Integrated Assessment of Circulating Cell-Free MicroRNA Signatures in Plasma of Patients with Melanoma Brain Metastasis
Abstract
1. Introduction
2. Results
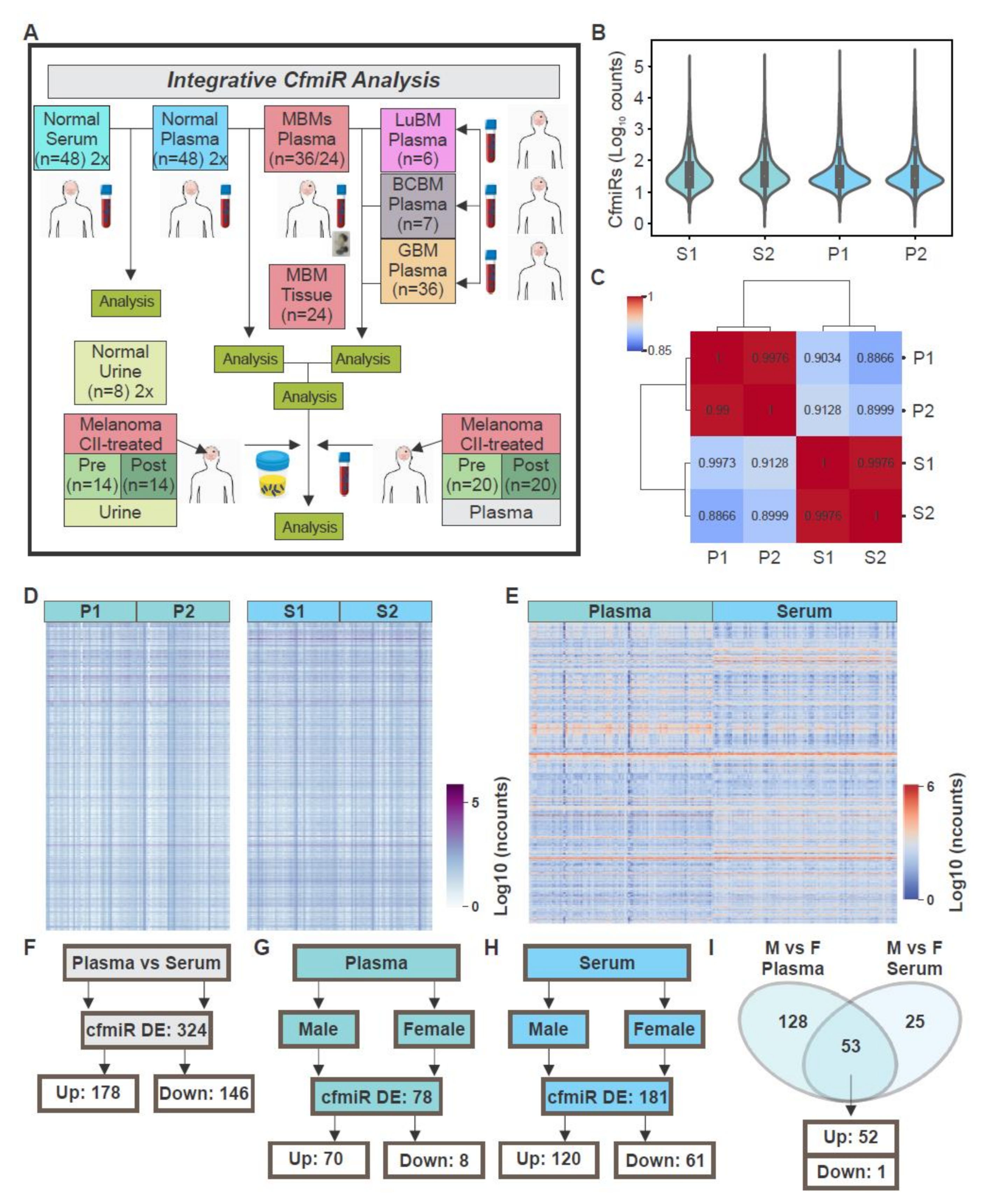
2.1. CfmiRs That Differentiate Plasma from Serum in Normal Healthy Donors
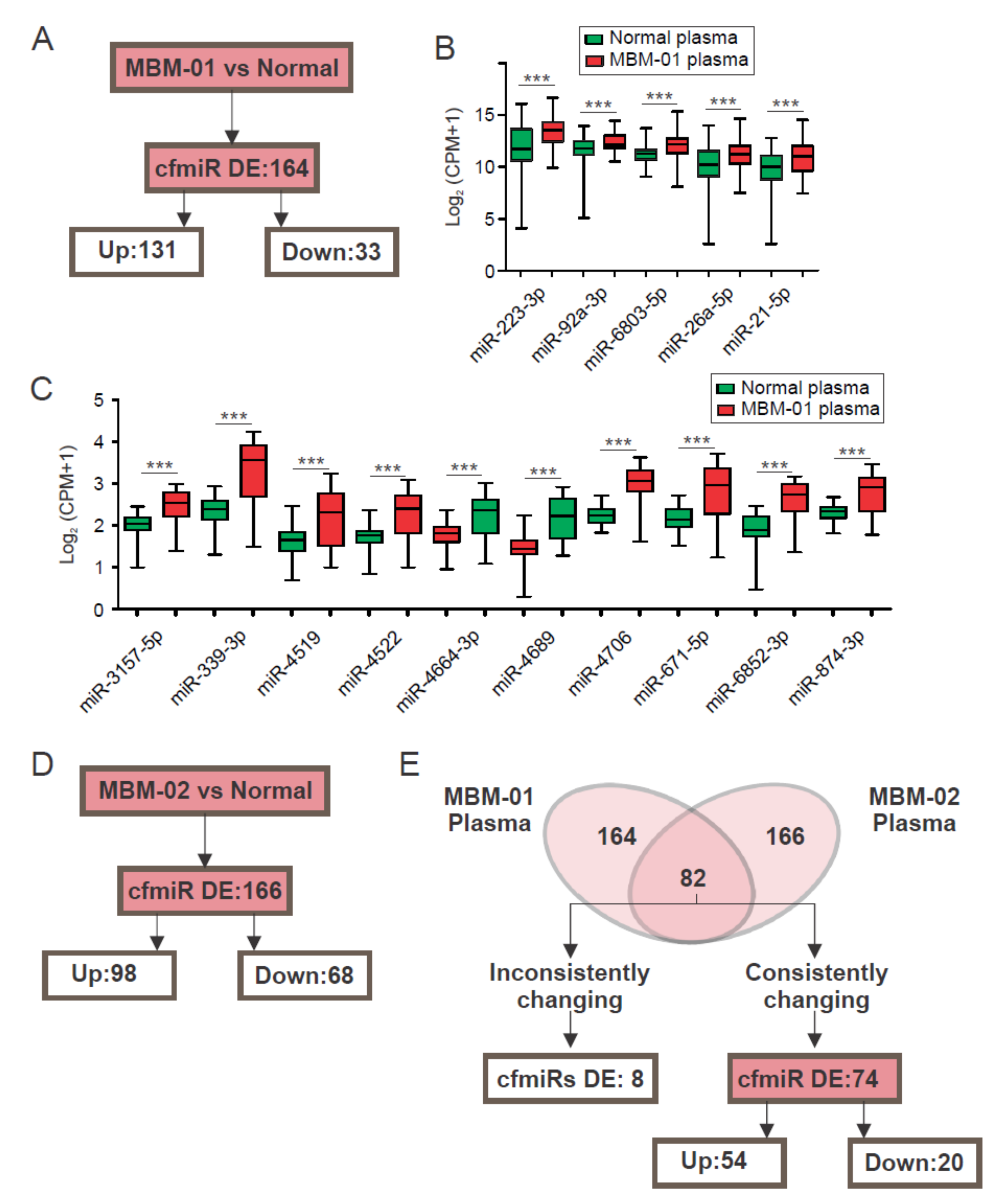
2.2. A CfmiR Signature That Identifies MBM Patients from Normal Healthy Donors’ Plasma
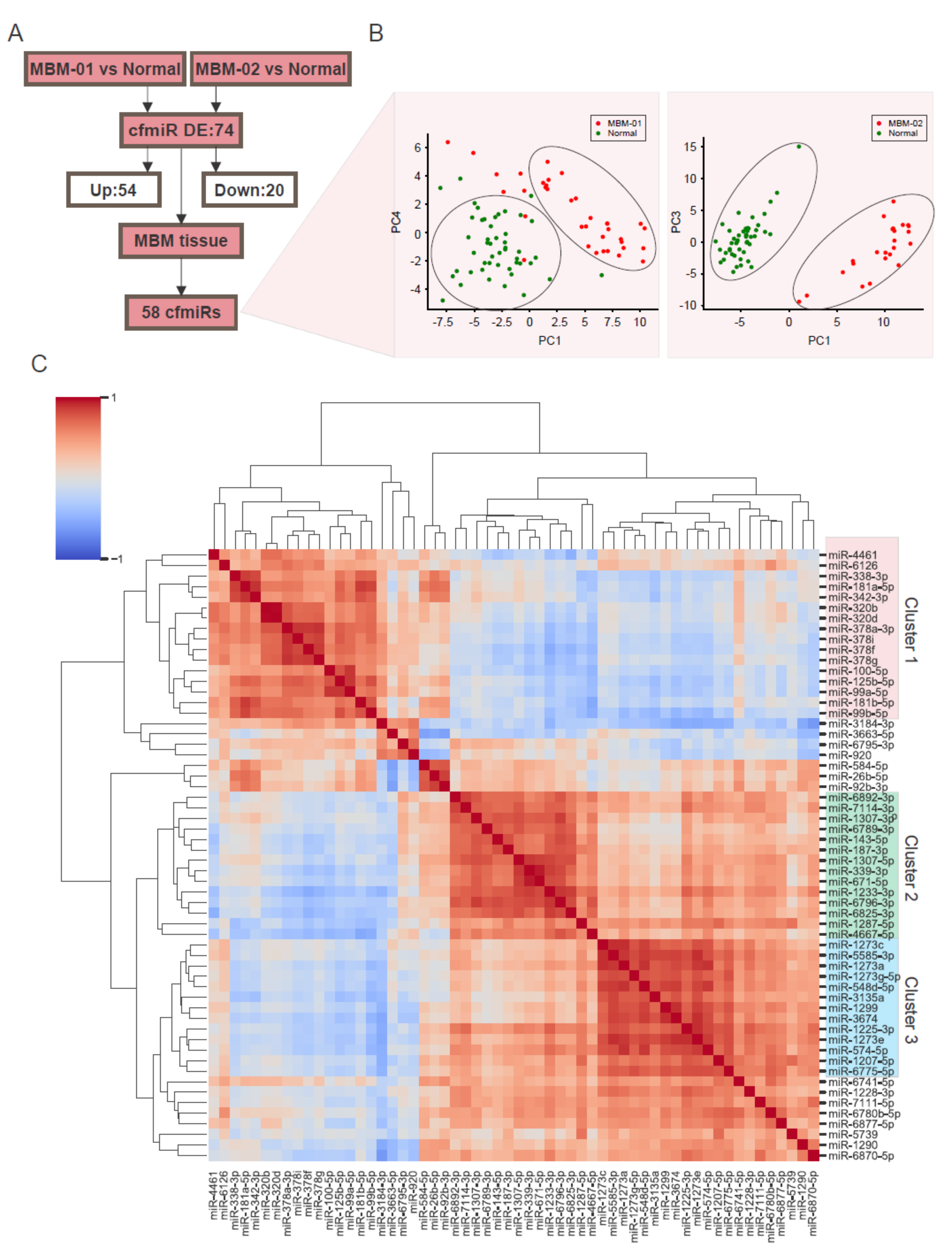
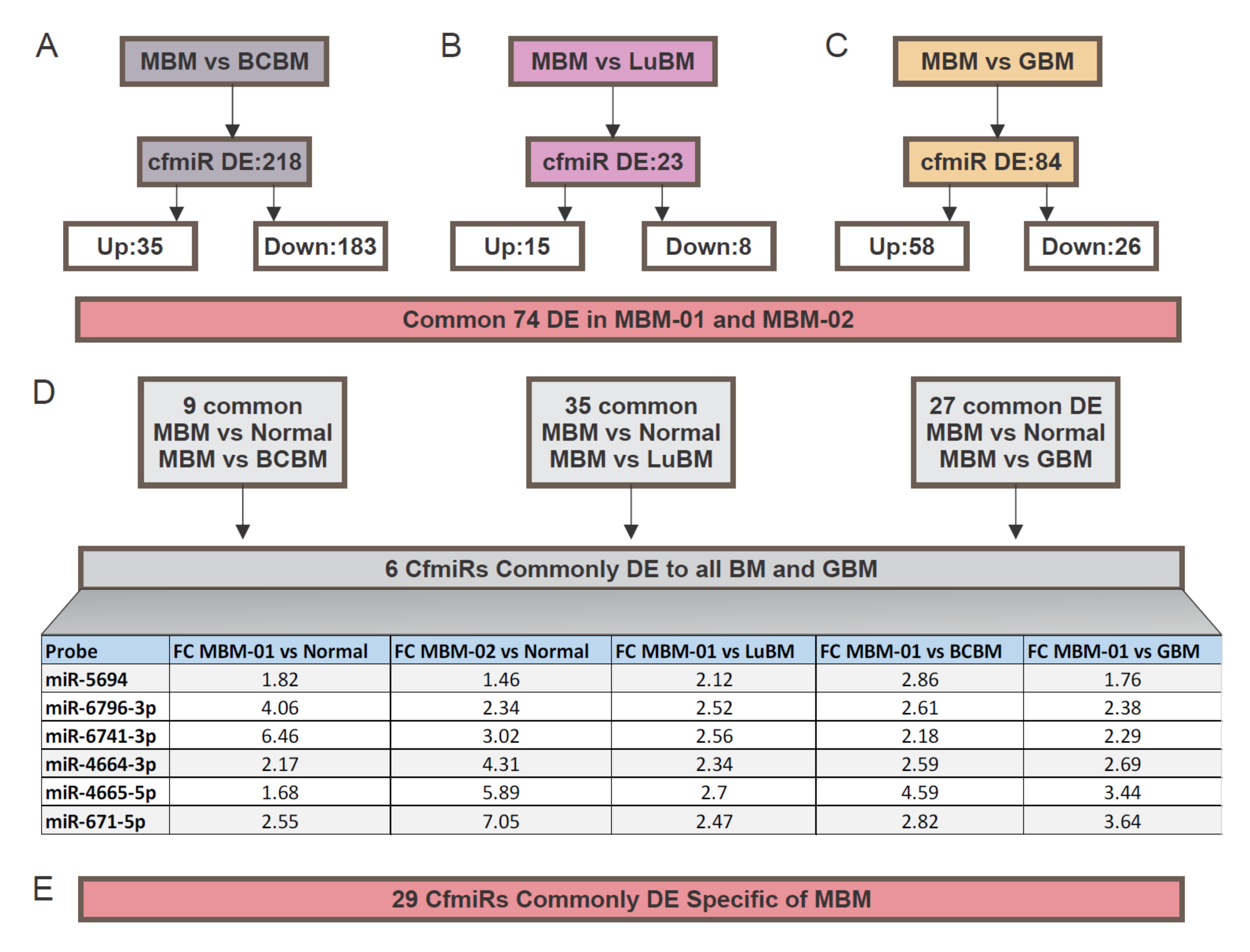
2.3. CfmiRs That Differentiate MBM from Other Brain Tumors
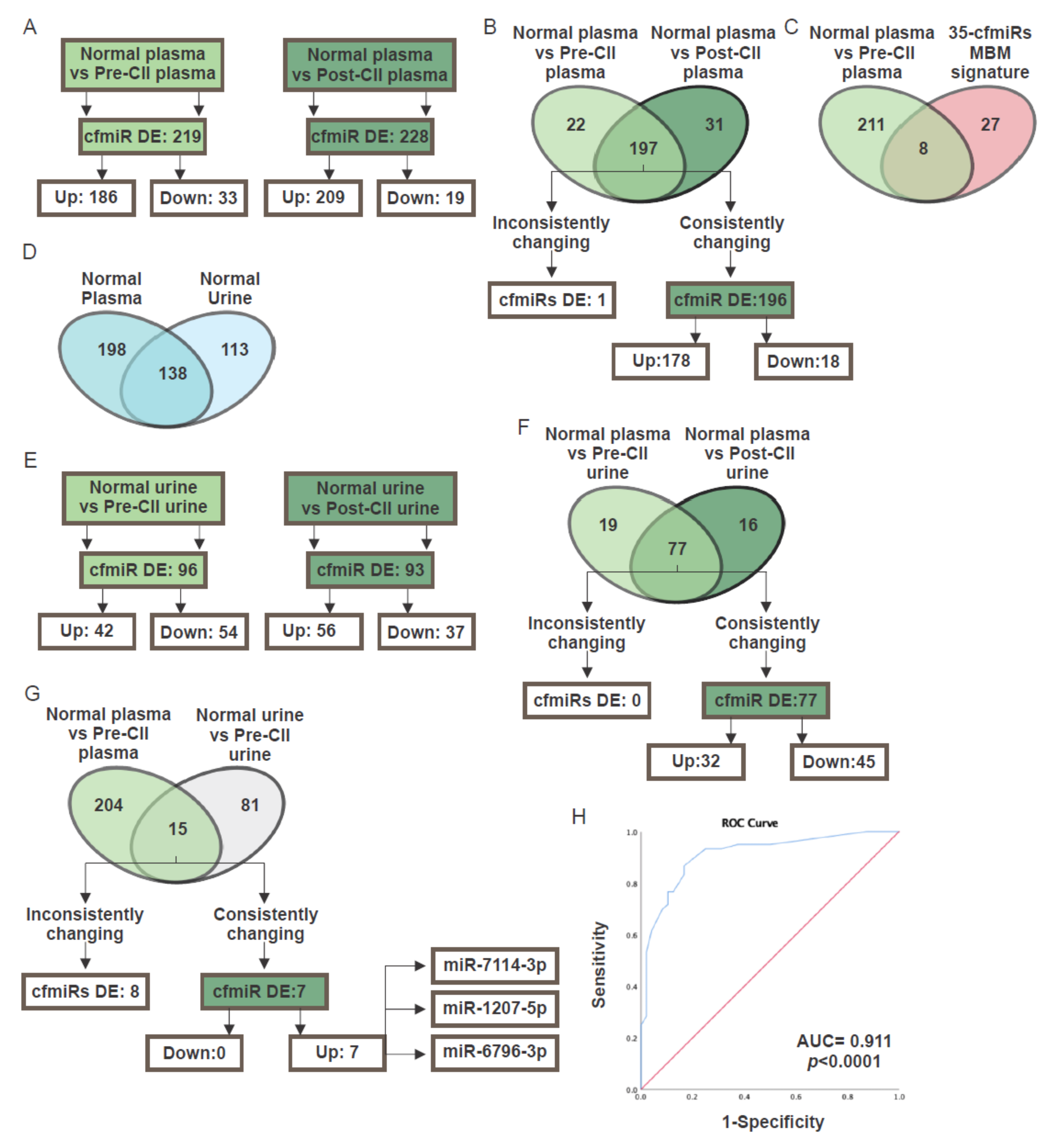
2.4. CfmiR in Patients Treated with Checkpoint Inhibitor Immunotherapy
2.5. CfmiR in Urine
3. Discussion
4. Materials and Methods
4.1. Consent to Participate and Patient Specimen Accrual
4.1.1. Blood and Urine
4.1.2. MBM FFPE Tissues
4.2. Sample Processing for HTG miRNA WTA
4.2.1. Serum and Plasma Processing for HTG miRNA WTA
4.2.2. Serum and Plasma Library Preparation
4.2.3. MBM Tissue Processing for HTG miRNA WTA
4.2.4. MBM Tissue Library Preparation
4.2.5. MBM Urine Processing for HTG miRNA WTA
4.2.6. NGS Library Quality Check
4.2.7. NGS Library Normalization and Pooling
4.2.8. NGS Profiling of the Libraries
4.2.9. HTG NGS Arrays
4.3. Biostatistical Analysis
4.4. Data Deposit
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| AUC | Areas Under Curves |
| BCBM | Breast Cancer Brain Metastasis |
| cfmiR | Cell-free miR |
| cfDNA | Cell-free DNA |
| cfNA | Cell-free Nucleic Acids |
| CII | Checkpoint Inhibitor Immunotherapy |
| CLIA | Clinical Laboratory Improvement Amendments |
| dNTP | Deoxyribonucleotide Triphosphate |
| EDTA | Ethylenediamine Tetraacetic Acid |
| IRAE | Immune-Related Adverse Events |
| DE | Differentially Expressed |
| FC | Fold-Change |
| FDA | Food and Drug Adminstration |
| FDR | False Discovery Rate |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| GBM | Glioblastoma |
| GEO | Gene Expression Omnibus |
| H&E | Hematoxylin & Eosin |
| LuBM | Lung Cancer Brain Metastasis |
| MBM | Melanoma Brain Metastasis |
| miR | MicroRNA |
| PCA | Principal Component Analysis |
| PC | Principal Components |
| PCR | Polymerase Chain Reaction |
| QC | Quality Check |
| NS | Non Significant |
| RECIST | Response Evaluation Criteria In Solid Tumors 1.1 |
| REMARK | Reporting Recommendations For Tumour Marker |
| ROC | Receiver Operating Characteristic |
| RT | Room Temperature |
| SD | Standard Deviation |
| sLDH | Serum Lactate Dehydrogenase |
| WTA | Whole Transcriptome Assay |
References
- Matthews, N.H.; Li, W.Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Balch, C.M.; Soong, S.J.; Thompson, J.F. Prognostic factors and natural history of melanoma. In Cutaneous Melanoma, 5th ed.; Balch, C.M., Houghton, A.N.A.J.S., Soong, S.J., Atkins, M.B., Thompson, J.F., Eds.; Quality Medical Publishing: St. Louis, MO, USA, 2009; pp. 35–64. [Google Scholar]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Izraely, S.; Sagi-Assif, O.; Klein, A.; Meshel, T.; Tsarfaty, G.; Pasmanik-Chor, M.; Nahmias, C.; Couraud, P.O.; Ateh, E.; Bryant, J.L.; et al. The metastatic microenvironment: Brain-residing melanoma metastasis and dormant micrometastasis. Int. J. Cancer 2012, 131, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.; Glitza Oliva, I.C.; Niessner, H. Molecular insights into melanoma brain metastases. Cancer 2017, 123, 2163–2175. [Google Scholar] [CrossRef]
