Identification of Clinical and Socioeconomic Predictors of Adjuvant Therapy after Trans-Oral Robotic Surgery in Patients with Oropharyngeal Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
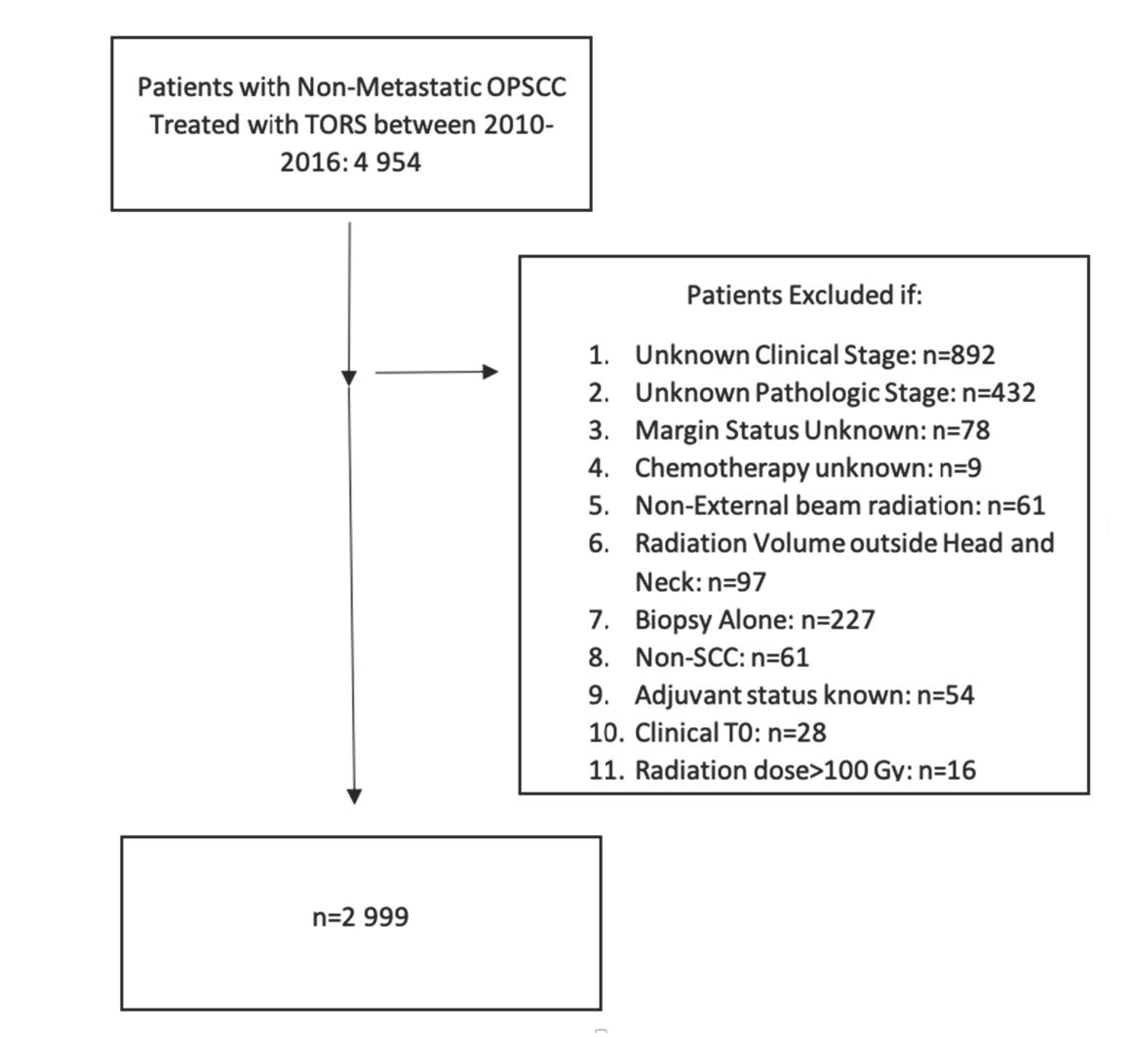
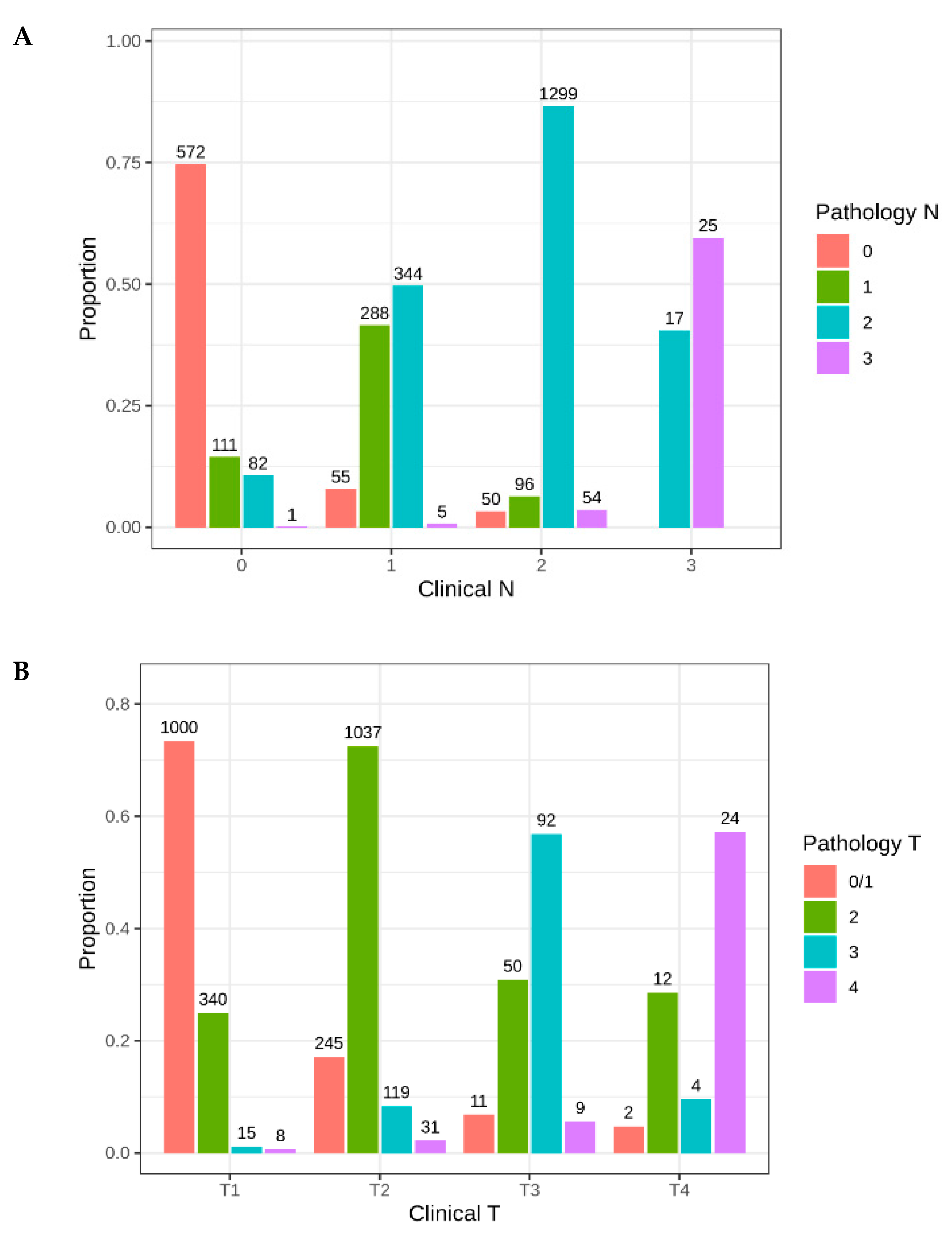
2.1. Patient Characteristics
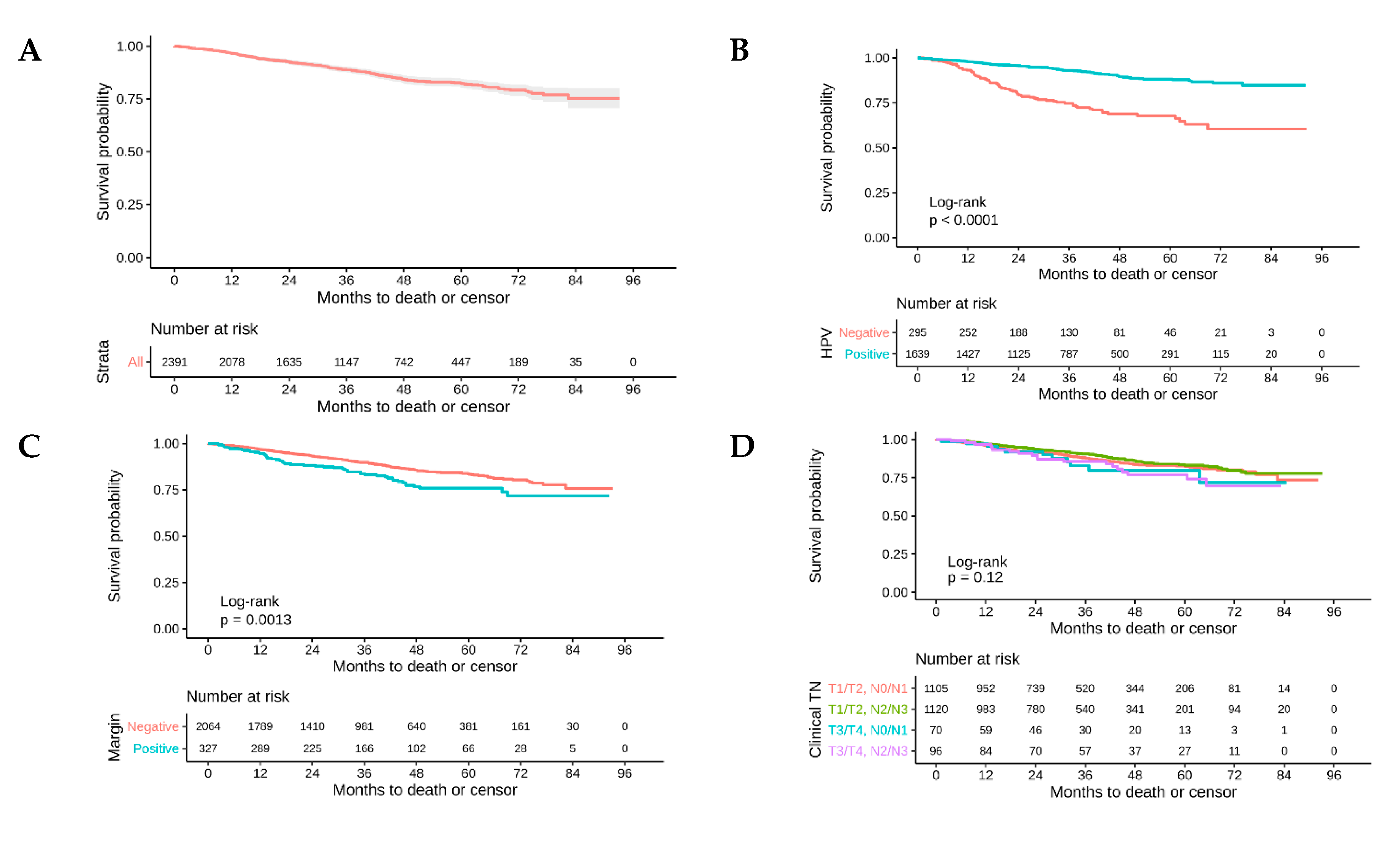
2.2. Survival Analysis
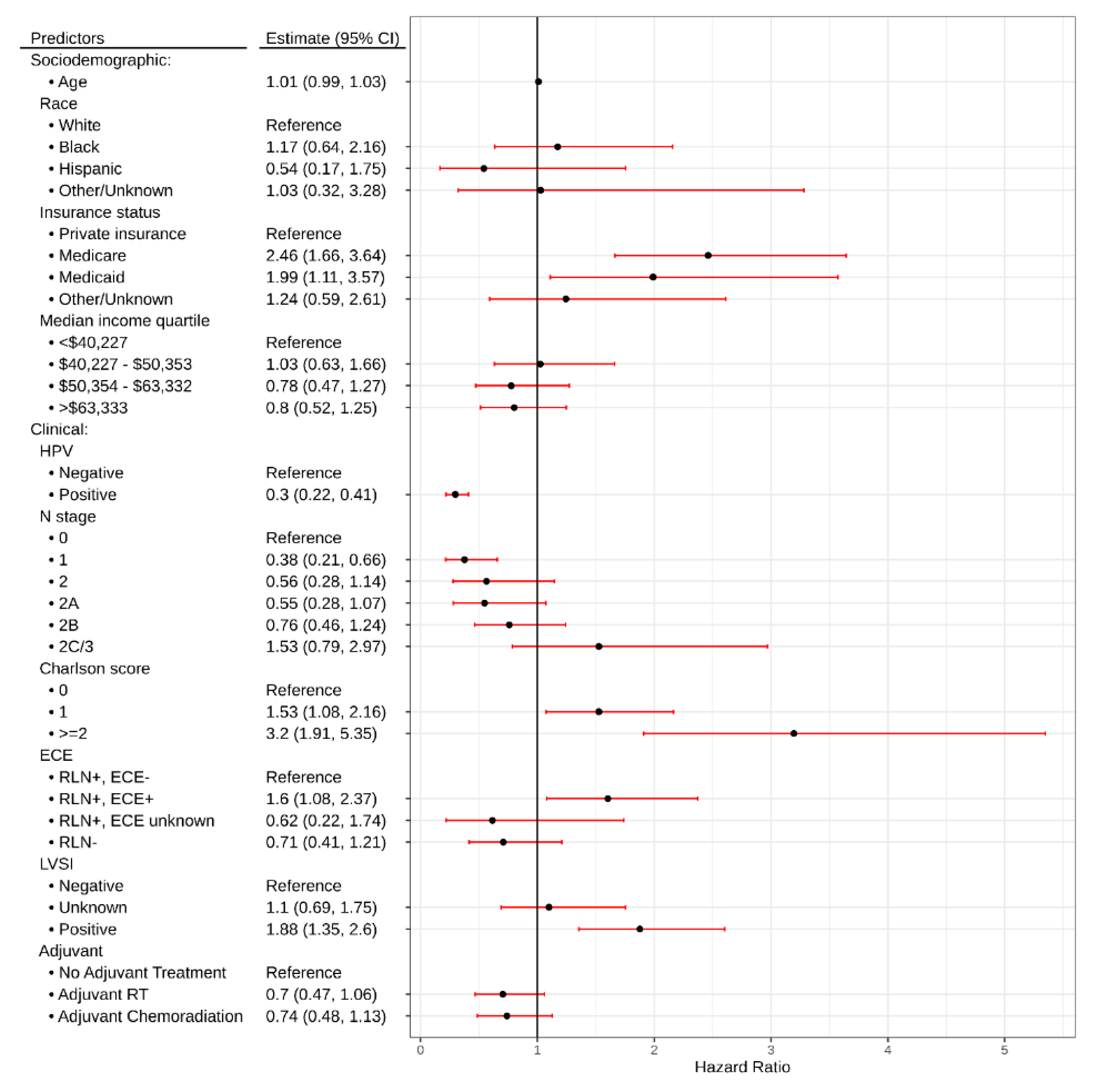
2.3. Utilization/Predictors of Adjuvant Treatment
2.4. Predictors of Radiation Dose
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Bs, A.U.F.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, K.A.; Holsinger, F.C.; Kupferman, M.E.; Lewin, J. Functional outcomes after TORS for oropharyngeal cancer: A systematic review. Eur. Arch. Oto Rhino Laryngol. 2014, 272, 463–471. [Google Scholar] [CrossRef]
- Dziegielewski, P.T.; Teknos, T.N.; Durmus, K.; Old, M.; Agrawal, A.; Kakarala, K.; Marcinow, A.; Ozer, E.; Agrawalm, A. Transoral robotic surgery for oropharyngeal cancer: Long-term quality of life and functional outcomes. JAMA Otolaryngol. Neck Surg. 2013, 139, 1099–1108. [Google Scholar] [CrossRef]
- Baliga, S.; Kabarriti, R.; Jiang, J.; Mehta, V.; Guha, C.; Kalnicki, S.; Smith, R.V.; Garg, M. Utilization of Transoral Robotic Surgery (TORS) in patients with oropharyngeal squamous cell carcinoma and its impact on survival and use of chemotherapy. Oral Oncol. 2018, 86, 75–80. [Google Scholar] [CrossRef]
