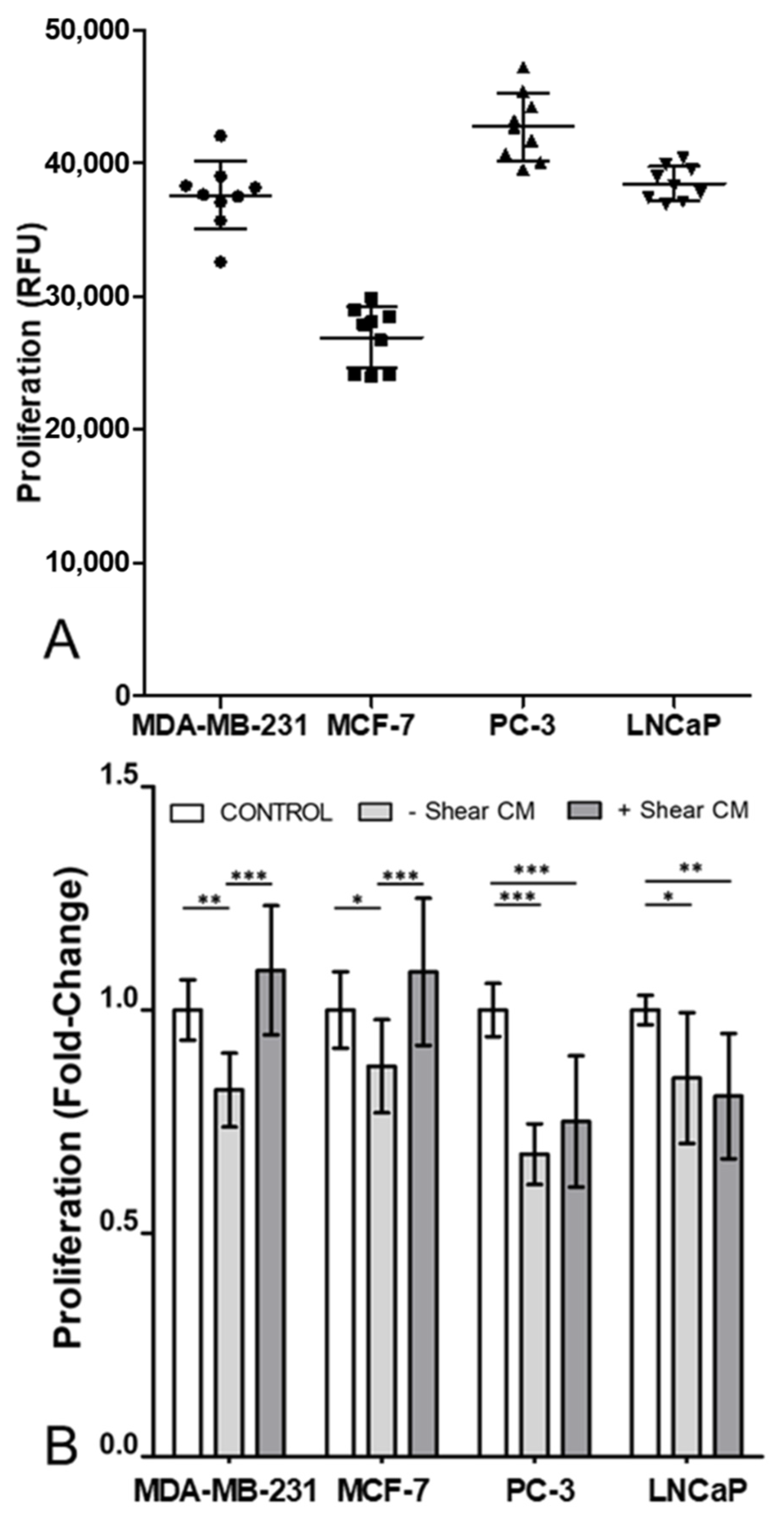
3.1. Proliferation in All Cancer Cell Lines Was Decreased by Osteocyte Conditioned Media, with Mechanical Stimulation Reversing This Effect in Breast Cancer Cells
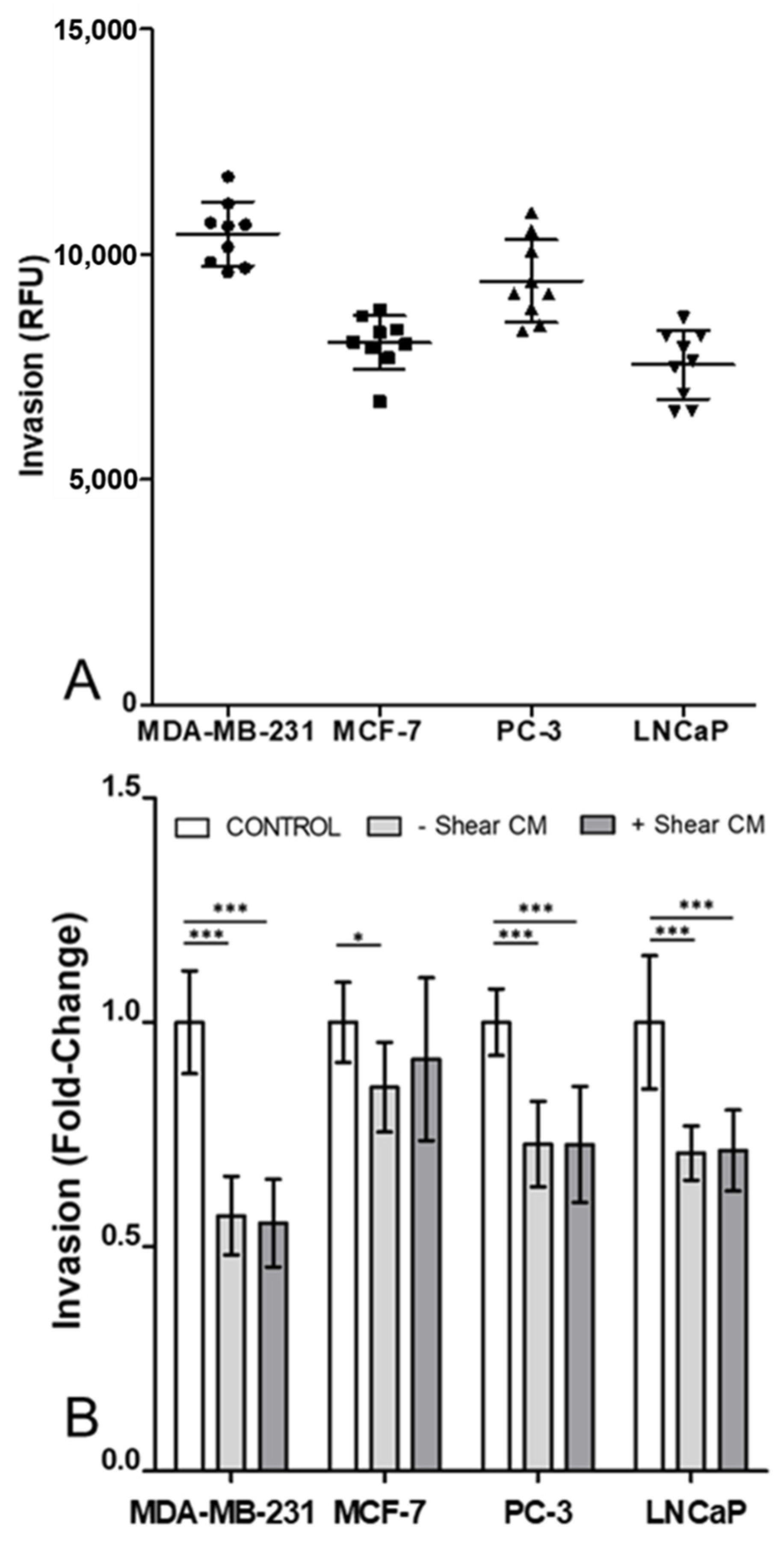
3.2. Invasion of Cancer Cells Was Decreased by Osteocyte Conditioned Media with and without Mechanical Stimulation
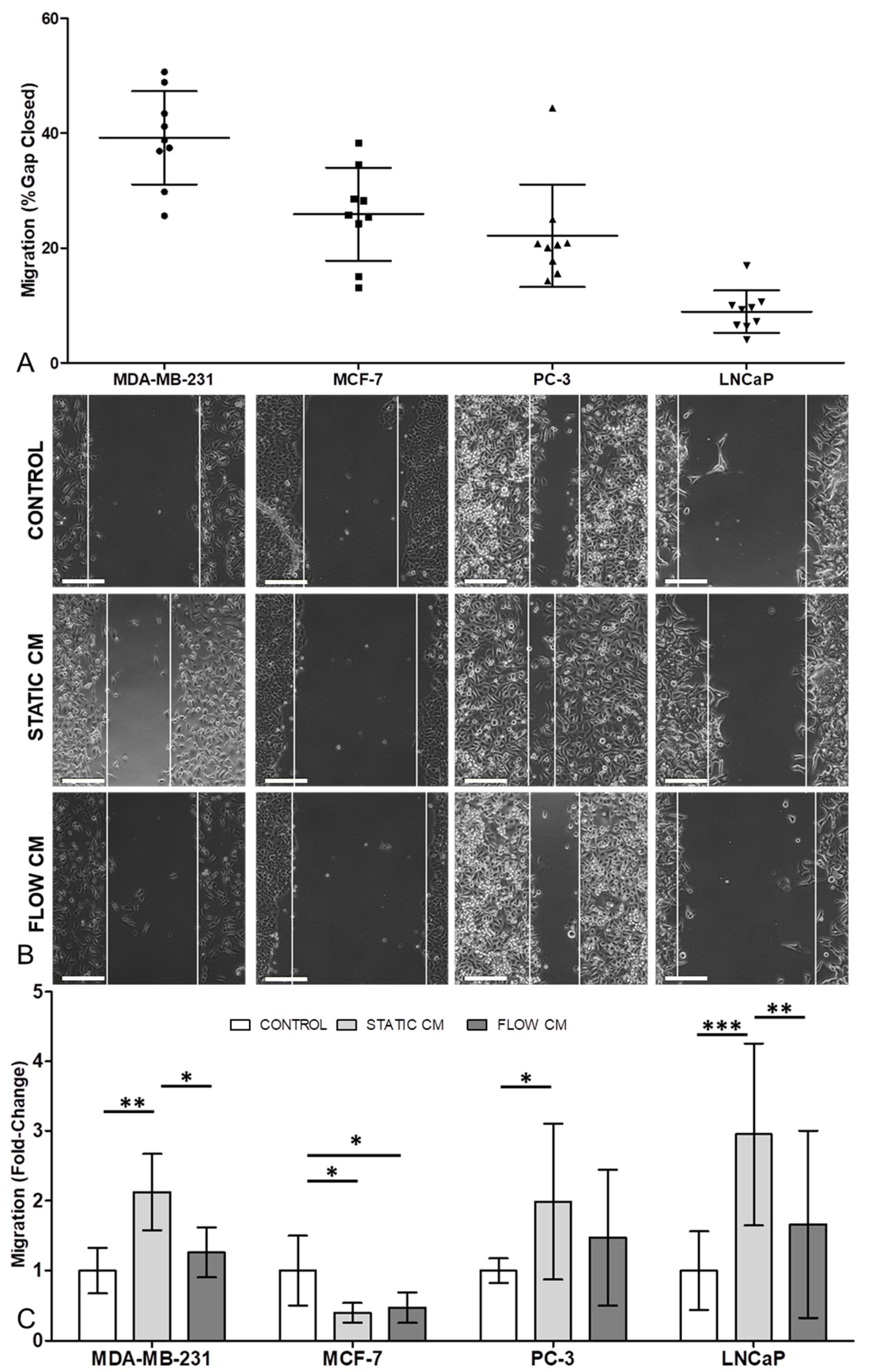
3.3. Migration in Three of the Four Cancer Cell Lines Was Increased by Osteocyte Conditioned Media, with Mechanical Stimulation of Osteocytes Reversing this Effect
3.4. An Organ-Chip Co-culture Model Replicated Effect of Conditioned Media on Cancer Cell Migration but Not Proliferation or Invasion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CLOBOCAN 2020: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. 2020. Available online: https://gco.iarc.fr/ (accessed on 14 April 2020).
- Roodman, D.G. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- David Roodman, G.; Silbermann, R. Mechanisms of osteolytic and osteoblastic skeletal lesions. Bonekey Rep. 2015, 4, 753. [Google Scholar] [CrossRef]
- Hosseini, H.; Obradovic, M.M.S.; Hoffmann, M.; Harper, K.L.; Sosa, M.S.; Werner-Klein, M.; Nanduri, L.K.; Werno, C.; Ehrl, C.; Maneck, M.; et al. Early dissemination seeds metastasis in breast cancer. Nature 2016, 540, 552–558. [Google Scholar] [CrossRef]
- Hüsemann, Y.; Geigl, J.B.; Schubert, F.; Musiani, P.; Meyer, M.; Burghart, E.; Forni, G.; Eils, R.; Fehm, T.; Riethmüller, G.; et al. Systemic spread is an early step in breast cancer. Cancer Cell 2008, 13, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Charles Coombes, R.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G.; et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research, UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk (accessed on 4 January 2020).