- Boire, A.; Brastianos, P.K.; Garzia, L.; Valiente, M. Brain metastasis. Nat. Rev. Cancer 2020, 20, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.A.; Wolchok, J.D.; Sznol, M. Immunotherapy of Melanoma: Facts and Hopes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5191–5201. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, D.; Leininger, J.; Hamby, C.; Safai, B. Diagnostic and prognostic biomarkers in melanoma. J. Clin. Aesthet. Dermatol. 2014, 7, 13–24. [Google Scholar]
- Van Wilpe, S.; Koornstra, R.; Den Brok, M.; De Groot, J.W.; Blank, C.; De Vries, J.; Gerritsen, W.; Mehra, N. Lactate dehydrogenase: A marker of diminished antitumor immunity. Oncoimmunology 2020, 9, 1731942. [Google Scholar] [CrossRef]
- Lin, S.Y.; Linehan, J.A.; Wilson, T.G.; Hoon, D.S.B. Emerging Utility of Urinary Cell-free Nucleic Acid Biomarkers for Prostate, Bladder, and Renal Cancers. Eur. Urol. Focus 2017, 3, 265–272. [Google Scholar] [CrossRef]
- Bax, C.; Lotesoriere, B.J.; Sironi, S.; Capelli, L. Review and Comparison of Cancer Biomarker Trends in Urine as a Basis for New Diagnostic Pathways. Cancers 2019, 11, 1244. [Google Scholar] [CrossRef]
- Diefenbach, R.J.; Lee, J.H.; Rizos, H. Monitoring Melanoma Using Circulating Free DNA. Am. J. Clin. Dermatol. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Huang, S.K.; Huynh, K.T.; Salomon, M.P.; Chang, S.-C.; Marzese, D.M.; Lanman, R.B.; Talasaz, A.; Hoon, D.S.B. Multiplex Gene Profiling of Cell-Free DNA in Patients With Metastatic Melanoma for Monitoring Disease. JCO Precis. Oncol. 2018, 2, 1–30. [Google Scholar] [CrossRef]
- Goh, J.Y.; Feng, M.; Wang, W.; Oguz, G.; Yatim, S.; Lee, P.L.; Bao, Y.; Lim, T.H.; Wang, P.; Tam, W.L.; et al. Chromosome 1q21.3 amplification is a trackable biomarker and actionable target for breast cancer recurrence. Nat. Med. 2017, 23, 1319–1330. [Google Scholar] [CrossRef]
- Leung, F.; Kulasingam, V.; Diamandis, E.P.; Hoon, D.S.; Kinzler, K.; Pantel, K.; Alix-Panabieres, C. Circulating Tumor DNA as a Cancer Biomarker: Fact or Fiction? Clin. Chem. 2016, 62, 1054–1060. [Google Scholar] [CrossRef]
- Huynh, K.; Hoon, D.S. Liquid Biopsies for Assessing Metastatic Melanoma Progression. Crit. Rev. Oncog. 2016, 21, 141–154. [Google Scholar] [CrossRef]
- Huang, S.K.; Hoon, D.S. Liquid biopsy utility for the surveillance of cutaneous malignant melanoma patients. Mol. Oncol. 2016, 10, 450–463. [Google Scholar] [CrossRef]
- Fleischhacker, M.; Schmidt, B. Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys. Acta 2007, 1775, 181–232. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Fleming, N.H.; Zhong, J.; da Silva, I.P.; Vega-Saenz de Miera, E.; Brady, B.; Han, S.W.; Hanniford, D.; Wang, J.; Shapiro, R.L.; Hernando, E.; et al. Serum-based miRNAs in the prediction and detection of recurrence in melanoma patients. Cancer 2015, 121, 51–59. [Google Scholar] [CrossRef]
- Mumford, S.L.; Towler, B.P.; Pashler, A.L.; Gilleard, O.; Martin, Y.; Newbury, S.F. Circulating MicroRNA Biomarkers in Melanoma: Tools and Challenges in Personalised Medicine. Biomolecules 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.M.; Barczak, A.J.; DeHoff, P.; Srinivasan, S.; Etheridge, A.; Galas, D.; Das, S.; Erle, D.J.; Laurent, L.C. Comparison of Reproducibility, Accuracy, Sensitivity, and Specificity of miRNA Quantification Platforms. Cell Rep. 2019, 29, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Perakis, S.; Geigl, J.B.; Speicher, M.R. The potential of liquid biopsies for the early detection of cancer. NPJ Precis. Oncol. 