Identifying High-Risk Tumors within AJCC Stage IB–III Melanomas Using a Seven-Marker Immunohistochemical Signature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Immunohistochemical Expression Analysis
2.3. Calculation of the 7-Marker-Signature Risk Score
2.4. Statistical Analysis
2.5. REMARK
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCCv8 | American Joint Committee on Cancer 8th Edition Cancer Staging |
| Bax | Bcl-2-associated X protein |
| CI | confidence interval |
| COX-2 | Cyclooxygenase-2 |
| DMFS | distant metastasis-free survival |
| FFPE | formalin-fixed paraffin-embedded |
| HR | hazard ratio |
| IHC | Immunohistochemistry |
| MTAP | S-methyl-5′-thioadenosine phosphorylase |
| NPV | negative predictive value |
| PPV | positive predictive value |
| OS | overall survival |
| PTEN | Phosphatase and Tensin Homolog |
| REMARK | Reporting Recommendations for Tumor Marker Prognostic Studies |
| RFS | relapse-free survival |
References
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef] [PubMed]
- Azzola, M.F.; Shaw, H.M.; Thompson, J.F.; Soong, S.J.; Scolyer, R.A.; Watson, G.F.; Colman, M.H.; Zhang, Y. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: An analysis of 3661 patients from a single center. Cancer 2003, 97, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Poklepovic, A.S.; Luke, J.J. Considering adjuvant therapy for stage II melanoma. Cancer 2020, 126, 1166–1174. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Kanaki, T.; Stang, A.; Gutzmer, R.; Zimmer, L.; Chorti, E.; Sucker, A.; Ugurel, S.; Hadaschik, E.; Gräger, N.S.; Satzger, I.; et al. Impact of American Joint Committee on Cancer 8th edition classification on staging and survival