Contrast-Enhanced Ultrasound (CEUS) for Follow-Up of Bosniak 2F Complex Renal Cystic Lesions—A 12-Year Retrospective Study in a Specialized European Center
Abstract
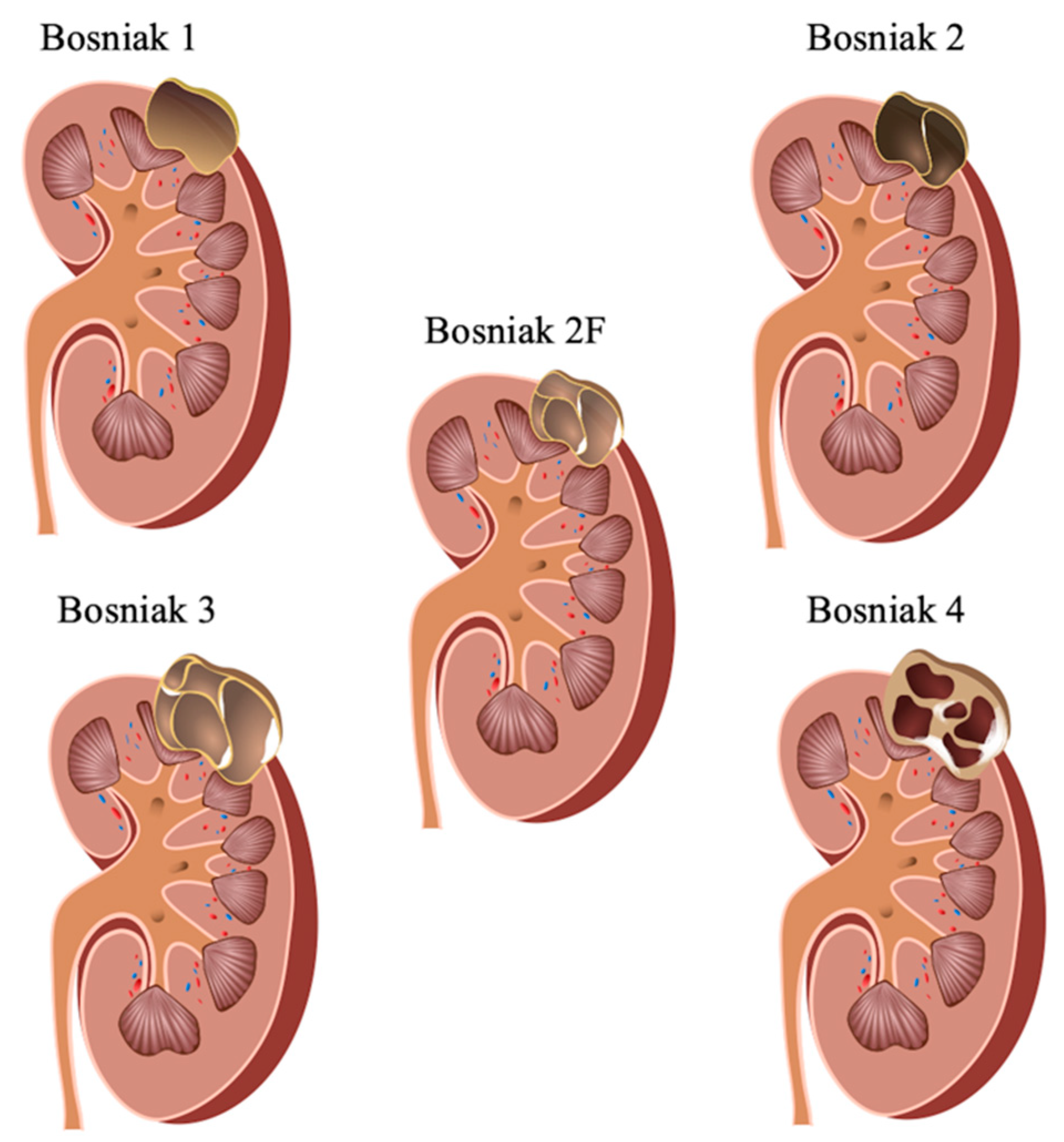
1. Introduction
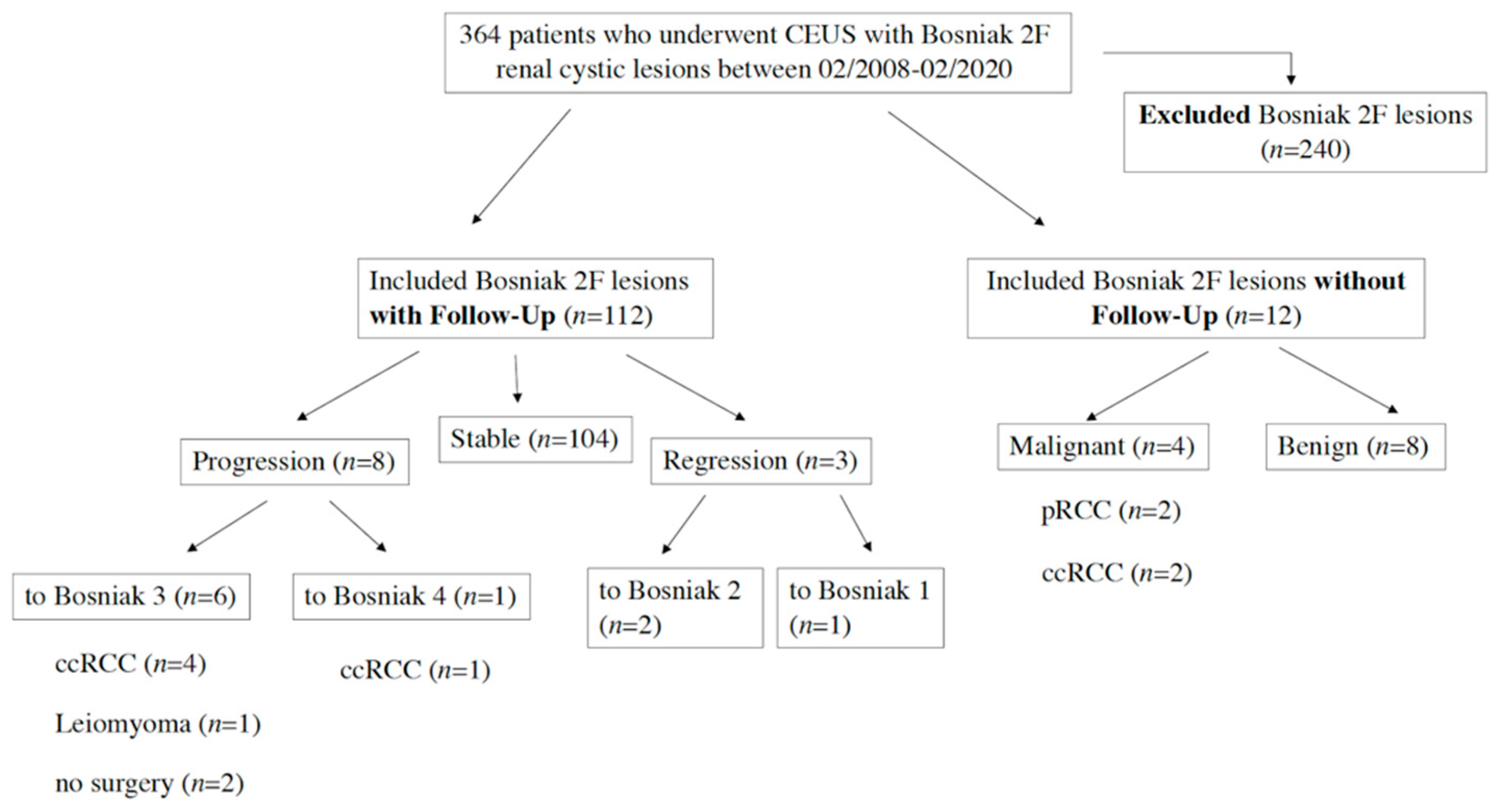
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhatt, J.R.; Finelli, A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat. Rev. Urol. 2014, 11, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Seppala, N.; Kielar, A.; Dabreo, D.; Duigenan, S. Inter-rater agreement in the characterization of cystic renal lesions on contrast-enhanced MRI. Abdom. Imaging 2014, 39, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Bosniak, M.A. Problems in the radiologic diagnosis of renal parenchymal tumors. Urol. Clin. N. Am. 1993, 20, 217–230. [Google Scholar]
- Bosniak, M.A. The current radiological approach to renal cysts. Radiology 1986, 158, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bosniak, M.A. Diagnosis and management of patients with complicated cystic lesions of the kidney. AJR Am. J. Roentgenol. 1997, 169, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Bosniak, M.A. The use of the Bosniak classification system for renal cysts and cystic tumors. J. Urol. 1997, 157, 1852–1853. [Google Scholar] [CrossRef]
- O’Malley, R.L.; Godoy, G.; Hecht, E.M.; Stifelman, M.D.; Taneja, S.S. Bosniak category IIF designation and surgery for complex renal cysts. J. Urol. 2009, 182, 1091–1095. [Google Scholar] [CrossRef]
- Tames, A.V.C.; Fonseca, E.; Yamauchi, F.I.; Arrais, G.; de Andrade, T.C.M.; Baroni, R.H. Progression rate in Bosniak category IIF complex renal cysts. Radiol. Bras 2019, 52, 155–160. [Google Scholar] [CrossRef]
- Bata, P.; Tarnoki, A.D.; Tarnoki, D.L.; Szasz, A.M.; Poloskei, G.; Fejer, B.; Gyebnar, J.; Nyirady, P.; Berczi, V.; Karlinger, K.; et al. Bosniak category III cysts are more likely to be malignant than we expected in the era of multidetector computed tomography technology. J. Res. Med. Sci. 2014, 19, 634–638. [Google Scholar]
- Curry, N.S.; Cochran, S.T.; Bissada, N.K. Cystic renal masses: Accurate Bosniak classification requires adequate renal, C.T. AJR Am. J. Roentgenol. 2000, 175, 339–342. [Google Scholar] [CrossRef]
- Hartman, D.S.; Choyke, P.L.; Hartman, M.S. From the RSNA refresher courses: A practical approach to the cystic renal mass. RadioGraphics 2004, 24 (Suppl. 1), S101–S115. [Google Scholar] [CrossRef]
