Optimal Androgen Deprivation Therapy Combined with Proton Beam Therapy for Prostate Cancer: Results from a Multi-Institutional Study of the Japanese Radiation Oncology Study Group
Abstract
1. Introduction
2. Results
2.1. Biochemical Relapse
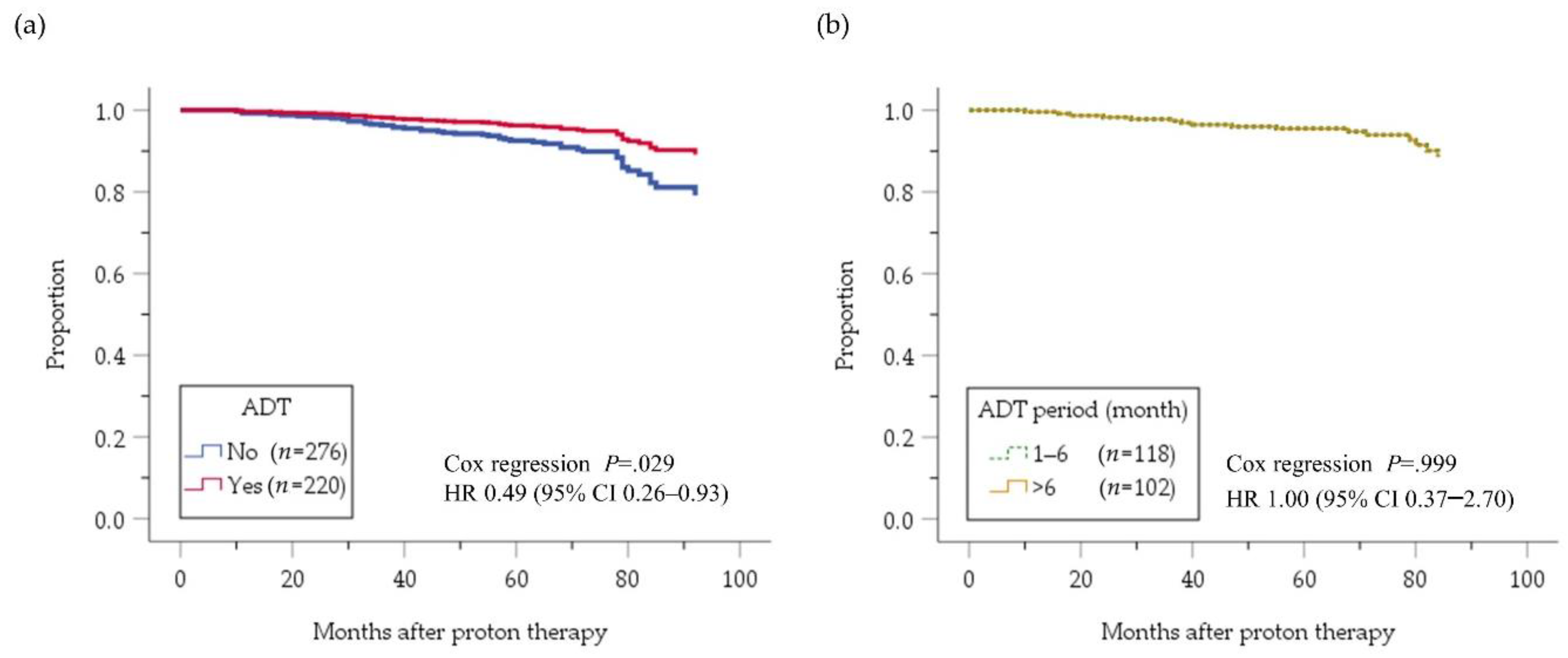
