Mutation Profile of Aggressive Pheochromocytoma and Paraganglioma with Comparison of TCGA Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Tissue Sample
2.2. DNA Extraction
2.3. Targeted Next-Generation Sequencing (NGS) and Data Processing
2.4. Variant Calling and Filtering
2.5. Copy Number Variants Analysis
2.6. Tumor Mutation Burden Analysis
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Characteristics of Mutation Profile of AMC Cohort
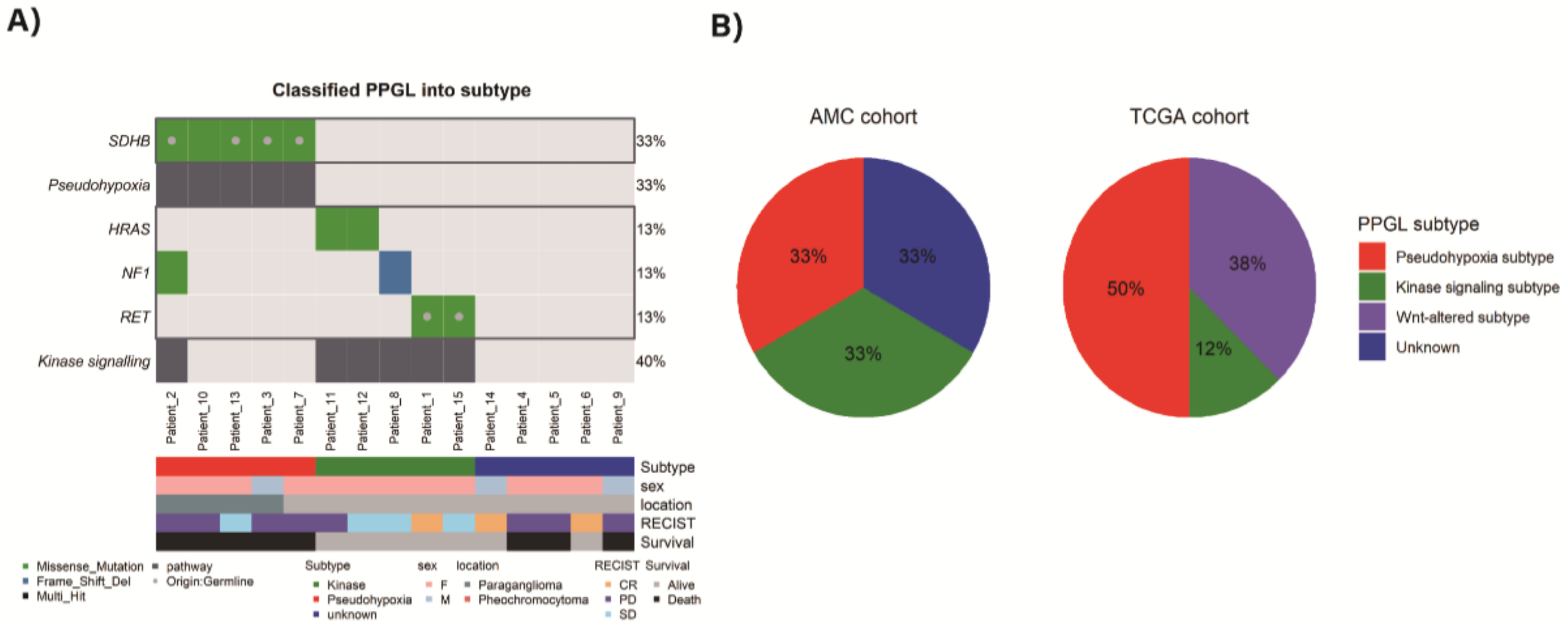
3.3. Classification of PPGL Based on the Pattern of Mutation Profile
3.4. Copy Number Variation
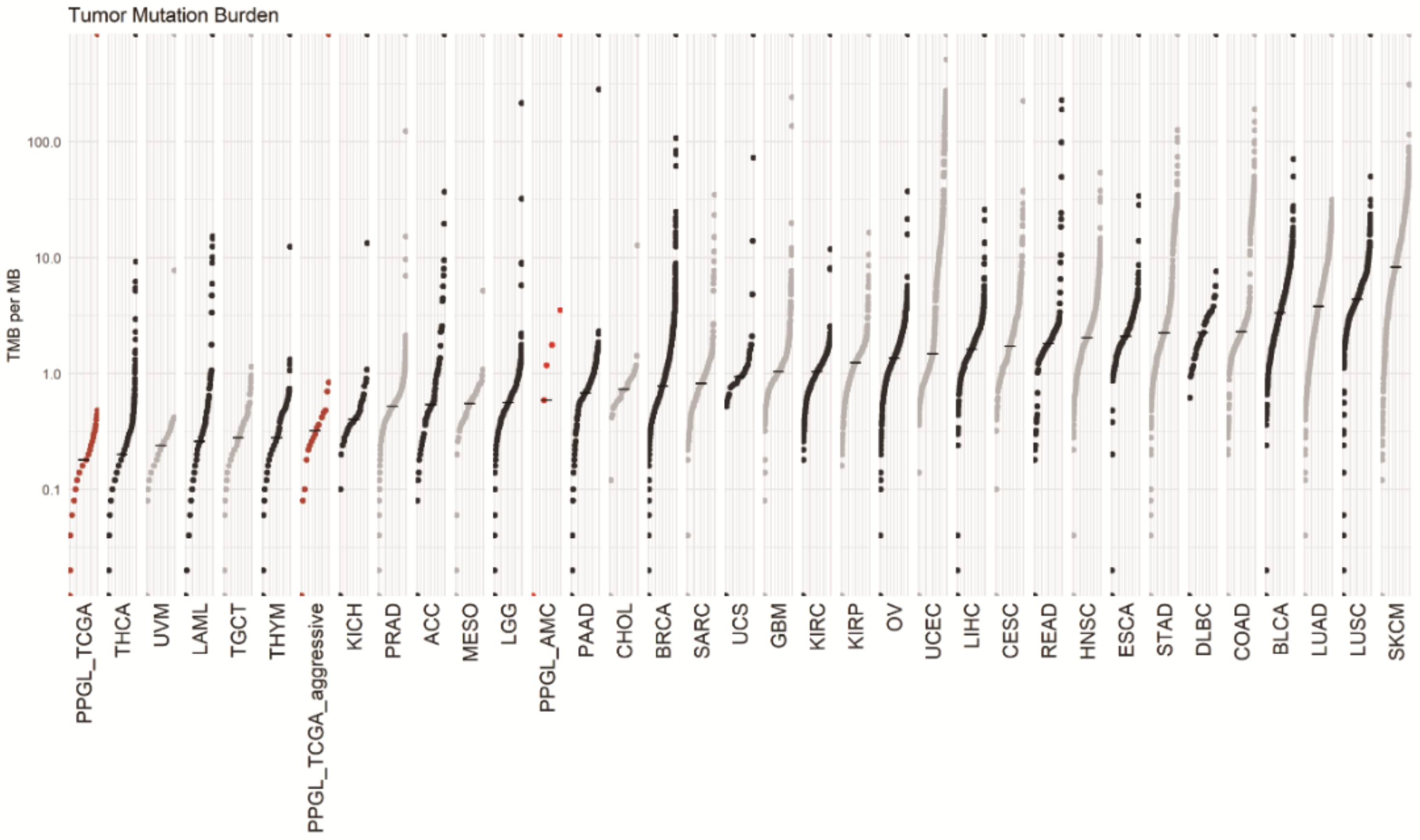
3.5. Tumor Mutation Burden
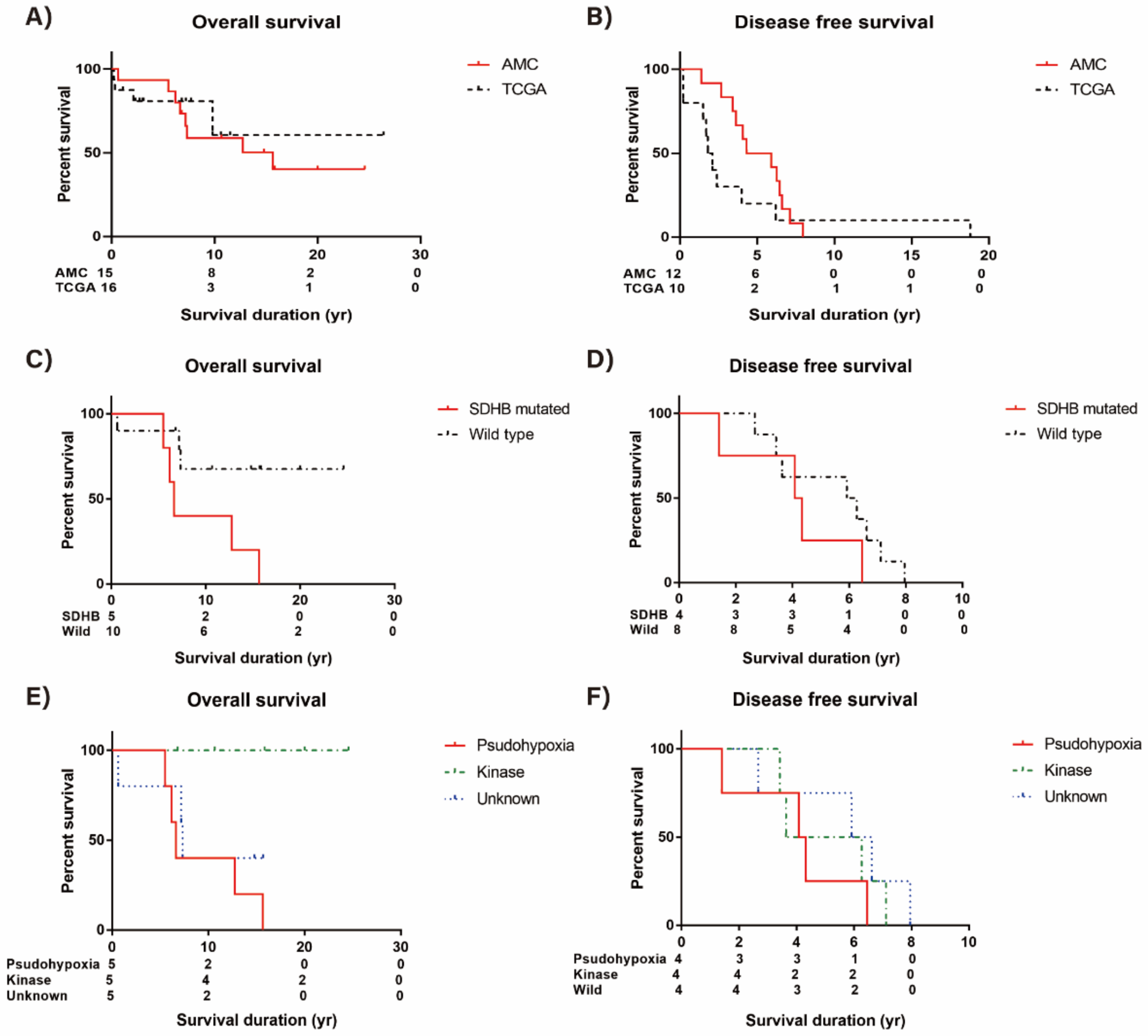
3.6. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, A.K. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Lenders, J.W.; Eisenhofer, G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol. Metab. 2017, 32, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Crona, J.; Taieb, D.; Pacak, K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr. Rev. 2017, 38, 489–515. [Google Scholar] [CrossRef] [PubMed]
- Dahia, P.L. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat. Rev. Cancer 2014, 14, 108. [Google Scholar] [CrossRef]
- Burnichon, N.; Buffet, A.; Gimenez-Roqueplo, A.-P. Pheochromocytoma and paraganglioma: Molecular testing and personalized medicine. Curr. Opin. Oncol. 2016, 28, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Asis, L.B.; Wolf, K.I.; Jochmanova, I.; Taïeb, D. Pheochromocytoma: A genetic and diagnostic update. Endocr. Pract. 2017, 24, 78–90. [Google Scholar] [CrossRef]
- Dahia, P.L. Pheochromocytomas and paragangliomas, genetically diverse and minimalist, all at once! Cancer Cell 2017, 31, 159–161. [Google Scholar] [CrossRef]
- Favier, J.; Buffet, A.; Gimenez-Roqueplo, A.-P. HIF2A mutations in paraganglioma with polycythemia. N. Engl. J. Med. 2012, 367, 2161. [Google Scholar]