2017, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- Leidinger, P.; Keller, A.; Borries, A.; Reichrath, J.; Rass, K.; Jager, S.U.; Lenhof, H.P.; Meese, E. High-throughput miRNA profiling of human melanoma blood samples. BMC Cancer 2010, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, R.; Lincoln, M.; Van Laar, B. Development and validation of a plasma-based melanoma biomarker suitable for clinical use. Br. J. Cancer 2018, 118, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Margue, C.; Reinsbach, S.; Philippidou, D.; Beaume, N.; Walters, C.; Schneider, J.G.; Nashan, D.; Behrmann, I.; Kreis, S. Comparison of a healthy miRNome with melanoma patient miRNomes: Are microRNAs suitable serum biomarkers for cancer? Oncotarget 2015, 6, 12110–12127. [Google Scholar] [CrossRef]
- Fogli, S.; Polini, B.; Carpi, S.; Pardini, B.; Naccarati, A.; Dubbini, N.; Lanza, M.; Breschi, M.C.; Romanini, A.; Nieri, P. Identification of plasma microRNAs as new potential biomarkers with high diagnostic power in human cutaneous melanoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2017, 39. [Google Scholar] [CrossRef]
- Philippidou, D.; Schmitt, M.; Moser, D.; Margue, C.; Nazarov, P.V.; Muller, A.; Vallar, L.; Nashan, D.; Behrmann, I.; Kreis, S. Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Res. 2010, 70, 4163–4173. [Google Scholar] [CrossRef]
- Greenberg, E.; Besser, M.J.; Ben-Ami, E.; Shapira-Frommer, R.; Itzhaki, O.; Zikich, D.; Levy, D.; Kubi, A.; Eyal, E.; Onn, A.; et al. A comparative analysis of total serum miRNA profiles identifies novel signature that is highly indicative of metastatic melanoma: A pilot study. Biomarkers 2013, 18, 502–508. [Google Scholar] [CrossRef]
- Bustos, M.A.; Ono, S.; Marzese, D.M.; Oyama, T.; Iida, Y.; Cheung, G.; Nelson, N.; Hsu, S.C.; Yu, Q.; Hoon, D.S.B. MiR-200a Regulates CDK4/6 Inhibitor Effect by Targeting CDK6 in Metastatic Melanoma. J. Investig. Dermatol. 2017, 137, 1955–1964. [Google Scholar] [CrossRef]
- Iida, Y.; Ciechanover, A.; Marzese, D.M.; Hata, K.; Bustos, M.; Ono, S.; Wang, J.; Salomon, M.P.; Tran, K.; Lam, S.; et al. Epigenetic Regulation of KPC1 Ubiquitin Ligase Affects the NF-kappaB Pathway in Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4831–4842. [Google Scholar] [CrossRef] [PubMed]
- Asaga, S.; Hoon, D.S. Direct serum assay for microRNA in cancer patients. Methods Mol. Biol. 2013, 1024, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Oyama, T.; Lam, S.; Chong, K.; Foshag, L.J.; Hoon, D.S. A direct plasma assay of circulating microRNA-210 of hypoxia can identify early systemic metastasis recurrence in melanoma patients. Oncotarget 2015, 6, 7053–7064. [Google Scholar] [CrossRef] [PubMed]
- Hanniford, D.; Zhong, J.; Koetz, L.; Gaziel-Sovran, A.; Lackaye, D.J.; Shang, S.; Pavlick, A.; Shapiro, R.; Berman, R.; Darvishian, F.; et al. A miRNA-Based Signature Detected in Primary Melanoma Tissue Predicts Development of Brain Metastasis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4903–4912. [Google Scholar] [CrossRef] [PubMed]
- Gajos-Michniewicz, A.; Czyz, M. Role of miRNAs in Melanoma Metastasis. Cancers 2019, 11, 326. [Google Scholar] [CrossRef]
- Peltier, H.J.; Latham, G.J. Normalization of microRNA expression levels in quantitative RT-PCR assays: Identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA (New York, N.Y.) 2008, 14, 844–852. [Google Scholar] [CrossRef]