- Gillison, M.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Meccariello, G.; Montevecchi, F.; D’Agostino, G.; Iannella, G.; Calpona, S.; Parisi, E.; Costantini, M.; Cammaroto, G.; Gobbi, R.; Firinu, E.; et al. Trans-oral robotic surgery for the management of oropharyngeal carcinomas: A 9-year institutional experience. Acta Otorhinolaryngol. Ital. 2019, 39, 75–83. [Google Scholar] [CrossRef]
- Park, Y.M.; Kang, M.S.; Koh, Y.W.; Choi, E.C.; Kim, S.-H. Does p16+ predict a favorable prognosis for oropharyngeal cancer? Risk factors for treatment failure for patients who underwent surgery-based therapy. Ann. Surg. Oncol. 2018, 26, 547–554. [Google Scholar] [CrossRef]
- Moore, E.J.; Van Abel, K.M.; Price, D.L.; Lohse, C.M.; Olsen, K.D.; Jackson, R.S.; Martin, E.J. Transoral robotic surgery for oropharyngeal carcinoma: Surgical margins and oncologic outcomes. Head Neck 2018, 40, 747–755. [Google Scholar] [CrossRef]
- Ling, D.; Chapman, B.; Kim, J.; Choby, G.; Kabolizadeh, P.; Clump, D.; Ferris, R.; Kim, S.; Beriwal, S.; Heron, D.; et al. Oncologic outcomes and patient-reported quality of life in patients with oropharyngeal squamous cell carcinoma treated with definitive transoral robotic surgery versus definitive chemoradiation. Oral Oncol. 2016, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sethia, R.; Yumusakhuylu, A.C.; Ozbay, I.; Diavolitsis, V.; Brown, N.V.; Zhao, S.; Wei, L.; Old, M.; Agrawal, A.; Teknos, T.N.; et al. Quality of life outcomes of transoral robotic surgery with or without adjuvant therapy for oropharyngeal cancer. Laryngoscope 2017, 128, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Hurtuk, A.; Agrawal, A.; Old, M.; Teknos, T.N.; Ozer, E.; Marcinow, A. Outcomes of transoral robotic surgery: A preliminary