- Comino-Méndez, I.; de Cubas, A.A.; Bernal, C.; Álvarez-Escolá, C.; Sanchez-Malo, C.; Ramírez-Tortosa, C.L.; Pedrinaci, S.; Rapizzi, E.; Ercolino, T.; Bernini, G. Tumoral EPAS1 (HIF2A) mutations explain sporadic pheochromocytoma and paraganglioma in the absence of erythrocytosis. Hum. Mol. Genet. 2013, 22, 2169–2176. [Google Scholar] [CrossRef]
- Burnichon, N.; Buffet, A.; Parfait, B.; Letouzé, E.; Laurendeau, I.; Loriot, C.; Pasmant, E.; Abermil, N.; Valeyrie-Allanore, L.; Bertherat, J. Somatic NF1 inactivation is a frequent event in sporadic pheochromocytoma. Hum. Mol. Genet. 2012, 21, 5397–5405. [Google Scholar] [CrossRef] [PubMed]
- Crona, J.; Delgado Verdugo, A.; Maharjan, R.; Stålberg, P.; Granberg, D.; Hellman, P.; Björklund, P. Somatic mutations in H-RAS in sporadic pheochromocytoma and paraganglioma identified by exome sequencing. J. Clin. Endocrinol. Metab. 2013, 98, E1266–E1271. [Google Scholar] [CrossRef]
- Luchetti, A.; Walsh, D.; Rodger, F.; Clark, G.; Martin, T.; Irving, R.; Sanna, M.; Yao, M.; Robledo, M.; Neumann, H.P. Profiling of somatic mutations in phaeochromocytoma and paraganglioma by targeted next generation sequencing analysis. Int. J. Endocrinol. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Burnichon, N.; Vescovo, L.; Amar, L.; Libé, R.; de Reynies, A.; Venisse, A.; Jouanno, E.; Laurendeau, I.; Parfait, B.; Bertherat, J. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 2011, 20, 3974–3985. [Google Scholar] [CrossRef]
- Flynn, A.; Benn, D.; Clifton-Bligh, R.; Robinson, B.; Trainer, A.H.; James, P.; Hogg, A.; Waldeck, K.; George, J.; Li, J. The genomic landscape of phaeochromocytoma. J. Pathol. 2015, 236, 78–89. [Google Scholar] [CrossRef]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef]
- Jochmanova, I.; Pacak, K. Genomic landscape of pheochromocytoma and paraganglioma. Trends Cancer 2018, 4, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, O. Metastatic pheochromocytoma and paraganglioma: Recent advances in prognosis and management. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Parenti, G.; Zampetti, B.; Rapizzi, E.; Ercolino, T.; Giache, V.; Mannelli, M. Updated and new perspectives on diagnosis, prognosis, and therapy of malignant pheochromocytoma/paraganglioma. J. Oncol. 2012, 2012. [Google Scholar] [CrossRef]
