Tolerance and Effectiveness of Targeted Therapies in Aged Patients with Metastatic Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Study Design
2.3. Data Collection Methodology
2.3.1. Toxicity Assessment
2.3.2. Efficacy Assessment
2.4. Statistical Analyses
2.5. Funding Sources
3. Results
3.1. Patients
3.2. Tolerance
3.2.1. Univariate Analyses
3.2.2. Multivariate Analyses
3.3. Effectiveness
4. Discussion
4.1. Tolerance
4.2. Effectiveness
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Skin and Sub Cutaneous Disorders: | Investigations |
|---|---|
| Photosensitivity | GGT increased |
| Palmar-plantar erythrodysesthesia syndrome | Alanine aminotransferase increased |
| Rash maculo-papular | Aspartate aminotransferase increased |
| Rash acneiform | Alkaline phosphatase increased |
| Erythema multiforme | CPK increased |
| Alopecia | Lipase increased |
| Dry skin | Renal and urinary disorders |
| Pruritus | Renal and urinary disorders - Other |
| Dysgeusia | Creatinine increased |
| Papulopustular rash | Chronic kidney disease |
| Erythroderma | Acute kidney injury |
| Skin hypopigmentation | Proteinuria |
| Keratitis | Renal calculi |
| Edema | Nervous system disorders |
| Dry mouth | Paresthesia |
| Urticaria | Peripheral motor neuropathy |
| Pain in extremity | Facial nerve disorder |
| Burn | Encephalopathy |
| Skin Others | Peripheral sensory neuropathy |
| Localized edema | Eyelid function disorder |
| Skin ulceration | Nervous system disorders - Other |
| Purpura | Dysesthesia |
| Rash pustular | Vascular disorder |
| Toxic epidermal necrolysis | Ejection fraction decreased |
| Skin hyperpigmentation | Left ventricular systolic dysfunction |
| Allergic reaction | Cardiac disorders - Other |
| Hypertrichosis | Hypotension |
| Mucositis oral | Sinus tachycardia |
| Musculoskeletal and systemic disorders | Hypertension |
| Arthralgia | Conduction disorder |
| Myalgia | Chest pain – cardiac |
| Back pain | Atrioventricular block complete |
| Musculoskeletal Other | Electrocardiogram QT corrected interval prolonged |
| Arthritis | Atrial fibrillation |
| Bone pain | Palpitations |
| Lordosis | Gastrointestinal disorders |
| Muscle weakness left-sided | Diarrhea |
| Joint range of motion decreased | Abdominal pain |
| General Disorders and Administration Site Conditions | Constipation |
| Fatigue | Bloating |
| Fever | Abdominal distension |
| Headache | Gastrointestinal disorders – Other |
| Pain | Colitis Gastric hemorrhage |
| Chills | Non precised, malignant and benign tumors |
| Anorexia | Treatment related secondary malignancy |
| Weight loss | Neoplasms benign, malignant and unspecified |
| Generalized muscle weakness | Hepatobilary disorders |
| General disorders and administration site conditions - Other | Vomiting |
| Anxiety | Bile duct stenosis |
| Depression | Stomach pain |
| Insomnia | Pancreatitis |
| Malaise | Ocular manifestations |
| Autoimmune disorder | Eye disorders |
| Hypothermia | Retinal detachment |
| Weight gain | Blurred vision |
| Hearing impaired | Uveitis |
| Concentration impairment | Dry eye |
| Ear and labyrinth disorders | Eye pain |
| Infection and infestations | Papilledema |
| Sepsis | Retinal tear |
| Skin infection | Mediastinal, respiratory and thoracic disorders |
| Infusion site extravasation | Dyspnea |
| Joint infection | Thorax cough |
| Pneumonitis | Metabolism and nutrition disorders |
| Urinary tract infection | Hyperglycemia |
| Lip infection | Adrenal insufficiency |
| Hematologic and lymphatic disorders | Metabolism and nutrition disorders, others |
| Anemia | |
| Neutrophil count decreased | |
| Lymphocyte count decreased | |
| Platelet count decreased | |
| Febrile neutropenia | |
| Hyponatremia | |
| Blood and lymphatic system disorders - Other | |
| White blood cell decreased | |
| Lymphedema |
References