of patients with melanoma. Eur. J. Cancer 2019, 119, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Baade, P.D.; Olsen, C.M. More people die from thin melanomas (⩽1 mm) than from thick melanomas (>4 mm) in Queensland, Australia. J. Investig. Dermatol. 2015, 135, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Scholl, L.; Bechara, F.G.; Stockfleth, E.; Stücker, M. Worse outcome for patients with recurrent melanoma after negative sentinel lymph biopsy as compared to sentinel-positive patients. Eur. J. Surg Oncol. 2016, 42, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.C.; Han, G.; Leong, S.P.; Kashani-Sabet, M.; Vetto, J.; Pockaj, B.; White, R.L.; Faries, M.B.; Schneebaum, S.; Mozzillo, N.; et al. Recurrence of Melanoma After a Negative Sentinel Node Biopsy: Predictors and Impact of Recurrence Site on Survival. Ann. Surg. Oncol. 2019, 26, 2254–2262. [Google Scholar] [CrossRef]
- Weiss, S.A.; Hanniford, D.; Hernando, E.; Osman, I. Revisiting determinants of prognosis in cutaneous melanoma. Cancer 2015, 121, 4108–4123. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Fuchs, T.J.; Bosserhoff, A.K.; Hofstädter, F.; Pauer, A.; Roth, V.; Buhmann, J.M.; Moll, I.; Anagnostou, N.; Brandner, J.M.; et al. A seven-marker signature and clinical outcome in malignant melanoma: A large-scale tissue-microarray study with two independent patient cohorts. PLoS ONE 2012, 7, e38222. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur. J. Cancer 2005, 41, 1690–1696. [Google Scholar] [CrossRef]