- Mu, J.; Mao, Y.; Li, F.; Xin, X.; Zhang, S. Superb microvascular imaging is a rational choice for accurate Bosniak classification of renal cystic masses. Br. J. Radiol. 2019, 92, 20181038. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, J.; Gloersen, K.; Osman, H.A.; Dimmen, M.; Berg, G.V. The role of contrast-enhanced ultrasound in the classification of CT-indeterminate renal lesions. Scand. J. Urol. 2016, 50, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H.; Chong, W.K.; Kasoji, S.K.; Dayton, P.A.; Rathmell, W.K. Management of Indeterminate Cystic Kidney Lesions: Review of Contrast-enhanced Ultrasound as a Diagnostic Tool. Urology 2016, 87, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, G.; Sarno, A.; Rizzo, G.; Grecchi, A.; Testa, I.; Giannotti, G.; D’Onofrio, M.; Beleù, A. Clinical use of contrast-enhanced ultrasound beyond the liver: A focus on renal, splenic, and pancreatic applications. Ultrasonography 2019, 38, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Rubenthaler, J.; Wilson, S.; Clevert, D.A. Multislice computed tomography/contrast-enhanced ultrasound image fusion as a tool for evaluating unclear renal cysts. Ultrasonography 2019, 38, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaumer, M.H.; Putz, F.J.; Rubenthaler, J.; Rogasch, J.; Jung, E.M.; Clevert, D.A.; Hamm, B.; Makowski, M.; Fischer, T. Contrast-enhanced ultrasound (CEUS) of cystic renal lesions in comparison to CT and MRI in a multicenter setting. Clin. Hemorheol. Microcirc. 2020, 1–11, prepress. [Google Scholar] [CrossRef]
- Rubenthaler, J.; Bogner, F.; Reiser, M.; Clevert, D.A. Contrast-Enhanced Ultrasound (CEUS) of the Kidneys by Using the Bosniak Classification. Ultraschall Med. 2016, 37, 234–251. [Google Scholar] [CrossRef]
- Piscaglia, F.; Bolondi, L.; Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: Retrospective analysis of 23188 investigations. Ultrasound Med. Biol. 2006, 32, 1369–1375. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, B.; Kim, S.H.; Ko, K.; Lee, H.M.; Choi, H.Y. Assessment of cystic renal masses based on Bosniak classification: Comparison of CT and contrast-enhanced, U.S. Eur. J. Radiol. 2007, 61, 310–314. [Google Scholar] [CrossRef]