- Lanidou, E.; Hoon, D. Circulating Tumor Cells and Circulating Tumor DNA as a real time liquid biopsy approach. In Tietz Textbook Clinical Chemistry and Moelcular Diagnostics, 6th ed.; Wittner, C., Rafai, N., Horvath, R., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 1145–1155. [Google Scholar]
- Lanidou, E.; Hoon, D. Circulating Tumor Cells and Circulating Tumor DNA. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th ed.; Wittner, C., Park, S., Rafai, N., Horvath, R., Eds.; Elsevier: St. Louis, MO, USA, 2018; pp. 1111–1144. [Google Scholar]
- Nziza, N.; Jeziorski, E.; Delpont, M.; Cren, M.; Chevassus, H.; Carbasse, A.; Mahe, P.; Abassi, H.; Joly-Monrigal, P.; Schordan, E.; et al. Synovial-Fluid miRNA Signature for Diagnosis of Juvenile Idiopathic Arthritis. Cells 2019, 8, 326. [Google Scholar] [CrossRef]
- Trejo, C.L.; Babić, M.; Imler, E.; Gonzalez, M.; Bibikov, S.I.; Shepard, P.J.; VanSteenhouse, H.C.; Yeakley, J.M.; Seligmann, B.E. Extraction-free whole transcriptome gene expression analysis of FFPE sections and histology-directed subareas of tissue. PLoS ONE 2019, 14, e0212031. [Google Scholar] [CrossRef]
- Max, K.E.A.; Bertram, K.; Akat, K.M.; Bogardus, K.A.; Li, J.; Morozov, P.; Ben-Dov, I.Z.; Li, X.; Weiss, Z.R.; Azizian, A.; et al. Human plasma and serum extracellular small RNA reference profiles and their clinical utility. Proc. Natl. Acad. Sci. USA 2018, 115, E5334–E5343. [Google Scholar] [CrossRef]
- Huang, S.; Hoon, D. Liquid Biopsy Utility for the Surveillance of Cutaneous Malignant Melanoma Patients. Mol. Oncol. 2016, 10, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Hoon, D. Liquid Biopsies for Assessing Metastatic Melanoma Progression. Crit. Rev. Onco. 2016, 21, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Nolte, F.; Best, H.; Kelley, T.; Patel, J.; Lanidou, E.; Hoon, D.; Chiu, R.; Lo, D. Molecular Applications. In Tietz Fundamentals of Clinical Chemistry; Wittner, C., Park, S., Rafai, N., Horvath, R., Eds.; Elsevier: St. Louis, MO, USA, 2018; pp. 961–987. [Google Scholar]
- Ono, S.; Lam, S.; Nagahara, M.; Hoon, D. Circulating MicroRNA Biomarkers as Liquid Biopsy for Cancer Patients: Pros and Cons of Current Assays. J. Clin. Med. 2015, 4, 1890–1907. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, J.; Sun, Y. Circulating Tumor DNA as Biomarkers for Cancer Detection. Genom. Proteom. Bioinform. 2017, 15, 59–72. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical utility of circulating non-coding RNAs—an update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef]
- Yeri, A.; Courtright, A.; Reiman, R.; Carlson, E.; Beecroft, T.; Janss, A.; Siniard, A.; Richholt, R.; Balak, C.; Rozowsky, J.; et al. Total Extracellular Small RNA Profiles from Plasma, Saliva, and Urine of Healthy Subjects. Sci. Rep. 2017, 7, 44061. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Seashols-Williams, S.; Lewis, C.; Calloway, C.; Peace, N.; Harrison, A.; Hayes-Nash, C.; Fleming, S.; Wu, Q.; Zehner, Z.E. High-throughput miRNA sequencing and identification of biomarkers for forensically relevant biological fluids. Electrophoresis 2016, 37, 2780–2788. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M.; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Taube, S.E.; McShane, L.M.; Cavenagh, M.M.; Altman, D.G. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J. Natl. Cancer Inst. 2018, 110, 803–811. [Google Scholar] [CrossRef]