clinical experience. Otolaryngol. Neck Surg. 2011, 145, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; De Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): An open-label, phase 2, randomised trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Gildener-Leapman, N.; Kim, J.; Abberbock, S.; Choby, G.; Mandal, R.; Duvvuri, U.; Ferris, R.L.; Kim, S. Utility of up-front transoral robotic surgery in tailoring adjuvant therapy. Head Neck 2016, 38, 1201–1207. [Google Scholar] [CrossRef]
- McMullen, C.P.; Garneau, J.; Weimar, E.; Ali, S.; Farinhas, J.M.; Yu, E.; Som, P.M.; Sarta, C.; Goldstein, D.P.; Su, S.; et al. Occult nodal disease and occult extranodal extension in patients with oropharyngeal squamous cell carcinoma undergoing primary transoral robotic surgery with neck dissection. JAMA Otolaryngol. Neck Surg. 2019, 145, 701–707. [Google Scholar] [CrossRef]
- Kann, B.H.; Hicks, D.F.; Payabvash, S.; Mahajan, A.; Du, J.; Gupta, V.; Park, H.S.; Yu, J.B.; Yarbrough, W.G.; Burtness, B.A.; et al. Multi-institutional validation of deep learning for pretreatment identification of extranodal extension in head and neck squamous cell carcinoma. J. Clin. Oncol. 2019, 38. [Google Scholar] [CrossRef]
- Gorphe, P.; Simon, C. A systematic review and meta-analysis of margins in transoral surgery for oropharyngeal carcinoma. Oral Oncol. 2019, 98, 69–77. [Google Scholar] [CrossRef]
- Hanna, J.; Morse, E.; Brauer, P.R.; Judson, B.; Mehra, S. Positive margin rates and predictors in transoral robotic surgery after federal approval: A national quality study. Head Neck 2019, 41, 3064–3072. [Google Scholar] [CrossRef]
