Primary Thromboprophylaxis in Patients with Malignancies: Daily Practice Recommendations by the Hemostasis Working Party of the German Society of Hematology and Medical Oncology (DGHO), the Society of Thrombosis and Hemostasis Research (GTH), and the Austrian Society of Hematology and Oncology (ÖGHO)
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results and Recommendations: General Recommendations for Primary Thromboprophylaxis in Patients with Malignancies
3.1. Studies in Unselected Cancer Patient Cohorts
3.1.1. VTE Prevention in Patients with Cancer Hospitalized Due to Medical Reasons
3.1.2. VTE Prevention in Ambulatory Patients with Cancer Receiving Anti-Cancer Therapy
- Based on recommendations for hospitalized patients due to medical illness in general, we recommend prophylactic anticoagulation for cancer patients in an in-patient setting, except for fit/mobile cancer patients admitted due to non-surgical procedures. In these cases, recommendations for outpatients should be followed (see below).
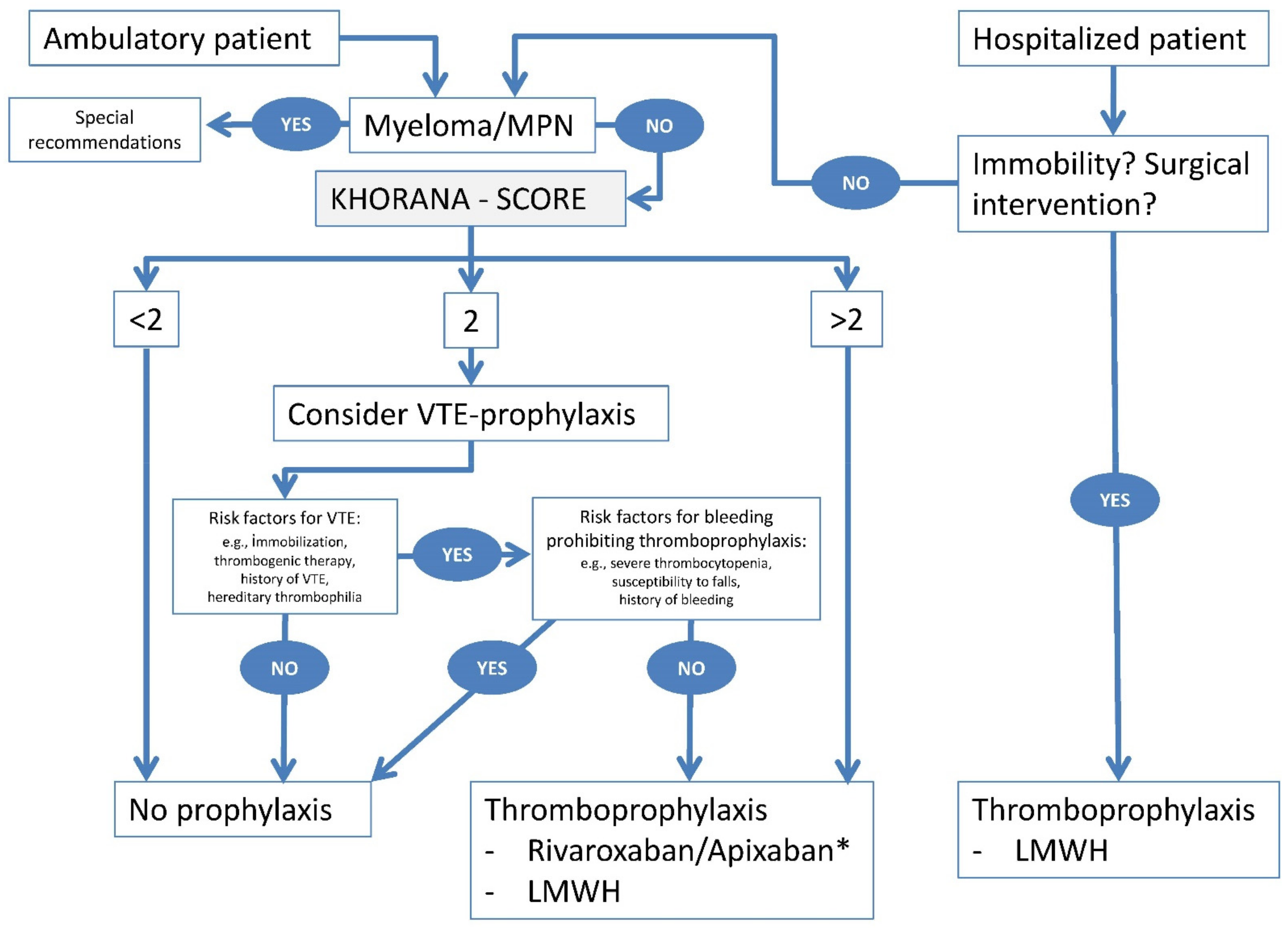
- Due to the wide variability of VTE risk in ambulatory cancer patients, we do not recommend primary pharmacological prophylaxis in general. This topic will be discussed in the following paragraphs. Refer also to Figure 1.
3.2. Studies in Selected Cancer Patient Cohorts
3.2.1. Low-Molecular-Weight Heparins (LMWHs)
3.2.2. Direct Oral Factor Xa Inhibitors (DOACs)
- (i)
- Mortality due to VTE complications in cancer patients is much higher than mortality due to bleeding complications [1].
- (ii)
- The International Society on Thrombosis and Haemostasis (ISTH) definition for major bleeding [49] applied in clinical trials has never been validated in cancer patients undergoing therapy. This is important, as the two main criteria to categorize a major bleeding event according to ISTH, namely a 2 g/dL drop of hemoglobin and transfusion requirement, are well-known events in non-bleeding cancer patients undergoing systemic cancer treatment. It should thus be realized that bleeding events in this patient population are up-graded in most of the events from “minor” to “major”.
- (iii)
- Despite the KS being validated for many cancer patients, this scoring system is not discriminative in all cancers [50], and it does not include well-known thrombogenic risk factors such as the history of VTE, highly elevated D-dimer levels, age, degree of mobilization, presence of thrombocytopenia, or thrombogenic or pro-hemorrhagic anti-cancer therapies. One other prediction model has been demonstrated in a post-hoc analysis of an interventional study to allow an improved risk prediction and decreased NNT compared to the Khorana score (Table 2).
| Patients’ Characteristics | Score |
|---|---|
| Site of cancer: | |
| – Very high risk (pancreas or stomach) – High risk (lung, lymphoma, gynecological, bladder, or testicular) | +2 +1 |
| Prechemotherapy platelet count 350 × 109/L or higher | +1 |
| Hemoglobin level less than 100 g/L or use of red cell growth factors | +1 |
| Prechemotherapy leucocyte count more than 11 × 109/L | +1 |
| BMI 35 kg/m2 or more | +1 |
- The available evidence is in favor of primary pharmacological prevention (LMWH either prophylactic or half-therapeutic dose in certain cancer types such as pancreatic cancer, or apixaban 2 × 2.5 mg or rivaroxaban 1 × 10 mg) in ambulatory cancer patients undergoing systemic anti-cancer therapy with a Khorana score (KS) of > 2 or, although less evidence-based, those identified with other risk scores or with other high-risk factors for VTE. However, given the limited available data, the panel agreed that any such recommendation must be made on an individual basis, and it should take into account the patient´s preference.
3.3. Drug Interactions in Patients with Malignancies Receiving Prophylactic Anticoagulation
- If prophylactic anticoagulation is indicated, the choice of optimal oral drug should be considered on an individual basis taking into account drug interactions and the bioavailability (e.g., patients after abdominal surgery). In uncertain cases, LMWH should be preferred to DOACs.
4. Specific situations of Prophylactic Anticoagulation in Patients with Malignancies
4.1. Peri-Interventional Thromboprophylaxis in Patients with Cancer Undergoing Elective Invasive Procedures
- Prophylactic anticoagulation in cancer patients undergoing major abdominal or pelvic surgery should be extended to 4–5 weeks after surgery.
- Thromboprophylaxis should be performed with LMWH, UFH (second choice), or fondaparinux. The exact peri-operative timing of these drugs is described in the respective Summary of Product Characteristics (SmPC).
- Mechanical prophylaxis (compression stockings or intermittent pneumatic compression) may be performed in patients with contraindications for anticoagulation.
4.2. Thromboprophylaxis in Patients with Cancer and Renal Insufficiency
- In patients with renal dysfunction, the risk of bleeding during thromboprophylaxis is increased. However, no firm clinical evidence exists on how to adjust the dose of thromboprophylactic agents. Therefore, we recommend a very thorough assessment of the necessity for and the dose of pharmacologic thromboprophylaxis in patients with reduced eGFR, particularly those with an eGFR below 30 mL/min. The risk of bleeding has to be weighed against the risk of thromboembolism.
- Anti-Xa plasma level monitoring during LMWH thromboprophylaxis is helpful in patients with stage 4 to 5 chronic kidney disease, with optimal levels (3–4 h after injection) being 0.1 to 0.3 IU/mL for prophylactic dosing [78]. However, clinical judgment currently remains the main factor in deciding on the dose of these agents.
4.3. Thromboprophylaxis in Patients with Cancer and Liver Disease
- There are no evidence-based recommendations on prophylaxis of cancer-associated VTE in patients with severe liver disease. We recommend individualized treatment regimens after shared decision-making with patients.
- Prophylactic dose anticoagulation may usually be offered since the bleeding risk is low. Careful clinical and laboratory monitoring is suggested, especially in patients with prolonged prothrombin time.