- McNeely, M.L.; Campbell, K.L.; Rowe, B.H.; Klassen, T.P.; Mackey, J.R.; Courneya, K.S. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. Can. Med. Assoc. J. 2006, 175, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.L.; Winters-Stone, K.; Gallucci, B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol. Nurs. Forum 2007, 34, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E.; Brooks, D.; Mohanan, S.; Lee, M.J.; Polamraju, P.; Dent, K.; Bonassar, L.J.; van der Meulen, M.C.H.; Fischbach, C. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J. Bone Miner. Res. 2013, 28, 2357–2367. [Google Scholar] [CrossRef]
- Bourke, L.; Smith, D.; Steed, L.; Hooper, R.; Carter, A.; Catto, J.; Albertsen, P.C.; Tombal, B.; Payne, H.A.; Rosario, D.J. Exercise for men with prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2016, 69, 693–703. [Google Scholar] [CrossRef]
- Uth, J.; Hornstrup, T.; Schmidt, J.F.; Christensen, J.F.; Frandsen, C.; Christensen, K.B.; Helge, E.W.; Brasso, K.; Rørth, M.; Midtgaard, J.; et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scand. J. Med. Sci. Sports 2014, 24, 105–112. [Google Scholar] [CrossRef]
- Liverani, C.; Mercatali, L.; Cristofolini, L.; Giordano, E.; Minardi, S.; Porta, G.D.; De Vita, A.; Miserocchi, G.; Spadazzi, C.; Tasciotti, E.; et al. Investigating the mechanobiology of cancer cell–ECM interaction through collagen-based 3D scaffolds. Cell. Mol. Bioeng. 2017, 10, 223–234. [Google Scholar] [CrossRef]
- Liverani, C.; De Vita, A.; Minardi, S.; Kang, Y.; Mercatali, L.; Amadori, D.; Bongiovanni, A.; La Manna, F.; Ibrahim, T.; Tasciotti, E. A biomimetic 3D model of hypoxia-driven cancer progression. Sci. Rep. 2019, 9, 12263. [Google Scholar] [CrossRef]
- Chen, F.; Han, Y.; Kang, Y. Bone marrow niches in the regulation of bone metastasis. Br. J. Cancer 2021, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Guise, T.A.; Mccauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11. [Google Scholar] [CrossRef]
- Esposito, M.; Mondal, N.; Greco, T.M.; Wei, Y.; Spadazzi, C.; Lin, S.-C.; Zheng, H.; Cheung, C.; Magnani, J.L.; Lin, S.-H.; et al. Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 2019, 21, 627–639. [Google Scholar] [CrossRef]
- Scimeca, M.; Antonacci, C.; Toschi, N.; Giannini, E.; Bonfiglio, R.; Buonomo, C.O.; Pistolese, C.A.; Tarantino, U.; Bonanno, E. Breast osteoblast-like cells: A reliable early marker for bone metastases from breast cancer. Clin. Breast Cancer 2018, 18, e659–e669. [Google Scholar] [CrossRef]
- Scimeca, M.; Urbano, N.; Rita, B.; Mapelli, S.N.; Catapano, C.V.; Carbone, G.M.; Ciuffa, S.; Tavolozza, M.; Schillaci, O.; Mauriello, A.; et al. Prostate osteoblast-like cells: A reliable prognostic marker of bone metastasis in prostate cancer patients. Contrast Media Mol. Imaging 2018, 2018, 9840962. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Kennedy, O.D. Osteocyte signaling in bone. Curr. Osteoporos. Rep. 2012, 10, 118–125. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Buenzli, P.R.; Sims, N.A. Quantifying the osteocyte network in the human skeleton. Bone 2015, 75, 144–150. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Cheung, W.-Y.; Majeska, R.; Kennedy, O. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; Mcnamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cells Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Vaughan, T.J.; Verbruggen, S.W.; McNamara, L.M. Are all osteocytes equal? Multiscale modelling of cortical bone to characterise the mechanical stimulation of osteocytes. Int. J. Numer. Method. Biomed. Eng. 2013, 29, 1361–1372. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Vaughan, T.J.; McNamara, L.M. Strain amplification in bone mechanobiology: A computational investigation of the in vivo mechanics of osteocytes. J. R. Soc. Interface 2012, 9, 2735–2744. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Mc Garrigle, M.J.; Haugh, M.G.; Voisin, M.C.; McNamara, L.M. Altered mechanical environment of bone cells in an animal model of short- and long-term osteoporosis. Biophys. J. 2015, 108, 1587–1598. [Google Scholar] [CrossRef]
