In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
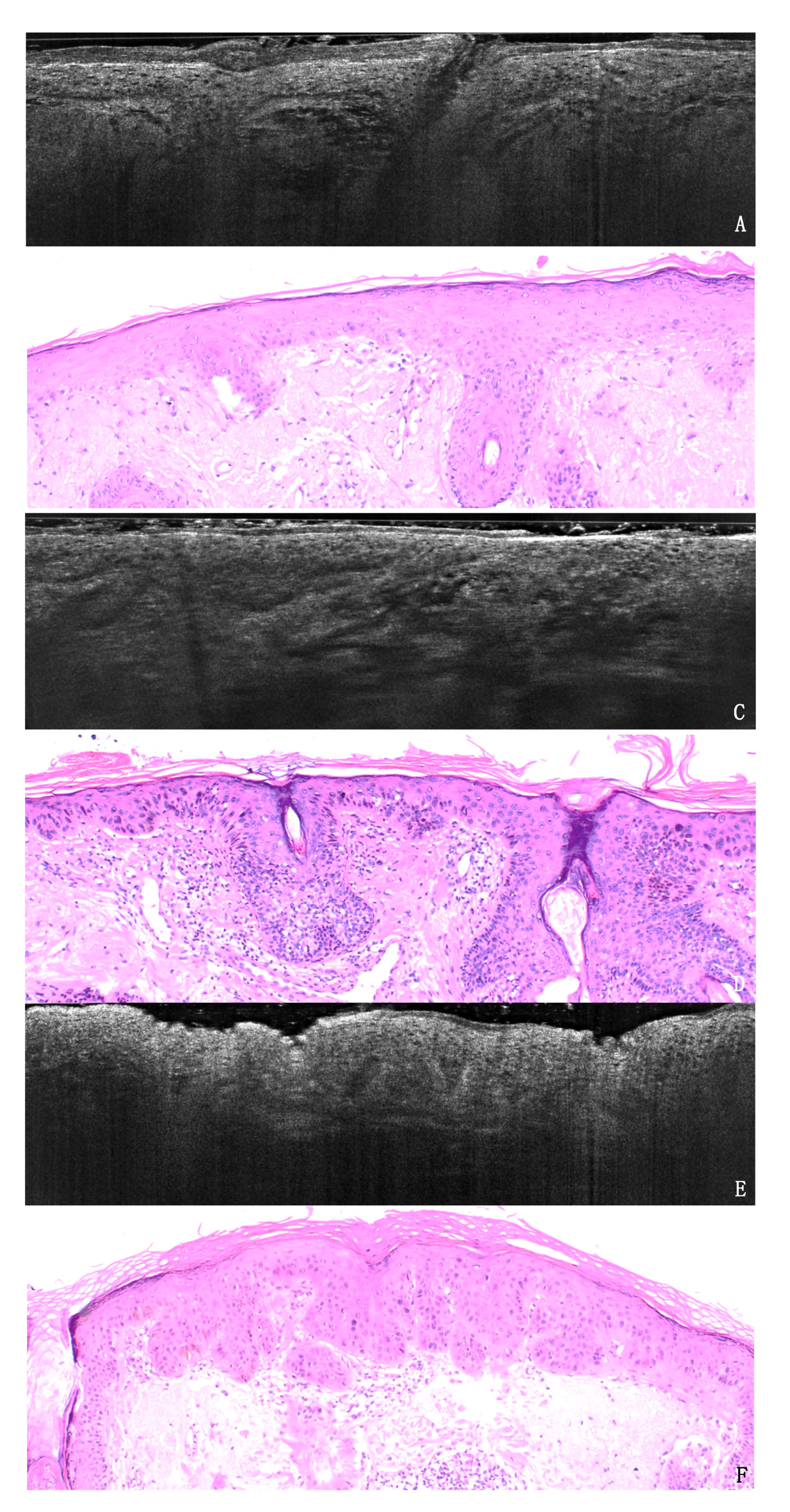
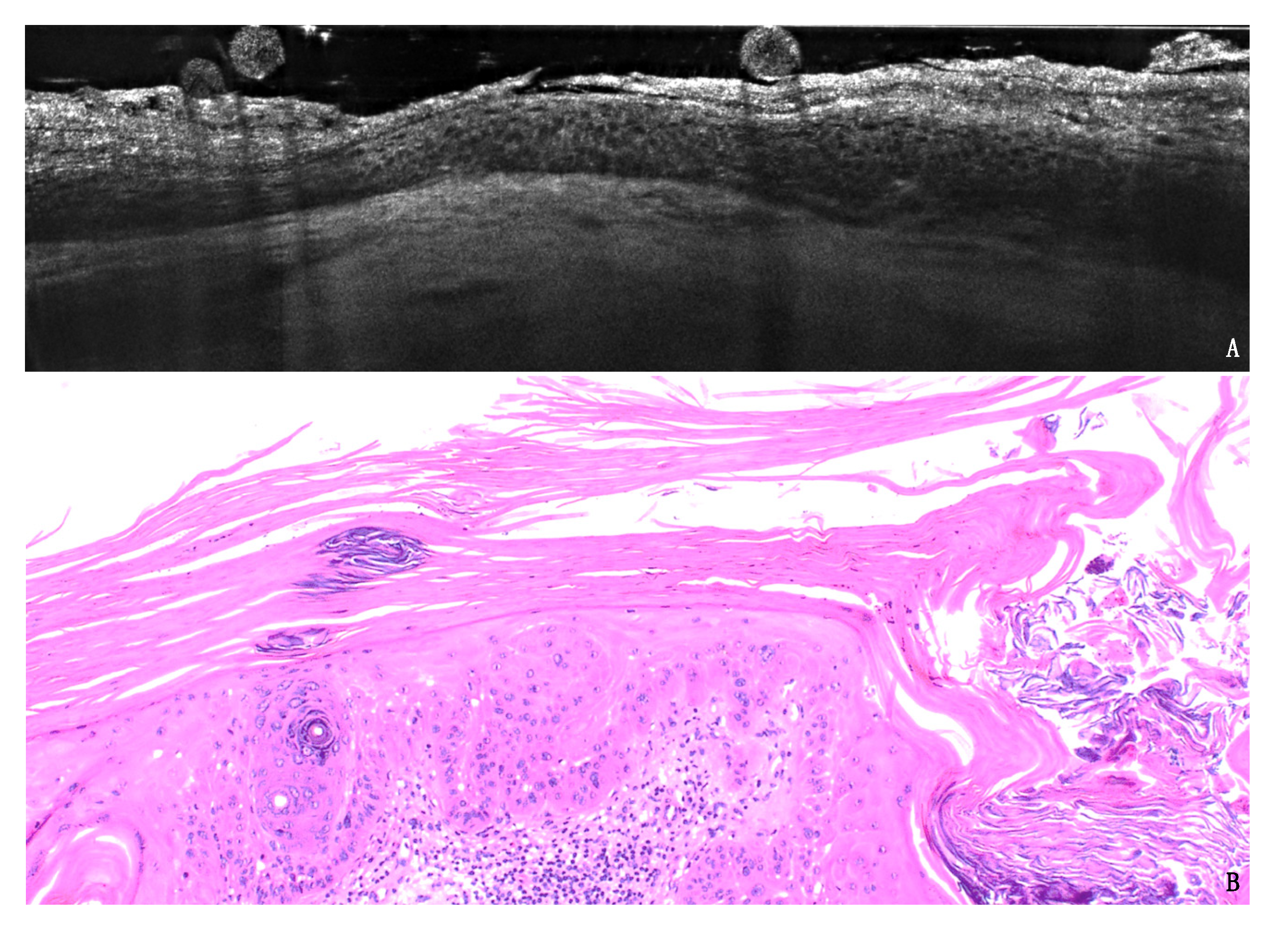
3. Results
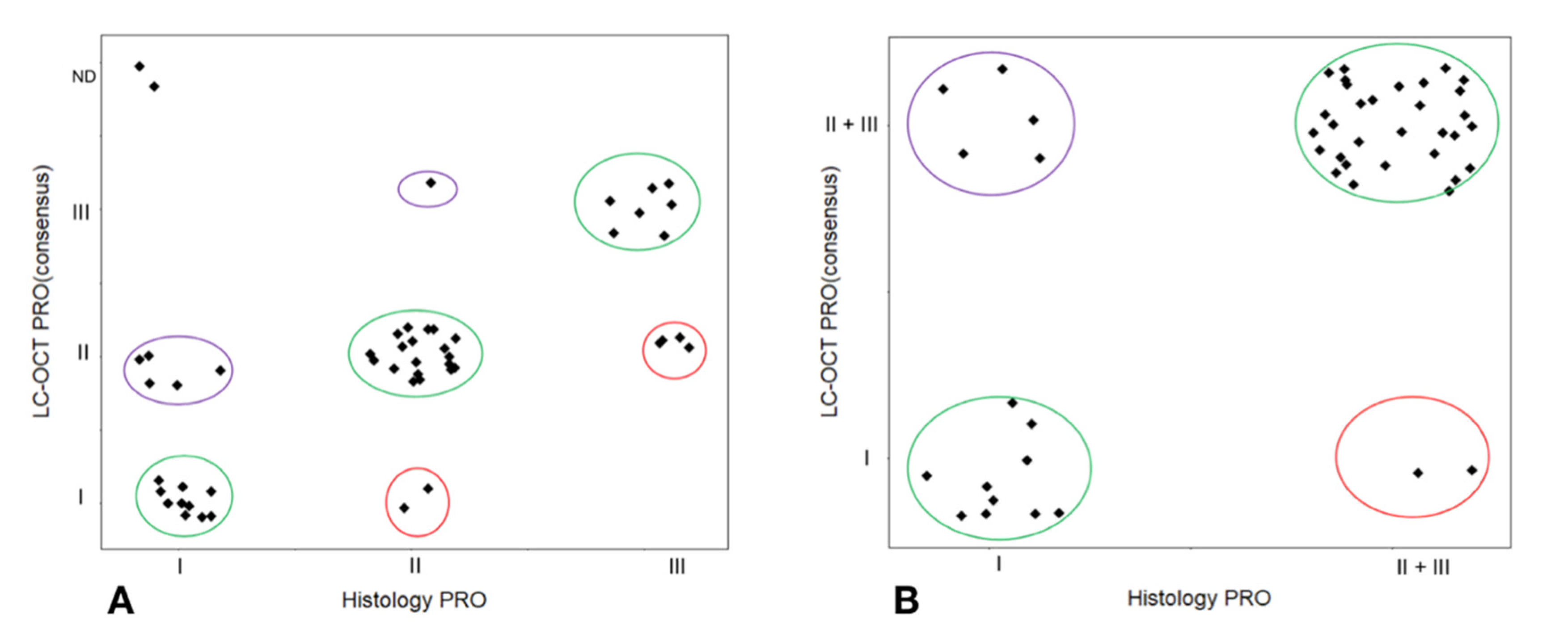
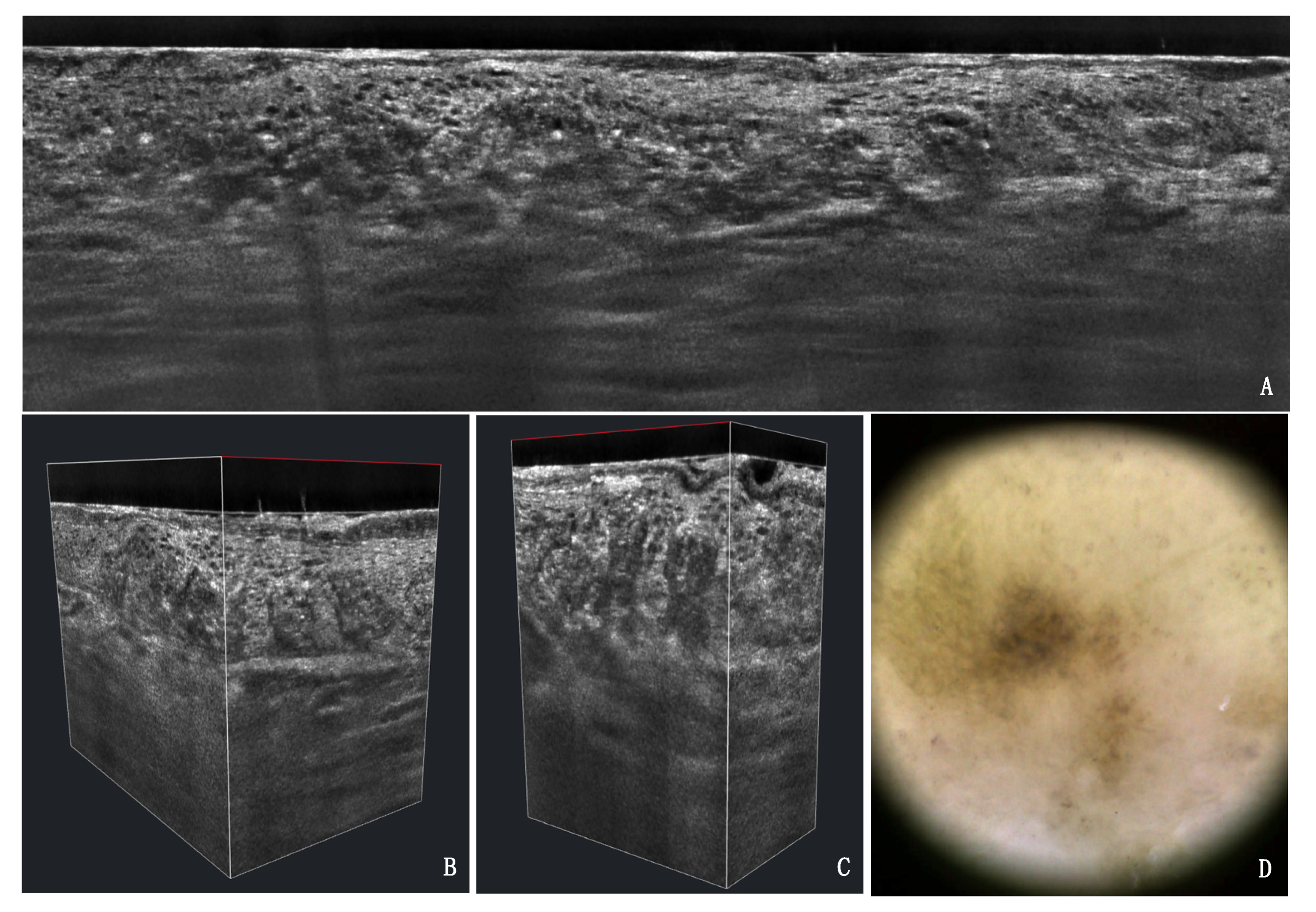
3.1. PRO Grading
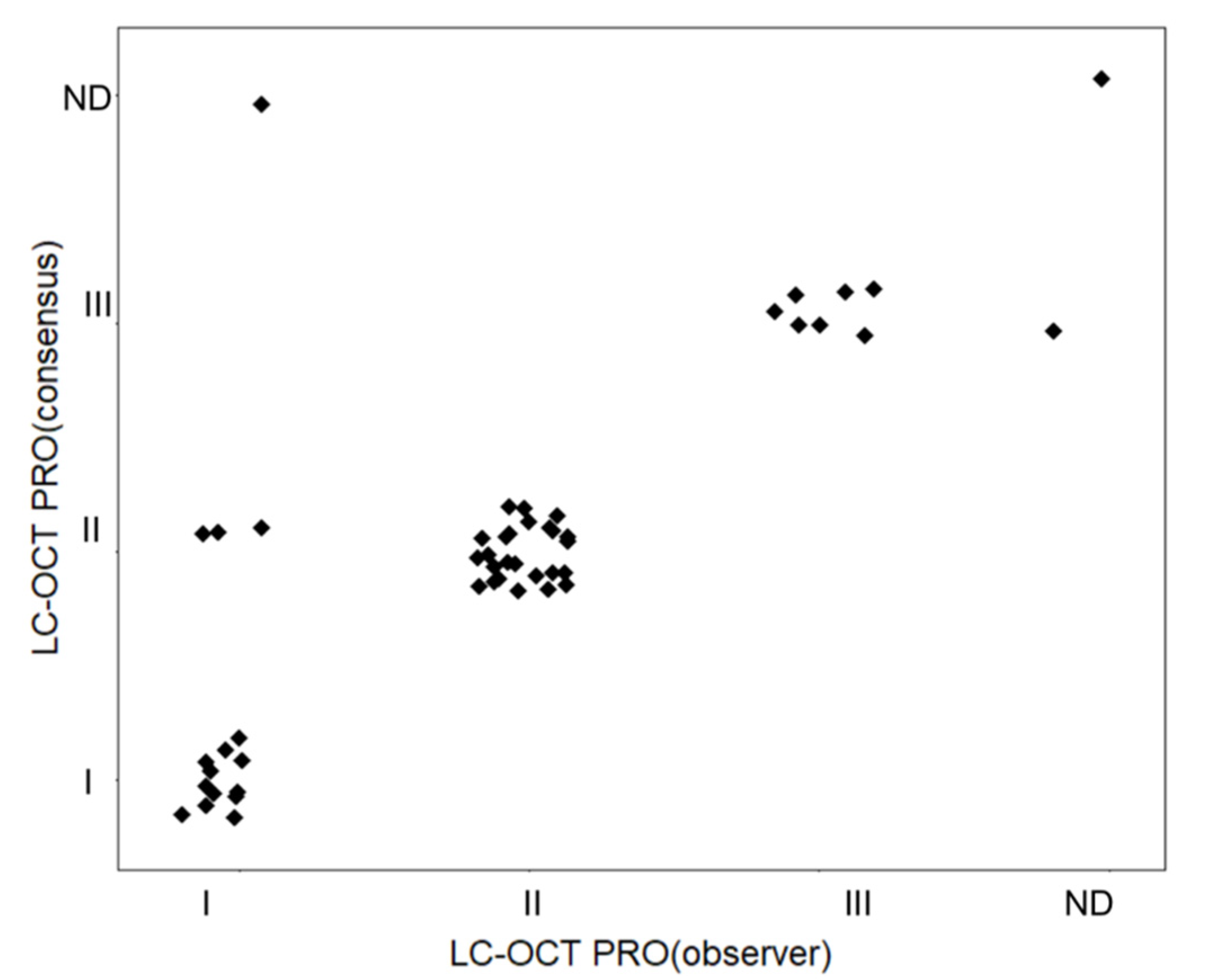
3.2. Agreement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, E.A.; Abernethy, M.L.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E., Jr. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J. Am. Acad. Dermatol. 1991, 24, 738–743. [Google Scholar] [CrossRef]
- Pinkus, H. Keratosis senilis; a biologic concept of its pathogenesis and diagnosis based on the study of normal epidermis and 1730 seborrheic and senile keratoses. Am. J. Clin. Pathol. 1958, 29, 193–207. [Google Scholar] [CrossRef]
- Frost, C.; Williams, G.; Green, A. High incidence and regression rates of solar keratoses in a queensland community. J. Investig. Dermatol. 2000, 115, 273–277. [Google Scholar] [CrossRef]
- Traianou, A.; Ulrich, M.; Apalla, Z.; De Vries, E.; Bakirtzi, K.; Kalabalikis, D.; Ferrandiz, L.; Ruiz-de-Casas, A.; Moreno-Ramirez, D.; Sotiriadis, D.; et al. Risk factors for actinic keratosis in eight European centres: A case-control study. Br. J. Dermatol. 2012, 167 (Suppl. 2), 36–42. [Google Scholar] [CrossRef]
- Stockfleth, E.; Ulrich, C.; Meyer, T.; Christophers, E. Epithelial malignancies in organ transplant patients: Clinical presentation and new methods of treatment. Recent Results Cancer Res. 2002, 160, 251–258. [Google Scholar]
