Successful Patient-Derived Organoid Culture of Gynecologic Cancers for Disease Modeling and Drug Sensitivity Testing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Features
2.2. Generation of Patient-Derived Organoid Models Using Tumor and Normal Cells
2.3. Organoid Viability Assay
2.4. Statistical Analysis
3. Results
3.1. Feasibility of Organoid Culture Creation from Freshly Resected Endometrial and Ovarian Cancer
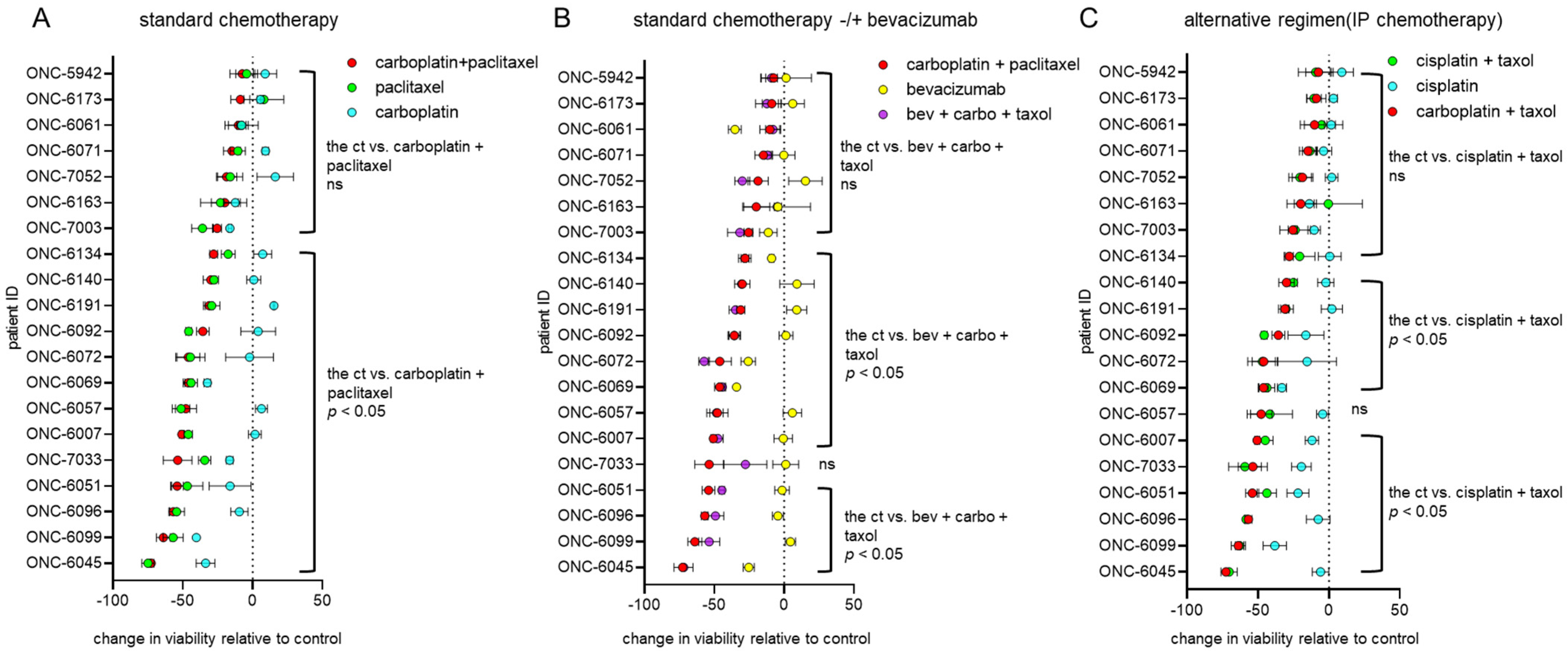
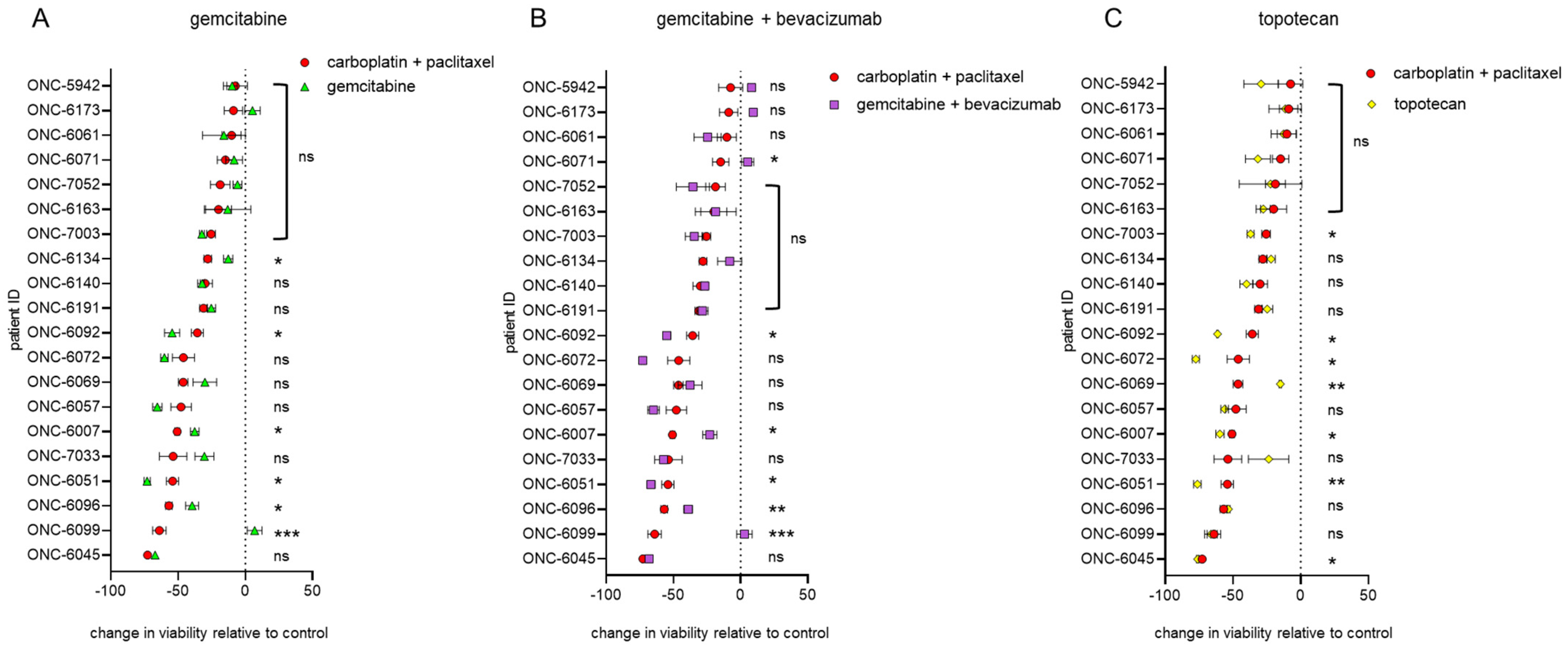
3.2. Endometrial and Ovarian Cancer PDO Drug Response
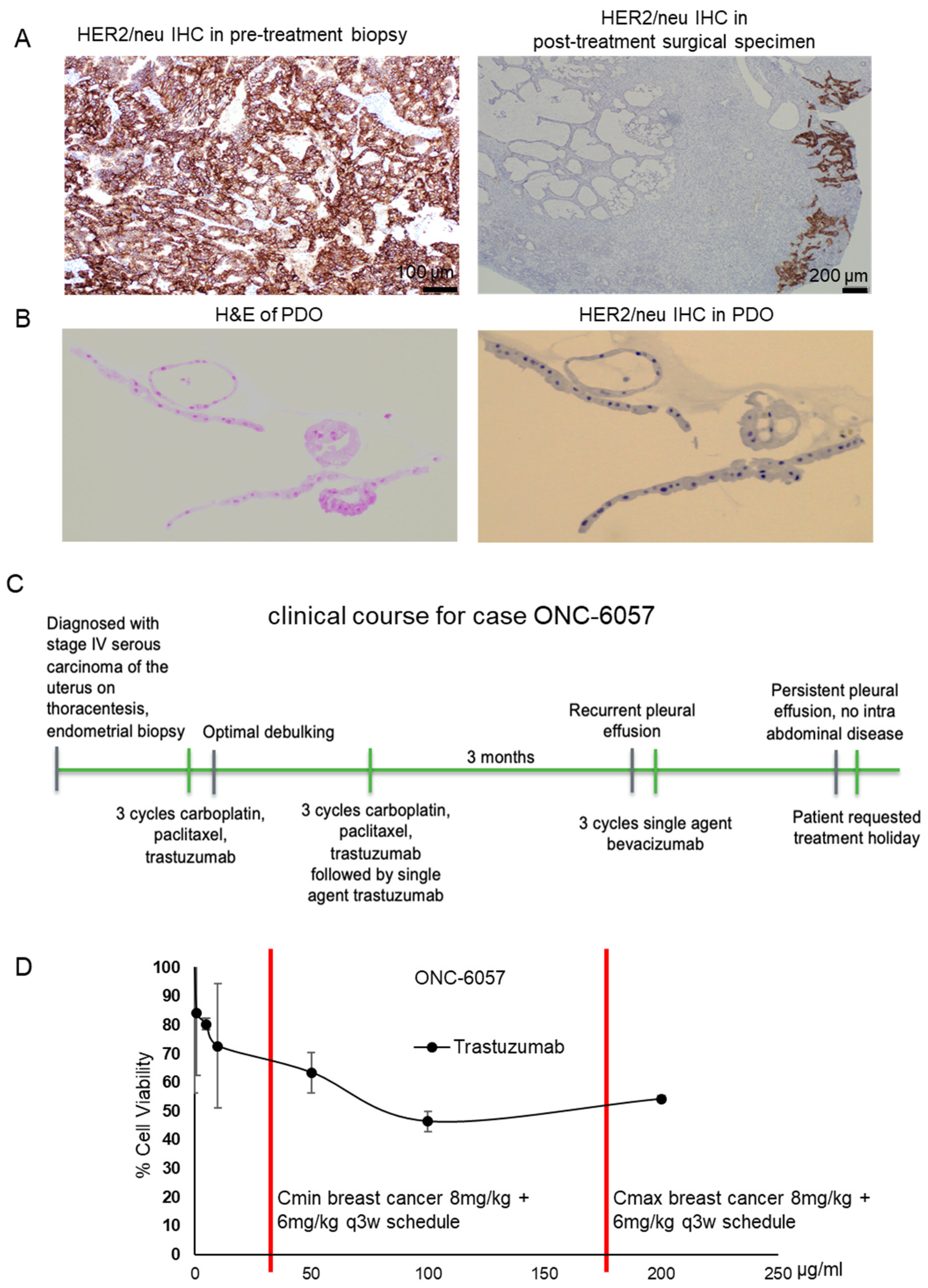
3.3. Proof-of-Concept: A PDO Model Reflects Treatment Response
3.3.1. Patient Characteristics
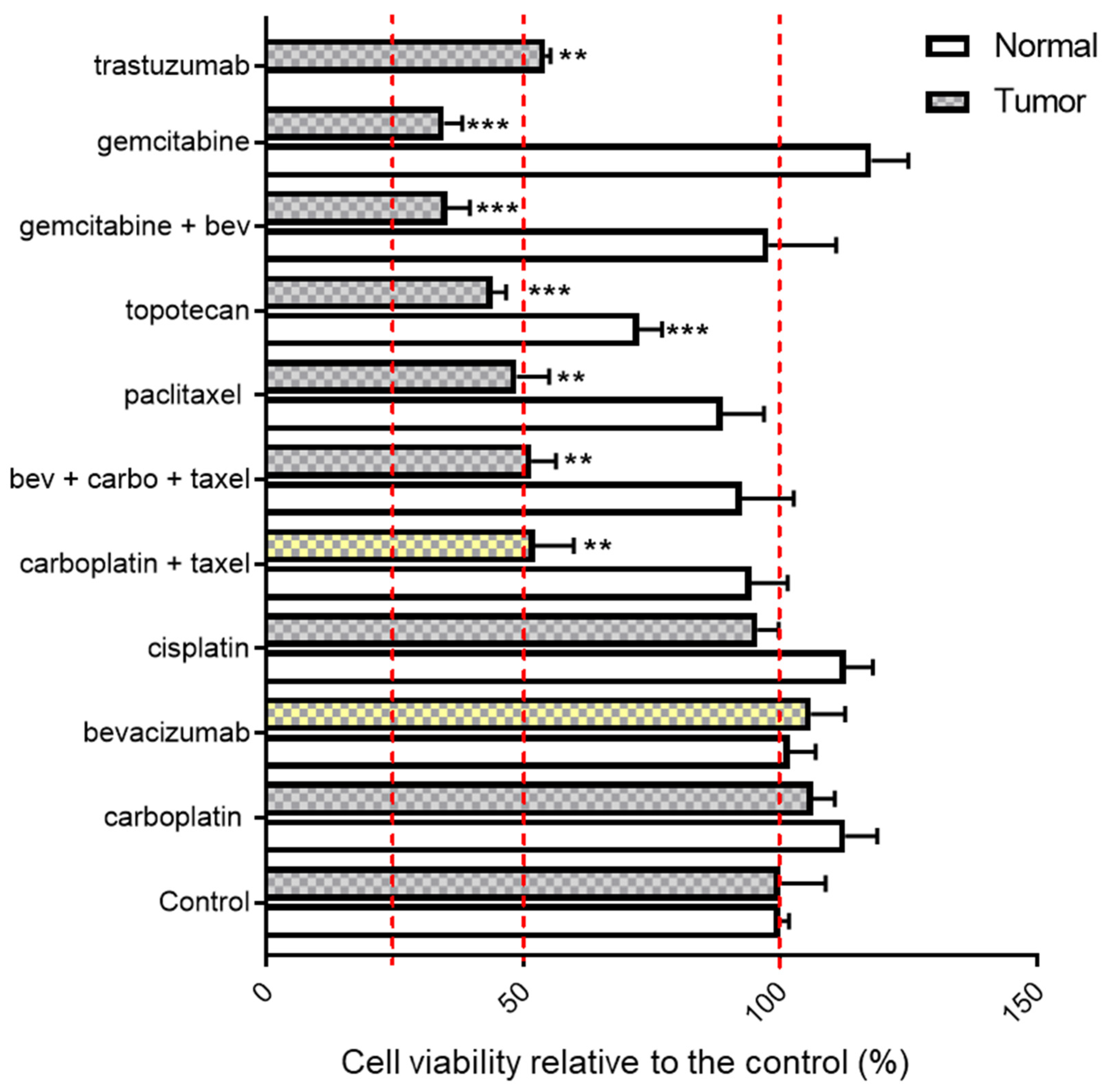
3.3.2. Trastuzumab Response in the Patient-Derived Tumor Organoid Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornelison, R.; Llaneza, D.C.; Landen, C.N. Emerging Therapeutics to Overcome Chemoresistance in Epithelial Ovarian Cancer: A Mini-Review. Int. J. Mol. Sci. 2017, 18, 2171. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, S.; Kim, Y.T.; Lim, M.C.; Lee, B.; Jung, K.-W.; Kim, J.W.; Park, S.-Y.; Won, Y.-J. Changes in ovarian cancer survival during the 20 years before the era of targeted therapy. BMC Cancer 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Viale, R.P.H. The American Cancer Society’s Facts & Figures: 2020 Edition. J. Adv. Pract. Oncol. 2020, 11, 135–136. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Althouse, A.D.; Freese, K.E.; Soisson, S.; Edwards, R.P.; Welburn, S.; Sukumvanich, P.; Comerci, J.; Kelley, J.; LaPorte, R.E.; et al. USA Endometrial Cancer Projections to 2030: Should we be concerned? Future Oncol. 2014, 10, 2561–2568. [Google Scholar] [CrossRef]