- Schoots, I.G.; Zaccai, K.; Hunink, M.G.; Verhagen, P. Bosniak Classification for Complex Renal Cysts Reevaluated: A Systematic Review. J. Urol. 2017, 198, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.O.; Violette, P.D.; Jewett, M.A.; Pouliot, F.; Leveridge, M.; So, A.; Whelan, T.F.; Rendon, R.; Finelli, A. CUA guideline on the management of cystic renal lesions. Can. Urol. Assoc. J. 2017, 11, E66–E73. [Google Scholar] [CrossRef] [PubMed]
- Herts, B.R.; Silverman, S.G.; Hindman, N.M.; Uzzo, R.G.; Hartman, R.P.; Israel, G.M.; Baumgarten, D.A.; Berland, L.L.; Pandharipande, P.V. Management of the Incidental Renal Mass on CT: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll Radiol. 2018, 15, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Israel, G.M.; Hindman, N.; Bosniak, M.A. Evaluation of cystic renal masses: Comparison of CT and MR imaging by using the Bosniak classification system. Radiology 2004, 231, 365–371. [Google Scholar] [CrossRef]
- Bosniak, M.A. The Bosniak renal cyst classification: 25 years later. Radiology 2012, 262, 781–785. [Google Scholar] [CrossRef]
- Pedrosa, I.; Chou, M.T.; Ngo, L.; Baroni, R.H.; Genega, E.M.; Galaburda, L.; DeWolf, W.C.; Rofsky, N.M. MR classification of renal masses with pathologic correlation. Eur. Radiol. 2008, 18, 365–375. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Reis, R.B.; Kajiwara, P.P.; Silva, G.E.; Elias, J., Jr.; Muglia, V.F. MRI evaluation of complex renal cysts using the Bosniak classification: A comparison to, C.T. Abdom. Radiol. 2016, 41, 2011–2019. [Google Scholar] [CrossRef]
- Silverman, S.G.; Pedrosa, I.; Ellis, J.H.; Hindman, N.M.; Schieda, N.; Smith, A.D.; Remer, E.M.; Shinagare, A.B.; Curci, N.E.; Raman, S.S.; et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology 2019, 292, 475–488. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B.; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, H.; Li, H.F.; Rao, Y.W.; Liu, J.H.; Tian, S.F.; Ju, Y.; Li, Y.; Chen, A.-L.; Chen, L.-H.; et al. Diagnostic Significance of Diffusion-Weighted MRI in Renal Cancer. BioMed Res. Int. 2015, 2015, 172165. [Google Scholar] [CrossRef]
- Saremi, F.; Knoll, A.N.; Bendavid, O.J.; Schultze-Haakh, H.; Narula, N.; Sarlati, F. Characterization of genitourinary lesions with diffusion-weighted imaging. RadioGraphics 2009, 29, 1295–1317. [Google Scholar] [CrossRef] [PubMed]