- Zager, J.S.; Gastman, B.R.; Leachman, S.; Gonzalez, R.C.; Fleming, M.D.; Ferris, L.K.; Ho, J.; Miller, A.R.; Cook, R.W.; Covington, K.R.; et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Bellomo, D.; Arias-Mejias, S.M.; Quattrocchi, E.; Sominidi-Damodaran, S.; Bridges, A.G.; Lehman, J.S.; Hieken, T.J.; Jakub, J.W.; Murphree, D.H.; et al. Identification of stage I/IIA melanoma patients at high risk for disease relapse using a clinicopathologic and gene expression model. Eur. J. Cancer 2020, 140, 11–18. [Google Scholar] [CrossRef]
- Schoffer, O.; Schülein, S.; Arand, G.; Arnholdt, H.; Baaske, D.; Bargou, R.C.; Becker, N.; Beckmann, M.W.; Bodack, Y.; Böhme, B.; et al. Tumour stage distribution and survival of malignant melanoma in Germany 2002–2011. BMC Cancer 2016, 16, 936. [Google Scholar] [CrossRef] [PubMed]
- Svedman, F.C.; Pillas, D.; Taylor, A.; Kaur, M.; Linder, R.; Hansson, J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—A systematic review of the literature. Clin. Epidemiol. 2016, 8, 109–122. [Google Scholar] [CrossRef]
- Criscione, V.D.; Weinstock, M.A. Melanoma thickness trends in the United States, 1988–2006. J. Investig. Dermatol. 2010, 130, 793–797. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, B.E.; Schafer, C.N.; Han, S.W.; Osman, I.; Zhong, H.; Brinster, N. Computer-assisted measurement of primary tumor area is prognostic of recurrence-free survival in stage IB melanoma patients. Mod. Pathol. 2017, 30, 1402–1410. [Google Scholar] [CrossRef]
- Satzger, I.; Leiter, U.; Gräger, N.; Keim, U.; Garbe, C.; Gutzmer, R. Melanoma-specific survival in patients with positive sentinel lymph nodes: Relevance of sentinel tumor burden. Eur. J. Cancer 2019, 123, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kunte, C.; Geimer, T.; Baumert, J.; Konz, B.; Volkenandt, M.; Flaig, M.; Ruzicka, T.; Berking, C.; Schmid-Wendtner, M.H. Prognostic factors associated with sentinel lymph node positivity and effect of sentinel status on survival: An analysis of 1049 patients with cutaneous melanoma. Melanoma Res. 2010, 20, 330–337. [Google Scholar] [CrossRef]