- Binder-Foucard, F.; Bossard, N.; Delafosse, P.; Belot, A.; Woronoff, A.-S.; Remontet, L. Cancer incidence and mortality in France over the 1980–2012 period: Solid tumors. Rev. D’épidémiologie Et De St. Publique 2014, 62, 95–108. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Kendal, W.S. Dying with cancer: The influence of age, comorbidity, and cancer site. Cancer 2008, 112, 1354–1362. [Google Scholar] [CrossRef]
- Tas, F.; Erturk, K. Patient age and cutaneous malignant melanoma: Elderly patients are likely to have more aggressive histological features and poorer survival. Mol. Clin. Oncol. 2017, 7, 1083–1088. [Google Scholar] [CrossRef]
- Marosi, C.; Köller, M. Challenge of cancer in the elderly. ESMO Open 2016, 1, e000020. [Google Scholar] [CrossRef]
- Pallis, A.G.; Ring, A.; Fortpied, C.; Penninckx, B.; Van Nes, M.C.; Wedding, U.; Vonminckwitz, G.; Johnson, C.D.; Wyld, L.; Timmer-Bonte, A.; et al. EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann. Oncol. 2011, 22, 1922–1926. [Google Scholar] [CrossRef]
- Trimble, E.L.; Cain, D.; Ungerleider, R.S.; Friedman, M.A.; Carter, C.L.; Freidlin, B. Representation of older patients in cancer treatment trials. Cancer 1994, 74, 2208–2214. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; De Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.G.M.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.-J.; et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Grob, J.J.; Amonkar, M.M.; Karaszewska, B.; Schachter, J.; Dummer, R.; Mackiewicz, A.; Stroyakovskiy, D.; Drucis, K.; Grange, F.; Sileni, V.C.; et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): Results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015, 16, 1389–1398. [Google Scholar] [CrossRef]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Larkin, J. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Dréno, B.; Ribas, A.; Larkin, J.; Ascierto, P.A.; Hauschild, A.; Thomas, L.; Grob, J.-J.; Koralek, D.O.; Rooney, I.; Hsu, J.J.; et al. Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann. Oncol. 2017, 28, 1137–1144. [Google Scholar] [CrossRef]
- Daste, A.; Chakiba, C.; Domblides, C.; Gross-Goupil, M.; Quivy, A.; Ravaud, A.; Soubeyran, P. Targeted therapy and elderly people: A review. Eur. J. Cancer 2016, 69, 199–215. [Google Scholar] [CrossRef]
- Quinten, C.; Coens, C.; Ghislain, I.; Zikos, E.; Sprangers, M.A.; Ringash, J.; Martinelli, F.; Ediebah, D.E.; Maringwa, J.; Reeve, B.B.; et al. The effects of age on health-related quality of life in cancer populations: A pooled analysis of randomized controlled trials using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur. J. Cancer 2015, 51, 2808–2819. [Google Scholar] [CrossRef]
- Menzies, A.M.; Haidj, L.E.; Visintin, L.; Carlino, M.S.; Howle, J.R.; Thompson, J.F.; Kefford, R.F.; Xcolyer, R.A.; Long, G.V. Distinguishing clinicopathologic featrures of patients with V600E and V600K BRAF-Mutant metastatic melanoma. Clin. Cancer Res. 2012, 18, 3242–3249. [Google Scholar] [CrossRef]
- Pallis, A.; Fortpied, C.; Wedding, U.; Van Nes, M.; Penninckx, B.; Ring, A.; Lacombe, D.; Monfardini, S.; Scalliet, P.; Wildiers, H. EORTC elderly task force position paper: Approach to the older cancer patient. Eur. J. Cancer 2010, 46, 1502–1513. [Google Scholar] [CrossRef]
- Larkin, J.; Del Vecchio, M.; Ascierto, P.A.; Krajsová, I.; Schachter, J.; Neyns, B.; Espinosa, E.; Garbe, C.; Sileni, V.C.; Gogas, H.; et al. Vemurafenib in patients with BRAFV600 mutated metastatic melanoma: An open-label, multicentre, safety study. Lancet Oncol. 2014, 15, 436–444. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; McArthur, G.A. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Hauschild, A.; Grob, J.-J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- McArthur, G.A.; Chapman, P.B.; Robert, C.; Larkin, J.; Haanen, J.B.; Dummer, R.; Hauschild, A. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomized, open-label study. Lancet Oncol. 2014, 15, 323–332. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600–Mutant Advanced Melanoma Treated with Vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef]