- Cockerell, C.J.; Wharton, J.R. New histopathological classification of actinic keratosis (incipient intraepidermal squamous cell carcinoma). J. Drugs Dermatol. JDD 2005, 4, 462–467. [Google Scholar]
- Heaphy, M.R., Jr.; Ackerman, A.B. The nature of solar keratosis: A critical review in historical perspective. J. Am. Acad. Dermatol. 2000, 43, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.B.; Mones, J.M. Solar (actinic) keratosis is squamous cell carcinoma. Br. J. Dermatol. 2006, 155, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Rowert-Huber, J.; Patel, M.J.; Forschner, T.; Ulrich, C.; Eberle, J.; Kerl, H.; Sterry, W.; Stockfleth, E. Actinic keratosis is an early in situ squamous cell carcinoma: A proposal for reclassification. Br. J. Dermatol. 2007, 156, 8–12. [Google Scholar] [CrossRef]
- de Berker, D.; McGregor, J.M.; Mohd Mustapa, M.F.; Exton, L.S.; Hughes, B.R. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br. J. Dermatol. 2017, 176, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Tokez, S.; Alblas, M.; Nijsten, T.; Pardo, L.; Wakkee, M. Predicting keratinocyte carcinoma in patients with actinic keratosis: Development and internal validation of a multivariable risk-prediction model. Br. J. Dermatol. 2020, 183, 495–502. [Google Scholar] [CrossRef]