- Cova, M.; Squillaci, E.; Stacul, F.; Manenti, G.; Gava, S.; Simonetti, G.; Mucelli, R.S.P. Diffusion-weighted MRI in the evaluation of renal lesions: Preliminary results. Br. J. Radiol. 2004, 77, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Inci, E.; Hocaoglu, E.; Aydin, S.; Cimilli, T. Diffusion-weighted magnetic resonance imaging in evaluation of primary solid and cystic renal masses using the Bosniak classification. Eur. J. Radiol. 2012, 81, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Taouli, B.; Thakur, R.K.; Mannelli, L.; Babb, J.S.; Kim, S.; Hecht, E.M.; Lee, V.S.; Israel, G.M. Renal lesions: Characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology 2009, 251, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Qu, H.C.; Li, N.; Zhu, X.W.; Liu, Y.L.; Liu, C.L. The Value of Contrast-Enhanced Ultrasonography and Contrast-Enhanced CT in the Diagnosis of Malignant Renal Cystic Lesions: A Meta-Analysis. PLoS ONE 2016, 11, e0155857. [Google Scholar] [CrossRef]
- Sevcenco, S.; Spick, C.; Helbich, T.H.; Heinz, G.; Shariat, S.F.; Klingler, H.C.; Rauchenwald, M.; Baltzer, P. Malignancy rates and diagnostic performance of the Bosniak classification for the diagnosis of cystic renal lesions in computed tomography—A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 2239–2247. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed tomography--an increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef]
- Little, M.P.; Wakeford, R.; Tawn, E.J.; Bouffler, S.D.; Berrington de Gonzalez, A. Risks associated with low doses and low dose rates of ionizing radiation: Why linearity may be (almost) the best we can do. Radiology 2009, 251, 6–12. [Google Scholar] [CrossRef]
- Kang, S.K.; Turan, E.A.; Eisenberg, J.D.; Lee, P.A.; Kong, C.Y.; Pandharipande, P.V. Microsimulation model of CT versus MRI surveillance of Bosniak IIF renal cystic lesions: Should effects of radiation exposure affect selection of imaging strategy? AJR Am. J. Roentgenol. 2014, 203, W629–W636. [Google Scholar] [CrossRef]
- Siracusano, S.; Bertolotto, M.; Ciciliato, S.; Valentino, M.; Liguori, G.; Visalli, F. The current role of contrast-enhanced ultrasound (CEUS) imaging in the evaluation of renal pathology. World J. Urol. 2011, 29, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, M.; Cicero, C.; Perrone, R.; Degrassi, F.; Cacciato, F.; Cova, M.A. Renal Masses with Equivocal Enhancement at CT: Characterization with Contrast-Enhanced Ultrasound. AJR Am. J. Roentgenol. 2015, 204, W557–W565. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tang, L.; Yang, T.; Chen, W. Comparison of contrast-enhanced ultrasound with MRI in the diagnosis of complex cystic renal masses: A meta-analysis. Acta Radiol. 2018, 59, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Rubenthaler, J.; Paprottka, K.J.; Marcon, J.; Reiser, M.; Clevert, D.A. MRI and contrast enhanced ultrasound (CEUS) image fusion of renal lesions. Clin. Hemorheol. Microcirc. 2016, 64, 457–466. [Google Scholar] [CrossRef] [PubMed]
- European Society of R. Abdominal applications of ultrasound fusion imaging technique: Liver, kidney, and pancreas. Insights Imaging 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Rubenthaler, J.; Negrao de Figueiredo, G.; Mueller-Peltzer, K.; Clevert, D.-A. Evaluation of renal lesions using contrast-enhanced ultrasound (CEUS); a 10-year retrospective European single-centre analysis. Eur. Radiol. 2018, 28, 4542–4549. [Google Scholar] [CrossRef]
- Mueller-Peltzer, K.; Negrao de Figueiredo, G.; Graf, T.; Rubenthaler, J.; Clevert, D.-A. Papillary renal cell carcinoma in contrast-enhanced ultrasound (CEUS)—A diagnostic performance study. Clin. Hemorheol. Microcirc. 2018, 71, 159–164. [Google Scholar] [CrossRef]
- Schwarze, V.; Marschner, C.; Negrao de Figueiredo, G.; Rubenthaler, J.; Clevert, D.-A. Single-Center Study: Evaluating the Diagnostic Performance and Safety of Contrast-Enhanced Ultrasound (CEUS) in Pregnant Women to Assess Hepatic Lesions. Ultraschall Med. 2019, 41, 29–35. [Google Scholar] [CrossRef]
- Schwarze, V.; Marschner, C.; Negrao de Figueiredo, G.; Mueller-Peltzer, K.; Neumann, J.; Rubenthaler, J.; Clevert, D.-A. SonoVue(R) Does Not Appear to Cross the Placenta as Observed During an Examination Aimed at Confirming a Diagnosis of Liver Echinococcosis in a Pregnant Woman. Ultraschall Med. 2019, 41, 146–147. [Google Scholar] [CrossRef]
- Gassert, F.; Schnitzer, M.; Kim, S.H.; Kunz, W.G.; Ernst, B.P.; Clevert, D.A.; Nörenberg, D.; Rübenthaler, J.; Froelich, M.F. Comparison of Magnetic Resonance Imaging and Contrast-Enhanced Ultrasound as Diagnostic Options for Unclear Cystic Renal Lesions: A Cost-Effectiveness Analysis. Ultraschall Med. 2020. prepress. [Google Scholar] [CrossRef]
- Shaish, H.; Ahmed, F.; Schreiber, J.; Hindman, N.M. Active Surveillance of Small (<4 cm) Bosniak Category 2F, 3, and 4 Renal Lesions: What Happens on Imaging Follow-Up? AJR Am. J. Roentgenol. 2019, 212, 1215–1222. [Google Scholar] [PubMed]