- Keller, J.; Schwartz, T.L.; Lizalek, J.M.; Chang, E.S.; Patel, A.D.; Hurley, M.Y.; Hsueh, E.C. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019, 8, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Greenhaw, B.N.; Zitelli, J.A.; Brodland, D.G. Estimation of Prognosis in Invasive Cutaneous Melanoma: An Independent Study of the Accuracy of a Gene Expression Profile Test. Dermatol. Surg. 2018, 44, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, E.C.; DeBloom, J.R.; Lee, J.; Sussman, J.J.; Covington, K.R.; Middlebrook, B.; Johnson, C.; Cook, R.W.; Slingluff, C.L.; McMasters, K.M. Interim analysis of survival in a prospective, multi-center registry cohort of cutaneous melanoma tested with a prognostic 31-gene expression profile test. J. Hematol. Oncol. 2017, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.A.; Coit, D.G.; Dusza, S.W.; Yu, A.; McLean, L.; Hu, Y.; Nanda, J.K.; Matsoukas, K.; Mancebo, S.E.; Bartlett, E.K. Performance of Gene Expression Profile Tests for Prognosis in Patients With Localized Cutaneous Melanoma: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 953–962. [Google Scholar] [CrossRef]
- Amaral, T.M.S.; Hoffmann, M.C.; Sinnberg, T.; Niessner, H.; Sülberg, H.; Eigentler, T.K.; Garbe, C. Clinical validation of a prognostic 11-gene expression profiling score in prospectively collected FFPE tissue of patients with AJCC v8 stage II cutaneous melanoma. Eur. J. Cancer 2020, 125, 38–45. [Google Scholar] [CrossRef]