- Perez, F.; Granger, B.E. IPython: A System for Interactive Scientific Computing. Computing in Science and Engg. 2007, 9, 21–29. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Oliphant, T.E. A guide to NumPy (Vol. 1); Trelgol Publishing: Spanish Fork, UT, USA, 2006. [Google Scholar]
- Oliphant, T.E. Python for Scientific Computing. Comput. Sci. Eng. 2007, 9, 10–20. [Google Scholar] [CrossRef]
| (A) Top 10 DE cfmiRs expressed in duplicated plasma samples P1 and P2 | |||||
|---|---|---|---|---|---|
| Probe | Mean Normalized Expression P1 | Mean Normalized Expression P2 | FC P1 vs. P2 | p-Value | Adjusted p-Value |
| miR-6873-3p | 99 | 133 | 1.21 | 0.030 | 0.999 |
| miR-92b-3p | 269 | 369 | 1.17 | 0.081 | 0.999 |
| miR-1273e | 350 | 454 | 1.16 | 0.09 | 0.999 |
| miR-4274 | 120 | 109 | −1.09 | 0.138 | 0.999 |
| miR-1909-5p | 144 | 163 | 1.11 | 0.142 | 0.999 |
| miR-584-5p | 103 | 141 | 1.14 | 0.147 | 0.999 |
| miR-3157-5p | 101 | 115 | 1.1 | 0.179 | 0.999 |
| miR-6852-3p | 95 | 115 | 1.12 | 0.185 | 0.999 |
| miR-572 | 171 | 192 | 1.1 | 0.187 | 0.999 |
| miR-1287-5p | 371 | 430 | 1.11 | 0.190 | 0.999 |
| (B) Top 10 DE cfmiRs expressed in duplicated serum samples S1 and S2 | |||||
| Probe | Mean Normalized Expression S1 | Mean Normalized Expression S2 | FC S1 vs. S2 | p-Value | Adjusted p-Value |
| miR-6819-5p | 149 | 103 | 1.36 | 0.0001 | 0.027 |
| miR-670-3p | 189 | 123 | 1.39 | 0.0001 | 0.030 |
| miR-1303 | 181 | 118 | 1.37 | 0.000 | 0.064 |
| miR-1273e | 2776 | 2058 | 1.26 | 0.009 | 0.418 |
| miR-193a-5p | 117 | 89 | 1.24 | 0.012 | 0.478 |
| miR-1290 | 229 | 160 | 1.26 | 0.017 | 0.555 |
| miR-1299 | 527 | 381 | 1.25 | 0.023 | 0.562 |
| miR-4769-3p | 120 | 102 | 1.15 | 0.027 | 0.588 |
| miR-6852-5p | 106 | 121 | −1.13 | 0.028 | 0.588 |
| miR-3674 | 473 | 357 | 1.21 | 0.048 | 0.703 |
| (A) Plasma | (B) Serum | ||||
|---|---|---|---|---|---|
| Probe | FC M vs. F | Adjusted P-value | Probe | FC M vs. F | Adjusted p-Value |
| miR-451a | 2.29 | 2.94 × 10−5 | miR-143-5p | 1.34 | 1.50 × 10−3 |
| miR-150-5p | 1.84 | 1.10 × 10−3 | miR-3943 | 1.45 | 2.20 × 10−3 |
| miR-6796-3p | 1.45 | 1.90 × 10−3 | miR-8071 | 1.45 | 2.80 × 10−3 |
| miR-4726-3p | 1.37 | 2.00 × 10−3 | miR-4746-5p | 1.38 | 3.30 × 10−3 |
| miR-3940-5p | 1.59 | 2.00 × 10−3 | miR-217 | 1.38 | 3.30 × 10−3 |
| miR-3155b | 1.42 | 2.10 × 10−3 | miR-4758-5p | −1.33 | 4.40 × 10−3 |
| miR-6798-5p | 1.61 | 2.10 × 10−3 | miR-6787-5p | 1.38 | 4.40 × 10−3 |
| miR-486-5p | 1.70 | 2.10 × 10−3 | miR-34b-3p | 1.34 | 5.20 × 10−3 |
| miR-3912-5p | 1.39 | 2.50 × 10−3 | miR-4522 | 1.42 | 5.20 × 10−3 |
| miR-6789-3p | 1.30 | 2.70 × 10−3 | miR-193a-3p | 1.43 | 5.20 × 10−3 |
| Probe | FC Plasma M vs. F | Adjusted p-Value | FC Serum M vs. F | Adjusted p-Value |
|---|---|---|---|---|
| miR-451a | 2.29 | 2.94 × 10−5 | 1.41 | 0.047 |
| miR-150-5p | 1.84 | 1.10 × 10−3 | 1.44 | 0.023 |
| miR-8071 | 1.64 | 3.80 × 10−3 | 1.45 | 0.003 |
| miR-363-3p | 1.58 | 1.16 × 10−2 | 1.35 | 0.047 |
| miR-6852-3p | 1.56 | 4.60 × 10−3 | 1.47 | 0.007 |
| miR-6870-3p | 1.52 | 8.70 × 10−3 | 1.31 | 0.035 |
| miR-92b-3p | 1.49 | 3.57 × 10−2 | 1.39 | 0.0387 |
| miR-764 | 1.48 | 4.70 × 10−3 | 1.26 | 0.044 |
| miR-339-3p | 1.48 | 1.75 × 10−2 | 1.37 | 0.025 |
| miR-6825-3p | 1.48 | 1.05 × 10−2 | 1.43 | 0.015 |
| miR-6085 | 1.47 | 1.17 × 10−2 | 1.38 | 0.015 |
| miR-6796-3p | 1.45 | 1.90 × 10−3 | 1.24 | 0.046 |
| miR-3155b | 1.42 | 2.10 × 10−3 | 1.28 | 0.015 |