- Hwang, J.H.; Lee, C.K.; Yu, H.S.; Cho, K.S.; Choi, Y.D.; Ham, W.S. Clinical Outcomes of Bosniak Category IIF Complex Renal Cysts in Korean Patients. Korean J. Urol. 2012, 53, 386–390. [Google Scholar] [CrossRef]
- Goenka, A.H.; Remer, E.M.; Smith, A.D.; Obuchowski, N.A.; Klink, J.; Campbell, S.C. Development of a clinical prediction model for assessment of malignancy risk in Bosniak III renal lesions. Urology 2013, 82, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Remer, E.M.; Cox, K.L.; Lieber, M.L.; Allen, B.C.; Shah, S.N.; Herts, B.R. Bosniak category IIF and III cystic renal lesions: Outcomes and associations. Radiology 2012, 262, 152–160. [Google Scholar] [CrossRef]
- Defortescu, G.; Cornu, J.N.; Bejar, S.; Giwerc, A.; Gobet, F.; Werquin, C.; Pfister, C.; Nouhaud, F. Diagnostic performance of contrast-enhanced ultrasonography and magnetic resonance imaging for the assessment of complex renal cysts: A prospective study. Int. J. Urol. 2017, 24, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Cho, J.Y. Imaging findings of common benign renal tumors in the era of small renal masses: Differential diagnosis from small renal cell carcinoma: Current status and future perspectives. Korean J. Radiol. 2015, 16, 99–113. [Google Scholar] [CrossRef][Green Version]
- Schwarze, V.; Marschner, C.; Negrao de Figueiredo, G.; Knosel, T.; Rubenthaler, J.; Clevert, D.A. Single-center study: The diagnostic performance of contrast-enhanced ultrasound (CEUS) for assessing renal oncocytoma. Scand. J. Urol. 2020, 54, 135–140. [Google Scholar] [CrossRef]
- Bielsa, O.; Lloreta, J.; Gelabert-Mas, A. Cystic renal cell carcinoma: Pathological features, survival and implications for treatment. Br. J. Urol. 1998, 82, 16–20. [Google Scholar] [CrossRef]
- Weibl, P.; Hora, M.; Kollarik, B.; Shariat, S.F.; Klatte, T. Management, pathology and outcomes of Bosniak category IIF and III cystic renal lesions. World J. Urol. 2015, 33, 295–300. [Google Scholar] [CrossRef]
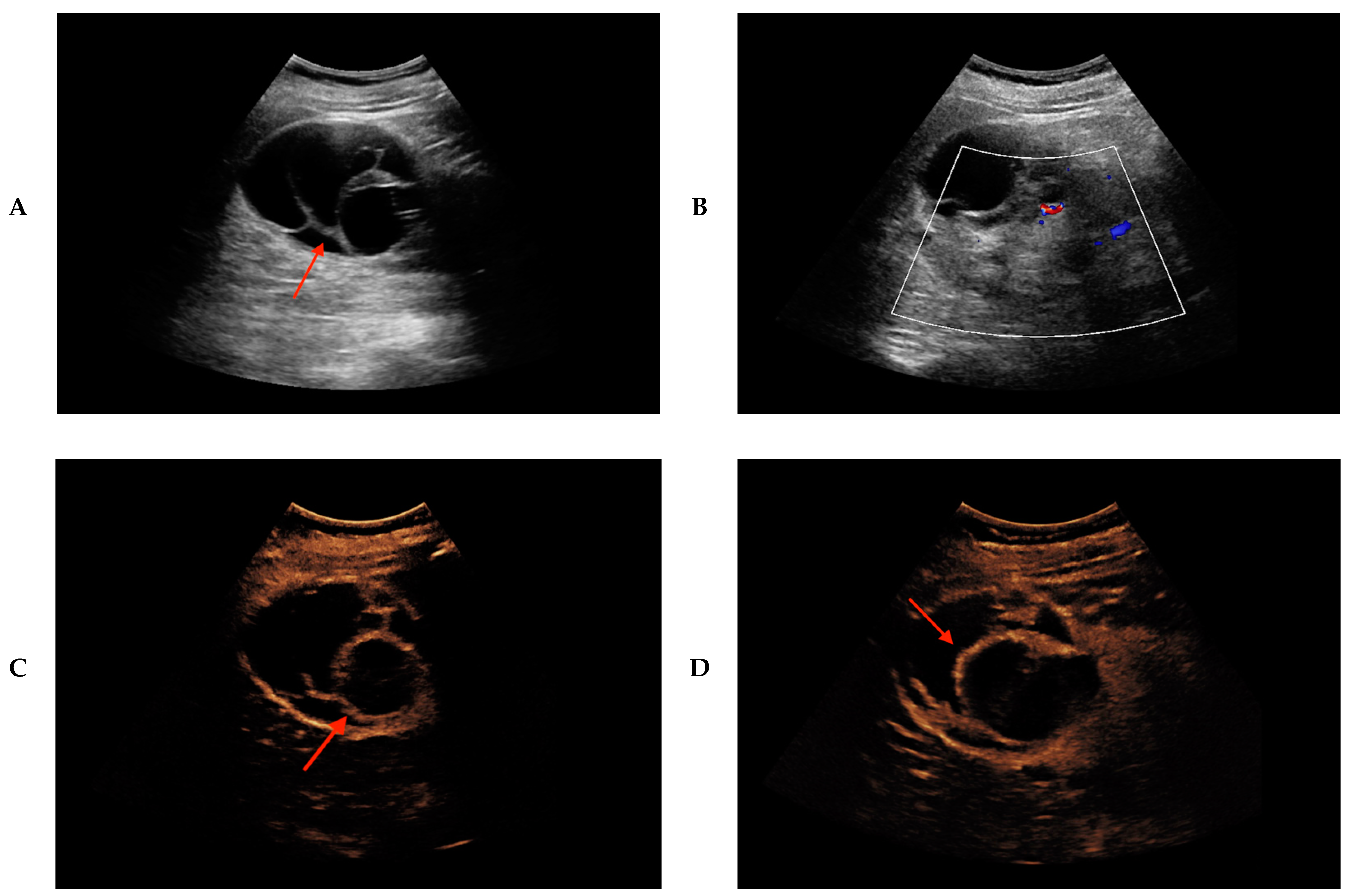
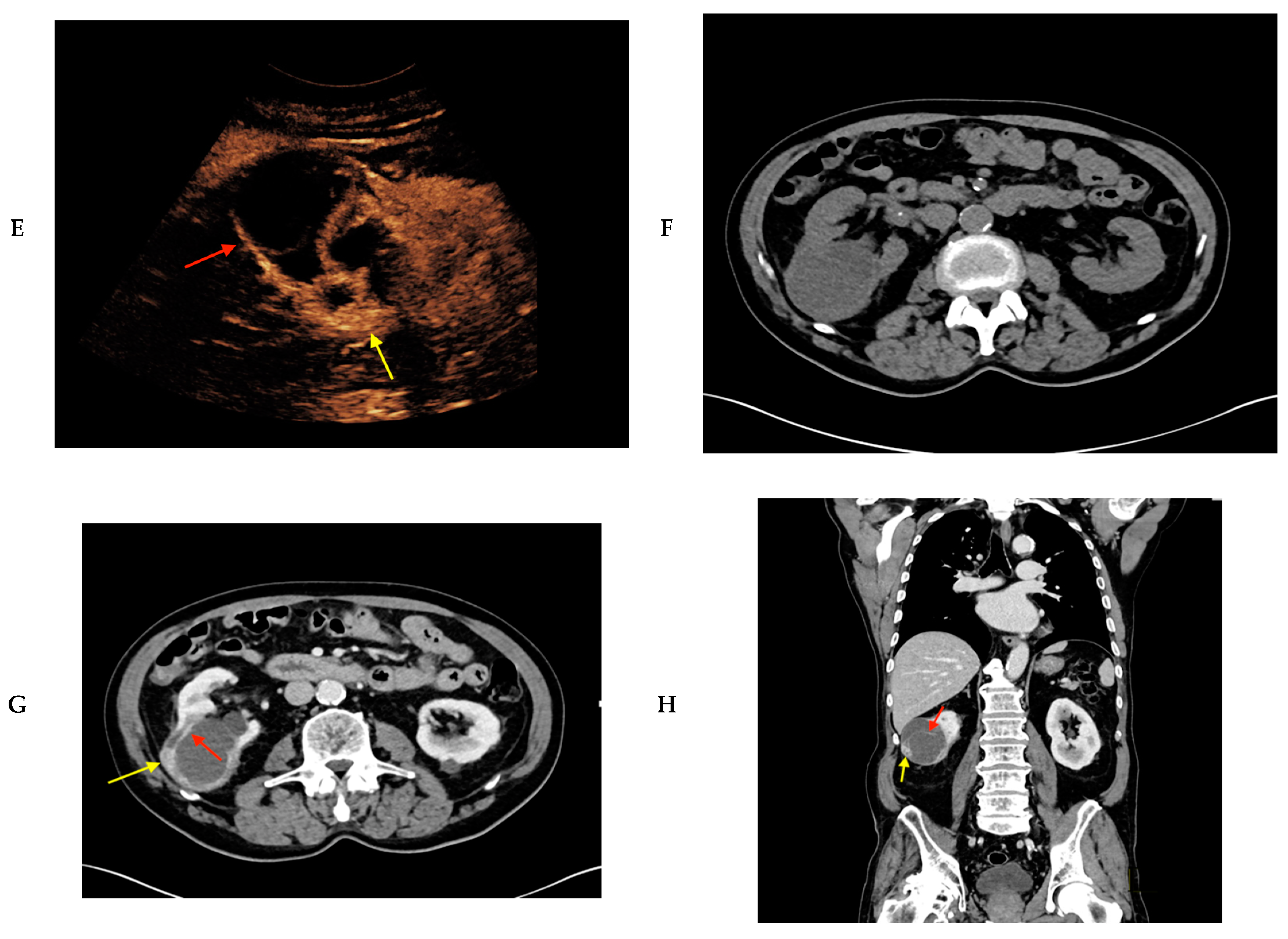
| Patients | Age | Sex | Size (cm) | Location | Native B-mode | Vascularization (CD) | CEUS | CT/MRI | Treatment | Histopathology |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | 1.0 | L | Cystic | - | septated, no contrast enhancement (Bosniak 2F) | CT: thin intracystic septa, (Bosniak 2F) | Partial nephrectomy | ccRCC |