- Suh, Y.J.; Choe, J.-Y.; Park, H.J. Malignancy in pheochromocytoma or paraganglioma: Integrative analysis of 176 cases in TCGA. Endocr. Pathol. 2017, 28, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, F.M.; Eisenhofer, G.; Tao, J.J.; Kant, J.A.; Adams, K.T.; Linehan, W.M.; Pacak, K. High Frequency of SDHB Germline Mutations in Patients with Malignant Catecholamine-Producing Paragangliomas: Implications for Genetic Testing. J. Clin. Endocrinol. Metab. 2006, 91, 4505–4509. [Google Scholar] [CrossRef] [PubMed]
- Van Hulsteijn, L.T.; Dekkers, O.M.; Hes, F.J.; Smit, J.W.; Corssmit, E. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: A systematic review and meta-analysis. J. Med Genet. 2012, 49, 768–776. [Google Scholar] [CrossRef]
- Fishbein, L.; Merrill, S.; Fraker, D.L.; Cohen, D.L.; Nathanson, K.L. Inherited mutations in pheochromocytoma and paraganglioma: Why all patients should be offered genetic testing. Ann. Surg. Oncol. 2013, 20, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Comino-Méndez, I.; Tejera, Á.M.; Currás-Freixes, M.; Remacha, L.; Gonzalvo, P.; Tonda, R.; Letón, R.; Blasco, M.A.; Robledo, M.; Cascón, A. ATRX driver mutation in a composite malignant pheochromocytoma. Cancer Genet. 2016, 209, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vega, L.J.; Buffet, A.; De Cubas, A.A.; Cascón, A.; Menara, M.; Khalifa, E.; Amar, L.; Azriel, S.; Bourdeau, I.; Chabre, O. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum. Mol. Genet. 2013, 23, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Comino-Méndez, I.; Gracia-Aznárez, F.J.; Schiavi, F.; Landa, I.; Leandro-García, L.J.; Letón, R.; Honrado, E.; Ramos-Medina, R.; Caronia, D.; Pita, G. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat. Genet. 2011, 43, 663. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Sung, T.Y.; Kim, W.G.; Lee, J.J.; Ryu, J.S.; Kim, T.Y.; Kim, W.B.; Hong, S.J.; Song, D.E.; Shong, Y.K. Clinical course and prognostic factors in patients with malignant pheochromocytoma and paraganglioma: A single institution experience. J. Surg. Oncol. 2015, 112, 815–821. [Google Scholar] [CrossRef]