| miR-6742-5p | 1.42 | 7.00 × 10−3 | 1.29 | 0.018 |
| miR-4309 | 1.42 | 5.10 × 10−3 | 1.29 | 0.014 |
| miR-1233-3p | 1.42 | 1.03 × 10−2 | 1.36 | 0.015 |
| miR-4793-5p | 1.42 | 3.20 × 10−3 | 1.37 | 0.009 |
| miR-497-3p | 1.41 | 7.00 × 10−3 | 1.32 | 0.014 |
| miR-6069 | 1.40 | 7.00 × 10−3 | 1.3 | 0.016 |
| miR-640 | 1.40 | 8.60 × 10−3 | 1.33 | 0.012 |
| miR-5694 | 1.39 | 9.80 × 10−3 | 1.24 | 0.039 |
| miR-193b-3p | 1.39 | 1.99 × 10−2 | 1.28 | 0.011 |
| miR-3912-5p | 1.39 | 2.50 × 10−3 | 1.31 | 0.011 |
| miR-4655-3p | 1.38 | 4.40 × 10−3 | 1.21 | 0.039 |
| miR-4726-3p | 1.37 | 2.00 × 10−3 | 1.29 | 0.010 |
| miR-6715b-3p | 1.37 | 5.10 × 10−3 | 1.31 | 0.009 |
| miR-3912-3p | 1.37 | 7.50 × 10−3 | 1.32 | 0.011 |
| miR-3124-3p | 1.36 | 6.10 × 10−3 | 1.26 | 0.015 |
| miR-33b-5p | 1.36 | 1.66 × 10−2 | 1.29 | 0.020 |
| miR-671-5p | 1.36 | 1.63 × 10−2 | 1.31 | 0.039 |
| miR-5587-3p | 1.35 | 1.16 × 10−2 | 1.26 | 0.027 |
| miR-541-3p | 1.35 | 7.90 × 10−3 | 1.27 | 0.036 |
| let-7g-3p | 1.35 | 7.00 × 10−3 | 1.3 | 0.011 |
| miR-4291 | 1.35 | 1.75 × 10−2 | 1.32 | 0.014 |
| miR-2115-5p | 1.33 | 5.40 × 10−3 | 1.23 | 0.039 |
| miR-1307-5p | 1.33 | 3.90 × 10−2 | 1.33 | 0.024 |
| miR-3157-5p | 1.33 | 1.15 × 10−2 | 1.36 | 0.009 |
| miR-217 | 1.33 | 1.40 × 10−2 | 1.38 | 0.003 |
| miR-937-3p | 1.32 | 6.50 × 10−3 | 1.2 | 0.040 |
| miR-187-5p | 1.32 | 1.16 × 10−2 | 1.3 | 0.010 |
| miR-4690-5p | 1.31 | 2.70 × 10−2 | 1.25 | 0.013 |
| miR-187-3p | 1.31 | 1.50 × 10−2 | 1.33 | 0.01 |
| miR-3907 | 1.30 | 2.90 × 10−3 | 1.22 | 0.03 |
| miR-6789-3p | 1.30 | 2.70 × 10−3 | 1.27 | 0.01 |
| miR-2115-3p | 1.30 | 7.70 × 10−3 | 1.28 | 0.011 |
| miR-4279 | 1.30 | 2.73 × 10−2 | 1.29 | 0.014 |
| miR-3714 | 1.30 | 1.30 × 10−2 | 1.34 | 0.005 |
| miR-1909-5p | 1.29 | 1.45 × 10−2 | 1.26 | 0.022 |
| miR-4537 | 1.29 | 7.00 × 10−3 | 1.27 | 0.011 |
| miR-3689d | 1.27 | 1.65 × 10−2 | 1.31 | 0.007 |
| miR-935 | 1.22 | 3.85 × 10−2 | 1.23 | 0.015 |
| miR-4746-5p | 1.21 | 4.53 × 10−2 | 1.38 | 0.003 |
| miR-1273h-5p | −1.51 | 3.56 × 10−2 | −1.33 | 0.034 |
| MBM-01 | MBM-02 | |
|---|---|---|
| Variables | n (%) | n (%) |
| Age at diagnosis, year, mean (SD) | 49 (26) | 55 (14) |
| Age at MBM diagnosis, year, mean (SD) | 52 (28) | 60 (13) |
| Gender | ||
| Male | 24 (66.7) | 14 (58.3) |
| Female | 12 (33.3) | 10 (41.7) |
| Probe | FC MBM-01 vs. Normal | Adjusted p-Value | FC MBM-02 vs. Normal | Adjusted p-Value |
|---|---|---|---|---|
| miR-6741-3p | 6.46 | 7.65 × 10−37 | 3.02 | 6.24 × 10−15 |
| miR-6852-3p | 5.34 | 2.41 × 10−22 | 4.9 | 7.63 × 10−26 |
| miR-4689 | 4.14 | 1.21 × 10−27 | 6.84 | 4.66 × 10−41 |
| miR-3943 | 3.56 | 6.57 × 10−21 | 3.01 | 2.44 × 10−21 |
| miR-541-3p | 3.34 | 8.31 × 10−18 | 1.56 | 7.64 × 10−4 |
| miR-3157-5p | 3.12 | 2.07 × 10−17 | 3.27 | 1.08 × 10−24 |
| miR-4784 | 2.44 | 1.27 × 10−13 | 1.73 | 3.33 × 10−7 |
| miR-4279 | 2.36 | 2.28 × 10−9 | 1.79 | 5.84 × 10−6 |
| miR-4664-3p | 2.17 | 1.96 × 10−10 | 4.31 | 7.29 × 10−24 |
| miR-4291 | 1.93 | 1.26 × 10−5 | 2.63 | 2.68 × 10−19 |
| miR-3912-5p | 1.88 | 7.34 × 10−7 | 1.71 | 1.14 × 10−7 |
| miR-5694 | 1.82 | 2.47 × 10−4 | 1.46 | 6.00 × 10−3 |
| miR-3912-3p | 1.79 | 1.35 × 10−5 | 1.57 | 4.39 × 10−5 |
| miR-670-3p | 1.78 | 3.53 × 10−5 | 2.66 | 3.32 × 10−22 |
| miR-4665-5p | 1.68 | 2.79 × 10−4 | 5.89 | 2.77 × 10−23 |
| miR-3937 | 1.67 | 1.10 × 10−3 | 2.98 | 3.76 × 10−8 |
| MBM-01 Versus Normal Plasma | MBM-02 Versus Normal Plasma | |||||
|---|---|---|---|---|---|---|