- Ledermann, J.A. First-line treatment of ovarian cancer: Questions and controversies to address. Ther. Adv. Med. Oncol. 2018, 10, 1758835918768232. [Google Scholar] [CrossRef]
- Gupta, D. Clinical Behavior and Treatment of Endometrial Cancer. Adv. Exp. Med. Biol. 2017, 943, 47–74. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef]
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA A Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, K.; Gévry, N.; Asselin, E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget 2016, 8, 4008–4042. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, J.A.; Liu, Y.; Kron, S.J. Small-molecule drug repurposing to target DNA damage repair and response pathways. Semin. Cancer Biol. 2021, 68, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Coleman, R.; González-Martín, A.; Moore, K.; Colombo, N.; Ray-Coquard, I.; Pignata, S. The forefront of ovarian cancer therapy: Update on PARP inhibitors. Ann. Oncol. 2020, 31, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.K.; Filiaci, V.L.; Mallen, A.R.; Thiel, K.W.; Devor, E.J.; Moxley, K.; Richardson, D.; Mutch, D.; Secord, A.A.; Tewari, K.S.; et al. Mutated p53 portends improvement in outcomes when bevacizumab is combined with chemotherapy in advanced/recurrent endometrial cancer: An NRG Oncology study. Gynecol. Oncol. 2021, 161, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017, 7, 462–477. [Google Scholar] [CrossRef]
- Leslie, K.K.; Thiel, K.W.; Benbrook, D.M.; Mutch, D. The Dawning of the Age of Personalized Medicine in Gynecologic Oncology. Cancers 2020, 12, 3135. [Google Scholar] [CrossRef] [PubMed]