- Chun, S.M.; Sung, C.O.; Jeon, H.; Kim, T.I.; Lee, J.Y.; Park, H.; Kim, Y.; Kim, D.; Jang, S.J. Next-Generation Sequencing Using S1 Nuclease for Poor-Quality Formalin-Fixed, Paraffin-Embedded Tumor Specimens. J. Mol. Diagn. 2018, 20, 802–811. [Google Scholar] [CrossRef]
- Chakravarty, D.; Gao, J.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Casanova, S.; Rosenberg-Bourgin, M.; Farkast, D.; Calmettes, C.; Feingold, N.; Heshmatl, H.M.; Cohen, R.; Conte-Devolx, B.; Guillausseau, P.J.; Houdent, C.; et al. Phaeochromocytoma in multiple endocrine neoplasia type 2 A: Survey of 100 cases. Clin. Endocrinol. 1993, 38, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Modigliani, E.; Vasen, H.M.; Raue, K.; Dralle, H.; Frilling, A.; Gheri, R.G.; Brandi, M.L.; Limbert, E.; Niederle, B.; Forgas, L.; et al. Pheochromocytoma in multiple endocrine neoplasia type 2: European study. The Euromen Study Group. J. Intern. Med. 1995, 238, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.A.; Burnichon, N.; Cascon, A.; Benn, D.E.; Bayley, J.-P.; Welander, J.; Tops, C.M.; Firth, H.; Dwight, T.; Ercolino, T. Consensus statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat. Rev. Endocrinol. 2017, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Hescot, S.; Curras-Freixes, M.; Deutschbein, T.; van Berkel, A.; Vezzosi, D.; Amar, L.; de la Fouchardière, C.; Valdes, N.; Riccardi, F.; Do Cao, C.; et al. European Network for the Study of Adrenal Tumors (ENS@T). Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J. Clin. Endocrinol. Metab. 2019, 104, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Van Nederveen, F.H.; Korpershoek, E.; Lenders, J.W.; de Krijger, R.R.; Dinjens, W.N. Somatic SDHB mutation in an extraadrenal pheochromocytoma. N. Engl. J. Med. 2007, 357, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Aim, L.B.; Pigny, P.; Castro-Vega, L.J.; Buffet, A.; Amar, L.; Bertherat, J.; Drui, D.; Guilhem, I.; Baudin, E.; Lussey-Lepoutre, C. Targeted next-generation sequencing detects rare genetic events in pheochromocytoma and paraganglioma. J. Med. Genet. 