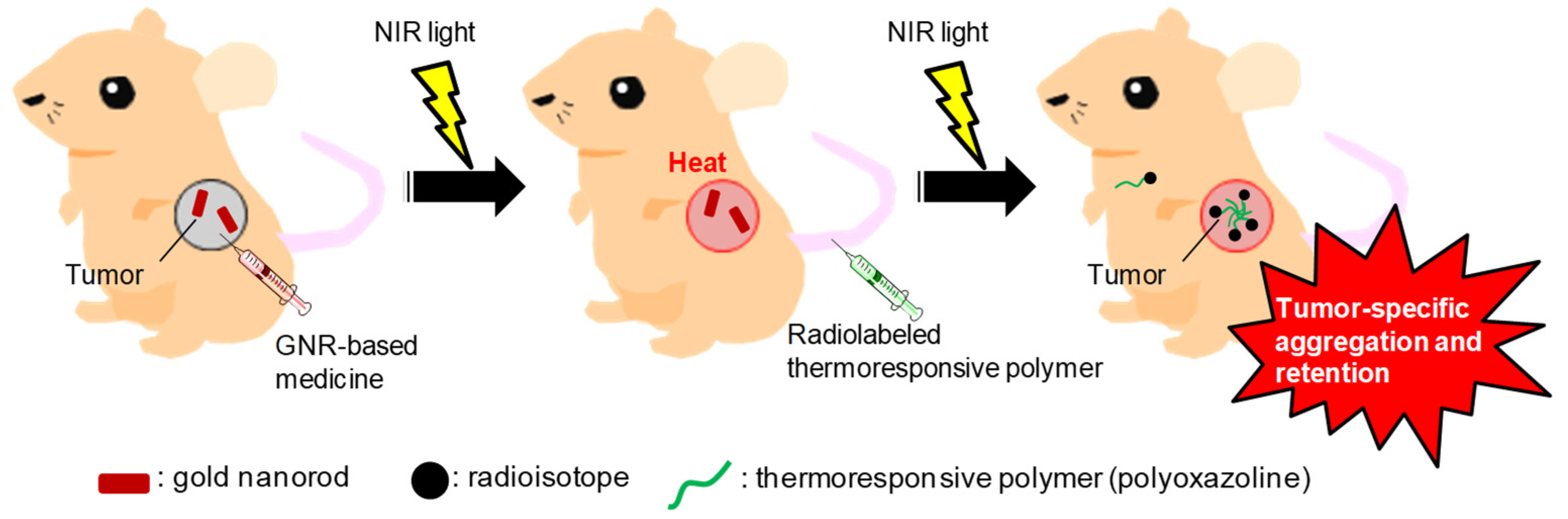
Enhanced Delivery of Thermoresponsive Polymer-Based Medicine into Tumors by Using Heat Produced from Gold Nanorods Irradiated with Near-Infrared Light
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Optical and Chemical Properties of GNR
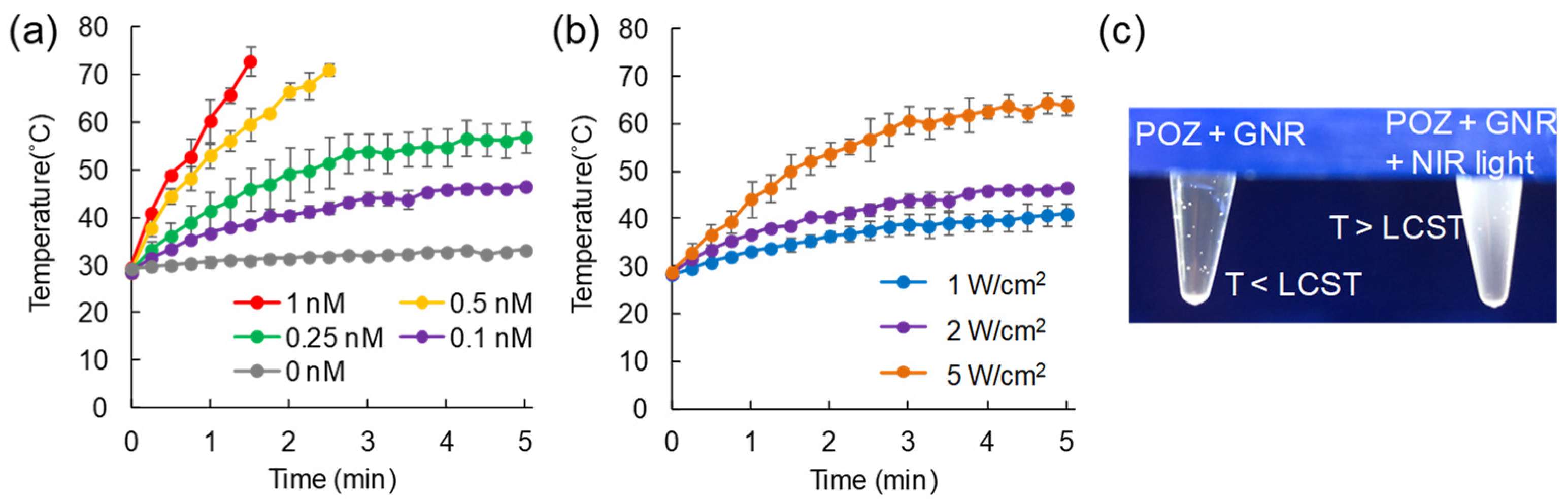
2.2. Evaluation on Temperature Change of GNR Solution upon NIR Light Irradiation
2.3. Aggregation of POZ via Heat Yielded from GNRs by NIR Light Irradiation
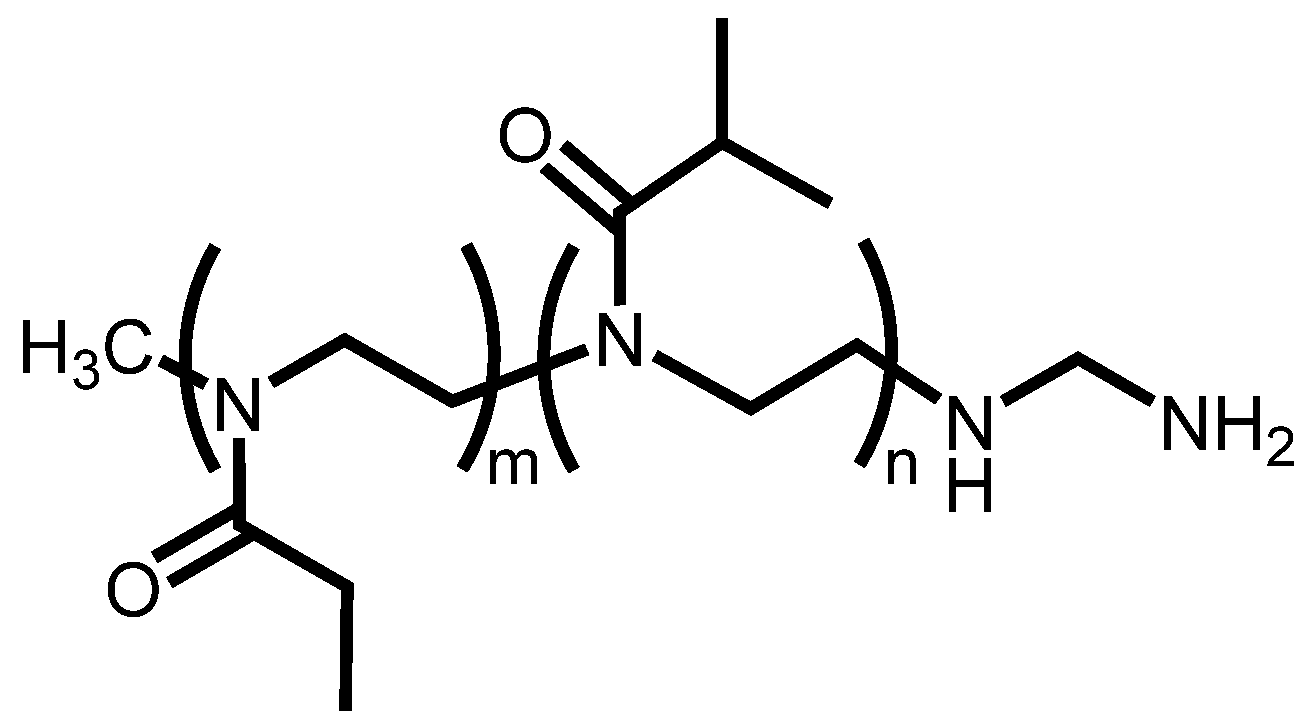
2.4. Synthesis of 111In-Labeled POZ and Fluorescence-Labeled POZ
2.5. Cell Culture and Animal Model
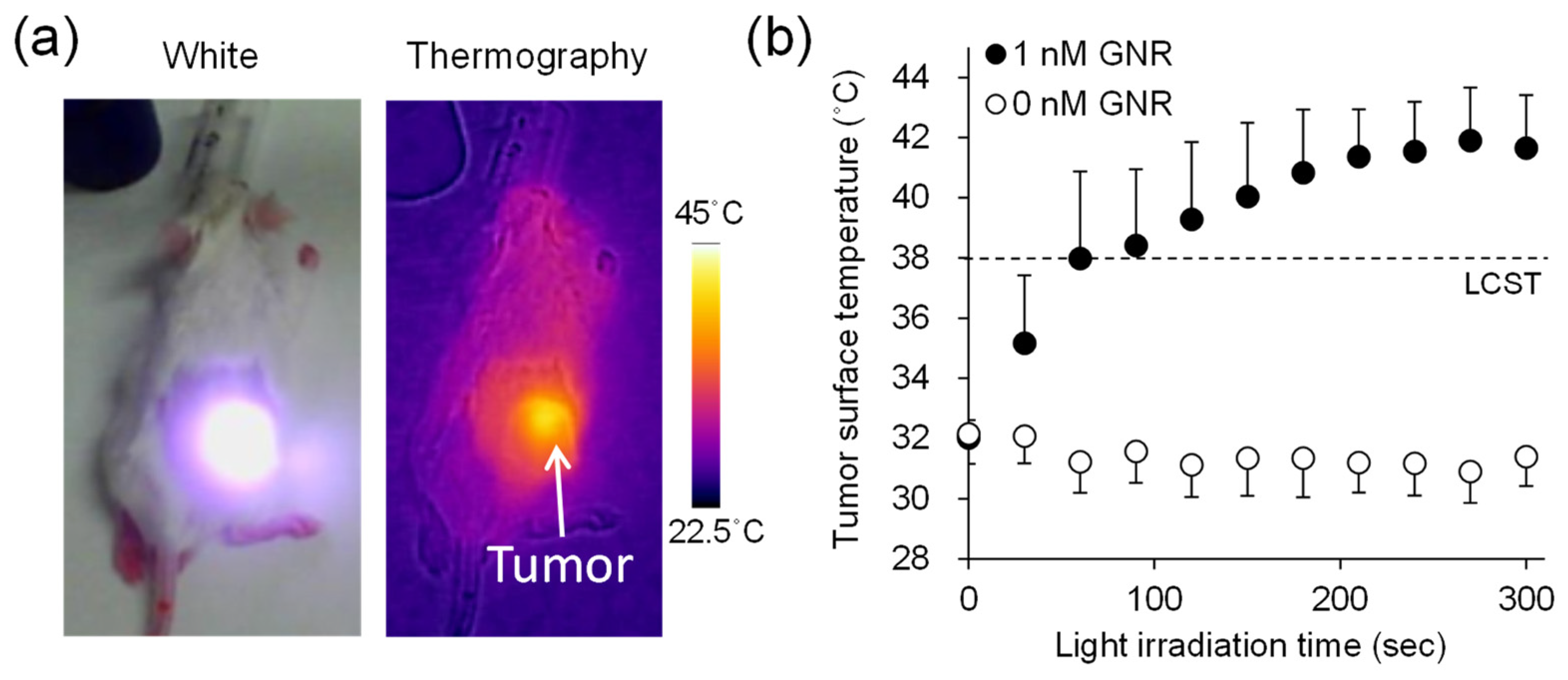
2.6. Biodistribution of 111In-Labeled POZ When Combined with GNR-Based Hyperthermia
2.7. Fibered Confocal Fluorescence Microscopic Imaging Studies
2.8. Statistical Analysis
3. Results
3.1. Optical and Chemical Properties of GNR
3.2. Evaluation on Temperature Change of GNR Solution upon NIR Light Irradiation
3.3. Aggregation of POZ via Heat Yielded from GNR by NIR Light Irradiation
3.4. Synthesis of FITC-Labeled POZ and 111In-Labeled POZ
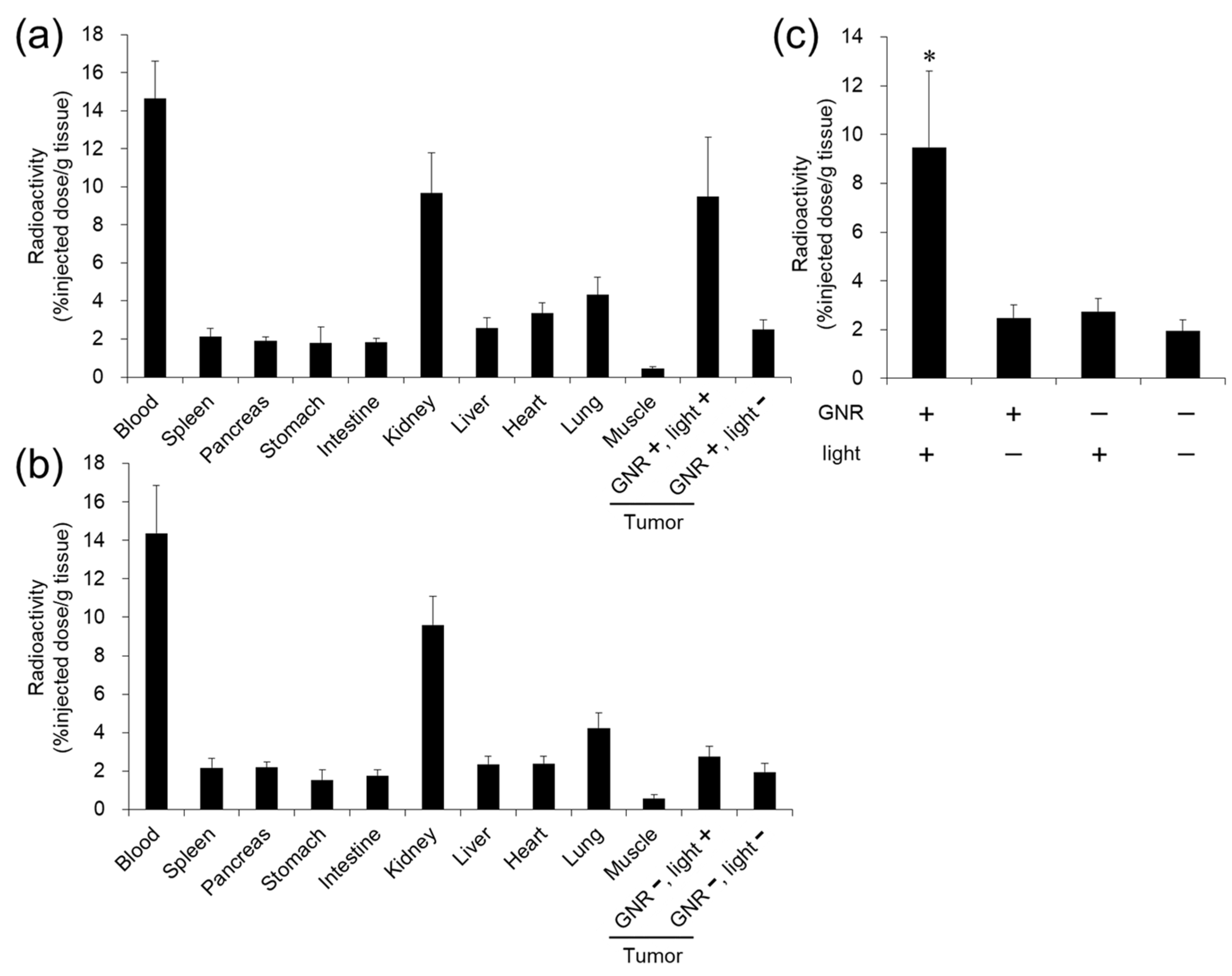
3.5. Evaluation of Distribution of 111In-Labeled POZ by Combination with GNR-Based Hyperthermia
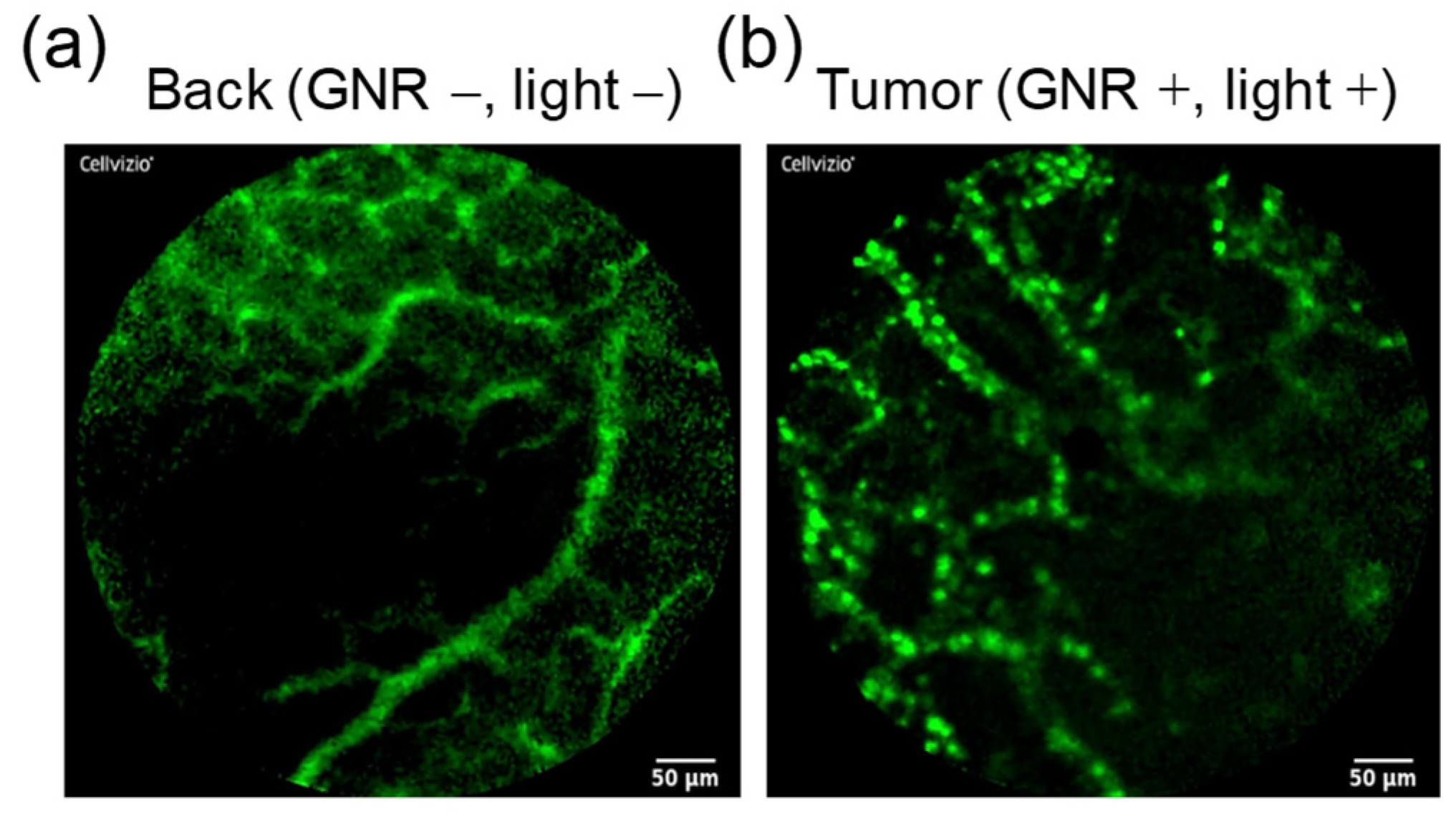