- Uda, Y.; Azab, E.; Sun, N.; Shi, C.; Pajevic, P.D. Osteocyte mechanobiology. Curr. Osteoporos. Rep. 2017, 15, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, T.J.; Mullen, C.A.; Verbruggen, S.W.; McNamara, L.M. Bone cell mechanosensation of fluid flow stimulation: A fluid–structure interaction model characterising the role integrin attachments and primary cilia. Biomech. Model. Mechanobiol. 2015, 14, 703–718. [Google Scholar] [CrossRef]
- Dole, N.S.; Mazur, C.M.; Acevedo, C.; Ritchie, R.O.; Mohammad, K.S.; Correspondence, T.A. Osteocyte-intrinsic TGF-b signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017, 21, 2585–2596. [Google Scholar] [CrossRef]
- Qing, H.; Ardeshirpour, L.; Pajevic, P.D.; Dusevich, V.; Jähn, K.; Kato, S.; Wysolmerski, J.; Bonewald, L.F. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 2012, 27, 1018–1029. [Google Scholar] [CrossRef]
- Nango, N.; Kubota, S.; Hasegawa, T.; Yashiro, W.; Momose, A.; Matsuo, K. Osteocyte-directed bone demineralization along canaliculi. Bone 2016, 84, 279–288. [Google Scholar] [CrossRef]
- Pitsillides, A.A.; Rawlinson, S.C.F.; Suswillo, R.F.L.; Bourrin, S.; Zaman, G.; Yon, L.E.L. Mechanical strain-induced NO production by bone cells: A possible role in adaptive bone (re)modeling? FASEB J. 1995, 9, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Smalt, R.; Mitchell, F.T.; Howard, R.L.; Chambers, T.J. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am. J. Physiol. Metab. 1997, 273, E751–E758. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Yellowley, C.E.; Donahue, H.J.; Zhang, Y.; Chen, Q.; Jacobs, C.R. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J. Biomech. Eng. 2000, 122, 387–393. [Google Scholar] [CrossRef]
- Mei, X.; Middleton, K.; Shim, D.; Wan, Q.; Xu, L.; Ma, Y.H.V.; Devadas, D.; Walji, N.; Wang, L.; Young, E.W.K.; et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr. Biol. Camb. 2019, 11, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.V.; Lam, C.; Dalmia, S.; Gao, P.; Young, J.; Middleton, K.; Liu, C.; Xu, H.; You, L. Mechanical regulation of breast cancer migration and apoptosis via direct and indirect osteocyte signaling. J. Cell. Biochem. 2018, 119, 5665–5675. [Google Scholar] [CrossRef]
- Cui, Y.X.; Evans, B.A.J.; Jiang, W.G. New roles of osteocytes in proliferation, migration and invasion of breast and prostate cancer cells. Anticancer. Res. 2016, 36, 1193–1202. [Google Scholar]
- Dwivedi, A.; Kiely, P.A.; Hoey, D.A. Mechanically stimulated osteocytes promote the proliferation and migration of breast cancer cells via a potential CXCL1/2 mechanism. Biochem. Biophys. Res. Commun. 2021, 534, 14–20. [Google Scholar] [CrossRef]
- Ma, Y.H.V.; Xu, L.; Mei, X.; Middleton, K.; You, L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J. Cell. Biochem. 2019, 120, 7590–7601. [Google Scholar] [CrossRef] [PubMed]
- Bahmaee, H.; Owen, R.; Boyle, L.; Perrault, C.M.; Garcia-Granada, A.A.; Reilly, G.C.; Claeyssens, F. Design and evaluation of an osteogenesis-on-a-chip microfluidic device incorporating 3D Cell culture. Front. Bioeng. Biotechnol. 2020, 8, 1042. [Google Scholar] [CrossRef]