2019, 56, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Currás-Freixes, M.; Inglada-Pérez, L.; Mancikova, V.; Montero-Conde, C.; Letón, R.; Comino-Méndez, I.; Apellániz-Ruiz, M.; Sánchez-Barroso, L.; Sánchez-Covisa, M.A.; Alcázar, V.; et al. Recommendations for somatic and germline genetic testing of single pheochromocytoma and paraganglioma based on findings from a series of 329 patients. J. Med. Genet. 2015, 52, 647–656. [Google Scholar] [CrossRef]
- Gniado, E.; Carracher, C.P.; Sharma, S. Simultaneous Occurrence of Germline Mutations of SDHB and TP53 in a patient with Metastatic Pheochromocytoma. J. Clin. Endocrinol. Metab. 2020, 105, 991–995. [Google Scholar] [CrossRef]
- Amar, L.; Baudin, E.; Burnichon, N.; Peyrard, S.; Silvera, S.; Bertherat, J.; Bertagna, X.; Schlumberger, M.; Jeunemaitre, X.; Gimenez-Roqueplo, A.-P. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J. Clin. Endocrinol. Metab. 2007, 92, 3822–3828. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.; Khare, S.; Wubbenhorst, B.; DeSloover, D.; D’Andrea, K.; Merrill, S.; Cho, N.W.; Greenberg, R.A.; Else, T.; Montone, K. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat. Commun. 2015, 6, 6140. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.A.; Qin, Y.; Cheng, Z.-M.; Gao, Q.; Iwata, S.; Silva, G.M.; Prasad, M.L.; Ocal, I.T.; Rao, S.; Aronin, N. Recurrent mutations of chromatin-remodeling genes and kinase receptors in pheochromocytomas and paragangliomas. Clin. Cancer Res. 2016, 22, 2301–2310. [Google Scholar] [CrossRef]
- Job, S.; Draskovic, I.; Burnichon, N.; Buffet, A.; Cros, J.; Lépine, C.; Venisse, A.; Robidel, E.; Verkarre, V.; Meatchi, T. Telomerase activation and ATRX mutations are independent risk factors for metastatic pheochromocytoma and paraganglioma. Clin. Cancer Res. 2019, 25, 760–770. [Google Scholar] [CrossRef] [PubMed]


| AMC Data (N = 15) | TCGA Data (N = 16) | ||
|---|---|---|---|
| Age, median [IQR] (year) | 37.8 [30.9–48.3] | 46.0 [42–62.3] | |
| Sex (Female), N (%) | 12 (80.0) | 8 (50.0) | |