3.6. Fibered Confocal Fluorescence Microscopic Imaging Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. EPR effect based drug design and clinical outlook for enhanced cancer chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Kizaka-Kondoh, S.; Yamahara, R.; Hara, I.; Kanzaki, T.; Ozeki, E.; Hiraoka, M.; Kimura, S. Near-infrared fluorescence tumor imaging using nanocarrier composed of poly(L-lactic acid)-block-poly(sarcosine) amphiphilic polydepsipeptide. Biomaterials 2009, 30, 5156–5160. [Google Scholar] [CrossRef] [PubMed]
- Zalipsky, S.; Hansen, C.B.; Oaks, J.M.; Allen, T.M. Evaluation of blood clearance rates and biodistribution of poly(2-oxazoline)-grafted liposomes. J. Pharm. Sci. 1996, 85, 133–137. [Google Scholar] [CrossRef]
- Drotleff, S.; Lungwitz, U.; Breunig, M.; Dennis, A.; Blunk, T.; Tessmar, J.; Göpferich, A. Biomimetic polymers in pharmaceutical and biomedical sciences. Eur. J. Pharm. Biopharm. 2004, 58, 385–407. [Google Scholar] [CrossRef]
- Kanazaki, K.; Sano, K.; Makino, A.; Yamauchi, F.; Takahashi, A.; Homma, T.; Ono, M.; Saji, H. Feasibility of poly(ethylene glycol) derivatives as diagnostic drug carriers for tumor imaging. J. Control. Release 2016, 226, 115–123. [Google Scholar] [CrossRef]
- Etrych, T.; Kovář, L.; Strohalm, J.; Chytil, P.; Ríhová, B.; Ulbrich, K. Biodegradable star HPMA polymer–drug conjugates: Biodegradability, distribution and anti-tumor efficacy. J. Control. Release 2011, 154, 241–248. [Google Scholar] [CrossRef]