- Liu, M.; Yang, X.; Liu, J.; Zhao, B.; Cai, W.; Li, Y.; Hu, D. Efficacy and safety of BRAF inhibition alone versus combined BRAF and MEK inhibition in melanoma: A meta-analysis of randomized controlled trials. Oncotarget 2017, 8, 32258–32269. [Google Scholar] [CrossRef]
- Carlos, G.; Anforth, R.; Clements, A.; Menzies, A.M.; Carlino, M.S.; Chou, S.; Fernandez-Peñas, P. Cutaneous Toxic Effects of BRAF Inhibitors Alone and in Combination With MEK Inhibitors for Metastatic Melanoma. JAMA Dermatol. 2015, 151, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Sanlorenzo, M.; Choudhry, A.; Vujic, I.; Posch, C.; Chong, K.; Johnston, K.; Meier, M.; Osella-Abate, S.; Quaglino, P.; Daud, A.; et al. Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. J. Am. Acad. Dermatol. 2014, 71, 1102–1109. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Schadendorf, D. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef]
- Russo, I.; Zorzetto, L.; Frigo, A.C.; Sileni, V.C.; Alaibac, M. A comparative study of the cutaneous side effects between BRAF monotherapy and BRAF/MEK inhibitor combination therapy in patients with advanced melanoma: A single-centre experience. Eur. J. Dermatol. EJD 2017, 27, 482–486. [Google Scholar] [CrossRef]
- Funck-Brentano, C.; Alvarez, J.C.; Longvert, C.; Abe, E.; Beauchet, A.; Saiag, P. Plasma vemurafenib concentrations in advanced BRAFV600mut melanoma patients: Impact on tumour response and tolerance. Ann. Oncol. 2015, 26, 1470–1475. [Google Scholar] [CrossRef]
- Manola, J.; Atkins, M.; Ibrahim, J.; Kirkwood, J. Prognostic Factors in Metastatic Melanoma: A Pooled Analysis of Eastern Cooperative Oncology Group Trials. J. Clin. Oncol. 2000, 18, 3782–3793. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Palmer, R.M.; Fortinsky, R.H.; Counsell, S.R.; Stewart, A.L.; Rn, D.K.; Ma, C.J.B.; Landefeld, C.S. Loss of Independence in Activities of Daily Living in Older Adults Hospitalized with Medical Illnesses: Increased Vulnerability with Age. J. Am. Geriatr. Soc. 2003, 51, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Trefzer, U.; Davies, M.A.; Kefford, R.; Ascierto, P.A.; Chapman, P.B.; Puzanov, I.; Hauschild, A.; Robert, C.; Algazi, A.; et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 1087–1095. [Google Scholar] [CrossRef]
- Márquez-Rodas, I.; Avilés-Izquierdo, J.-A.; Parra, V.; Álvarez-González, A.; Borrego, P.; Fernández-García, P.; Guzmán-De-Villoria, J.A.; Jerez, Y.; Martin, M. Exclusion Criteria vs Reality: Dual BRAF/MEK Inhibition and Radiotherapy in a Patient with Melanoma Metastatic to the Brain and ECOG 3. Tumori J. 2016, 102, S54–S56. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.; Long, G.V. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Dummer, R.; Goldinger, S.M.; Turtschi, C.P.; Eggmann, N.B.; Michielin, O.; Mitchell, L.; Rinderknecht, J.D. Vemurafenib in patients with BRAF (V600) mutation-positive melanoma with symptomatic brain metastases: Final results of an open-label pilot study. Eur. J. Cancer 2014, 50, 611–621. [Google Scholar] [CrossRef]
- Bastiaannet, E.; Battisti, N.; Loh, K.P.; de Glas, N.; Soto-Perez-De-Celis, E.; Baldini, C.; Kapiteijn, E.; Lichtman, S. Immunotherapy and targeted therapies in older patients with advanced melanoma; Young International Society of Geriatric Oncology review paper. J. Geriatr. Oncol. 2019, 10, 389–397. [Google Scholar] [CrossRef]
- Kramkimel, N.; Thomasschoemann, A.; Sakji, L.; Golmard, J.; Noe, G.; Regnier-Rosencher, E.; Chapuis, N.; Maubec, E.; Vidal, M.; Avril, M.; et al. Vemurafenib pharmacokinetics and its correlation with efficacy and safety in outpatients with advanced BRAF-mutated melanoma. Target. Oncol. 2016, 11, 59–69. [Google Scholar] [CrossRef]
- European Medicines Agency—Research and Development—Medicines for Older People [Internet]. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000249.jsp&mid=WC0b01ac058004cbb9 (accessed on 6 February 2018).