- In patients with liver disease and a history of severe bleeding, e.g., from esophageal varices, it might be prudent not to use pharmacological thromboprophylaxis.
- Make sure the patient has been informed about clinically relevant bleeding symptoms and about signs of thromboembolism. He/She should know whom to contact when developing such symptoms and where to go after office hours or on the weekend.
4.4. Thromboprophylaxis in Patients with Cancer and Elevated Bleeding Risk
- (i)
- Tumor-site specific bleeding risk: patients with glioblastoma/CNS tumors carry a high risk for the development of VTE in the first six-month period from diagnosis [94,95]. Prophylactic application of LMWH non-significantly decreases the VTE incidence but, in contrast, is associated with a higher risk of intracranial bleeding [96,97]. Nevertheless, prophylactic anticoagulation is generally recommended in this cohort [98]. In addition, individually risk-adapted algorithms addressing the safety of primary VTE prophylaxis (i.e., prevention of VTE on the one and clinically relevant major bleedings (CRMB) on the other hand) should be applied for lymphomas (depending on the stage and sites of lymphoma), lung tumors (with respect to the involvement of arterial vessels and/or mediastinal, pleural or pericardial manifestations) and tumors of the genitourinary tract in general.
- (ii)
- Individual/situational bleeding risk factors: renal impairment, liver dysfunction, and low platelet counts are addressed in separate sections. Moreover, platelet dysfunction, preexisting bleeding disorders (i.e., coagulopathy or (acquired) von Willebrand Syndrome), history of severe bleeding episodes, as well as poor functional status, and age of > 75 years may contribute to an increased bleeding risk. On the other hand, perioperative and periprocedural settings can implicate a higher risk of local (mucosal, intracranial, or intraspinal) or systemic bleeding.
- (iii)
- Drug-specific risk factors for bleeding: tumor-specific treatment (i.e., BTK inhibitor—ibrutinib or inhibitors of VEGFR-associated tyrosine kinases i.e., axitinib, cabozantinib, lenvatinib, nintedanib, pazopanib, regorafenib, sorafenib, sunitinib, tivozanib or vandetanib) and/or concomitant pain medication (e.g., NSAIDs) may contribute to an increased risk of bleeding. These drugs have to be handled carefully with frequent monitoring of clinical aspects indicating increased individual bleeding risk.
- Tumor-site specific risk: an individual assessment of bleeding risk has to be carried out, especially in patients with glioblastomas, lymphomas, gastrointestinal cancers, lung cancers, and tumors of the genitourinary tract.
- Individual/situational risk: laboratory tests reflecting organ function and platelet count as well as age, bleeding history have to be evaluated carefully. In the context of interventional procedures, management and scoring of bleeding risk are recommended for individual adaptation of thromboprophylaxis.
- Drug-specific risk factors: bleeding risk has to be assessed and monitored according to the anti-cancer medication and other co-medication.
4.5. Thromboprophylaxis in Patients with Cancer and Thrombocytopenia
- we recommend against thromboprophylaxis in cancer patients, if platelet counts are <25 G/L.
- pharmacologic thromboprophylaxis with LMWH may be considered if platelet counts are between 25 and 50 G/L.
- we recommend against mechanical prophylaxis (pneumatic intermittent compression or compression stockings) in patients with platelet counts <50 G/L.
4.6. Thromboprophylaxis in Patients with Cancer and Thrombophilia
- Patients with malignancies and co-existing thrombophilia require special attention and potentially alternative prophylactic anticoagulation. However, prospective data on prophylactic anticoagulation are lacking. We thus recommend adhering to guidelines for non-cancer patients with thrombophilia.
- Breast cancer patients receiving tamoxifen treatment have an increased VTE risk if they have a heterozygous factor V Leiden mutation, and we recommend genetic testing if this information is likely to change clinical management. There are no general guidelines on thromboprophylaxis in these patients.
- We recommend thromboprophylaxis in patients with cancer and homozygous or combined (compound) factor V and/or prothrombin mutations or antithrombin, protein C, or protein S deficiency if they have a Khorona-Score ≥ 2 or other thrombogenic risk factors.
- We recommend routine prophylactic anticoagulation with LMWH in high-risk situations for patients without previous thromboembolism who have a heterozygous factor V Leiden mutation or a heterozygous prothrombin mutation G20210A. Whether DOACs are equally efficacious, has yet to be determined, but they have been used in cancer patients, and the studies did not exclude patients with thrombophilia.
- Patients with thrombotic APS and cancer require long-term therapeutic anticoagulation with vitamin K antagonists or adjusted doses of LMWH +/- aspirin. DOACs must be avoided in high-risk APS (i.e., triple-positive APS) and should also be avoided in low-risk APS.
- We recommend thromboprophylaxis in patients with PNH and cancer. However, it has to be weighed against the risk of bleeding and the potential of other drugs (e.g., eculizumab) to prevent thromboembolism.
- Patients with certain Bcr-Abl negative MPN (all PV, int-/high-risk ET) should receive acetylsalicylic acid (ASA) and potentially additional primary VTE prophylaxis (individual decision for LMWH or DOAC).
4.7. Thromboprophylaxis in Patients with Cancer and Indwelling Catheters
- It is not generally recommended to administer prophylactic anticoagulation in ambulatory cancer patients solely because of implanted central venous catheters.
- The role of DOACs in this setting remains unclear.
4.8. Thromboprophylaxis in Patients with Multiple Myeloma
- VTE risk assessment is recommended in patients with MM at diagnosis and during the course of the disease.
- The SAVED score (for patients treated with IMiDs) and the IMPEDE-VTE score (independent of IMiD therapy) are recommended as validated VTE risk assessment tools and classified into low and high VTE risk. In low-risk patients, ASA or no intervention is recommended, whereas prophylaxis with LMWH is recommended in high-risk patients.
- The use of DOACs in high-risk patients is being examined in current studies.
4.9. Proposed Algorithm for Thromboprophylaxis in Individual Patients with Cancer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.T.; Mellemkjær, L.; Olsen, J.H.; Baron, J.A. Prognosis of Cancers Associated with Venous Thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, H.E.; Tafur, A.J.; Caprini, J.A.; Alatri, A.; Trujillo-Santos, J.; Farge-Bancel, D.; Rosa, V.; Font, C.; Vilaseca, A.; Monreal, M.; et al. Prediction of early mortality in patients with cancer-associated thrombosis in the RIETE Database. Int. Angiol. 2019, 38, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, H.E.; Oramas, D.M.; Paz, L.H.; Wang, Y.; Andrade, X.A.; Tafur, A.J. Venous Thromboembolism Is an Independent Predictor of Mortality Among Patients with Gastric Cancer. J. Gastrointest. Cancer 2018, 49, 415–421. [Google Scholar] [CrossRef] [PubMed]
- de Meis, E.; Pinheiro, V.R.; Zamboni, M.M.; Guedes, M.T.; Castilho, I.A.; Martinez, M.M.; Leda, M.S.; Silveira, N.P.; Rumjanek, V.M.; Levy, R.A. Clotting, immune system, and venous thrombosis in lung adenocarcinoma patients: A prospective study. Cancer Investig. 2009, 27, 989–997. [Google Scholar] [CrossRef]
- Fuentes, H.; Oramas, D.; Paz, L.; Casanegra, A.; Mansfield, A.; Tafur, A. Meta-analysis on anticoagulation and prevention of thrombosis and mortality among patients with lung cancer. Thromb. Res. 2017, 154, 28–34. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.J.; Zhou, H.; White, R.H. Incidence of Venous Thromboembolism and the Impact on Survival in Breast Cancer Patients. J. Clin. Oncol. 2007, 26, 70–76. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Sidana, S.; Elson, P.; Khorana, A.A.; McCrae, K.R. Symptomatic and Incidental Venous Thromboembolic Disease Are Both Associated with Mortality in Patients with Prostate Cancer. PLoS ONE 2014, 9, e94048. [Google Scholar] [CrossRef][Green Version]