- Dejonckheere, G.; Suppa, M.; Del Marmol, V.; Meyer, T.; Stockfleth, E. The actinic dysplasia syndrome—Diagnostic approaches defining a new concept in field carcinogenesis with multiple cSCC. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 8), 16–20. [Google Scholar] [CrossRef]
- Schmitz, L.; Stücker, M.; Gambichler, T.; Stockfleth, E.; Dirschka, T. Histological intralesional heterogeneity of actinic keratoses relates to field cancerization. J. Dtsch. Dermatol. Ges. 2018, 16, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Figueras, M.T. From actinic keratosis to squamous cell carcinoma: Pathophysiology revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. 2), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Witkowski, A.M.; Cesinaro, A.; Teixeira De Carvalho, N.; Pellacani, G. From actinic keratosis to squamous cell carcinoma: Evidence of morphologic and biologic progression. J. Am. Acad. Dermatol. 2015, 72, S8–S10. [Google Scholar] [CrossRef]
- Cockerell, C.J. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J. Am. Acad. Dermatol. 2000, 42, 11–17. [Google Scholar] [CrossRef]
- Cockerell, C.J. Pathology and pathobiology of the actinic (solar) keratosis. Br. J. Dermatol. 2003, 149 (Suppl. 66), 34–36. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Figueras, M.T.; Carrato, C.; Saenz, X.; Puig, L.; Musulen, E.; Ferrandiz, C.; Ariza, A. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 991–997. [Google Scholar] [CrossRef]