- De Witte, C.J.; Valle-Inclan, J.E.; Hami, N.; Lõhmussaar, K.; Kopper, O.; Vreuls, C.P.H.; Jonges, G.N.; van Diest, P.; Nguyen, L.; Clevers, H.; et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep. 2020, 31, 107762. [Google Scholar] [CrossRef]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; Van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; Van De Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, eaay2574. [Google Scholar] [CrossRef]
- Pasch, C.A.; Favreau, P.F.; Yueh, A.E.; Babiarz, C.P.; Gillette, A.A.; Sharick, J.T.; Karim, M.R.; Nickel, K.P.; DeZeeuw, A.K.; Sprackling, C.M.; et al. Patient-Derived Cancer Organoid Cultures to Predict Sensitivity to Chemotherapy and Radiation. Clin. Cancer Res. 2019, 25, 5376–5387. [Google Scholar] [CrossRef]
- Collins, A.; Miles, G.J.; Wood, J.; MacFarlane, M.; Pritchard, C.; Moss, E. Patient-derived explants, xenografts and organoids: 3-dimensional patient-relevant pre-clinical models in endometrial cancer. Gynecol. Oncol. 2020, 156, 251–259. [Google Scholar] [CrossRef]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Kopper, O.; de Witte, C.J.; Lõhmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.; Hong, J.J.; Tofig, B.; Mapua, M.; Elashoff, D.; Moatamed, N.; Huang, J.; Memarzadeh, S.; Damoiseaux, R.; Soragni, A. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Organoids for Drug Discovery and Personalized Medicine. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 447–462. [Google Scholar] [CrossRef]
- Bi, J.; Thiel, K.W.; Litman, J.M.; Zhang, Y.; Devor, E.J.; Newtson, A.M.; Schnieders, M.J.; Bosquet, J.G.; Leslie, K.K. Characterization of a TP53 Somatic Variant of Unknown Function from an Ovarian Cancer Patient Using Organoid Culture and Computational Modeling. Clin. Obstet. Gynecol. 2020, 63, 109–119. [Google Scholar] [CrossRef]