- Schermers, B.; Franke, V.; Rozeman, E.A.; van de Wiel, B.A.; Bruining, A.; Wouters, M.W.; van Houdt, W.J.; Ten Haken, B.; Muller, S.H.; Bierman, C.; et al. Surgical removal of the index node marked using magnetic seed localization to assess response to neoadjuvant immunotherapy in patients with stage III melanoma. Br. J. Surg. 2019, 106, 519–522. [Google Scholar] [CrossRef]
- Hodges, M.; Jones, E.; Jones, T.; Pearlman, N.; Gajdos, C.; Kounalakis, N.; McCarter, M. Analysis of melanoma recurrence following a negative sentinel lymph node biopsy. Melanoma Manag. 2015, 2, 285–294. [Google Scholar] [CrossRef]
- Moody, J.A.; Botham, S.J.; Dahill, K.E.; Wallace, D.L.; Hardwicke, J.T. Complications following completion lymphadenectomy versus therapeutic lymphadenectomy for melanoma—A systematic review of the literature. Eur. J. Surg. Oncol. 2017, 43, 1760–1767. [Google Scholar] [CrossRef]
- De Vries, M.; Hoekstra, H.J.; Hoekstra-Weebers, J.E. Quality of life after axillary or groin sentinel lymph node biopsy, with or without completion lymph node dissection, in patients with cutaneous melanoma. Ann. Surg. Oncol. 2009, 16, 2840–2847. [Google Scholar] [CrossRef]
- Hu, Y.; Briggs, A.; Gennarelli, R.L.; Bartlett, E.K.; Ariyan, C.E.; Coit, D.G.; Brady, M.S. Sentinel Lymph Node Biopsy for T1b Melanoma: Balancing Prognostic Value and Cost. Ann. Surg. Oncol. 2020, 27, 5248–5256. [Google Scholar] [CrossRef]
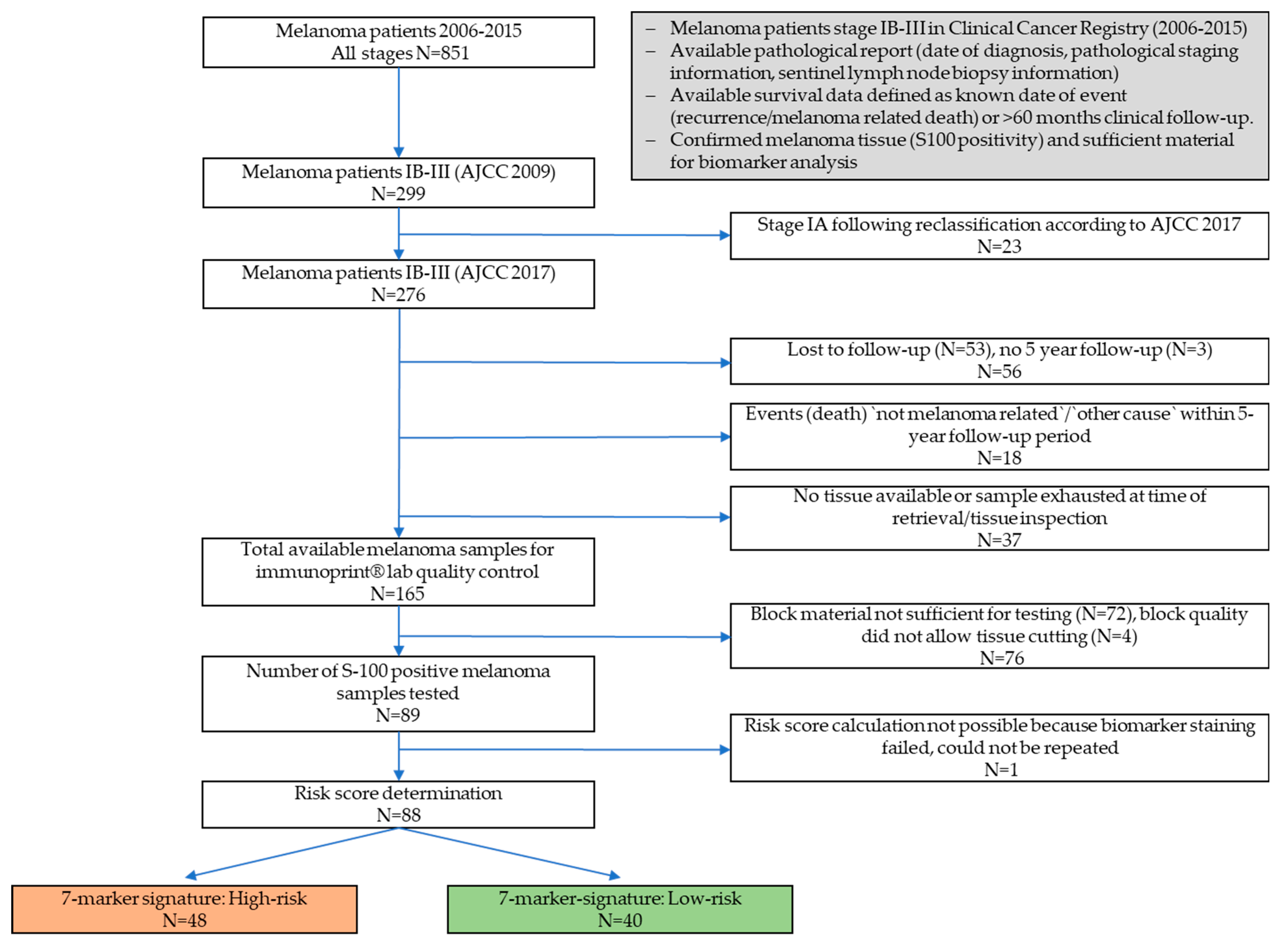

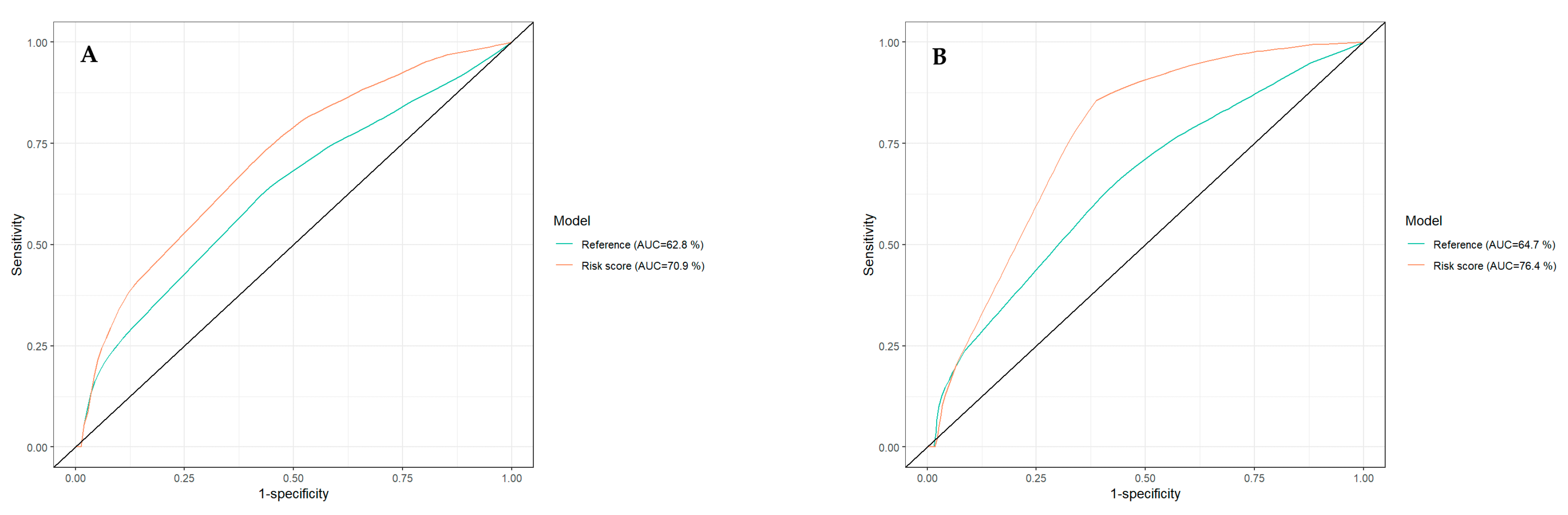
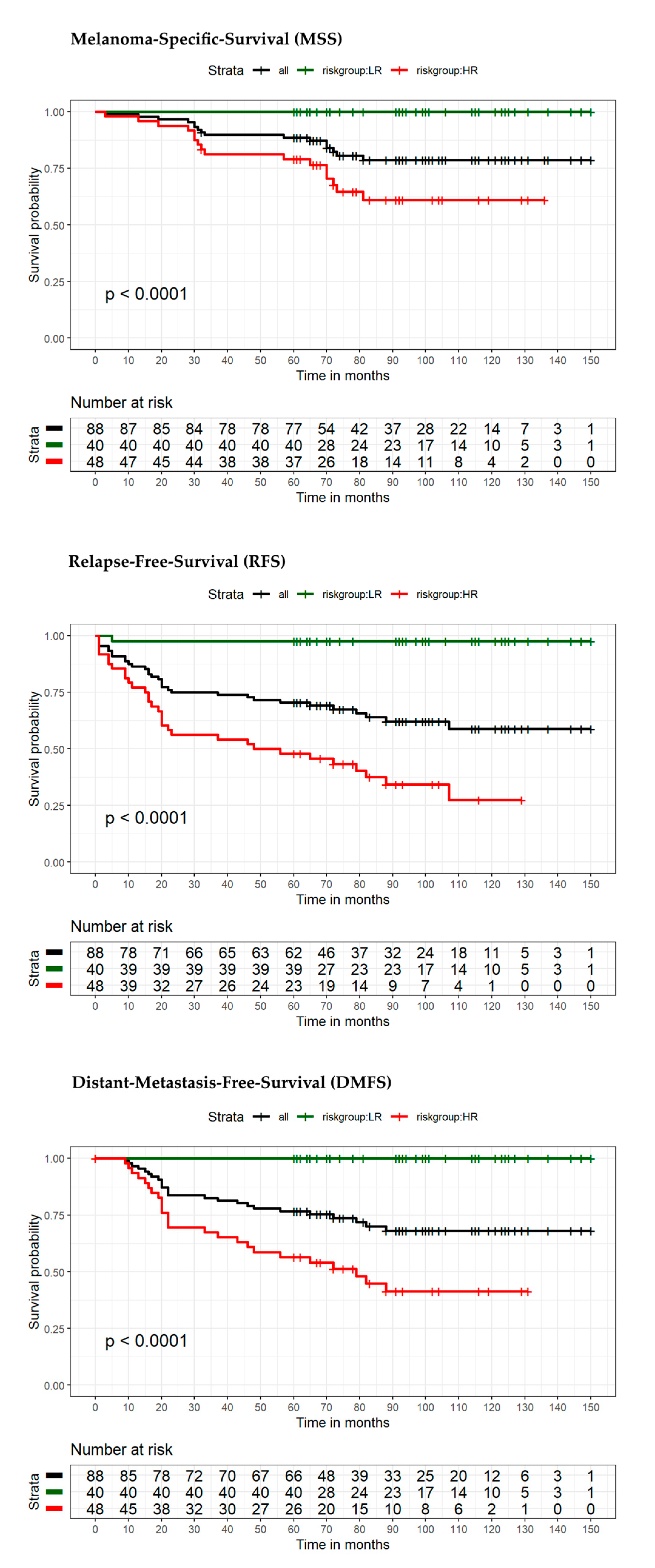
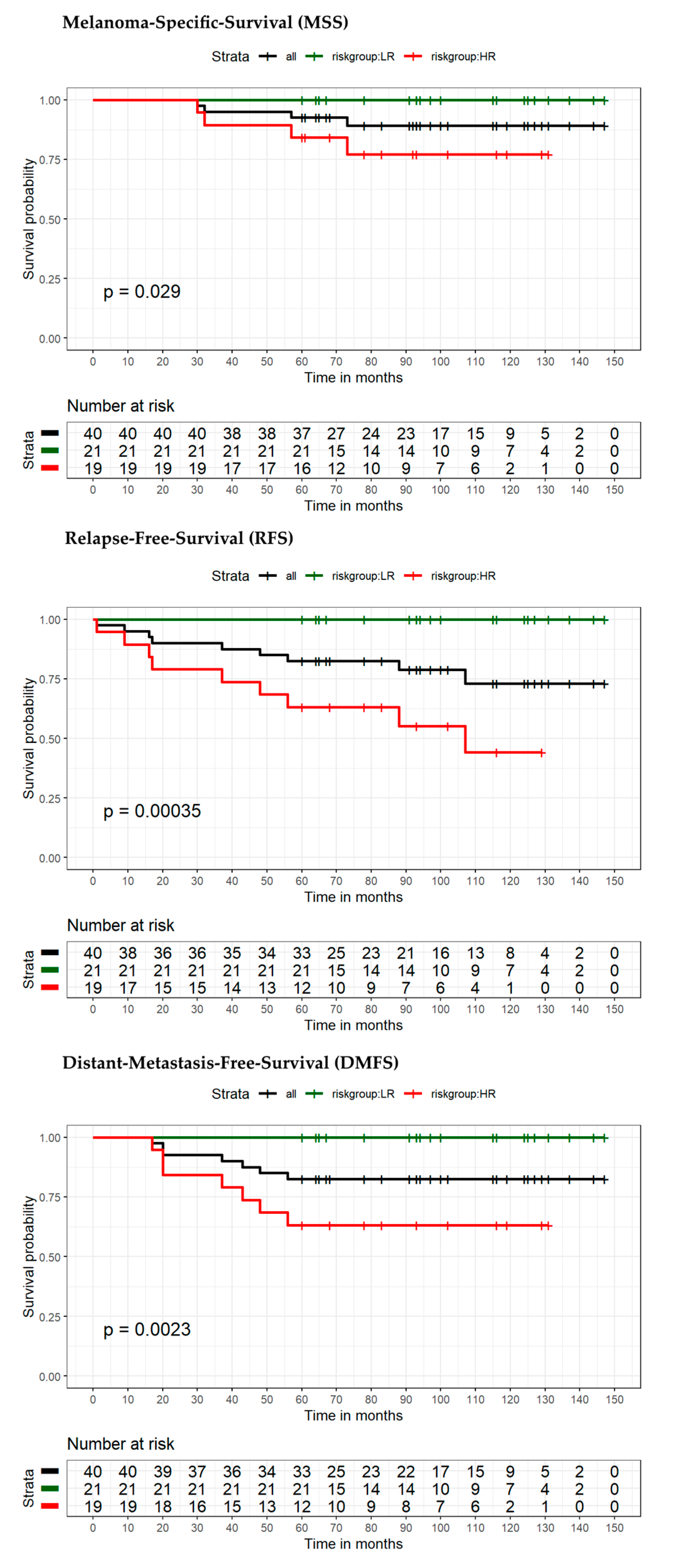
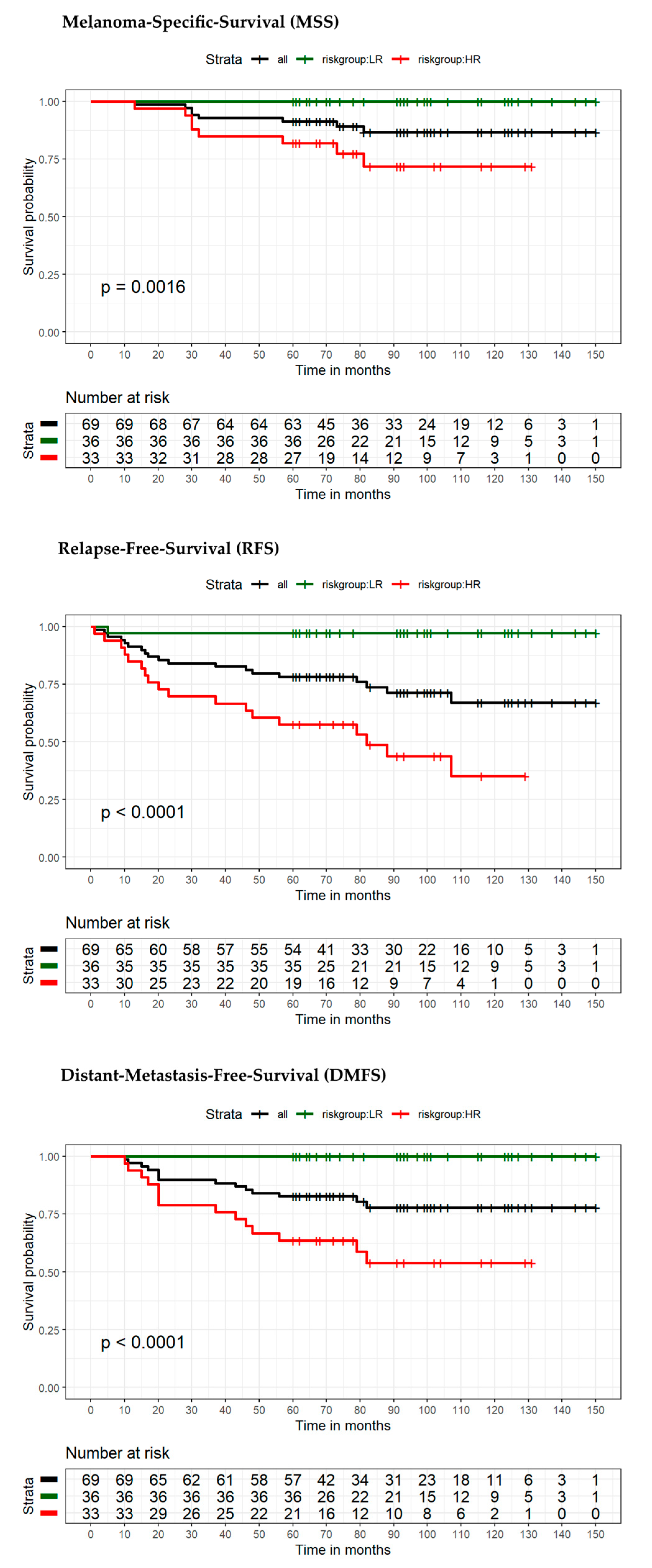
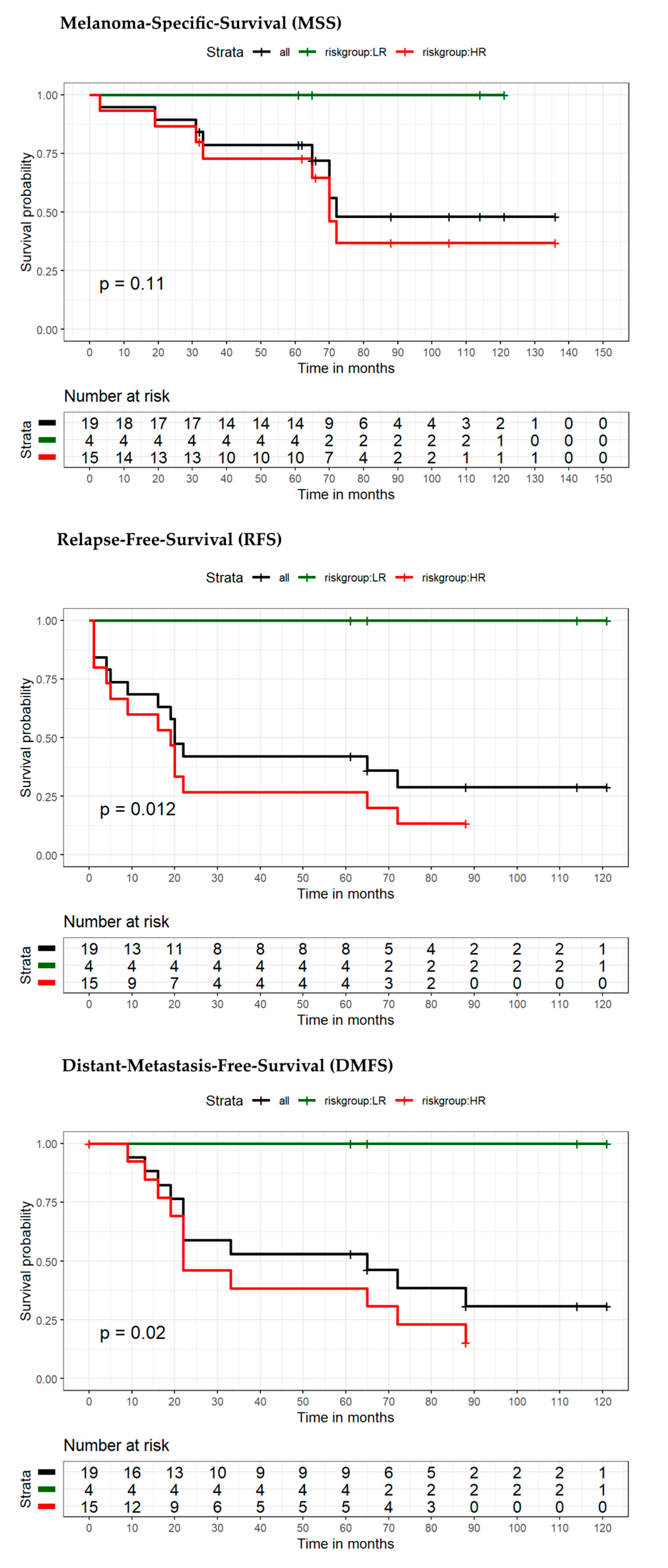
| Variable | Category | Overall Patients (n = 88) | Low-Risk Patients (n = 40) | High-Risk Patients (n = 48) | p-Value |