| FU (11 mo): Increasing size: 1.8 cm | - | no contrast enhancement (Bosniak 3) | ||||||||
| 2 | 67 | M | 7.0 | R | Septated cystic lesion | - | CEUS: Septal contrast enhancement (Bosniak 2F) | CT (8 mo): Contrast enhancing septated cystic lesion (Bosniak 4) | Partial nephrectomy | Well-differentiated, highly-regressive ccRCC |
| FU (3 mo): Same morphology | - | Bosniak 2F | ||||||||
| FU(14 mo) | - | Progressive and contrast enhancing septa (Bosniak 3) | ||||||||
| FU(21 mo) | - | Same morphology (Bosniak 3) | ||||||||
| FU(28 mo) | - | Progressive and contrast enhancing septa (Bosniak 4) | ||||||||
| 3 | 55 | M | 2.0 | L | Cystic | - | Visualization of intracystic septa; septal contrast enhancement (Bosniak 2F) | CT (12 mo): Nodular contras enhancement, partially solid | - | - |
| FU(10 mo): partial solid cystic lesion | - | Intraseptal + marginal contrast enhancements (Bosniak 3) | ||||||||
| 4 | 56 | M | 1.5 | 1 | Septated cystic | - | Septal contrast enhancement (Bosniak 2F) | CT (3 mo): Hypodenes, septated renal mass, nodular contrast-enhancing (Bosniak 3) | Partial nephrectomy | Moderately differentiated ccRCC |
| FU(2 mo): increasing size, 3.5 cm | - | Septal and nodular contrast enhancement (Bosniak 3) | - | - | - | |||||
| 5 | 66 | F | 1.6 | L | Cystic | - | Septated, septal contrast-enhancement (Bosniak 2F) | MRI (6 mo): Contrast-enhancing cystic lesion, delayed wash-out (Bosniak 4) | Partial nephrectomy | Leiomyoma |
| FU(6 mo) | - | Bosniak 3 | ||||||||
| 6 | 69 | M | 2.4 | R | Cystic | - | Visualization of intracystic septa, septal contrast-enhancement (Bosniak 2F) | MRI (6 mo): Wall thickened renal cystic lesion, no contrast enhancement (hemorrhagic cyst) | - | - |
| FU(6 mo) | - | Increasing septal and nodular contrast-enhancement (Bosniak 3) | ||||||||
| 7 | 79 | M | 5.0 | R | Cystic | - | Early and bright contrast-enhancement (Bosniak 2F) | CT: Inhomogeneously contrast-enhancing cystic lesion (Bosniak 3) MRI (8 mo): Inhomogeneously contrast-enhancing cystic lesion (Bosniak 3) | Nephrectomy | Moderately differentiated ccRCC |
| FU (30 mo) | - | Increasing contrast-enhancement of the cystic lesion (Bosniak 3) | ||||||||
| 8 | 79 | F | 1.7 | L | Cystic | - | Intracystic septa, intraseptal and marginal contrast-enhancement (Bosniak 2F) | - | Partial nephrectomy | Well-differentiated ccRCC |
| FU (12 mo) | Increasing contrast-enhancement of the septa (Bosniak 3) | - | - | - |