| Hereditary case, N (%) | 1 (6.7) | 0 (0) | |
| Tumor location (Adrenal PCC) | 11 (73.3) | 10 (62.5) | |
| Functioning tumor, N (%) | 13 (86.7) | 12 (92.3) | |
| AJCC staging a | I–II | 0 | 0 |
| III | 4 (26.7) | 5 (31.3) | |
| IV | 11 (73.3) | 11 (68.8) | |
| Distant metastasis | 11 (73.3) | 11 (68.8) | |
| RECIST | CR | 3 (20.0) | NA |
| PR | 0 | NA | |
| SD | 4 (26.7) | NA | |
| PD | 8 (53.3) | NA | |
| Survival status (Death) | 8 (53.3) | 4 (25.0) | |
| #Pt | Gene Symbol | HGVS.codon | HGVS.protein | Mutation Class | Clinically Actionable Mutation | Clinical Significance | |
|---|---|---|---|---|---|---|---|
| 1 | #6 | ABCC2 | c.3436C > T | p.Arg1146Cys | Germline | NA | Uncertain significance |
| 2 | #10 | ABCC2 | c.1177C > T | p.Arg393Trp | Germline | NA | Likely pathogenic |
| 3 | #14 | ACVR1B | c.865C > T | p.Pro289Ser | Germline | NA | NA |
| 4 | #6 | ACVR1B | c.865C > T | p.Pro289Ser | Germline | NA | NA |
| 5 | #4 | ALOX12B | c.1496G > A | p.Arg499His | Somatic | NA | NA |
| 6 | #9 | ALOX12B | c.1643G > A | p.Arg548Gln | Germline | NA | NA |
| 7 | #4 | ATRX | c.5400G > T | p.Met1800Ile | Somatic | NA | NA |
| 8 | #5 | ATRX | c.2965A > G | p.Thr989Ala | Germline | NA | NA |
| 9 | #4 | CDK12 | c.404A > G | p.Glu135Gly | Germline | NA | NA |
| 10 | #10 | CDK12 | c.2089C > T | p.Pro697Ser | Germline | NA | NA |
| 11 | #14 | CDKN2A | c.315C > A | p.Asp105Glu | Germline | NA | Uncertain significance |
| 12 | #4 | CDKN2A | c.197A > G | p.His66Arg | Germline | NA | Likely benign |
| 13 | #1 | CDKN2A | c.496C > T | p.His166Tyr | Germline | NA | Not provided |
| 14 | #4 | CLTCL1 | c.4597C > T | p.Leu1533Phe | Somatic | NA | NA |
| 15 | #9 | CLTCL1 | c.1453C > G | p.Pro485Ala | Germline | NA | NA |
| 16 | #4 | CUBN | c.3172A > T | p.Thr1058Ser | Germline | NA | NA |
| 17 | #10 | CUBN | c.4438A > C | p.Thr1480Pro | Germline | NA | NA |
| 18 | #5 | FLNB | c.3555C > A | p.Asn1185Lys | Somatic | NA | NA |
| 19 | #10 | FLNB | c.3792C > A | p.Asp1264Glu | Germline | NA | Uncertain significance |
| 20 | #11 | HRAS | c.182A > G | p.Gln61Arg | Somatic | likely Oncogenic | Likely pathogenic |