|---|---|---|---|---|---|
| Follow-up time (months) | Mean (SD) | 94.9 (28.5) | 73.1 (33) | 0.0013 | |
| Median (min, max) | 93.5 (60–150) | 71 (3–136) | |||
| Gender | 0.8304 | ||||
| Male | 48 (54.6%) | 21 (23.9%) | 27 (30.7%) | ||
| Female | 40 (45.5%) | 19 (21.6%) | 21 (23.9%) | ||
| Age (years) | Mean (SD) | 63.4 (17.6) | 64.1 (16.2) | 0.8556 | |
| Median (min, max) | 69.5 (17–90) | 66 (27–93) | |||
| Tumor thickness | Mean (SD) | 2.6 (1.7) | 3.9 (3) | 0.012 | |
| Median (min, max) | 2.1 (1.1–8) | 3 (1–14) | |||
| Ulceration | 0.0557 | ||||
| Yes | 41 (46.6%) | 14 (15.9%) | 27 (30.7%) | ||
| No | 47 (53.4%) | 26 (29.5%) | 21 (23.9%) | ||
| SLN status | n.c. | ||||
| Positive 1 | 19 (21.6%) | 4 (4.5%) | 15 (17%) | ||
| Negative 0 | 69 (78.4%) | 36 (40.9%) | 33 (37.5%) | ||
| -cN0 | 6 (6.8%) | 3 (3.4%) | 3 (3.4%) | ||
| -pN0 | 63 (71.6%) | 33 (37.5%) | 30 (34.1%) | ||
| pTNM (AJCC8) | n.c. | ||||
| IB | 24 (27.3%) | 16 (18.2%) | 8 (9.1%) | ||
| IIA | 16 (18.2%) | 5 (5.7%) | 11 (12.5%) | ||
| IIB | 20 (22.7%) | 13 (14.8%) | 7 (8%) | ||
| IIC | 9 (10.2%) | 2 (2.3%) | 7 (8%) | ||
| IIIA | 1 (1.1%) | 1 (1.1%) | 0 (0%) | ||
| IIIB | 5 (5.7%) | 1 (1.1%) | 4 (4.5%) | ||
| IIIC | 13 (14.8) | 2 (2.3%) | 11 (12.5%) |
| Univariate | Multivariable | ||||
|---|---|---|---|---|---|
| Patients/Cohort | Variables/ Prognostic Factors | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Entire cohort (n = 88) (Events: MSS: 16 RFS: 32) | MSS | ||||
| 7-mrs * | 2.05 (1.21–3.46) | 0.007 | 1.73 (0.98–3.05) | 0.060 | |
| Tumor thickness (mm) | 1.27 (1.10–1.46) | 0.001 | 1.21 (1.05–1.40) | 0.011 | |
| Ulceration | 2.12 (0.77–5.84) | 0.146 | 1.09 (0.37–3.26) | 0.874 | |