| PCA Components | Standard Deviation | Proportion of Variance | Cumulative Proportion | Standard Deviation | Proportion of Variance | Cumulative Proportion |
| PC1 | 4.906 | 0.310 | 0.310 | 7.011 | 0.457 | 0.457 |
| PC2 | 4.383 | 0.248 | 0.558 | 4.782 | 0.213 | 0.670 |
| PC3 | 3.438 | 0.152 | 0.711 | 3.676 | 0.126 | 0.795 |
| PC4 | 2.452 | 0.078 | 0.788 | 2.458 | 0.056 | 0.851 |
| PC5 | 1.686 | 0.037 | 0.825 | 2.061 | 0.039 | 0.891 |
| (A) Cluster 1 | |||
|---|---|---|---|
| Probe | Mean Normalized Expression MBM-01 Plasma | Mean Normalized Expression MBM-02 Plasma | Mean Normalized Expression MBM Tissue |
| miR-100-5p | 150 | 79 | 98 |
| miR-125b-5p | 235 | 134 | 126 |
| miR-181a-5p | 1499 | 842 | 969 |
| miR-181b-5p | 191 | 113 | 119 |
| miR-320b | 4407 | 2417 | 3146 |
| miR-320d | 2445 | 1617 | 1685 |
| miR-338-3p | 224 | 130 | 112 |
| miR-342-3p | 413 | 227 | 285 |
| miR-378a-3p | 210 | 139 | 126 |
| miR-378f | 136 | 91 | 82 |
| miR-378g | 181 | 130 | 118 |
| miR-378i | 217 | 152 | 133 |
| miR-4461 | 3221 | 1837 | 1113 |
| miR-6126 | 108,094 | 78,636 | 79,367 |
| miR-99a-5p | 381 | 146 | 195 |
| miR-99b-5p | 267 | 171 | 121 |
| (B) Cluster 2 | |||
| Probe | Mean Normalized Expression MBM-01 Plasma | Mean Normalized Expression MBM-02 Plasma | Mean Normalized Expression MBM Tissue |
| miR-1228-3p | 92 | 127 | 171 |
| miR-1233-3p | 234 | 997 | 1573 |
| miR-1307-3p | 1067 | 1554 | 2763 |
| miR-1307-5p | 787 | 2465 | 1792 |
| miR-143-5p | 90 | 114 | 239 |
| miR-187-3p | 135 | 185 | 282 |
| miR-339-3p | 289 | 4295 | 1437 |
| miR-4667-5p | 88 | 160 | 237 |
| miR-671-5p | 162 | 1274 | 539 |
| miR-6789-3p | 100 | 147 | 178 |
| miR-6796-3p | 283 | 681 | 1513 |
| miR-6825-3p | 173 | 438 | 586 |
| miR-6892-3p | 409 | 514 | 808 |
| (C) Cluster 3 | |||
| Probe | Mean Normalized Expression MBM-01 Plasma | Mean Normalized Expression MBM-02 Plasma | Mean Normalized Expression MBM Tissue |
| miR-1207-5p | 478 | 759 | 1354 |
| miR-1225-3p | 188 | 462 | 609 |
| miR-1273a | 109 | 208 | 308 |
| miR-1273c | 171 | 243 | 357 |
| miR-1273e | 428 | 1248 | 1914 |
| miR-1273g-5p | 96 | 129 | 176 |
| miR-1299 | 109 | 342 | 318 |
| miR-3135a | 81 | 126 | 216 |
| miR-3674 | 88 | 178 | 224 |
| miR-548d-5p | 144 | 248 | 337 |
| miR-5585-3p | 1372 | 2369 | 3112 |
| miR-574-5p | 1632 | 4042 | 7203 |
| miR-6775-5p | 143 | 263 | 279 |
| Probe | FC MBM-01 vs. Normal | Adjusted p-Value | FC MBM-02 vs. Normal | Adjusted p-Value |
|---|---|---|---|---|
| miR-3937 | 1.67 | 1.10 × 10−3 | 2.98 | 2.09 × 10−9 |
| miR-1299 | 2.25 | 1.07 × 10−4 | 2.95 | 2.54 × 10−10 |
| miR-1273e | 3.36 | 2.30 × 10−10 | 2.79 | 1.13 × 10−12 |
| miR-670-3p | 1.78 | 3.53 × 10−5 | 2.66 | 2.96 × 10−24 |
| miR-1225-3p | 2.55 | 2.52 × 10−10 | 2.42 | 3.85 × 10−16 |
| miR-574-5p | 3.27 | 2.52 × 10−9 | 2.36 | 3.05 × 10−7 |
| miR-6780b-5p | 1.90 | 1.20 × 10−3 | 2.01 | 6.58 × 10−7 |
| miR-3674 | 1.96 | 5.90 × 10−3 | 1.95 | 3.72 × 10−4 |
| miR-7111-5p | 1.69 | 6.50 × 10−3 | 1.89 | 1.30 × 10−6 |
| miR-1273a | 2.22 | 7.15 × 10−6 | 1.85 | 1.06 × 10−4 |
| miR-6877-5p | 1.53 | 2.92 × 10−2 | 1.84 | 2.85 × 10−5 |
| miR-6775-5p | 1.57 | 3.50 × 10−3 | 1.81 | 2.34 × 10−6 |
| miR-4279 | 2.36 | 2.28 × 10−9 | 1.79 | 7.18 × 10−7 |
| miR-5585-3p | 1.77 | 5.80 × 10−3 | 1.69 | 3.48 × 10−4 |
| miR-548d-5p | 1.81 | 1.29 × 10−2 | 1.66 | 7.80 × 10−3 |
| miR-7114-3p | 1.88 | 1.70 × 10−6 | 1.65 | 2.27 × 10−8 |
| miR-1207-5p | 2.23 | 1.83 × 10−7 | 1.58 | 1.71 × 10−5 |