- Sano, K.; Ohashi, M.; Kanazaki, K.; Makino, A.; Ding, N.; Deguchi, J.; Kanada, Y.; Ono, M.; Saji, H. Indocyanine green-labeled polysarcosine for in vivo photoacoustic tumor imaging. Bioconjug. Chem. 2017, 28, 1024–1030. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, W.; Zhou, J.; Pidhatika, B.; Xiong, B.; Huang, L.; Tian, Q.; Shu, Y.; Wen, W.; Hsing, I.M.; et al. Poly(l-lysine)-graft-folic acid-coupled poly(2-methyl-2-oxazoline) (PLL-g-PMOXA-c-FA): A bioactive copolymer for specific targeting to folate receptor-positive cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Kanazaki, K.; Sano, K.; Makino, A.; Homma, T.; Ono, M.; Saji, H. Polyoxazoline multivalently conjugated with indocyanine green for sensitive in vivo photoacoustic imaging of tumors. Sci. Rep. 2016, 6, 33798. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Bao, L.; Suzuno, N.; Kannaka, K.; Yamasaki, T.; Munekane, M.; Mukai, T. Development of cancer-targeted single photon emission computed tomography/fluorescence dual imaging probe based on polyoxazoline. ACS Appl. Polym. Mater. 2019, 1, 953–958. [Google Scholar] [CrossRef]