| Nodal status | 4.51 (1.68–12.07) | 0.003 | 2.66 (0.91–7.77) | 0.074 | |
| Age at diagnosis | 1.00 (0.97–1.03) | 0.880 | --- | --- | |
| Gender | 1.01 (0.38–2.71) | 0.988 | --- | --- | |
| RFS | |||||
| 7-mrs | 38.68 (5.27–284.15) | <0.001 | 30.60 (4.11–227.76) | <0.001 | |
| Tumor thickness (mm) | 1.25 (1.13–1.38) | <0.001 | 1.14 (1.02–1.27) | 0.022 | |
| Ulceration | 2.03 (1.00–4.12) | 0.051 | 1.11 (0.51–2.42) | 0.792 | |
| Nodal status | 3.79 (1.86–7.71) | <0.001 | 2.15 (1.01–4.56) | 0.046 | |
| Age at diagnosis | 1.00 (0.98–1.03) | 0.737 | --- | --- | |
| Gender | 0.60 (0.29–1.24) | 0.167 | --- | --- | |
| MSS | DMFS | RFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients/Cohort | High Risk | Low Risk | Total | High Risk | Low Risk | Total | High Risk | Low Risk | Total | |
| Entire cohort (n = 88) | No event | 32 | 40 | 72 | 23 | 40 | 63 | 16 | 39 | 55 |
| Event | 16 | 0 | 16 | 25 | 0 | 25 | 32 | 1 | 33 | |
| Total | 48 | 40 | 88 | 48 | 40 | 88 | 48 | 40 | 88 | |
| Sensitivity | 100% | PPV | 33.3% | 100% | PPV | 52.1% | 97% | PPV | 66.7% | |
| Specificity | 55.6% | NPV | 100% | 63.5% | NPV | 100% | 70.9% | NPV | 97.5% | |
| Increase in risk | 183.3% | 183.3% | 177.8% | |||||||
| Stage IB–IIC (n = 69) | No event | 25 | 36 | 61 | 19 | 36 | 55 | 15 | 35 | 50 |
| Event | 8 | 0 | 8 | 14 | 0 | 14 | 18 | 1 | 19 | |
| Total | 33 | 36 | 69 | 33 | 36 | 69 | 33 | 36 | 69 | |
| Sensitivity | 100% | PPV | 24.2% | 100% | PPV | 42.4% | 94.7% | PPV | 54.6% | |
| Specificity | 59% | NPV | 100% | 65.5% | NPV | 100% | 70% | NPV | 97.2% | |
| Increase in risk | 209.1% | 209.1% | 198.1% | |||||||
| Stage IB–IIA (n = 40) | No event | 15 | 21 | 36 | 12 | 21 | 33 | 10 | 21 | 31 |