| Demography | |||
|---|---|---|---|
| Characteristics | Group 1 | Group 2 | p |
| Age | |||
| Mean (standard deviation) years | 50 (10) | 75 (6) | |
| Median (Q1;Q3) years | 51 (43–57) | 73 (70–79) | |
| Gender, N (%) | 0.6 | ||
| Female | 96 (42) | 50 (41) | |
| Male | 135 (58) | 72 (59) | |
| Clinical characteristics | |||
| ECOG, N (%) | 0.8 | ||
| <2 | 202 (87) | 108 (89) | |
| ≥2 | 29 (13) | 14 (11) | |
| Stage T | 0.7 | ||
| 1 | 32 (14) | 9 (7) | |
| 2 | 45 (19) | 18 (15) | |
| 3 | 46 (20) | 37 (30) | |
| 4 | 62 (27) | 37 (30) | |
| Unknown primary | 37 (16) | 15 (12) | |
| Unknown Breslow | 9 (4) | 6 (5) | |
| Stage N | 0.6 | ||
| 0 | 67 (29) | 36 (30) | |
| 1 | 38 (16) | 13 (11) | |
| 2 | 29 (13) | 19 (16) | |
| 3 | 97 (42) | 54 (44) | |
| Stage M | 0.8 | ||
| 0 | 21 (9) | 10 (8) | |
| 1a | 22 (10) | 11 (9) | |
| 1b | 23 (10) | 16 (13) | |
| 1c | 165 (71) | 85 (70) | |
| Stage AJCC | 0.9 | ||
| III | 20 (9) | 10 (8) | |
| IV | 211 (91) | 112 (92) | |
| Nb of affected organs | 0.4 | ||
| <3 | 121 (52) | 58 (48) | |
| >3 | 110 (48) | 64 (52) | |
| Nb of patients with brain metastases | 61 (26) | 30 (25) | 0.5 |
| LDH | 0.2 | ||
| N missing | 28 (12) | 13 (11) | |
| N (%) > x ULN | 79 (34) | 54 (44) | |
| N (%) ≤ x ULN | 124 (54) | 55 (45) | |
| Treatment characteristics | |||
| Treatment line | 0.8 | ||
| First line | 185 (80) | 102 (84) | |
| Second line | 33 (14) | 12 (10) | |
| Third line | 13 (6) | 8 (7) | |
| Discontinuations, causes | 0,.6 | ||
| Progression | 96 (42) | 35 (29) | |
| Toxicity | 41 (18) | 34 (28) | |
| Medical choice | 16 (7) | 14 (11) | |
| Patient's choice | 7 (3) | 2 (2) | |