- Schmitz, L.; Gambichler, T.; Kost, C.; Gupta, G.; Stucker, M.; Stockfleth, E.; Dirschka, T. Cutaneous squamous cell carcinomas are associated with basal proliferating actinic keratoses. Br. J. Dermatol. 2019, 180, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Gupta, G.; Stücker, M.; Doerler, M.; Gambichler, T.; Welzel, J.; Szeimies, R.M.; Bierhoff, E.; Stockfleth, E.; Dirschka, T. Evaluation of two histological classifications for actinic keratoses—PRO classification scored highest inter-rater reliability. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1092–1097. [Google Scholar] [CrossRef]
- Schmitz, L.; Gambichler, T.; Gupta, G.; Stücker, M.; Stockfleth, E.; Szeimies, R.M.; Dirschka, T. Actinic keratoses show variable histological basal growth patterns—A proposed classification adjustment. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Kahl, P.; Majores, M.; Bierhoff, E.; Stockfleth, E.; Dirschka, T. Actinic keratosis: Correlation between clinical and histological classification systems. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1303–1307. [Google Scholar] [CrossRef]
- Pellacani, G.; Ulrich, M.; Casari, A.; Prow, T.W.; Cannillo, F.; Benati, E.; Losi, A.; Cesinaro, A.M.; Longo, C.; Argenziano, G.; et al. Grading keratinocyte atypia in actinic keratosis: A correlation of reflectance confocal microscopy and histopathology. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2216–2221. [Google Scholar] [CrossRef]