- Mahajan, A.; Wun, T.; Chew, H.; White, R.H. Lymphoma and venous thromboembolism: Influence on mortality. Thromb. Res. 2014, 133, S23–S28. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Ortel, T.L.; Francis, C.W. Impact of Venous Thromboembolism and Anticoagulation on Cancer and Cancer Survival. J. Clin. Oncol. 2009, 27, 4902–4911. [Google Scholar] [CrossRef]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O'Fallon, W.M.; Melton, L.J., 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef]
- Barbui, T.; Finazzi, G.; Falanga, A. Myeloproliferative neoplasms and thrombosis. Blood 2013, 122, 2176–2184. [Google Scholar] [CrossRef]
- Ahlbrecht, J.; Dickmann, B.; Ay, C.; Dunkler, D.; Thaler, J.; Schmidinger, M.; Quehenberger, P.; Haitel, A.; Zielinski, C.; Pabinger, I. Tumor Grade Is Associated With Venous Thromboembolism in Patients With Cancer: Results From the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2012, 30, 3870–3875. [Google Scholar] [CrossRef]
- Khorana, A.A.; Dalal, M.; Lin, J.; Connolly, G.C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2012, 119, 648–655. [Google Scholar] [CrossRef]
- Zhou, H.; Romano, P.S.; White, R.H. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb. Haemost. 2003, 90, 446–455. [Google Scholar] [CrossRef]
- Carrier, M.; Khorana, A.A.; Moretto, P.; Le Gal, G.; Karp, R.; Zwicker, J.I. Lack of Evidence to Support Thromboprophylaxis in Hospitalized Medical Patients with Cancer. Am. J. Med. 2014, 127, 82–86.e1. [Google Scholar] [CrossRef]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Kuderer, N.M.; Carrier, M.; Ortel, T.L.; Wun, T.; Rubens, D.; Hobbs, S.; Iyer, R.; Peterson, D.; et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Thromb. Res. 2017, 151, 89–95. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Ventresca, M.; Crowther, M.; Briel, M.; Zhou, Q.; Garcia, D.; Lyman, G.; Noble, S.; Macbeth, F.; Griffiths, G.; et al. Use of heparins in patients with cancer: Individual participant data meta-analysis of randomised trials study protocol. BMJ Open 2016, 6, e010569. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Ventresca, M.; Crowther, M.; Briel, M.; Zhou, Q.; Noble, S.; Macbeth, F.; Griffiths, G.; Garcia, D.; Lyman, G.H.; et al. Evaluating prophylactic heparin in ambulatory patients with solid tumours: A systematic review and individual participant data meta-analysis. Lancet Haematol. 2020, 7, e746–e755. [Google Scholar] [CrossRef]
- Xin, Z.; Liu, F.; Du, Y.; Mao, F.; Wang, X.; Xu, P.; Li, Z.; Qian, J.; Yao, J. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients: A systematic review and network meta-analysis. Ann. Palliat. Med. 2020, 9, 2970–2981. [Google Scholar] [CrossRef]
- Pelzer, U.; Opitz, B.; Deutschinoff, G.; Stauch, M.; Reitzig, P.C.; Hahnfeld, S.; Müller, L.; Grunewald, M.; Stieler, J.M.; Sinn, M.; et al. Efficacy of Prophylactic Low–Molecular Weight Heparin for Ambulatory Patients With Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J. Clin. Oncol. 2015, 33, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; George, D.J.; Kakkar, A.K.; Fisher, W.; Lassen, M.R.; Mismetti, P.; Mouret, P.; Chaudhari, U.; Lawson, F.; Turpie, A.G. Semuloparin for Thromboprophylaxis in Patients Receiving Chemotherapy for Cancer. N. Engl. J. Med. 2012, 366, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.V.; Balibrea, J.L.; Martínez-González, J.; Prandoni, P. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: The CANBESURE randomized study. J. Thromb. Haemost. 2010, 8, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Verso, M. Thromboprophylaxis during chemotherapy in patients with advanced cancer. Thromb. Res. 2010, 125 (Suppl. 2), S17–S20. [Google Scholar] [CrossRef]
- Maraveyas, A.; Waters, J.; Roy, R.; Fyfe, D.; Propper, D.; Lofts, F.; Sgouros, J.; Gardiner, E.; Wedgwood, K.; Ettelaie, C.; et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur. J. Cancer 2012, 48, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Samama, M.M.; Cohen, A.T.; Darmon, J.-Y.; Desjardins, L.; Eldor, A.; Janbon, C.; Leizorovicz, A.; Nguyen, H.; Olsson, C.-G.; Turpie, A.G.; et al. A Comparison of Enoxaparin with Placebo for the Prevention of Venous Thromboembolism in Acutely Ill Medical Patients. N. Engl. J. Med. 1999, 341, 793–800. [Google Scholar] [CrossRef]
- Leizorovicz, A.; Cohen, A.T.; Turpie, A.G.; Olsson, C.-G.; Vaitkus, P.T.; Goldhaber, S.Z. Randomized, Placebo-Controlled Trial of Dalteparin for the Prevention of Venous Thromboembolism in Acutely Ill Medical Patients. Circ. 2004, 110, 874–879. [Google Scholar] [CrossRef]
- Cohen, A.T.; Davidson, B.L.; Gallus, A.S.; Lassen, M.R.; Prins, M.H.; Tomkowski, W.; Turpie, A.G.G.; Egberts, J.F.M.; Lensing, A.W.A. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: Randomised placebo controlled trial. BMJ 2006, 332, 325–329. [Google Scholar] [CrossRef]
- Riess, H.; Haas, S.; Tebbe, U.; Gerlach, H.-E.; Abletshauser, C.; Sieder, C.; Rossol, S.; Pfeiffer, B.; Schellong, S.M. A randomized, double-blind study of certoparin vs. unfractionated heparin to prevent venous thromboembolic events in acutely ill, non-surgical patients: CERTIFY Study. J. Thromb. Haemost. 2010, 8, 1209–1215. [Google Scholar] [CrossRef]
- Haas, S.; Schellong, S.M.; Tebbe, U.; Gerlach, H.-E.; Bauersachs, R.; Melzer, N.; Abletshauser, C.; Sieder, C.; Bramlage, P.; Riess, H. Heparin based prophylaxis to prevent venous thromboembolic events and death in patients with cancer—A subgroup analysis of CERTIFY. BMC Cancer 2011, 11, 316. [Google Scholar] [CrossRef]
- Lyman, G.H.; Bohlke, K.; Khorana, A.A.; Kuderer, N.M.; Lee, A.Y.; Arcelus, J.I.; Balaban, E.P.; Clarke, J.M.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update 2014. J. Clin. Oncol. 2015, 33, 654–656. [Google Scholar] [CrossRef]
- Mandalà, M.; Labianca, R. Venous thromboembolism (VTE) in cancer patients. ESMO Clinical Recommendations for prevention and management. Thromb. Res. 2010, 125, S117–S119. [Google Scholar] [CrossRef]
- S3-Leitlinie Prophylaxe der Venösen Thromboembolie (VTE). Available online: https://www.awmf.org/uploads/tx_szleitlinien/003-001l_S3_VTE-Prophylaxe_2015-12.pdf (accessed on 31 July 2019).
- Riess, H.; Pabinger-Fasching, I.; Alt-Epping, B.; Demarmels Biasiutti, F.; Langer, F.; Wörmann, B. Venöse Thrombembolien (VTE) bei Tumorpatienten. Available online: https://www.onkopedia.com/de/onkopedia/guidelines/venoese-thrombembolien-vte-bei-tumorpatienten/@@guideline/html/index.html (accessed on 6 August 2019).
- Patell, R.; Rybicki, L.; McCrae, K.R.; Khorana, A.A. Predicting risk of venous thromboembolism in hospitalized cancer patients: Utility of a risk assessment tool. Am. J. Hematol. 2017, 92, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Gussoni, G.; Bianchini, C.; Verso, M.; Mandalà, M.; Cavanna, L.; Barni, S.; Labianca, R.; Buzzi, F.; Scambia, G.; et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009, 10, 943–949. [Google Scholar] [CrossRef]
- Ek, L.; Gezelius, E.; Bergman, B.; Bendahl, P.; Anderson, H.; Sundberg, J.; Wallberg, M.; Falkmer, U.; Verma, S.; Belting, M. Randomized phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: The RASTEN trial. Ann. Oncol. 2018, 29, 398–404. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
- Cohen, A.T.; Katholing, A.; Rietbrock, S.; Bamber, L.; Martinez, C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb. Haemost. 2017, 117, 57–65. [Google Scholar] [CrossRef]
- Di Nisio, M.; Porreca, E.; Candeloro, M.; De Tursi, M.; Russi, I.; Rutjes, A.W. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst. Rev. 2016, 12, Cd008500. [Google Scholar] [CrossRef]
- Akl, E.A.; Kahale, L.A.; Ballout, R.A.; Barba, M.; Yosuico, V.E.D.; Van Doormaal, F.F.; Middeldorp, S.; Bryant, A.; Schünemann, H. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst. Rev. 2014, 12, CD006652. [Google Scholar] [CrossRef]
- Meyer, G.; Besse, B.; Doubre, H.; Charles-Nelson, A.; Aquilanti, S.; Izadifar, A.; Azarian, R.; Monnet, I.; Lamour, C.; Descourt, R.; et al. Anti-tumour effect of low molecular weight heparin in localised lung cancer: A phase III clinical trial. Eur. Respir. J. 2018, 52, 1801220. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Dunkler, D.; Marosi, C.; Chiriac, A.-L.; Vormittag, R.; Simanek, R.; Quehenberger, P.; Zielinski, C.; Pabinger, I. Prediction of venous thromboembolism in cancer patients. Blood 2010, 116, 5377–5382. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Le Gal, G.; Wells, P.S. Preventing Venous Thromboembolism in Patients with Cancer. Reply. N. Engl. J. Med. 2019, 380, 2181. [Google Scholar] [CrossRef]
- Kumar, V.; Shaw, J.R.; Key, N.S.; Ilich, A.; Mallick, R.; Wells, P.S.; Carrier, M. D-Dimer Enhances Risk-Targeted Thromboprophylaxis in Ambulatory Patients with Cancer. Oncologist 2020, 25, 1075–1083. [Google Scholar] [CrossRef]