- Gupta, S.; Provenzale, D.; Llor, X.; Halverson, A.L.; Grady, W.; Chung, D.C.; Haraldsdottir, S.; Markowitz, A.J.; Slavin, T.P., Jr.; Hampel, H.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 2.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton-Thirsk, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Van Leeuwen, J.H.S.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef]
- Francis, J.-A.; Coakley, N.; Elit, L.; Mackay, H. Systemic Therapy for Recurrent Epithelial Ovarian Cancer: A Clinical Practice Guideline. Curr. Oncol. 2017, 24, 540–546. [Google Scholar] [CrossRef]
- Barcellini, A.; Roccio, M.; Laliscia, C.; Zanellini, F.; Pettinato, D.; Valvo, F.; Mirandola, A.; Orlandi, E.; Gadducci, A. Endometrial Cancer: When Upfront Surgery Is Not an Option. Oncology 2021, 99, 65–71. [Google Scholar] [CrossRef]
- Charo, L.M.; Plaxe, S.C. Recent advances in endometrial cancer: A review of key clinical trials from 2015 to 2019. F1000Research 2019, 8, 849. [Google Scholar] [CrossRef]
- Shapiro, J.D.; Millward, M.J.; Rischin, D.; Michael, M.; Walcher, V.; Francis, P.A.; Toner, G.C. Activity of Gemcitabine in Patients with Advanced Ovarian Cancer: Responses Seen Following Platinum and Paclitaxel. Gynecol. Oncol. 1996, 63, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Huinink, W.T.B.; Gore, M.; Carmichael, J.; Gordon, A.; Malfetano, J.; Hudson, I.; Broom, C.; Scarabelli, C.; Davidson, N.; Spanczynski, M.; et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J. Clin. Oncol. 1997, 15, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J. Clin. Oncol. 2018, 36, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Itami, M.; Hippo, Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol. Oncol. 2019, 154, 189–198. [Google Scholar] [CrossRef]