- Hruby, M.; Filippov, S.K.; Panek, J.; Novakova, M.; Mackova, H.; Kucka, J.; Vetvicka, D.; Ulbrich, K. Polyoxazoline thermoresponsive micelles as radionuclide delivery systems. Macromol. Biosci. 2010, 10, 916–924. [Google Scholar] [CrossRef]
- Sano, K.; Kanada, Y.; Takahashi, K.; Ding, N.; Kanazaki, K.; Mukai, T.; Ono, M.; Saji, H. Enhanced delivery of radiolabeled polyoxazoline into tumors via self-aggregation under hyperthermic conditions. Mol. Pharm. 2018, 15, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.E.; Murphy, C.J. The quest for shape control: A history of gold nanorod synthesis. Chem. Mater. 2013, 25, 1250–1261. [Google Scholar] [CrossRef]
- Chen, Y.S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef]
- An, L.; Wang, Y.; Lin, J.; Tian, Q.; Xie, Y.; Hu, J.; Yang, S. Macrophages-mediated delivery of small gold nanorods for tumor hypoxia photoacoustic imaging and enhanced photothermal therapy. ACS Appl. Mater. Interfaces 2019, 11, 15251–15261. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Lian, J.; Shen, Q.; Wang, C.; Ma, B.; Zhang, Y.; Xu, T.; Li, J.; Shao, Y.; et al. Engineering the surface of smart nanocarriers using a pH-/thermal-/GSH-responsive polymer zipper for precise tumor targeting therapy in vivo. Adv. Mater. 2017, 29, 28719022. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, C.X.; Chen, L.G.; Yan, X.P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat. Commun. 