- Schuh, S.; Kaestle, R.; Sattler, E.C.; Welzel, J. Optical coherence tomography of actinic keratoses and basal cell carcinomas—differentiation by quantification of signal intensity and layer thickness. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1321–1326. [Google Scholar] [CrossRef]
- Ruini, C.; Hartmann, D.; Bastian, M.; Ruzicka, T.; French, L.E.; Berking, C.; von Braunmühl, T. Non-invasive monitoring of subclinical and clinical actinic keratosis of face and scalp under topical treatment with ingenol mebutate gel 150 mcg/g by means of reflectance confocal microscopy and optical coherence tomography: New perspectives and comparison of diagnostic techniques. J. Biophotonics 2019, 12, e201800391. [Google Scholar] [CrossRef]
- Ogien, J.; Daures, A.; Cazalas, M.; Perrot, J.-L.; Dubois, A. Line-field confocal optical coherence tomography for three-dimensional skin imaging. Front. Optoelectron. 2020, 13, 381–392. [Google Scholar] [CrossRef]
- Dubois, A.; Levecq, O.; Azimani, H.; Davis, A.; Ogien, J.; Siret, D.; Barut, A. Line-field confocal time-domain optical coherence tomography with dynamic focusing. Opt. Express 2018, 26, 33534–33542. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Petrie, A. Method agreement analysis: A review of correct methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Heppt, M.V.; Leiter, U.; Steeb, T.; Amaral, T.; Bauer, A.; Becker, J.C.; Breitbart, E.; Breuninger, H.; Diepgen, T.; Dirschka, T.; et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma—Short version, part 1: Diagnosis, interventions for actinic keratoses, care structures and quality-of-care indicators. J. Dtsch. Dermatol. Ges. 2020, 18, 275–294. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography-Practical applications in dermatology and comparison with established imaging methods. Skin Res. Technol. 2020. [Google Scholar] [CrossRef]
- Nguyen, K.P.; Peppelman, M.; Hoogedoorn, L.; Van Erp, P.E.; Gerritsen, M.P. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: A systematic review. Eur. J. Dermatol. 2016, 26, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Themstrup, L.; De Carvalho, N.; Mogensen, M.; Pellacani, G.; Jemec, G.B. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn. Ther. 2016, 16, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.; Suppa, M.; Pellacani, G.; Marneffe, A.; Miyamoto, M.; Alarcon, I.; Ruini, C.; Hofmann-Wellenhof, R.; Malvehy, J.; Jemec, G.B.; et al. High-definition optical coherence tomography algorithm for discrimination of basal cell carcinoma from clinical BCC imitators and differentiation between common subtypes. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1771–1780. [Google Scholar] [CrossRef]
- Pedrazzani, M.; Breugnot, J.; Rouaud-Tinguely, P.; Cazalas, M.; Davis, A.; Bordes, S.; Dubois, A.; Closs, B. Comparison of line-field confocal optical coherence tomography images with histological sections: Validation of a new method for in vivo and non-invasive quantification of superficial dermis thickness. Skin Res. Technol. 