| Death | 7 (3) | 7 (6) | |
| Unknown | 16 (7) | 6 (5) | |
| Whole Population (n = 353) | Group 1 (n = 231) | Group 2 (n = 122) | p | |
|---|---|---|---|---|
| AE (all grade) (n event (% of concerned patients)) | 281 (80) | 184 (80) | 97 (80) | 0.8 |
| AE grade <3 (n event (% of concerned patients)) | 255 (72) | 172 (75) | 83 (68) | 0.5 |
| AE grade ≥3 (n event (% of concerned patients)) | 112 (31) | 65 (28) | 47 (39) | <0.05 |
| Number of patients who have 0 AE | 72 (20) | 47 (20) | 25 (20) | 0.8 |
| Number of patients who have 1 AE | 71 (20) | 49 (21) | 22 (18) | 0.7 |
| Number of patients who have 2 AE | 46 (13) | 28 (12) | 18 (15) | 0.7 |
| Number of patients who have >2 AE | 164 (47) | 107 (46) | 57 (47) | 0.8 |
| Dose modification (n event (% of concerned patients)) | 76 (22) | 46 (20) | 30 (25) | 0.6 |
| Treatment interruption (n event (% of concerned patients)) | 95 (27) | 57 (25) | 38 (31) | 0.4 |
| Treatment discontinuation (n event (% of concerned patients)) | 72 (20) | 41 (18) | 31 (25) | 0.6 |
| Number of hospitalization for toxicity (n event (% of concerned patients)) | 99 (18) | 69 (19) | 23 (19) | 0.8 |
| AE time to onset (months) | 3.9 | 4 | 3.6 | 0.7 |
| Adverse Effects Profile | ||
|---|---|---|
| Group 1 (n Event (% of Concerned Patients)) | Group 2 (n Event (% of Concerned Patients)) | |
| All Grade Adverse Effects | ||
| Skin and sub cutaneous disorders | 265 (50) | 141 (57) |
| General disorders and administration site conditions | 166 (40) | 87 (33) |
| Gastrointestinal disorders | 102 (27) | 64 (30) |
| Musculoskeletal and systemic disorders | 67 (20) | 21 (15) |