| Patients | Age | Sex | Size (cm) | Location | Native B-mode | Vascularization (CD) | CEUS | CT/MRI | Treatment | Histopathology |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | 7.0 | R | Partial solid | - | No enhancement (Bosniak 2F) | - | Partial nephrectomy | Well-differentiated ccRCC |
| 2 | 67 | M | 1.0 | R | Partial solid cystic | - | No enhancement (Bosniak 2F) | - | Nephrectomy | Well-differentiated pRCC, WHO Type I |
| 3 | 59 | M | 1.0 | L | Cystic | - | Moderate contrast enhancement of the cystic lesion (Bosniak 2F) | - | Partial nephrectomy | Well-differentiated ccRCC |
| 4 | 70 | M | 2.5 | R | Cystic | - | Early contrast-enhancement, venous wash-out (Bosniak2F) | - | Partial nephrectomy | pRCC, WHO Type II |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rübenthaler, J.; Čečatka, S.; Froelich, M.F.; Stechele, M.; Marschner, C.; Sabel, B.O.; Bogner, F.; Schnitzer, M.L.; Overhoff, D.; Große Hokamp, N.; et al. Contrast-Enhanced Ultrasound (CEUS) for Follow-Up of Bosniak 2F Complex Renal Cystic Lesions—A 12-Year Retrospective Study in a Specialized European Center. Cancers 2020, 12, 2170. https://doi.org/10.3390/cancers12082170
Rübenthaler J, Čečatka S, Froelich MF, Stechele M, Marschner C, Sabel BO, Bogner F, Schnitzer ML, Overhoff D, Große Hokamp N, et al. Contrast-Enhanced Ultrasound (CEUS) for Follow-Up of Bosniak 2F Complex Renal Cystic Lesions—A 12-Year Retrospective Study in a Specialized European Center. Cancers. 2020; 12(8):2170. https://doi.org/10.3390/cancers12082170
Chicago/Turabian StyleRübenthaler, Johannes, Saša Čečatka, Matthias Frank Froelich, Matthias Stechele, Constantin Marschner, Bastian Oliver Sabel, Florian Bogner, Moritz Ludwig Schnitzer, Daniel Overhoff, Nils Große Hokamp, and et al. 2020. "Contrast-Enhanced Ultrasound (CEUS) for Follow-Up of Bosniak 2F Complex Renal Cystic Lesions—A 12-Year Retrospective Study in a Specialized European Center" Cancers 12, no. 8: 2170. https://doi.org/10.3390/cancers12082170
APA StyleRübenthaler, J., Čečatka, S., Froelich, M. F., Stechele, M., Marschner, C., Sabel, B. O., Bogner, F., Schnitzer, M. L., Overhoff, D., Große Hokamp, N., Staehler, M., Schwarze, V., & Clevert, D.-A. (2020). Contrast-Enhanced Ultrasound (CEUS) for Follow-Up of Bosniak 2F Complex Renal Cystic Lesions—A 12-Year Retrospective Study in a Specialized European Center. Cancers, 12(8), 2170. https://doi.org/10.3390/cancers12082170