| 21 | #12 | HRAS | c.182A > G | p.Gln61Arg | Somatic | likely Oncogenic | Likely pathogenic |
| 22 | #15 | IL7R | c.332T > C | p.Val111Ala | Germline | NA | NA |
| 23 | #2 | IL7R | c.460C > T | p.His154Tyr | Germline | NA | Uncertain significance |
| 24 | #12 | KMT2C | c.3485_3486del | p.Lys1162SerfsTer19 | Somatic | NA | NA |
| 25 | #5 | KMT2C | c.11665A > C | p.Lys3889Gln | Germline | NA | NA |
| 26 | #8 | KMT2C | c.12112C > T | p.Pro4038Ser | Germline | NA | NA |
| 27 | #5 | LRP1B | c.11483G > T | p.Arg3828Leu | Germline | NA | NA |
| 28 | #12 | LRP1B | c.10597T > C | p.Trp3533Arg | Germline | NA | NA |
| 29 | #2 | NF1 | c.2407C > A | p.Gln803Lys | Somatic | NA | Pathogenic |
| 30 | #8 | NF1 | c.3454_3455del | p.Leu1152ThrfsTer42 | Somatic | NA | NA |
| 31 | #14 | NF2 | c.1397G > A | p.Arg466Gln | Germline | NA | Uncertain significance |
| 32 | #11 | NF2 | c.1439C > T | p.Thr480Met | Germline | NA | Benign |
| 33 | #14 | NOTCH4 | c.5522C > T | p.Pro1841Leu | Germline | NA | NA |
| 34 | #1 | NOTCH4 | c.1753C > T | p.Arg585Cys | Germline | NA | NA |
| 35 | #2 | NOTCH4 | c.3774C > A | p.Tyr1258Ter | Germline | NA | NA |
| 36 | #4 | PER1 | c.1421C > T | p.Pro474Leu | Somatic | NA | NA |
| 37 | #8 | PER1 | c.1114G > A | p.Asp372Asn | Germline | NA | NA |
| 38 | #4 | PKHD1 | c.3241C > T | p.Arg1081Cys | Germline | NA | Uncertain significance |
| 39 | #12 | PKHD1 | c.2347C > T | p.Arg783Trp | Germline | NA | Uncertain significance |
| 40 | #4 | PRKDC | c.8265A > C | p.Glu2755Asp | Germline | NA | NA |
| 41 | #7 | PRKDC | c.874T > C | p.Ser292Pro | Germline | NA | NA |
| 42 | #1 | RET | c.2897C > T | p.Thr966Ile | Germline | NA | Uncertain significance |
| 43 | #15 | RET | c.1900T > C | p.Cys634Arg | Germline | Oncogenic | Pathogenic |
| 44 | #10 | SDHB | c.599G > T | p.Trp200Leu | Somatic | NA | NA |
| 45 | #13 | SDHB | c.194T > C | p.Leu65Pro | Germline | NA | Uncertain significance |
| 46 | #2 | SDHB | c.689G > A | p.Arg230His | Germline | NA | Pathogenic |
| 47 | #7 | SDHB | c.137G > A | p.Arg46Gln | Germline | Likely Oncogenic | Likely pathogenic |