| Investigations | 55 (11) | 19 (8) |
| Nervous system disorders | 28 (10) | 4 (2) |
| Hematologic and lymphatic disorders | 23 (7) | 16 (7) |
| Ocular manifestations | 21 (9) | 18 (11) |
| Renal and urinary disorders | 17 (6) | 25 (13) |
| Non-precised malignant and benign tumors | 9 (3) | 20 (7) |
| Vascular Disorders | 17 (6) | 14 (9) |
| Group 1 n = 231 | Group 2 n = 72 | p | |
|---|---|---|---|
| Best response N (%) | |||
| Complete Response (CR) | 45(19) | 20 (16) | 0.8 |
| Partial Response (PR) | 91 (39) | 41 (34) | 0.7 |
| Stable Disease (SD) | 55 (24) | 33 (27) | 0.8 |
| Progressive Disease (PD) | 40 (17) | 28 (23) | 0.4 |
| Objective response (CR+ PR) N(%) | 136 (59) | 61 (50) | 0.6 |
| Disease control (R + PR + SD) N (%) | 191 (83) | 94 (77) | 0.7 |
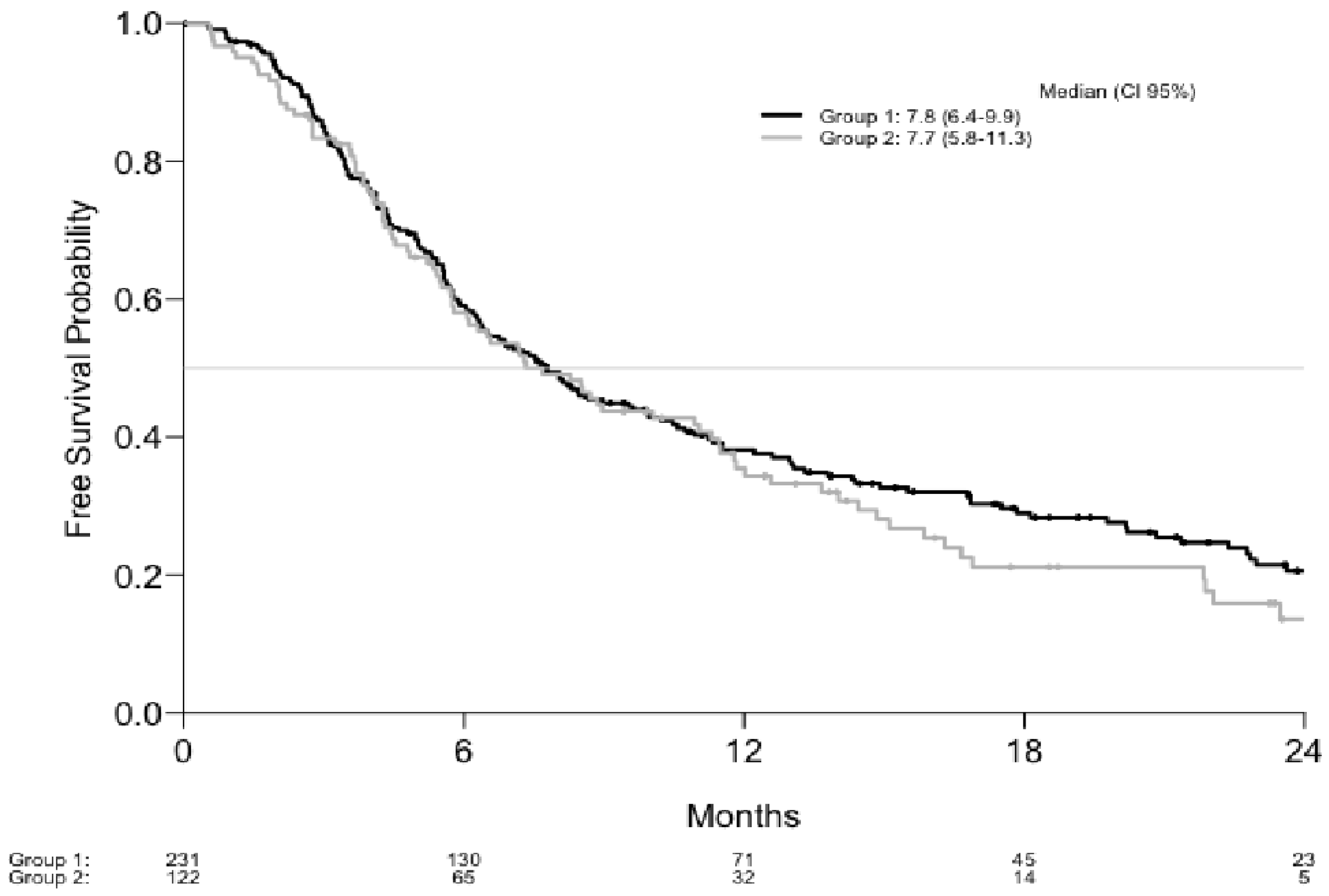
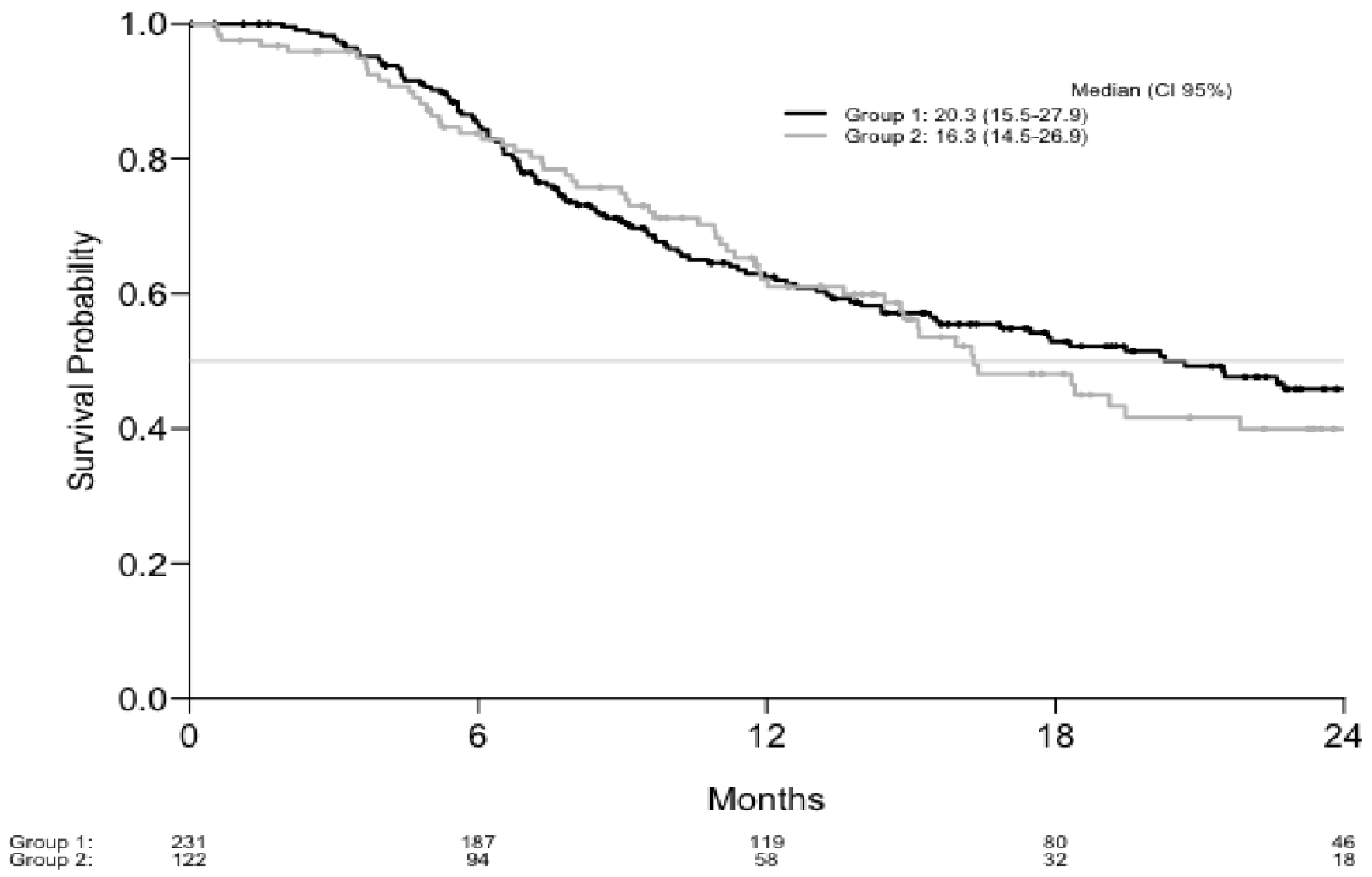
| Progression Free Survival Estimations | Overall Survival Estimations | ||||
|---|---|---|---|---|---|
| Months | Estimation (%) | CI 95% | Months | Estimation (%) | CI 95% |
| Group 1 (%) | Group 1 (%) | ||||
| 6 | 59.1 | 53.0–65.9 | 6 | 85.2 | 80.7–90.0 |
| 12 | 38.1 | 32.0–45.2 | 12 | 62.4 | 56.2–69.4 |
| 18 | 29.0 | 23.3–36.1 | 18 | 52.9 | 46.3–60.3 |
| 24 | 20.6 | 15.3–27.8 | 24 | 45.9 | 39.0–53.9 |
| Group 2 (%) | Group 2 (%) | ||||
| 6 | 58.1 | 49.7–67.8 | 6 | 83.8 | 77.4–90.7 |
| 12 | 35.4 | 27.5–45.7 | 12 | 62.1 | 53.6–71.9 |
| 18 | 21.1 | 14.1–31.6 | 18 | 48.1 | 38.9–59.5 |
| 24 | 13.6 | 7.5–24.8 | 24 | 39.9 | 30.5–52.2 |
| Total (%) | Total (%) | ||||
| 6 | 58.7 | 53.7–64.2 | 6 | 84.7 | 81.0–88.6 |
| 12 | 37.2 | 32.3–42.9 | 12 | 62.3 | 57.2–67.9 |