- Pabinger, I.; van Es, N.; Heinze, G.; Posch, F.; Riedl, J.; Reitter, E.-M.; Di Nisio, M.; Cesarman-Maus, G.; Kraaijpoel, N.; Zielinski, C.C.; et al. A clinical prediction model for cancer-associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol. 2018, 5, e289–e298. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee Of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Tafur, A.J.; Wang, C.E.; Kourelis, T.V.; Wysokinska, E.M.; Yang, P. Predictors of active cancer thromboembolic outcomes: Validation of the Khorana score among patients with lung cancer. J. Thromb. Haemost. 2016, 14, 1773–1778. [Google Scholar] [CrossRef]
- Preissner, S.; Kroll, K.; Dunkel, M.; Senger, C.; Goldsobel, G.; Kuzman, D.; Guenther, S.; Winnenburg, R.; Schroeder, M.; Preissner, R. SuperCYP: A comprehensive database on Cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions. Nucleic Acids Res. 2009, 38, D237–D243. [Google Scholar] [CrossRef]
- Kim, S.-A.; Yhim, H.-Y.; Bang, S.-M. Current Management of Cancer-associated Venous Thromboembolism: Focus on Direct Oral Anticoagulants. J. Korean Med. Sci. 2019, 34, e52. [Google Scholar] [CrossRef]
- Vazquez, S.R. Drug-drug interactions in an era of multiple anticoagulants: A focus on clinically relevant drug interactions. Blood 2018, 132, 2230–2239. [Google Scholar] [CrossRef]
- Hakeam, H.A.; Al-Sanea, N. Effect of major gastrointestinal tract surgery on the absorption and efficacy of direct acting oral anticoagulants (DOACs). J. Thromb. Thrombolysis 2017, 43, 343–351. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Perlstein, I.; Wang, J.; Badawy, S.; Frost, C.; LaCreta, F. Relative Bioavailability of Apixaban Solution or Crushed Tablet Formulations Administered by Mouth or Nasogastric Tube in Healthy Subjects. Clin. Ther. 2015, 37, 1703–1712. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007, 110, 2339–2346. [Google Scholar] [CrossRef]
- Cronin, M.; Dengler, N.; Krauss, E.S.; Segal, A.; Wei, N.; Daly, M.; Mota, F.; Caprini, J.A. Completion of the Updated Caprini Risk Assessment Model (2013 Version). Clin. Appl. Thromb. 2019, 25. [Google Scholar] [CrossRef]
- Clagett, G.; Reisch, J. Prevention of Venous Thromboembolism in General, Surgical Patients: Results of a Meta-Analysis. J. Urol. 1989, 141, 1051–1052. [Google Scholar] [CrossRef]
- Akl, E.A.; Labedi, N.; Terrenato, I.; Barba, M.; Sperati, F.; Sempos, E.V.; Muti, P.; Cook, D.; Schünemann, H. Low molecular weight heparin versus unfractionated heparin for perioperative thromboprophylaxis in patients with cancer. Cochrane Database Syst. Rev. 2011, 2014. [Google Scholar] [CrossRef]
- Wang, T.-F.; Li, A.; Garcia, D. Managing thrombosis in cancer patients. Res. Pr. Thromb. Haemost. 2018, 2, 429–438. [Google Scholar] [CrossRef]
- Bergqvist, D.; Agnelli, G.; Cohen, A.T.; Eldor, A.; Nilsson, P.E.; Le Moigne-Amrani, A.; Dietrich-Neto, F. Duration of Prophylaxis against Venous Thromboembolism with Enoxaparin after Surgery for Cancer. N. Engl. J. Med. 2002, 346, 975–980. [Google Scholar] [CrossRef]
- Rasmussen, M.S.; Jorgensen, L.N.; Wille-Jørgensen, P. Prolonged thromboprophylaxis with Low Molecular Weight heparin for abdominal or pelvic surgery. Cochrane Database Syst. Rev. 2009, 2009, CD004318. [Google Scholar] [CrossRef]
- Felder, S.; Rasmussen, M.S.; King, R.; Sklow, B.; Kwaan, M.; Madoff, R.; Jensen, C. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst. Rev. 2019, 8. [Google Scholar] [CrossRef]
- Akl, E.A.; Terrenato, I.; Barba, M.; Sperati, F.; Muti, P.; Schünemann, H.J. Extended perioperative thromboprophylaxis in patients with cancer. A systematic review. Thromb. Haemost. 2008, 100, 1176–1180. [Google Scholar]
- Bottaro, F.J.; Elizondo, M.C.; Doti, C.; Bruetman, J.E.; Perez Moreno, P.D.; Bullorsky, E.O.; Ceresetto, J.M. Efficacy of extended thrombo-prophylaxis in major abdominal surgery: What does the evidence show? A meta-analysis. Thromb. Haemost. 2008, 99, 1104–1111. [Google Scholar]
- Rasmussen, M.S.; Jorgensen, L.N.; Wille-Jørgensen, P.; Nielsen, J.D.; Horn, A.; Mohn, A.C.; Sømod, L.; Olsen, B. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: A multicenter randomized open-label study. J. Throm. Haemost. 2006, 4, 2384–2390. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, B.; Zhao, J.; Ma, Y.; Yuan, D.; Yang, Y.; Du, X. Perioperative Pharmacological Thromboprophylaxis in Patients With Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2017, 265, 1087–1093. [Google Scholar] [CrossRef]
- Fagarasanu, A.; Alotaibi, G.S.; Hrimiuc, R.; Lee, A.Y.Y.; Wu, C. Role of Extended Thromboprophylaxis After Abdominal and Pelvic Surgery in Cancer Patients: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 1422–1430. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Akl, E.A.; Crowther, M.; Gutterman, D.D.; Schuünemann, H.J. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, 7s–47s. [Google Scholar] [CrossRef]
- Khorana, A.A.; Carrier, M.; Garcia, D.A.; Lee, A.Y.Y. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 81–91. [Google Scholar] [CrossRef]
- Kitchens, C.S.; Alving, B.M.; Kessler, C.M. Consultative Hemostasis and Thrombosis; Elsevier BV: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Schwahn-Schreiber, C.; Breu, F.X.; Rabe, E.; Buschmann, I.; Döller, W.; Lulay, G.R.; Miller, A.; Valesky, E.; Reich-Schupke, S. S1 guideline on intermittent pneumatic compression (IPC). Der Hautarzt Zeitschrift Dermatologie Venerologie Verwandte Gebiete 2018, 69, 662–673. [Google Scholar] [CrossRef]
- Launay-Vacher, V.; Oudard, S.; Janus, N.; Gligorov, J.; Pourrat, X.; Rixe, O.; Morere, J.F.; Beuzeboc, P.; Deray, G. Prevalence of Renal Insufficiency in cancer patients and implications for anticancer drug management: The renal insufficiency and anticancer medications (IRMA) study. Cancer 2007, 110, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, B.K.; Gansevoort, R.T.; Naess, I.A.; Lutsey, P.L.; Braekkan, S.K.; Veeger, N.J.; Brodin, E.E.; Meijer, K.; Sang, Y.; Matsushita, K.; et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: Pooled analysis of five prospective general population cohorts. Circulation 2012, 126, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Yamashita, Y.; Kim, K.; Morimoto, T.; Saga, S.; Amano, H.; Takase, T.; Hiramori, S.; Oi, M.; Akao, M.; et al. Risk Factors for Major Bleeding During Anticoagulation Therapy in Cancer-Associated Venous Thromboembolism—From the COMMAND VTE Registry. Circ. J. 2020, 84, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Adhikari, N.K.J.; Ostermann, M.; Heels-Ansdell, D.; Douketis, J.D.; Skrobik, Y.; Qushmaq, I.; Meade, M.; Guyatt, G.; Geerts, W.; et al. Low-molecular-weight heparin venous thromboprophylaxis in critically ill patients with renal dysfunction: A subgroup analysis of the PROTECT trial. PLoS ONE 2018, 13, e0198285. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.E.; Thadhani, R.I.; Maddux, F.W. No difference in bleeding risk between subcutaneous enoxaparin and heparin for thromboprophylaxis in end-stage renal disease. Kidney Int. 2013, 84, 555–561. [Google Scholar] [CrossRef]
- Hughes, S.; Szeki, I.; Nash, M.J.; Thachil, J. Anticoagulation in chronic kidney disease patients--the practical aspects. Clin. Kidney J. 2014, 7, 442–449. [Google Scholar] [CrossRef]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.; Webber, L.; Sheron, N. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Drug Hepatotoxicity: Newer Agents. Clin. Liver Dis. 2017, 21, 115–134. [Google Scholar] [CrossRef]
- Khoury, T.; Ayman, A.R.; Cohen, J.; Daher, S.; Shmuel, C.; Mizrahi, M. The Complex Role of Anticoagulation in Cirrhosis: An Updated Review of Where We Are and Where We Are Going. Digestion 2016, 93, 149–159. [Google Scholar] [CrossRef]
- Tripodi, A.; Mannucci, P.M. The Coagulopathy of Chronic Liver Disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef]
- Ogren, M.; Bergqvist, D.; Bjorck, M.; Acosta, S.; Eriksson, H.; Sternby, N.H. Portal vein thrombosis: Prevalence, patient characteristics and lifetime risk: A population study based on 23,796 consecutive autopsies. World J. Gastroenterol. 2006, 12, 2115–2119. [Google Scholar] [CrossRef]
- Rodriguez-Castro, K.I.; Simioni, P.; Burra, P.; Senzolo, M. Anticoagulation for the treatment of thrombotic complications in patients with cirrhosis. Liver Int. 2012, 32, 1465–1476. [Google Scholar] [CrossRef]