2017, 8, 14998. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Yue, W.; Cai, S.; Tang, Q.; Lu, W.; Huang, L.; Qi, T.; Liao, J. Improvement of gold nanorods in photothermal therapy: Recent progress and perspective. Front. Pharmacol. 2021, 12, 664123. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [Green Version]
- Sano, K.; Kanada, Y.; Kanazaki, K.; Ding, N.; Ono, M.; Saji, H. Brachytherapy with intratumoral injections of radiometal-labeled polymers that thermoresponsively self-aggregate in tumor tissues. J. Nucl. Med. 2017, 58, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, X.; Ji, Y.; Bai, R.; Zhao, Y.; Wu, X.; Chen, C. Surface chemistry of gold nanorods: Origin of cell membrane damage and cytotoxicity. Nanoscale 2013, 5, 8384–8391. [Google Scholar] [CrossRef]
- Nedrow, J.R.; Josefsson, A.; Park, S.; Ranka, S.; Roy, S.; Sgouros, G. Imaging of programmed cell death ligand 1: Impact of protein concentration on distribution of anti-PD-L1 SPECT agents in an immunocompetent murine model of melanoma. J. Nucl. Med. 2017, 58, 1560–1566. [Google Scholar] [CrossRef] [Green Version]
- van der Zee, J.; González González, D.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Kawano, T.; Niidome, Y.; Mori, T.; Katayama, Y.; Niidome, T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjug. Chem. 2009, 20, 209–212. [Google Scholar] [CrossRef]