| 48 | #3 | SDHB | c.725G > A | p.Arg242His | Germline | Likely Oncogenic | Pathogenic |
| 49 | #1 | SETD2 | c.7143dup | p.Ser2382LeufsTer47 | Somatic | NA | NA |
| 50 | #4 | SETD2 | c.401del | p.Lys134SerfsTer18 | Somatic | NA | NA |
| 51 | #1 | SETD2 | c.6895G > A | p.Gly2299Arg | Germline | NA | NA |
| 52 | #2 | SMARCA4 | c.929G > A | p.Arg310His | Somatic | NA | Uncertain significance |
| 53 | #9 | SMARCA4 | c.602A > T | p.Gln201Leu | Germline | NA | Likely benign |
| 54 | #12 | SYNE1 | c.7968C > A | p.Ser2656Arg | Somatic | NA | NA |
| 55 | #8 | SYNE1 | c.8686C > T | p.Arg2896Cys | Germline | NA | NA |
| 56 | #1 | TP53 | c.326dup | p.Arg110ProfsTer39 | Somatic | NA | NA |
| 57 | #11 | TP53 | c.31G > C | p.Glu11Gln | Germline | NA | Uncertain significance |
| 58 | #10 | TP53 | c.725G > A | p.Cys242Tyr | Germline | Likely Oncogenic | Pathogenic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.M.; Lim, J.; Jeon, M.J.; Lee, Y.-M.; Sung, T.-Y.; Hong, E.-G.; Lee, J.-Y.; Jang, S.J.; Kim, W.G.; Song, D.E.; et al. Mutation Profile of Aggressive Pheochromocytoma and Paraganglioma with Comparison of TCGA Data. Cancers 2021, 13, 2389. https://doi.org/10.3390/cancers13102389
Choi YM, Lim J, Jeon MJ, Lee Y-M, Sung T-Y, Hong E-G, Lee J-Y, Jang SJ, Kim WG, Song DE, et al. Mutation Profile of Aggressive Pheochromocytoma and Paraganglioma with Comparison of TCGA Data. Cancers. 2021; 13(10):2389. https://doi.org/10.3390/cancers13102389
Chicago/Turabian StyleChoi, Yun Mi, Jinyeong Lim, Min Ji Jeon, Yu-Mi Lee, Tae-Yon Sung, Eun-Gyoung Hong, Ji-Young Lee, Se Jin Jang, Won Gu Kim, Dong Eun Song, and et al. 2021. "Mutation Profile of Aggressive Pheochromocytoma and Paraganglioma with Comparison of TCGA Data" Cancers 13, no. 10: 2389. https://doi.org/10.3390/cancers13102389
APA StyleChoi, Y. M., Lim, J., Jeon, M. J., Lee, Y.-M., Sung, T.-Y., Hong, E.-G., Lee, J.-Y., Jang, S. J., Kim, W. G., Song, D. E., & Chun, S.-M. (2021). Mutation Profile of Aggressive Pheochromocytoma and Paraganglioma with Comparison of TCGA Data. Cancers, 13(10), 2389. https://doi.org/10.3390/cancers13102389