- Girda, E.; Huang, E.C.; Leiserowitz, G.S.; Smith, L.H. The Use of Endometrial Cancer Patient–Derived Organoid Culture for Drug Sensitivity Testing Is Feasible. Int. J. Gynecol. Cancer 2017, 27, 1701–1707. [Google Scholar] [CrossRef]
- Chen, H.; Gotimer, K.; De Souza, C.; Tepper, C.G.; Karnezis, A.N.; Leiserowitz, G.S.; Chien, J.; Smith, L.H. Short-term organoid culture for drug sensitivity testing of high-grade serous carcinoma. Gynecol. Oncol. 2020, 157, 783–792. [Google Scholar] [CrossRef]
- Hill, S.J.; Decker, B.; Roberts, E.A.; Horowitz, N.S.; Muto, M.G.; Worley, M.J.; Feltmate, C.M.; Nucci, M.R.; Swisher, E.M.; Nguyen, H.; et al. Prediction of DNA Repair Inhibitor Response in Short-Term Patient-Derived Ovarian Cancer Organoids. Cancer Discov. 2018, 8, 1404–1421. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Es, H.A.; Montazeri, L.; Aref, A.R.; Vosough, M.; Baharvand, H. Personalized Cancer Medicine: An Organoid Approach. Trends Biotechnol. 2018, 36, 358–371. [Google Scholar] [CrossRef]
- Van De Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; Van Houdt, W.; Van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Fujii, M.; Shimokawa, M.; Date, S.; Takano, A.; Matano, M.; Nanki, K.; Ohta, Y.; Toshimitsu, K.; Nakazato, Y.; Kawasaki, K.; et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell 2016, 18, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Gaiti, F.; Chaligne, R.; Gu, H.; Brand, R.M.; Kothen-Hill, S.; Schulman, R.C.; Grigorev, K.; Risso, D.; Kim, K.-T.; Pastore, A.; et al. Epigenetic evolution and lineage histories of chronic lymphocytic leukaemia. Nat. Cell Biol. 2019, 569, 576–580. [Google Scholar] [CrossRef] [PubMed]
| Patient ID | Cancer Type | Stage | Neoadjuvant Treatment | Adjuvant Treatment | Platinum-Sensitive? | Disease Status | |
|---|---|---|---|---|---|---|---|