- Villa, E.; Cammà, C.; Marietta, M.; Luongo, M.; Critelli, R.; Colopi, S.; Tata, C.; Zecchini, R.; Gitto, S.; Petta, S.; et al. Enoxaparin Prevents Portal Vein Thrombosis and Liver Decompensation in Patients With Advanced Cirrhosis. Gastroenterology 2012, 143, 1253–1260.e4. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Liakoni, E.; Bravo, A.E.R.; Terracciano, L.; Heim, M.; Krähenbühl, S. Symptomatic Hepatocellular Liver Injury With Hyperbilirubinemia in Two Patients Treated With Rivaroxaban. JAMA Intern. Med. 2014, 174, 1683–1686. [Google Scholar] [CrossRef]
- Anastasia, E.J.; Rosenstein, R.S.; Bergsman, J.A.; Parra, D. Use of apixaban after development of suspected rivaroxaban-induced hepatic steatosis; a case report. Blood Coagul. Fibrinolysis 2015, 26, 699–702. [Google Scholar] [CrossRef]
- Rossel, A.; Robert-Ebadi, H.; Combescure, C.; Grosgurin, O.; Stirnemann, J.; Addeo, A.; Garin, N.; Agoritsas, T.; Reny, J.-L.; Marti, C. Anticoagulant therapy for acute venous thrombo-embolism in cancer patients: A systematic review and network meta-analysis. PLoS ONE 2019, 14, e0213940. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, H.; Li, D.; Chen, C.; Gu, G.; Sun, Y.; Yang, X.; Liu, Y.; Fang, F.; Liu, J.; et al. Efficacy and Safety of Direct Oral Anticoagulants for Secondary Prevention of Cancer-Associated Thrombosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Frere, C.; Benzidia, I.; Marjanovic, Z.; Farge, D. Recent Advances in the Management of Cancer-Associated Thrombosis: New Hopes but New Challenges. Cancers 2019, 11, 71. [Google Scholar] [CrossRef]
- Schlichtig, K.; Dürr, P.; Dörje, F.; Fromm, M.F. New Oral Anti-Cancer Drugs and Medication Safety. Dtsch. Aerzteblatt Online 2019, 116, 775–782. [Google Scholar] [CrossRef]
- Brandes, A.; Scelzi, E.; Salmistraro, G.; Ermani, M.; Carollo, C.; Berti, F.; Zampieri, P.; Baiocchi, C.; Fiorentino, M. Incidence and risk of thromboembolism during treatment of high-grade gliomas: A prospective study. Eur. J. Cancer 1997, 33, 1592–1596. [Google Scholar] [CrossRef]
- Yust-Katz, S.; Mandel, J.J.; Wu, J.; Yuan, Y.; Webre, C.; Pawar, T.A.; Lhadha, H.S.; Gilbert, M.R.; Armstrong, T.S. Venous thromboembolism (VTE) and glioblastoma. J. Neuro-Oncol. 2015, 124, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.D.; Miller, L.D.; Patel, C.P.; Gupta, S.K. Enoxaparin Increases the Incidence of Postoperative Intracranial Hemorrhage when Initiated Preoperatively for Deep Venous Thrombosis Prophylaxis in Patients with Brain Tumors. Neurosurgery 1998, 43, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.R.; Julian, J.A.; Laperriere, N.J.; Geerts, W.; Agnelli, G.; Rogers, L.R.; Malkin, M.G.; Sawaya, R.; Baker, R.; Falanga, A.; et al. PRODIGE: A randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J. Thromb. Haemost. 2010, 8, 1959–1965. [Google Scholar] [CrossRef]
- Taillibert, S.; Taillandier, L.; Le Rhun, E. Venous thrombosis in patients with high-grade glioma. Curr. Opin. Oncol. 2015, 27, 516–521. [Google Scholar] [CrossRef]
- Ay, C.; Kamphuisen, P.; Agnelli, G. Antithrombotic therapy for prophylaxis and treatment of venous thromboembolism in patients with cancer: Review of the literature on current practice and emerging options. ESMO Open 2017, 2, e000188. [Google Scholar] [CrossRef]
- Samuelson Bannow, B.T.; Lee, A.; Khorana, A.A.; Zwicker, J.I.; Noble, S.; Ay, C.; Carrier, M. Management of cancer-associated thrombosis in patients with thrombocytopenia: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1246–1249. [Google Scholar] [CrossRef]
- Samuelson Bannow, B.T.; Walter, R.B.; Gernsheimer, T.B.; Garcia, D.A. Patients treated for acute VTE during periods of treatment-related thrombocytopenia have high rates of recurrent thrombosis and transfusion-related adverse outcomes. J. Thromb. Thrombolysis 2017, 44, 442–447. [Google Scholar] [CrossRef]
- Cortelezzi, A.; Moia, M.; Falanga, A.; Pogliani, E.M.; Agnelli, G.; Bonizzoni, E.; Gussoni, G.; Barbui, T.; Mannucci, P.M. Incidence of thrombotic complications in patients with haematological malignancies with central venous catheters: A prospective multicentre study. Br. J. Haematol. 2005, 129, 811–817. [Google Scholar] [CrossRef]
- Easaw, J.C.; Shea-Budgell, M.A.; Wu, C.M.; Czaykowski, P.M.; Kassis, J.; Kuehl, B.; Lim, H.J.; MacNeil, M.; Martinusen, D.; McFarlane, P.A.; et al. Canadian consensus recommendations on the management of venous thromboembolism in patients with cancer. Part 1: Prophylaxis. Curr. Oncol. 2015, 22, 133–143. [Google Scholar] [CrossRef][Green Version]
- Herishanu, Y.; Misgav, M.; Kirgner, I.; Ben-Tal, O.; Eldor, A.; Naparstek, E. Enoxaparin can be used safely in patients with severe thrombocytopenia due to intensive chemotherapy regimens. Leuk. Lymphoma 2004, 45, 1407–1411. [Google Scholar] [CrossRef]
- Chalayer, E.; Cavalieri, D.; Martignoles, J.A.; Genthon, A.; Tavernier, E.; Tardy, B. Antithrombotic therapy and platelet transfusions in hematologic malignancy patients presenting chemotherapy-induced thrombocytopenia: A French survey. Transfusion 2017, 57, 1717–1723. [Google Scholar] [CrossRef]
- Pabinger-Fasching, I.; Alt-Epping, B.; Demarmels Biasiutti, F.; Langer, F.; Riess, H.; Wörrmann, B. Thrombembolien (VTE) bei Tumorpatienten. Available online: https://www.onkopedia.com/de/onkopedia/archive/guidelines/venoese-thrombembolien-vte-bei-tumorpatienten/version-09042019T082542/@@guideline/html/index.html (accessed on 6 August 2019).
- Watson, H.G.; Keeling, D.M.; Laffan, M.; Tait, R.C.; Makris, M. Guideline on aspects of cancer-related venous thrombosis. Br. J. Haematol. 2015, 170, 640–648. [Google Scholar] [CrossRef]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Bockenstedt, P.L.; Chesney, C.; Fanikos, J.; Fenninger, R.B.; Fogerty, A.E.; Gao, S.; et al. NCCN Guidelines Insights: Cancer-Associated Venous Thromboembolic Disease, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 1289–1303. [Google Scholar] [CrossRef]
- Di Nisio, M.; Carrier, M.; Lyman, G.H.; Khorana, A.A. Prevention of venous thromboembolism in hospitalized medical cancer patients: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 1746–1749. [Google Scholar] [CrossRef]
- Farge, D.; Debourdeau, P.; Beckers, M.; Baglin, C.; Bauersachs, R.M.; Brenner, B.; Brilhante, D.; Falanga, A.; Gerotzafias, G.T.; Haim, N.; et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J. Thromb. Haemost. 2013, 11, 56–70. [Google Scholar] [CrossRef]
- Farge, D.; Frere, C.; Connors, J.M.; Ay, C.; Khorana, A.A.; Munoz, A.; Brenner, B.; Kakkar, A.; Rafii, H.; Solymoss, S.; et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet. Oncol. 2019, 20, e566–e581. [Google Scholar] [CrossRef]
- Annibali, O.; Napolitano, M.; Avvisati, G.; Siragusa, S. Incidence of venous thromboembolism and use of anticoagulation in hematological malignancies: Critical review of the literature. Crit. Rev. Oncol. Hematol. 2018, 124, 41–50. [Google Scholar] [CrossRef]
- Connors, J.M. Thrombophilia Testing and Venous Thrombosis. N. Engl. J. Med. 2017, 377, 2298. [Google Scholar] [CrossRef]
- Elsebaie, M.A.T.; van Es, N.; Langston, A.; Buller, H.R.; Gaddh, M. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: A systematic review and meta-analysis. J. Thromb. Haemost. 2019, 17, 645–656. [Google Scholar] [CrossRef]
- Garber, J.E.; Halabi, S.; Tolaney, S.M.; Kaplan, E.; Archer, L.; Atkins, J.N.; Edge, S.; Shapiro, C.L.; Dressler, L.; Paskett, E.D.; et al. Factor V Leiden mutation and thromboembolism risk in women receiving adjuvant tamoxifen for breast cancer. J. Natl. Cancer Inst. 2010, 102, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Skeith, L. Anticoagulating patients with high-risk acquired thrombophilias. Hematol. Am. Soc. Hematol. Educ. Program Book 2018, 2018, 439–449. [Google Scholar] [CrossRef]
- Munoz-Linares, C.; Ojeda, E.; Fores, R.; Pastrana, M.; Cabero, M.; Morillo, D.; Bautista, G.; Banos, I.; Monteserin, C.; Bravo, P.; et al. Paroxysmal nocturnal hemoglobinuria: A single Spanish center's experience over the last 40 yr. Eur. J. Haematol. 2014, 93, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Richards, S.; Hillmen, P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 2003, 102, 3587–3591. [Google Scholar] [CrossRef]
- Schubert, J.R.; Röth, A.; Bettelheim, P.; Stüssi, G.; Höchsmann, B.; Panse, J.; Brümmendorf, T.H.; Schrezenmeier, H. Paroxysmale nächtliche Hämoglobinurie (PNH). Available online: https://www.onkopedia.com/de/onkopedia/guidelines/paroxysmale-naechtliche-haemoglobinurie-pnh/@@guideline/html/index.html (accessed on 15 August 2020).