2020, 26, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Monnier, J.; Tognetti, L.; Miyamoto, M.; Suppa, M.; Cinotti, E.; Fontaine, M.; Perez, J.; Orte Cano, C.; Yélamos, O.; Puig, S.; et al. In vivo characterization of healthy human skin with a novel, non-invasive imaging technique: Line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zwinderman, A.H.; Glas, A.S.; Bossuyt, P.M.; Florie, J.; Bipat, S.; Stoker, J. Statistical models for quantifying diagnostic accuracy with multiple lesions per patient. Biostatistics 2008, 9, 513–522. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruini, C.; Schuh, S.; Gust, C.; Hartmann, D.; French, L.E.; Sattler, E.C.; Welzel, J. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers 2021, 13, 2856. https://doi.org/10.3390/cancers13122856
Ruini C, Schuh S, Gust C, Hartmann D, French LE, Sattler EC, Welzel J. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers. 2021; 13(12):2856. https://doi.org/10.3390/cancers13122856
Chicago/Turabian StyleRuini, Cristel, Sandra Schuh, Charlotte Gust, Daniela Hartmann, Lars Einar French, Elke Christina Sattler, and Julia Welzel. 2021. "In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study" Cancers 13, no. 12: 2856. https://doi.org/10.3390/cancers13122856
APA StyleRuini, C., Schuh, S., Gust, C., Hartmann, D., French, L. E., Sattler, E. C., & Welzel, J. (2021). In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers, 13(12), 2856. https://doi.org/10.3390/cancers13122856