| miR-3135a | 2.12 | 1.08 × 10−5 | 1.55 | 6.26 × 10−4 |
| miR-6789-3p | 1.44 | 2.20 × 10−3 | 1.46 | 1.23 × 10−5 |
| miR-1273c | 1.67 | 6.20 × 10−3 | 1.41 | 1.63 × 10−2 |
| miR-1228-3p | 1.50 | 2.80 × 10−3 | 1.38 | 5.43 × 10−4 |
| miR-1273g-5p | 1.48 | 2.29 × 10−2 | 1.35 | 1.77 × 10−2 |
| miR-143-5p | 2.12 | 5.05 × 10−11 | 1.26 | 4.60 × 10−3 |
| miR-6795-3p | −1.31 | 1.43 × 10−2 | −1.26 | 7.06 × 10−4 |
| miR-6126 | –1.63 | 2.06 × 10−2 | −1.36 | 1.90 × 10−2 |
| miR-920 | –1.64 | 1.51 × 10−8 | −1.42 | 1.18 × 10−4 |
| miR-378f | –2.02 | 4.64 × 10−10 | −1.5 | 1.53 × 10−5 |
| miR-3663-5p | –2.33 | 4.44 × 10−12 | −1.7 | 2.23 × 10−5 |
| miR-3184-3p | −1430.32 | 1.02 × 10−105 | −2.07 | 2.78 × 10−11 |
| Plasma (n = 20) | Urine (n = 14) | Paired Plasma and Urine (n = 11) | |
|---|---|---|---|
| Variables | n (%) | n (%) | n (%) |
| Age, year, mean (SD) | 61.8 (21.1) | 59.7 (24.9) | 55 (13) |
| Gender | |||
| Male | 13 (66.7) | 9 (64.3) | 6 (54.5) |
| Female | 7 (33.3) | 5 (35.4) | 5 (45.5) |
| AJCC 8th stages | |||
| III b/c | 3 (15) | 4 (28.6) | 2 (18.2) |
| IV b/c/d | 17 (85) | 10 (71.4) | 9 (81.8) |
| BRAF mutations | |||
| Positive | 12 (60) | 9 (64.3) | 7 (63.6) |
| Negative | 8 (40) | 5 (35.7) | 4 (36.4) |
| Number of metastasis | |||
| 1 | 17 (85) | 13 (92.9) | 10 (85) |
| 2–3 | 3 (15) | 1 (7.1) | 1 (15) |
| Probe | FC MBM-01 vs. Normal | FC MBM-02 vs. Normal | FC MBM vs. LuBM | FC MBM vs. BCBM | FC MBM vs. GBM | FC Pre-CII vs. Normal | FC Post-CII vs. Normal |
|---|---|---|---|---|---|---|---|
| miR-3184-3p | −2.07 | −1430.32 | NDE | NDE | NDE | −2669.64 | −3258.79 |
| miR-143-5p | 1.26 | 2.12 | NDE | NDE | NDE | 1.81 | 2.96 |
| miR-6789-3p | 1.46 | 1.44 | NDE | NDE | NDE | 1.6 | 2.94 |
| miR-1207-5p | 1.58 | 2.23 | NDE | NDE | NDE | 1.54 | 1.62 |
| miR-7114-3p | 1.65 | 1.88 | NDE | NDE | NDE | 3.07 | 5 |
| miR-4279 | 1.79 | 2.36 | NDE | NDE | NDE | 2.87 | 4.74 |
| miR-1225-3p | 2.42 | 2.55 | NDE | NDE | NDE | 1.4 | 2.01 |
| miR-670-3p | 2.66 | 1.78 | NDE | NDE | NDE | 1.45 | 1.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos, M.A.; Tran, K.D.; Rahimzadeh, N.; Gross, R.; Lin, S.Y.; Shoji, Y.; Murakami, T.; Boley, C.L.; Tran, L.T.; Cole, H.; et al. Integrated Assessment of Circulating Cell-Free MicroRNA Signatures in Plasma of Patients with Melanoma Brain Metastasis. Cancers 2020, 12, 1692. https://doi.org/10.3390/cancers12061692
Bustos MA, Tran KD, Rahimzadeh N, Gross R, Lin SY, Shoji Y, Murakami T, Boley CL, Tran LT, Cole H, et al. Integrated Assessment of Circulating Cell-Free MicroRNA Signatures in Plasma of Patients with Melanoma Brain Metastasis. Cancers. 2020; 12(6):1692. https://doi.org/10.3390/cancers12061692
Chicago/Turabian StyleBustos, Matias A., Kevin D. Tran, Negin Rahimzadeh, Rebecca Gross, Selena Y. Lin, Yoshiaki Shoji, Tomohiro Murakami, Christine L. Boley, Linh T. Tran, Hunter Cole, and et al. 2020. "Integrated Assessment of Circulating Cell-Free MicroRNA Signatures in Plasma of Patients with Melanoma Brain Metastasis" Cancers 12, no. 6: 1692. https://doi.org/10.3390/cancers12061692
APA StyleBustos, M. A., Tran, K. D., Rahimzadeh, N., Gross, R., Lin, S. Y., Shoji, Y., Murakami, T., Boley, C. L., Tran, L. T., Cole, H., Kelly, D. F., O’Day, S., & Hoon, D. S. B. (2020). Integrated Assessment of Circulating Cell-Free MicroRNA Signatures in Plasma of Patients with Melanoma Brain Metastasis. Cancers, 12(6), 1692. https://doi.org/10.3390/cancers12061692