- Rathke, H.; Flechsig, P.; Mier, W.; Bronzel, M.; Mavriopoulou, E.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Kratochwil, C. Dosimetry estimate and initial clinical experience with 90Y-PSMA-617. J. Nucl. Med. 2019, 60, 806–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutetium Lu 177 Dotatate Approved by FDA. Cancer Discov. 2018, 8, OF2. [CrossRef] [PubMed] [Green Version]
- Akiyama, Y.; Mori, T.; Katayama, Y.; Niidome, T. Conversion of rod-shaped gold nanoparticles to spherical forms and their effect on biodistribution in tumor-bearing mice. Nanoscale Res. Lett. 2012, 7, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Bian, X.; Aliru, M.; Deorukhkar, A.A.; Ekpenyong, O.; Liang, S.; John, J.; Ma, J.; Gao, X.; Schwartz, J.; et al. Hypoxia-targeted gold nanorods for cancer photothermal therapy. Oncotarget 2018, 9, 26556–26571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, K.; Miki, M.; Tanaka, T.; Munemura, M.; Munekane, M.; Yamasaki, T.; Mukai, T. Electrostatically self-assembled gold nanorods with chondroitin sulfate for targeted photothermal therapy for melanoma. Photodiagn. Photodyn. Ther. 2021, 35, 102402. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sano, K.; Ishida, Y.; Tanaka, T.; Mizukami, T.; Nagayama, T.; Haratake, Y.; Munekane, M.; Yamasaki, T.; Mukai, T. Enhanced Delivery of Thermoresponsive Polymer-Based Medicine into Tumors by Using Heat Produced from Gold Nanorods Irradiated with Near-Infrared Light. Cancers 2021, 13, 5005. https://doi.org/10.3390/cancers13195005
Sano K, Ishida Y, Tanaka T, Mizukami T, Nagayama T, Haratake Y, Munekane M, Yamasaki T, Mukai T. Enhanced Delivery of Thermoresponsive Polymer-Based Medicine into Tumors by Using Heat Produced from Gold Nanorods Irradiated with Near-Infrared Light. Cancers. 2021; 13(19):5005. https://doi.org/10.3390/cancers13195005
Chicago/Turabian StyleSano, Kohei, Yumi Ishida, Toshie Tanaka, Tatsuya Mizukami, Tomono Nagayama, Yoshie Haratake, Masayuki Munekane, Toshihide Yamasaki, and Takahiro Mukai. 2021. "Enhanced Delivery of Thermoresponsive Polymer-Based Medicine into Tumors by Using Heat Produced from Gold Nanorods Irradiated with Near-Infrared Light" Cancers 13, no. 19: 5005. https://doi.org/10.3390/cancers13195005
APA StyleSano, K., Ishida, Y., Tanaka, T., Mizukami, T., Nagayama, T., Haratake, Y., Munekane, M., Yamasaki, T., & Mukai, T. (2021). Enhanced Delivery of Thermoresponsive Polymer-Based Medicine into Tumors by Using Heat Produced from Gold Nanorods Irradiated with Near-Infrared Light. Cancers, 13(19), 5005. https://doi.org/10.3390/cancers13195005