- Kwok, J.; Langevin, S.M.; Argiris, A.; Grandis, J.R.; Gooding, W.E.; Taioli, E. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer 2010, 116, 476–485. [Google Scholar] [CrossRef]
- Bates, J.E.; Hitchcock, K.E.; Mendenhall, W.M.; Dziegielewski, P.T.; Amdur, R.J. Comparing national practice versus standard guidelines for the use of adjuvant treatment following robotic surgery for oropharyngeal squamous cell carcinoma. Head Neck 2020, 42. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.J.; Clark, G.M.; Von Hoff, D.D. Hispanic patients with head and neck cancer do not have a worse prognosis than Anglo-American patients. Cancer 1992, 69, 1003–1007. [Google Scholar] [CrossRef]
- Schrank, T.P.; Han, Y.; Weiss, H.; Resto, V.A.; Ba, T.P.S.; Resto, V.A. Case-matching analysis of head and neck squamous cell carcinoma in racial and ethnic minorities in the United States-Possible role for human papillomavirus in survival disparities. Head Neck 2011, 33, 45–53. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.; Seikaly, H.; Murphy, R.; Fung, C.; Cooper, T.; Knox, A.; Scrimger, R.; Harris, J. Primary surgery versus chemoradiotherapy for advanced oropharyngeal cancers: A longitudinal population study. J. Otolaryngol. Head Neck Surg. 2013, 42, 31. [Google Scholar] [CrossRef]


| Patient Characteristics | Total Number (%) |
|---|---|
| Total | 2999 |
| Age (years) | |
| Median | 59 |
| IQR Range | 53–66 |
| Sex | |
| Male | 2550 (85%) |
| Female | 449 (15%) |
| Race | |
| White | 2704 (90.2%) |
| Black | 136 (4.5%) |
| Hispanic | 83 (2.8%) |