| Ovarian Cancer | ONC-5942 | High-grade serous | IVB | Six cycles of carboplatin and paclitaxel followed by two cycles of carboplatin | Three cycles of doxorubicin and bevacizumab followed by bevacizumab | No | NED |
| ONC-6007 * | High-grade serous | IVB | No | Six cycles of IV/IP cisplatin and paclitaxel | Yes | Alive with disease | |
| ONC-6045 * | High-grade serous | IIIB | No | One cycle of a single agent, carboplatin, followed by six cycles of carboplatin and paclitaxel | Yes | NED | |
| ONC-6069 | High-grade serous | Recurrent | Six cycles of carboplatin and paclitaxel followed by olaparib | Olaparib followed by gemcitabine | No | Dead of disease | |
| ONC-6072 | High-grade serous | IC | No | None | N/A | Lost to follow-up | |
| ONC-6134 | High-grade serous | IIIA | No | Six cycles of carboplatin and paclitaxel | Yes | NED | |
| ONC-6163 | High-grade serous | Recurrent | No | Six cycles of carboplatin and doxorubicin followed by olaparib | TBD | NED | |
| ONC-7052 | High-grade serous | IIIC | No | Six cycles of IV/IP cisplatin and paclitaxel | TBD | NED | |
| ONC-6061 | Low-grade serous | IIIC | No | Two cycles of carboplatin and paclitaxel followed by seven cycles of a single agent, carboplatin, followed by letrozole | No | Alive with disease | |
| ONC-7063 | Clear cell | IC1 | No | Three cycles of carboplatin and paclitaxel | TBD | NED | |
| ONC-6092 | Clear cell | IC | No | Three cycles of carboplatin and paclitaxel | Yes | NED | |
| Endometrial Cancer | ONC-6096 | Endometrioid, grade 1 | IA | No | Observation | N/A | NED |
| ONC-6191 | Endometrioid, grade 1 | IB | No | N/A | N/A | NED | |
| ONC-6051 | Endometrioid, grade 2 | IA | No | Observation | N/A | NED | |
| ONC-6173 | Endometrioid, grade 2 | IA | No | Observation | N/A | NED | |