| 18 | 26.6 | 21.9–32.2 | 18 | 51.5 | 46.1–57.6 |
| 24 | 18.5 | 14.1–24.2 | 24 | 44.2 | 38.5–50.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becquart, O.; Oriano, B.; Dalle, S.; Mortier, L.; Leccia, M.T.; Dutriaux, C.; Dalac, S.; Montaudié, H.; De Quatrebarbes, J.; Brunet-Possenti, F.; et al. Tolerance and Effectiveness of Targeted Therapies in Aged Patients with Metastatic Melanoma. Cancers 2021, 13, 3042. https://doi.org/10.3390/cancers13123042
Becquart O, Oriano B, Dalle S, Mortier L, Leccia MT, Dutriaux C, Dalac S, Montaudié H, De Quatrebarbes J, Brunet-Possenti F, et al. Tolerance and Effectiveness of Targeted Therapies in Aged Patients with Metastatic Melanoma. Cancers. 2021; 13(12):3042. https://doi.org/10.3390/cancers13123042
Chicago/Turabian StyleBecquart, Ondine, Bastien Oriano, Stéphane Dalle, Laurent Mortier, Marie Thérèse Leccia, Caroline Dutriaux, Sophie Dalac, Henri Montaudié, Julie De Quatrebarbes, Florence Brunet-Possenti, and et al. 2021. "Tolerance and Effectiveness of Targeted Therapies in Aged Patients with Metastatic Melanoma" Cancers 13, no. 12: 3042. https://doi.org/10.3390/cancers13123042
APA StyleBecquart, O., Oriano, B., Dalle, S., Mortier, L., Leccia, M. T., Dutriaux, C., Dalac, S., Montaudié, H., De Quatrebarbes, J., Brunet-Possenti, F., Saiag, P., Lesimple, T., Beylot-Barry, M., Aubin, F., Stoebner, P.-E., Arnault, J.-P., Dreno, B., Porcher, R., Lebbe, C., & Guillot, B. (2021). Tolerance and Effectiveness of Targeted Therapies in Aged Patients with Metastatic Melanoma. Cancers, 13(12), 3042. https://doi.org/10.3390/cancers13123042