| Other/Unknown | 76 (2.5%) |
| Hispanic | |
| No | 2866 (95.6%) |
| Yes | 83 (2.8%) |
| Unknown | 50 (1.7%) |
| Comorbidity Score | |
| 0 | 2367 (78.9%) |
| 1 | 495 (16.5%) |
| ≥2 | 137 (4.6%) |
| Subsite | |
| Tonsil | 1937 (64.6%) |
| Base of Tongue | 1062 (35.4%) |
| HPV Status | |
| Positive | 2076 (69.2%) |
| Negative | 563 (18.8%) |
| Unknown | 360 (12.0%) |
| Clinical T Stage AJCC 7th edition | |
| T1 | 1363 (45.4%) |
| T2 | 1432 (47.7%) |
| T3 | 162 (5.4%) |
| T4 | 42 (1.4%) |
| Clinical N Stage AJCC 7th edition | |
| N0 | 766 (25.5%) |
| N1 | 692 (23.1%) |
| N2* | 212 (7.1%) |
| N2a | 331 (11%) |
| N2b | 862 (28.7%) |
| N2c | 94 (3.3%) |
| N3 | 42 (1.4%) |
| Surgical Margin Status | |
| Negative | 2595 (86.5%) |
| Positive | 404 (13.5%) |
| LVSI | |
| Negative | 1911 (63.7%) |
| Positive | 708 (23.6%) |
| Unknown | 380 (12.7%) |
| Extracapsular Extension (ECE) | |
| Yes | 840 (28.0%) |
| No | 2049 (68.3%) |
| Unknown | 110 (3.7%) |
| Adjuvant Therapy | |
| None | 1054 (35.1%) |
| Radiation Alone | 1005 (33.5%) |
| Chemotherapy and Radiation | 940 (31.3%) |
| Insurance Status | |
| Private | 1854 (61.8%) |
| Medicare | 852 (28.4%) |
| Medicaid | 162 (5.4%) |
| Other Government | 62 (2.1%) |
| Not Insured | 48 (1.6%) |
| Unknown | 21 (0.7%) |
| Facility | |
| Academic | 2512 (83.8%) |
| Community Center | 320 (9.8%) |
| Integrated Network | 157 (5.2%) |
| Unknown | 35 (1.2%) |
| Median Income Quartiles | |
| <40,227 | 390 (13.0%) |
| 40,227–50,353 | 580 (19.3%) |
| 50,354–63,332 | 648 (21.6%) |
| >63,333 | 1337 (44.6%) |
| Unknown | 44 (1.5%) |