- Agnelli, G.; Bergqvist, D.; Cohen, A.T.; Gallus, A.S.; Gent, M.; investigators, P. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br. J. Surg. 2005, 92, 1212–1220. [Google Scholar] [CrossRef]
- Opatrny, L.; Warner, M.N. Risk of thrombosis in patients with malignancy and heparin-induced thrombocytopenia. Am. J. Hematol. 2004, 76, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, M.; Zhou, W.; Wang, Y. Heparin-induced thrombocytopenia with hematoma necrosis and persistent high fever after gastric cancer surgery: A case report. Asian J. Surg. 2020, 43, 387–388. [Google Scholar] [CrossRef]
- Kreher, S.; Ochsenreither, S.; Trappe, R.U.; Pabinger, I.; Bergmann, F.; Petrides, P.E.; Koschmieder, S.; Matzdorff, A.; Tiede, A.; Griesshammer, M.; et al. Prophylaxis and management of venous thromboembolism in patients with myeloproliferative neoplasms: Consensus statement of the Haemostasis Working Party of the German Society of Hematology and Oncology (DGHO), the Austrian Society of Hematology and Oncology (ÖGHO) and Society of Thrombosis and Haemostasis Research (GTH e.V.). Ann. Hematol. 2014, 93, 1953–1963. [Google Scholar]
- Barbui, T.; Ghirardi, A.; Masciulli, A.; Carobbio, A.; Palandri, F.; Vianelli, N.; De Stefano, V.; Betti, S.; Di Veroli, A.; Iurlo, A.; et al. Second cancer in Philadelphia negative myeloproliferative neoplasms (MPN-K). A nested case-control study. Leukemia 2019, 33, 1996–2005. [Google Scholar] [CrossRef]
- Mustafa, S.; Stein, P.D.; Patel, K.C.; Otten, T.R.; Holmes, R.; Silbergleit, A. Upper extremity deep venous thrombosis. Chest 2003, 123, 1953–1956. [Google Scholar] [CrossRef]
- Flinterman, L.E.; van Hylckama Vlieg, A.; Rosendaal, F.R.; Doggen, C.J. Venous thrombosis of the upper extremity: Effect of blood group and coagulation factor levels on risk. Br. J. Haematol. 2010, 149, 118–123. [Google Scholar] [CrossRef]
- Saber, W.; Moua, T.; Williams, E.C.; Verso, M.; Agnelli, G.; Couban, S.; Young, A.; De Cicco, M.; Biffi, R.; van Rooden, C.J.; et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: A patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J. Thromb. Haemost. 2011, 9, 312–319. [Google Scholar] [CrossRef]
- Evans, R.S.; Sharp, J.H.; Linford, L.H.; Lloyd, J.F.; Tripp, J.S.; Jones, J.P.; Woller, S.C.; Stevens, S.M.; Elliott, C.G.; Weaver, L.K. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest 2010, 138, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Bonizzoli, M.; Batacchi, S.; Cianchi, G.; Zagli, G.; Lapi, F.; Tucci, V.; Martini, G.; Di Valvasone, S.; Peris, A. Peripherally inserted central venous catheters and central venous catheters related thrombosis in post-critical patients. Intensive Care Med. 2011, 37, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Nyska, A. Drug-induced thrombosis–experimental, clinical, and mechanistic considerations. Toxicol. Pathol. 2007, 35, 208–225. [Google Scholar] [CrossRef]
- Ramot, Y.; Nyska, A.; Spectre, G. Drug-induced thrombosis: An update. Drug Saf. 2013, 36, 585–603. [Google Scholar] [CrossRef]
- Oppelt, P.; Betbadal, A.; Nayak, L. Approach to chemotherapy-associated thrombosis. Vasc. Med. Lond. Engl. 2015, 20, 153–161. [Google Scholar] [CrossRef]
- Bern, M.M.; Lokich, J.J.; Wallach, S.R.; Bothe, A., Jr.; Benotti, P.N.; Arkin, C.F.; Greco, F.A.; Huberman, M.; Moore, C. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann. Intern. Med. 1990, 112, 423–428. [Google Scholar] [CrossRef]
- Monreal, M.; Alastrue, A.; Rull, M.; Mira, X.; Muxart, J.; Rosell, R.; Abad, A. Upper extremity deep venous thrombosis in cancer patients with venous access devices–prophylaxis with a low molecular weight heparin (Fragmin). Thromb. Haemost. 1996, 75, 251–253. [Google Scholar] [CrossRef]
- Beckers, M.M.; Ruven, H.J.; Seldenrijk, C.A.; Prins, M.H.; Biesma, D.H. Risk of thrombosis and infections of central venous catheters and totally implanted access ports in patients treated for cancer. Thromb. Res. 2010, 125, 318–321. [Google Scholar] [CrossRef]
- Debourdeau, P.; Espié, M.; Chevret, S.; Gligorov, J.; Elias, A.; Dupré, P.F.; Desseaux, K.; Kalidi, I.; Villiers, S.; Giachetti, S.; et al. Incidence, risk factors, and outcomes of central venous catheter-related thromboembolism in breast cancer patients: The CAVECCAS study. Cancer Med. 2017, 6, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Lavau-Denes, S.; Lacroix, P.; Maubon, A.; Preux, P.M.; Genet, D.; Vénat-Bouvet, L.; Labourey, J.L.; Martin, J.; Slaouti, P.; Tubiana-Mathieu, N. Prophylaxis of catheter-related deep vein thrombosis in cancer patients with low-dose warfarin, low molecular weight heparin, or control: A randomized, controlled, phase III study. Cancer Chemother. Pharmacol. 2013, 72, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Karthaus, M.; Kretzschmar, A.; Kröning, H.; Biakhov, M.; Irwin, D.; Marschner, N.; Slabber, C.; Fountzilas, G.; Garin, A.; Abecasis, N.G.; et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: Final results of a double-blind, placebo-controlled phase III trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006, 17, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Gaitini, D.; Beck-Razi, N.; Haim, N.; Brenner, B. Prevalence of upper extremity deep venous thrombosis diagnosed by color Doppler duplex sonography in cancer patients with central venous catheters. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2006, 25, 1297–1303. [Google Scholar] [CrossRef]
- Akl, E.A.; Karmath, G.; Yosuico, V.; Kim, S.Y.; Barba, M.; Sperati, F.; Cook, D.; Schünemann, H.J. Anticoagulation for thrombosis prophylaxis in cancer patients with central venous catheters. Cochrane Database Syst. Rev. 2007, Cd006468. [Google Scholar] [CrossRef]
- D'Ambrosio, L.; Aglietta, M.; Grignani, G. Anticoagulation for central venous catheters in patients with cancer. N. Engl. J Med. 2014, 371, 1362–1363. [Google Scholar] [CrossRef]
- Heaton, D.C.; Han, D.Y.; Inder, A. Minidose (1 mg) warfarin as prophylaxis for central vein catheter thrombosis. Intern. Med. J. 2002, 32, 84–88. [Google Scholar] [CrossRef]
- Boraks, P.; Seale, J.; Price, J.; Bass, G.; Ethell, M.; Keeling, D.; Mahendra, P.; Baglin, T.; Marcus, R. Prevention of central venous catheter associated thrombosis using minidose warfarin in patients with haematological malignancies. Br. J. Haematol. 1998, 101, 483–486. [Google Scholar] [CrossRef]
- Kirkpatrick, A.; Rathbun, S.; Whitsett, T.; Raskob, G. Prevention of central venous catheter-associated thrombosis: A meta-analysis. Am. J. Med. 2007, 120, e901–e913. [Google Scholar] [CrossRef]
- Rawson, K.M.; Newburn-Cook, C.V. The use of low-dose warfarin as prophylaxis for central venous catheter thrombosis in patients with cancer: A meta-analysis. Oncol. Nurs. Forum 2007, 34, 1037–1043. [Google Scholar] [CrossRef]
- Akl, E.A.; Ramly, E.P.; Kahale, L.A.; Yosuico, V.E.; Barba, M.; Sperati, F.; Cook, D.; Schünemann, H. Anticoagulation for people with cancer and central venous catheters. Cochrane Database Syst. Rev. 2014, Cd006468. [Google Scholar] [CrossRef]
- Kahale, L.A.; Tsolakian, I.G.; Hakoum, M.B.; Matar, C.F.; Barba, M.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Schünemann, H.; Akl, E.A. Anticoagulation for people with cancer and central venous catheters. Cochrane Database Syst. Rev. 2018, 6, Cd006468. [Google Scholar] [CrossRef]
- Mismetti, P.; Mille, D.; Laporte, S.; Charlet, V.; Buchmuller-Cordier, A.; Jacquin, J.P.; Fournel, P.; Dutrey-Dupagne, C.; Decousus, H.; CIP Study Group. Low-molecular-weight heparin (nadroparin) and very low doses of warfarin in the prevention of upper extremity thrombosis in cancer patients with indwelling long-term central venous catheters: A pilot randomized trial. Haematologica 2003, 88, 67–73. [Google Scholar]