| ONC-6071 | Endometrioid, grade 3 | IA | No | Observation | N/A | NED | |
| ONC-6057 | Serous | IVB | Three cycles of carboplatin, paclitaxel and trastuzumab | Three cycles of carboplatin, paclitaxel and trastuzumab followed by a single agent, bevacizumab | No | Alive with disease | |
| ONC-6099 | Serous | IVB | Six cycles of carboplatin and paclitaxel | Three cycles of paclitaxel and bevacizumab | No | Dead of disease | |
| ONC-7003 | Mixed serous/endometrioid | IA | No | Patient declined adjuvant therapy | N/A | NED | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, J.; Newtson, A.M.; Zhang, Y.; Devor, E.J.; Samuelson, M.I.; Thiel, K.W.; Leslie, K.K. Successful Patient-Derived Organoid Culture of Gynecologic Cancers for Disease Modeling and Drug Sensitivity Testing. Cancers 2021, 13, 2901. https://doi.org/10.3390/cancers13122901
Bi J, Newtson AM, Zhang Y, Devor EJ, Samuelson MI, Thiel KW, Leslie KK. Successful Patient-Derived Organoid Culture of Gynecologic Cancers for Disease Modeling and Drug Sensitivity Testing. Cancers. 2021; 13(12):2901. https://doi.org/10.3390/cancers13122901
Chicago/Turabian StyleBi, Jianling, Andreea M. Newtson, Yuping Zhang, Eric J. Devor, Megan I. Samuelson, Kristina W. Thiel, and Kimberly K. Leslie. 2021. "Successful Patient-Derived Organoid Culture of Gynecologic Cancers for Disease Modeling and Drug Sensitivity Testing" Cancers 13, no. 12: 2901. https://doi.org/10.3390/cancers13122901
APA StyleBi, J., Newtson, A. M., Zhang, Y., Devor, E. J., Samuelson, M. I., Thiel, K. W., & Leslie, K. K. (2021). Successful Patient-Derived Organoid Culture of Gynecologic Cancers for Disease Modeling and Drug Sensitivity Testing. Cancers, 13(12), 2901. https://doi.org/10.3390/cancers13122901