| Predictors | Overall Survival | ||
|---|---|---|---|
| Hazard Ratio | CI | p | |
| Age | 1.01 | 0.99–1.03 | 0.32 |
| Race | |||
| White | Reference | ||
| Black | 1.17 | 0.64–2.16 | 0.608 |
| Hispanic | 0.54 | 0.17–1.75 | 0.307 |
| Other/Unknown | 1.03 | 0.32–3.28 | 0.962 |
| Insurance status | |||
| Private Insurance | Reference | ||
| Medicare | 2.46 | 1.66–3.64 | <0.001 |
| Medicaid | 1.99 | 1.11–3.57 | 0.021 |
| Other/Unknown | 1.24 | 0.59–2.61 | 0.564 |
| Median Income Quartile | |||
| <$40,227 | Reference | ||
| $40,227–$50,353 | 1.03 | 0.63–1.66 | 0.919 |
| $50,354–$63,332 | 0.78 | 0.47–1.27 | 0.314 |
| >$63,333 | 0.8 | 0.52–1.25 | 0.325 |
| HPV | |||
| Negative | Reference | ||
| Positive | 0.3 | 0.22–0.41 | <0.001 |
| N Stage | |||
| 0 | Reference | ||
| 1 | 0.38 | 0.21–0.66 | 0.001 |
| 2 | 0.56 | 0.28–1.14 | 0.113 |
| 2A | 0.55 | 0.28–1.07 | 0.08 |
| 2B | 0.76 | 0.46–1.24 | 0.271 |
| 2C/3 | 1.53 | 0.79–2.97 | 0.212 |
| Charlson score | |||
| 0 | Reference | ||
| 1 | 1.53 | 1.08 – 2.16 | 0.018 |
| ≥2 | 3.2 | 1.91–5.35 | <0.001 |
| ECE | |||
| RLN+, ECE- | Reference | ||
| RLN+, ECE+ | 1.6 | 1.08–2.37 | 0.019 |
| RLN+, ECE unknown | 0.62 | 0.22–1.74 | 0.36 |
| RLN- | 0.71 | 0.41–1.21 | 0.204 |
| LVSI | |||
| Negative | Reference | ||
| Unknown | 1.1 | 0.69–1.75 | 0.692 |
| Positive | 1.88 | 1.35–2.60 | <0.001 |
| Adjuvant | |||
| No adjuvant treatment | |||
| Adjuvant treatment | 0.7 | 0.47–1.06 | 0.094 |
| Adjuvant chemoradiation | 0.74 | 0.48–1.13 | 0.158 |
| Observations | 1840 | ||