- Sheyn, D.; Cohn-Yakubovich, D.; Ben-David, S.; De Mel, S.; Chan, V.; Hinojosa, C.; Wen, N.; Hamilton, G.A.; Gazit, D.; Gazit, Z. Bone-chip system to monitor osteogenic differentiation using optical imaging graphic abstract keywords organ-on-a-chip mesenchymal stem cells osteogenesis optical imaging. Microfluid. Nanofluidics 2019, 23, 99. [Google Scholar] [CrossRef]
- Torisawa, Y.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat. Methods 2014, 11, 663–669. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; You, L.; Wang, L. Quantifying fluid shear stress in a rocking culture dish. J. Biomech. 2010, 43, 1598–1602. [Google Scholar] [CrossRef]
- Hoey, D.A.; Kelly, D.J.; Jacobs, C.R. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2011, 412, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W.; Vaughan, T.J.; McNamara, L.M. Fluid flow in the osteocyte mechanical environment: A fluid-structure interaction approach. Biomech. Model. Mechanobiol. 2014, 13, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W.; Vaughan, T.J.; McNamara, L.M. Mechanisms of osteocyte stimulation in osteoporosis. J. Mech. Behav. Biomed. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Zhou, X.; Li, W.; Wang, L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: Direct evidence for load-induced fluid flow. J. Bone Miner. Res. 2011, 26, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef]
- Jain, A.; Barrile, R.; van der Meer, A.D.; Mammoto, A.; Mammoto, T.; De Ceunynck, K.; Aisiku, O.; Otieno, M.A.; Louden, C.S.; Hamilton, G.A.; et al. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin. Pharmacol. Ther. 2018, 103, 332–340. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. Vitr. Cell Migr. Invasion Assays. J. Vis. Exp. 2014, 88, 51046. [Google Scholar] [CrossRef]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy an introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.E.; Ottewell, P.D.; Rucci, N.; Peyruchaud, O.; Pagnotti, G.M.; Chiechi, A.; Buijs, J.T.; Sterling, J.A. Murine models of breast cancer bone metastasis. Bonekey Rep. 2016, 5, 804. [Google Scholar] [CrossRef]
- Wu, X.; Gong, S.; Roy-Burman, P.; Lee, P.; Culig, Z. Current mouse and cell models in prostate cancer research. Endocr. Relat. Cancer 2013, 20, R155–R170. [Google Scholar] [CrossRef]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell … and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef]
- Schurman Charles, A.; Verbruggen Stefaan, W.; Alliston, T. Degenerated lacunocanalicular networks, mass transport and osteocyte pericellular fluid flow in bone with aging and disrupted TGFB signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2023999118. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbruggen, S.W.; Thompson, C.L.; Duffy, M.P.; Lunetto, S.; Nolan, J.; Pearce, O.M.T.; Jacobs, C.R.; Knight, M.M. Mechanical Stimulation Modulates Osteocyte Regulation of Cancer Cell Phenotype. Cancers 2021, 13, 2906. https://doi.org/10.3390/cancers13122906
Verbruggen SW, Thompson CL, Duffy MP, Lunetto S, Nolan J, Pearce OMT, Jacobs CR, Knight MM. Mechanical Stimulation Modulates Osteocyte Regulation of Cancer Cell Phenotype. Cancers. 2021; 13(12):2906. https://doi.org/10.3390/cancers13122906
Chicago/Turabian StyleVerbruggen, Stefaan W., Clare L. Thompson, Michael P. Duffy, Sophia Lunetto, Joanne Nolan, Oliver M. T. Pearce, Christopher R. Jacobs, and Martin M. Knight. 2021. "Mechanical Stimulation Modulates Osteocyte Regulation of Cancer Cell Phenotype" Cancers 13, no. 12: 2906. https://doi.org/10.3390/cancers13122906
APA StyleVerbruggen, S. W., Thompson, C. L., Duffy, M. P., Lunetto, S., Nolan, J., Pearce, O. M. T., Jacobs, C. R., & Knight, M. M. (2021). Mechanical Stimulation Modulates Osteocyte Regulation of Cancer Cell Phenotype. Cancers, 13(12), 2906. https://doi.org/10.3390/cancers13122906