| Event | 4 | 0 | 4 | 7 | 0 | 7 | 9 | 0 | 9 | |
| Total | 19 | 21 | 40 | 19 | 21 | 40 | 19 | 21 | 40 | |
| Sensitivity | 100% | PPV | 21.1% | 100% | PPV | 36.8% | 100% | PPV | 47.4% | |
| Specificity | 58.3% | NPV | 100% | 63.6% | NPV | 100% | 67.7% | NPV | 100% | |
| Increase in risk | 210.5% | 210.5% | 210.5% | |||||||
| Stage III (n = 19) | No event | 7 | 4 | 11 | 4 | 4 | 8 | 2 | 4 | 6 |
| Event | 8 | 0 | 8 | 11 | 0 | 11 | 13 | 0 | 13 | |
| Total | 15 | 4 | 19 | 15 | 4 | 19 | 15 | 4 | 19 | |
| Sensitivity | 100% | PPV | 53.3% | 100% | PPV | 73.3% | 100% | PPV | 86.7% | |
| Specificity | 36.4% | NPV | 100% | 50% | NPV | 100% | 66.7% | NPV | 100% | |
| Increase in risk | 126.7% | 130.1% | 126.7% | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reschke, R.; Gussek, P.; Ziemer, M. Identifying High-Risk Tumors within AJCC Stage IB–III Melanomas Using a Seven-Marker Immunohistochemical Signature. Cancers 2021, 13, 2902. https://doi.org/10.3390/cancers13122902
Reschke R, Gussek P, Ziemer M. Identifying High-Risk Tumors within AJCC Stage IB–III Melanomas Using a Seven-Marker Immunohistochemical Signature. Cancers. 2021; 13(12):2902. https://doi.org/10.3390/cancers13122902
Chicago/Turabian StyleReschke, Robin, Philipp Gussek, and Mirjana Ziemer. 2021. "Identifying High-Risk Tumors within AJCC Stage IB–III Melanomas Using a Seven-Marker Immunohistochemical Signature" Cancers 13, no. 12: 2902. https://doi.org/10.3390/cancers13122902
APA StyleReschke, R., Gussek, P., & Ziemer, M. (2021). Identifying High-Risk Tumors within AJCC Stage IB–III Melanomas Using a Seven-Marker Immunohistochemical Signature. Cancers, 13(12), 2902. https://doi.org/10.3390/cancers13122902