| R2 Nagelkerke | 0.645 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baliga, S.; Klamer, B.; Jhawar, S.; Gamez, M.; Mitchell, D.; Blakaj, A.; Grecula, J.; Gardner, U.; Dibs, K.; Old, M.; et al. Identification of Clinical and Socioeconomic Predictors of Adjuvant Therapy after Trans-Oral Robotic Surgery in Patients with Oropharyngeal Squamous Cell Carcinoma. Cancers 2020, 12, 2474. https://doi.org/10.3390/cancers12092474
Baliga S, Klamer B, Jhawar S, Gamez M, Mitchell D, Blakaj A, Grecula J, Gardner U, Dibs K, Old M, et al. Identification of Clinical and Socioeconomic Predictors of Adjuvant Therapy after Trans-Oral Robotic Surgery in Patients with Oropharyngeal Squamous Cell Carcinoma. Cancers. 2020; 12(9):2474. https://doi.org/10.3390/cancers12092474
Chicago/Turabian StyleBaliga, Sujith, Brett Klamer, Sachin Jhawar, Mauricio Gamez, Darrion Mitchell, Adriana Blakaj, John Grecula, Ulysses Gardner, Khaled Dibs, Matthew Old, and et al. 2020. "Identification of Clinical and Socioeconomic Predictors of Adjuvant Therapy after Trans-Oral Robotic Surgery in Patients with Oropharyngeal Squamous Cell Carcinoma" Cancers 12, no. 9: 2474. https://doi.org/10.3390/cancers12092474
APA StyleBaliga, S., Klamer, B., Jhawar, S., Gamez, M., Mitchell, D., Blakaj, A., Grecula, J., Gardner, U., Dibs, K., Old, M., Seim, N., Kang, S., Carrau, R., Agrawal, A., Karivedu, V., Bhateja, P., Ozer, E., Rocco, J., Bonomi, M., & Blakaj, D. (2020). Identification of Clinical and Socioeconomic Predictors of Adjuvant Therapy after Trans-Oral Robotic Surgery in Patients with Oropharyngeal Squamous Cell Carcinoma. Cancers, 12(9), 2474. https://doi.org/10.3390/cancers12092474