- Couban, S.; Goodyear, M.; Burnell, M.; Dolan, S.; Wasi, P.; Barnes, D.; Macleod, D.; Burton, E.; Andreou, P.; Anderson, D.R. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 4063–4069. [Google Scholar] [CrossRef]
- Ruud, E.; Holmstrom, H.; De Lange, C.; Hogstad, E.M.; Wesenberg, F. Low-dose warfarin for the prevention of central line-associated thromboses in children with malignancies—A randomized, controlled study. Acta Paediatr. 2006, 95, 1053–1059. [Google Scholar] [CrossRef]
- Verso, M.; Agnelli, G.; Kamphuisen, P.W.; Ageno, W.; Bazzan, M.; Lazzaro, A.; Paoletti, F.; Paciaroni, M.; Mosca, S.; Bertoglio, S. Risk factors for upper limb deep vein thrombosis associated with the use of central vein catheter in cancer patients. Intern. Emerg. Med. 2008, 3, 117–122. [Google Scholar] [CrossRef]
- De Cicco, M.; Matovic, M.; Balestreri, L.; Steffan, A.; Pacenzia, R.; Malafronte, M.; Fantin, D.; Bertuzzi, C.A.; Fabiani, F.; Morassut, S.; et al. Early and short-term acenocumarine or dalteparin for the prevention of central vein catheter-related thrombosis in cancer patients: A randomized controlled study based on serial venographies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 1936–1942. [Google Scholar] [CrossRef]
- Young, A.M.; Billingham, L.J.; Begum, G.; Kerr, D.J.; Hughes, A.I.; Rea, D.W.; Shepherd, S.; Stanley, A.; Sweeney, A.; Wilde, J.; et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): An open-label randomised trial. Lancet 2009, 373, 567–574. [Google Scholar] [CrossRef]
- Niers, T.M.; Di Nisio, M.; Klerk, C.P.; Baarslag, H.J.; Buller, H.R.; Biemond, B.J. Prevention of catheter-related venous thrombosis with nadroparin in patients receiving chemotherapy for hematologic malignancies: A randomized, placebo-controlled study. J. Thromb. Haemost. 2007, 5, 1878–1882. [Google Scholar] [CrossRef]
- Debourdeau, P.; Farge, D.; Beckers, M.; Baglin, C.; Bauersachs, R.M.; Brenner, B.; Brilhante, D.; Falanga, A.; Gerotzafias, G.T.; Haim, N.; et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J. Thromb. Haemost. 2013, 11, 71–80. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Connolly, G.; Carrier, M.; Kamphuisen, P.W.; Lee, A.Y. Catheter-associated deep vein thrombosis of the upper extremity in cancer patients: Guidance from the SSC of the ISTH. J. Thromb. Haemos. 2014, 12, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Vanderschoot, J.P.; Oostindiër, M.J.; Osanto, S.; van der Meer, F.J.; Rosendaal, F.R. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: Results of a record linkage study. J. Thromb. Haemos. 2006, 4, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Russo, L. Venous thromboembolism in the hematologic malignancies. Curr. Opin. Oncol. 2012, 24, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Pfeiffer, R.M.; Björkholm, M.; Goldin, L.R.; Schulman, S.; Blimark, C.; Mellqvist, U.H.; Wahlin, A.; Turesson, I.; Landgren, O. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: A population-based study. Blood 2010, 115, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Tang, M.; Pfeiffer, R.M.; Björkholm, M.; Goldin, L.R.; Blimark, C.; Mellqvist, U.H.; Wahlin, A.; Turesson, I.; Landgren, O. Monoclonal gammopathy of undetermined significance and risk of infections: A population-based study. Haematologica 2012, 97, 854–858. [Google Scholar] [CrossRef]
- Zangari, M.; Saghafifar, F.; Mehta, P.; Barlogie, B.; Fink, L.; Tricot, G. The blood coagulation mechanism in multiple myeloma. Semin. Thromb. Hemost. 2003, 29, 275–282. [Google Scholar]
- Elice, F.; Fink, L.; Tricot, G.; Barlogie, B.; Zangari, M. Acquired resistance to activated protein C (aAPCR) in multiple myeloma is a transitory abnormality associated with an increased risk of venous thromboembolism. Br. J. Haematol. 2006, 134, 399–405. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Blood, E.; Vesole, D.; Fonseca, R.; Greipp, P.R. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 431–436. [Google Scholar] [CrossRef]
- Zangari, M.; Siegel, E.; Barlogie, B.; Anaissie, E.; Saghafifar, F.; Fassas, A.; Morris, C.; Fink, L.; Tricot, G. Thrombogenic activity of doxorubicin in myeloma patients receiving thalidomide: Implications for therapy. Blood 2002, 100, 1168–1171. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.E.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef]
- Baz, R.; Walker, E.; Karam, M.A.; Choueiri, T.K.; Jawde, R.A.; Bruening, K.; Reed, J.; Faiman, B.; Ellis, Y.; Brand, C.; et al. Lenalidomide and pegylated liposomal doxorubicin-based chemotherapy for relapsed or refractory multiple myeloma: Safety and efficacy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006, 17, 1766–1771. [Google Scholar] [CrossRef]
- Morgan, G.J.; Schey, S.A.; Wu, P.; Srikanth, M.; Phekoo, K.J.; Jenner, M.; Davies, F.E. Lenalidomide (Revlimid), in combination with cyclophosphamide and dexamethasone (RCD), is an effective and tolerated regimen for myeloma patients. Br. J. Haematol. 2007, 137, 268–269. [Google Scholar] [CrossRef]
- Palumbo, A.; Rajkumar, S.V.; Dimopoulos, M.A.; Richardson, P.G.; San Miguel, J.; Barlogie, B.; Harousseau, J.; Zonder, J.A.; Cavo, M.; Zangari, M.; et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008, 22, 414–423. [Google Scholar] [CrossRef]
- Li, A.; Wu, Q.; Luo, S.; Warnick, G.S.; Zakai, N.A.; Libby, E.N.; Gage, B.F.; Garcia, D.A.; Lyman, G.H.; Sanfilippo, K.M. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug-Associated Thrombosis Among Patients With Multiple Myeloma. J. Natl. Compr. Cancer Netw. 2019, 17, 840–847. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Luo, S.; Wang, T.F.; Fiala, M.; Schoen, M.; Wildes, T.M.; Mikhael, J.; Kuderer, N.M.; Calverley, D.C.; Keller, J.; et al. Predicting venous thromboembolism in multiple myeloma: Development and validation of the IMPEDE VTE score. Am. J. Hematol. 2019, 94, 1176–1184. [Google Scholar] [CrossRef]
- Carrier, M.; Le Gal, G.; Tay, J.; Wu, C.; Lee, A.Y. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: A systematic review and meta-analysis. J. Thromb. Haemost. 2011, 9, 653–663. [Google Scholar] [CrossRef]
- Palumbo, A.; Cavo, M.; Bringhen, S.; Zamagni, E.; Romano, A.; Patriarca, F.; Rossi, D.; Gentilini, F.; Crippa, C.; Galli, M.; et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: A phase III, open-label, randomized trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 986–993. [Google Scholar] [CrossRef]
- Larocca, A.; Cavallo, F.; Bringhen, S.; Di Raimondo, F.; Falanga, A.; Evangelista, A.; Cavalli, M.; Stanevsky, A.; Corradini, P.; Pezzatti, S.; et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012, 119, 933–939; quiz 1093. [Google Scholar] [CrossRef]
- Wang, T.F.; Zwicker, J.I.; Ay, C.; Pabinger, I.; Falanga, A.; Antic, D.; Noble, S.; Khorana, A.A.; Carrier, M.; Meyer, G. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2019, 17, 1772–1778. [Google Scholar] [CrossRef]
- Storrar, N.P.F.; Mathur, A.; Johnson, P.R.E.; Roddie, P.H. Safety and efficacy of apixaban for routine thromboprophylaxis in myeloma patients treated with thalidomide- and lenalidomide-containing regimens. Br. J. Haematol. 2019, 185, 142–144. [Google Scholar] [CrossRef]
| Name of the Study and Reference | n | Patient Cohorts (Cancer Type or Khorana Risk) | 1°EP | 1°EP Control | 1°EP Experimental | RRR | ARR |
|---|---|---|---|---|---|---|---|
| PROTECHT [37] | 1150 | Mixed | sVTE, sArtE. | Placebo 3.9% | Nadroparin (3800 U QD) 2.0% | 49% | 1.9% |
| SAVE-ONCO [23] | 3212 | Mixed | sDVT, PE, VTE-rD | Placebo 3.4% | Semuloparin (20 mg QD) 1.2% | 65% | 2.2% |
| PRH-HCTU-FRAGEM [26] | 123 | Pancreatic cancer | s/i VTE, s/i ArtE | Observation 23% | Dalteparin (200 IU ≥ 150/kg/d) 3.4% | 85% | 19.6% |
| CONKO-004 [22] | 312 | Pancreatic cancer | sVTE | Observation 10.2% | Enoxaparin (1 mg/kg/d) 1.3% | 87% | 8.9% |
| RASTEN [38] | 377 | Small cell lung cancer | sVTE | Observation 8.4% | Enoxaparin (1 mg/kg/d) 2.7% | 71% | 5.7% |
| PHACS [18] | 117 | Khorana Score ≥3 | all VTE | Observation 21% | Dalteparin (5000 U/d) 12% | 43% | 9.0% |
| CASSINI [39] | 841 | Khorana Score ≥2 | s/i VTE, VTE-rD | Placebo 8.8% | Rivaroxaban (10 mg QD) 6.0% | 32% | 2.8% |
| AVERT [17] | 563 | Khorana Score ≥2 | s/i VTE, VTE-rD | Placebo 10.2% | Apixaban 2.5 mg BID 4.2% | 59% | 6.0% |
| Anticoagulant | Relevant Indications | Dose | Dose Adjustment for Kidney Dysfunction (See Label for More Information) |
|---|---|---|---|
| Unfractionated heparin (UFH) | Prophylaxis of venous or arterial thromboembolism | Prophylaxis: 5000 IE s.c. q8–12 h or 7500 IE s.c. q12 h | Exert use possible in renal insufficiency, but requires close monitoring |
| Enoxaparin |
| Prophylaxis mod. risk: 20 mg s.c. QD Prophylaxis high risk: 40 mg s.c. QD | Mild to moderate kidney dysfunction (creatinine clearance >/= 30 mL/min): No dose adjustment, but careful clinical observation Severe kidney dysfunction (creatinine clearance 15–30 mL/min): Prophylaxis 20 mg s.c. QD Kidney failure (creatinine clearance < 15 mL/min): Use not recommended |
| Dalteparin |
| Prophylaxis low/mod. risk: 2500 IE s.c. QD Prophylaxis high risk: 2500–5000 IE s.c. QD (see label) Prophylaxis medical: 5000 IE s.c. QD | Kidney failure (creatinine clearance < 15 mL/min): Use only with close observation |
| Certoparin | Prophylaxis of VTE | 3000 IE s.c. QD | Severe kidney dysfunction (creatinine clearance </= 30 mL/min): Great care is necessary |
| Nadroparin | Prophylaxis of VTE | Prophylaxis: 2850–5700 IE s.c. QD | Mild to moderate kidney dysfunction (creatinine clearance 30–80 mL/min): Use with great care, monitor anti-Xa levels Severe kidney dysfunction (creatinine clearance </= 30 mL/min): Use is contraindicated |
| Reviparin | Prophylaxis of VTE | Prophylaxis: 1750 IE s.c. QD | Mild to moderate kidney dysfunction (creatinine clearance 30–80 mL/min): Use with great care, monitor anti-Xa levels Severe kidney dysfunction (creatinine clearance </= 30 mL/min): Use is contraindicated |
| Fondaparinux | Prophylaxis of VTE | Prophylaxis: 2.5 mg s.c. QD | Moderate kidney dysfunction (creatinine clearance 30–50 mL/min): Use with great care Severe kidney dysfunction (creatinine clearance </= 30 mL/min): Use is contraindicated |
| Apixaban | Prophylaxis of VTE (off-label) * | Prophylaxis: 2.5 mg BID | Moderate kidney dysfunction (creatinine clearance 30–60 mL/min): no dose reduction Severe kidney dysfunction (creatinine clearance </= 30 mL/min): Use with great care Kidney failure (creatinine clearance < 15 mL/min): Use not recommended |
| Rivaroxaban | Prophylaxis of VTE (off-label) * | Prophylaxis: 10 mg QD | Moderate kidney dysfunction (creatinine clearance 30–60 mL/min): no dose reduction Severe kidney dysfunction (creatinine clearance </= 30 mL/min): Use with great care Kidney failure (creatinine clearance < 15 mL/min): Use not recommended |
| Hereditary | Acquired |
|---|---|
| Factor V Leiden mutation • heterozygous • homozygous | Antiphospholipid syndrome |
| Prothrombin mutation • heterozygous • homozygous | Medication-induced thrombophilia (see also sections below) • Lenalidomide, thalidomide, pomalidomide(?), all particularly in combination with corticosteroids) (see the section on multiple myeloma below) • Estrogens • Heparin-induced thrombocytopenia (HIT) |
| Antithrombin deficiency | Paroxysmal nocturnal hemoglobinuria (PNH) |
| Protein C deficiency | Myeloproliferative neoplasms (e.g., PV, ET) |
| Protein S deficiency | |
| Combined thrombophilias (e.g., factor V and prothrombin mutations) |
| Author | Year | n | Intervention | PE | PE Control | PE Experimental | p-Value |
|---|---|---|---|---|---|---|---|
| Bern et al. [133] | 1990 | 121 | VKA vs. placebo | DVT (VG) | 37.5% | 9.5% | <0.001 |
| Monreal et al. [134] | 1996 | 29 | LMWH vs. placebo | DVT (VG) | 62% | 6% | 0.002 |
| Boraks et al. [143] | 1998 | 223 | VKA vs. placebo | DVT (US, VG) | 13% | 5% | 0.03 |
| Heaton et al. [142] | 2002 | 88 | VKA vs. placebo | DVT (VG) | 11.6% | 17.8% | 0.42 |
| Mismetti et al. [148] | 2003 | 59 | VKA (v), LMWH (h) | DVT (VG) | 16.7% (v) | 28.6% (h) | 0.48 |
| Couban et al. [149] | 2005 | 255 | VKA vs. placebo | DVT (symptomatic) | 4.6% | 4.0% | ns |
| Ruud et al. * [150] | 2006 | 73 | VKA vs. placebo | DVT | 36% | 48% | 0.44 |
| Karthaus et al. [138] | 2006 | 439 | LMWH vs. placebo | DVT (VG or US) | 3.4% | 3.7% | 0.83 |
| Verso et al. [151] | 2008 | 385 | LMWH vs. placebo | DVT (VG) | 18% | 14.1% | 0.35 |
| De Cicco et al. [152] | 2009 | 450 | VKA (v), LWMH (h), placebo | DVT (VG) | 52.6% | 21.5%(v)/40% (h) | <0.01 (w); 0.05 (d) |
| Young et al. [153] | 2009 | 812 | VKAvs placebo | DVT (symptomatic) | 6% | 6% | 0.98 |
| Lavau-Denes et al. [137] | 2013 | 420 | VKA or LMWH vs. placebo | DVT | 14.8% | 8% | 0.0357 |
| Niers et al. [154] | 2013 | 113 | VKA vs. placebo | DVT (VG) | 9% | 17% | 0.49 |
| Variable | Point Score |
|---|---|
| IMiD therapy | +4 |
| BMI ≥25 kg/m2 | +1 |
| Pelvic, hip or femur fracture | +4 |
| Erythropoiesis-stimulating agent | +1 |
| Dexamethason (regimen dose) | |
| – Standard dose (≤160 mg/month) – High dose (>160 mg/month) | +2 +4 |
| Doxorubicin | +3 |
| Ethnicity/Race = Asian/Pacific Islander | -3 |
| History of VTE before multiple myeloma diagnosis | +5 |
| Tunneled line or central venous catheter | +2 |
| Existing thromboprophylaxis: therapeutic LMWH or Warfarin | −4 |
| Existing thromboprophylaxis: prophylactic LMWH or aspirin | −3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirschner, M.; do Ó Hartmann, N.; Parmentier, S.; Hart, C.; Henze, L.; Bisping, G.; Griesshammer, M.; Langer, F.; Pabinger-Fasching, I.; Matzdorff, A.; et al. Primary Thromboprophylaxis in Patients with Malignancies: Daily Practice Recommendations by the Hemostasis Working Party of the German Society of Hematology and Medical Oncology (DGHO), the Society of Thrombosis and Hemostasis Research (GTH), and the Austrian Society of Hematology and Oncology (ÖGHO). Cancers 2021, 13, 2905. https://doi.org/10.3390/cancers13122905
Kirschner M, do Ó Hartmann N, Parmentier S, Hart C, Henze L, Bisping G, Griesshammer M, Langer F, Pabinger-Fasching I, Matzdorff A, et al. Primary Thromboprophylaxis in Patients with Malignancies: Daily Practice Recommendations by the Hemostasis Working Party of the German Society of Hematology and Medical Oncology (DGHO), the Society of Thrombosis and Hemostasis Research (GTH), and the Austrian Society of Hematology and Oncology (ÖGHO). Cancers. 2021; 13(12):2905. https://doi.org/10.3390/cancers13122905
Chicago/Turabian StyleKirschner, Martin, Nicole do Ó Hartmann, Stefani Parmentier, Christina Hart, Larissa Henze, Guido Bisping, Martin Griesshammer, Florian Langer, Ingrid Pabinger-Fasching, Axel Matzdorff, and et al. 2021. "Primary Thromboprophylaxis in Patients with Malignancies: Daily Practice Recommendations by the Hemostasis Working Party of the German Society of Hematology and Medical Oncology (DGHO), the Society of Thrombosis and Hemostasis Research (GTH), and the Austrian Society of Hematology and Oncology (ÖGHO)" Cancers 13, no. 12: 2905. https://doi.org/10.3390/cancers13122905
APA StyleKirschner, M., do Ó Hartmann, N., Parmentier, S., Hart, C., Henze, L., Bisping, G., Griesshammer, M., Langer, F., Pabinger-Fasching, I., Matzdorff, A., Riess, H., & Koschmieder, S. (2021). Primary Thromboprophylaxis in Patients with Malignancies: Daily Practice Recommendations by the Hemostasis Working Party of the German Society of Hematology and Medical Oncology (DGHO), the Society of Thrombosis and Hemostasis Research (GTH), and the Austrian Society of Hematology and Oncology (ÖGHO). Cancers, 13(12), 2905. https://doi.org/10.3390/cancers13122905