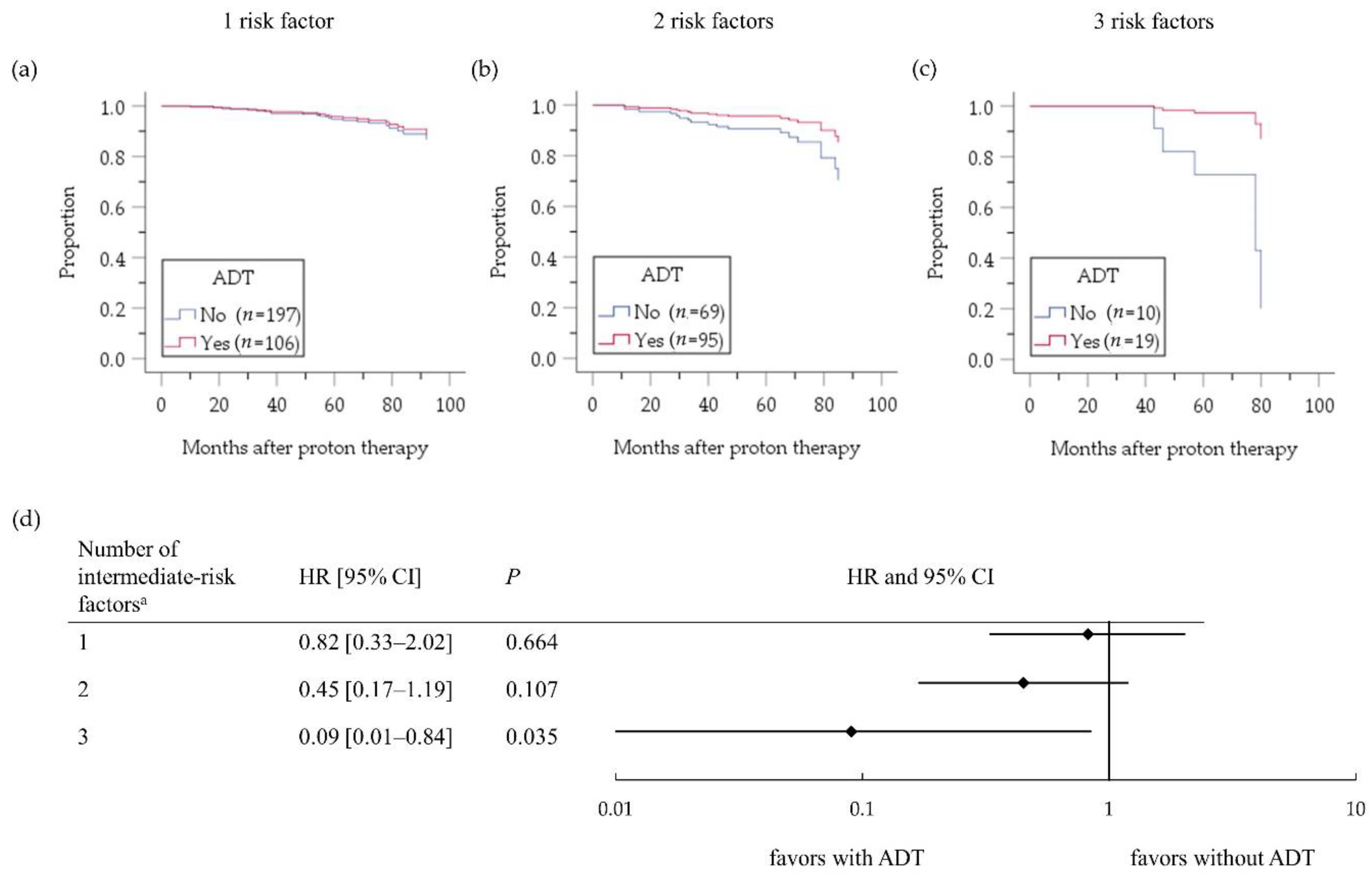
2.2. Intermediate-Risk PC Group
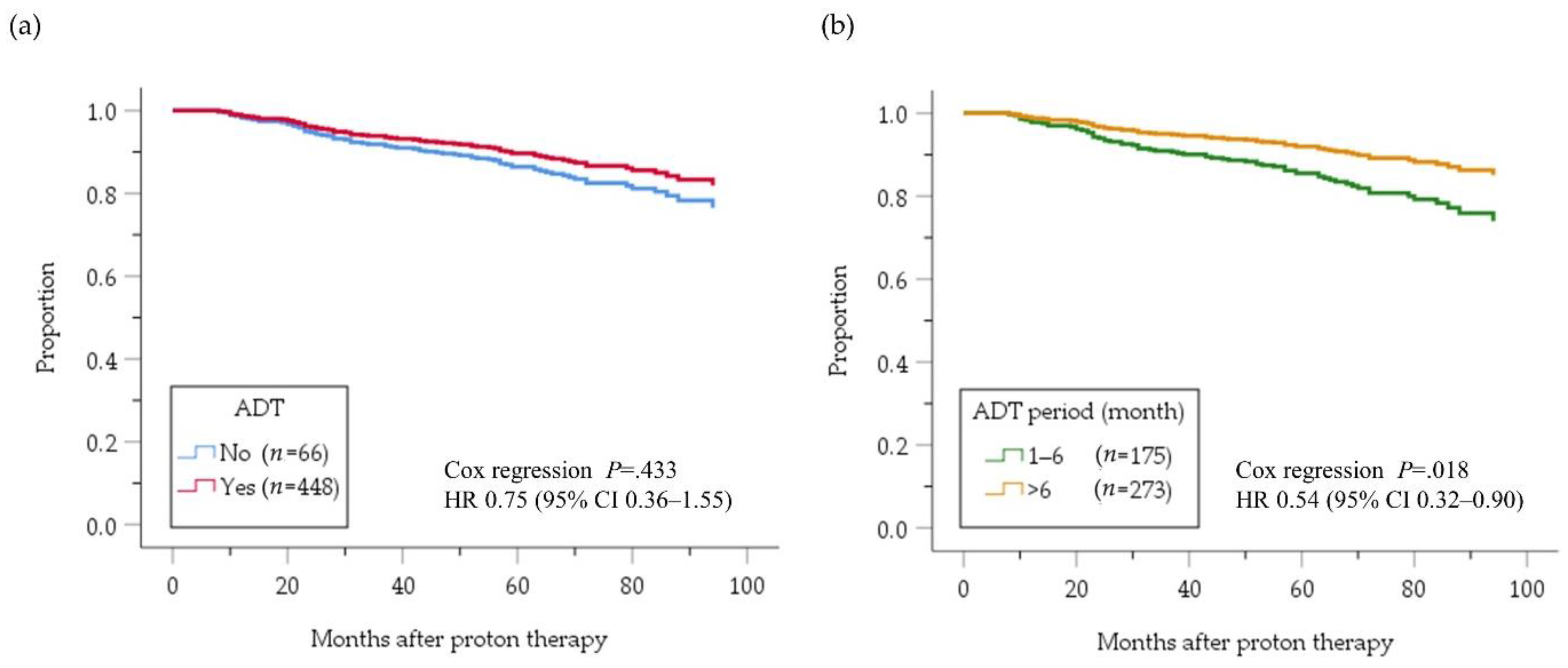
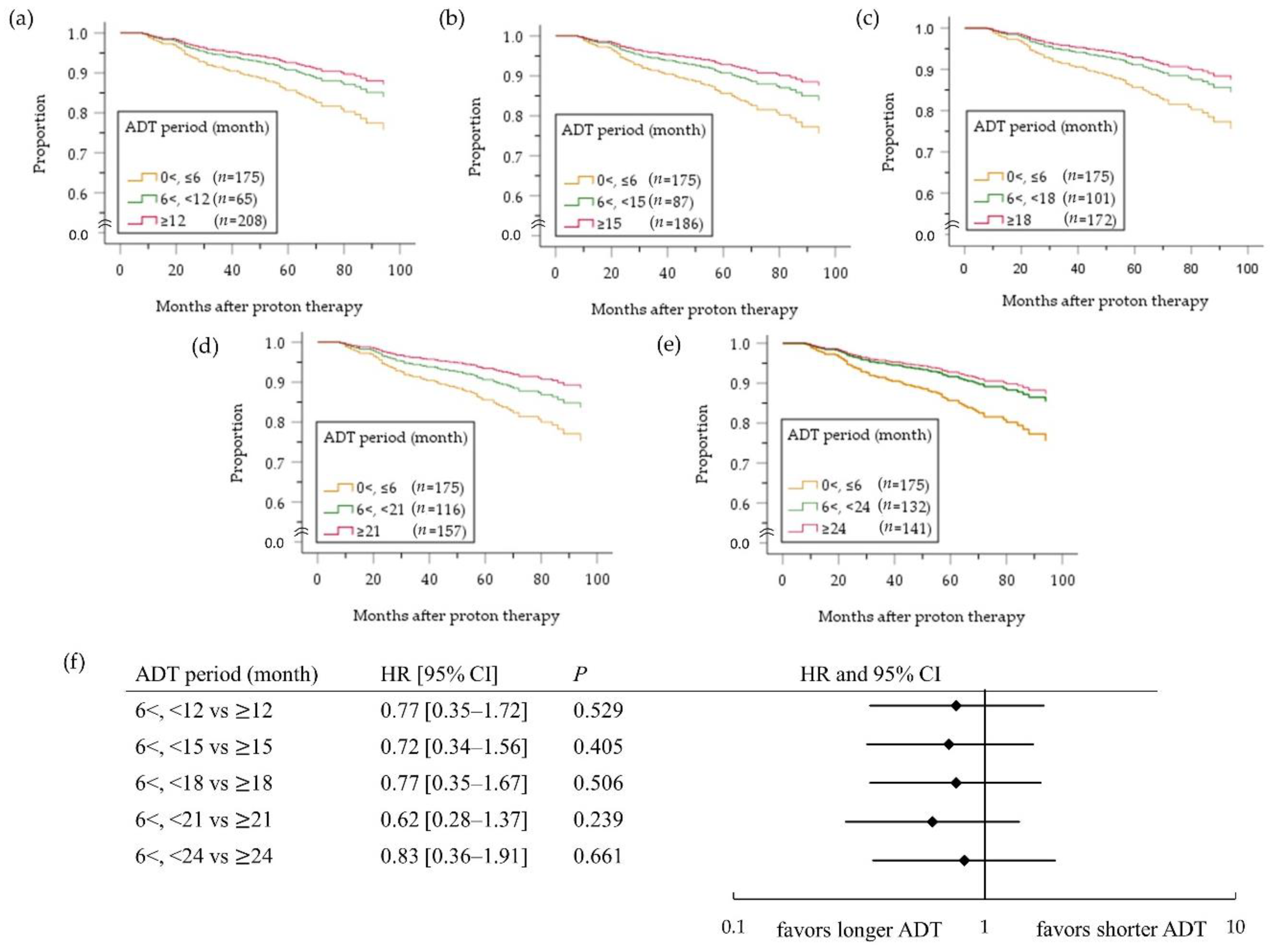
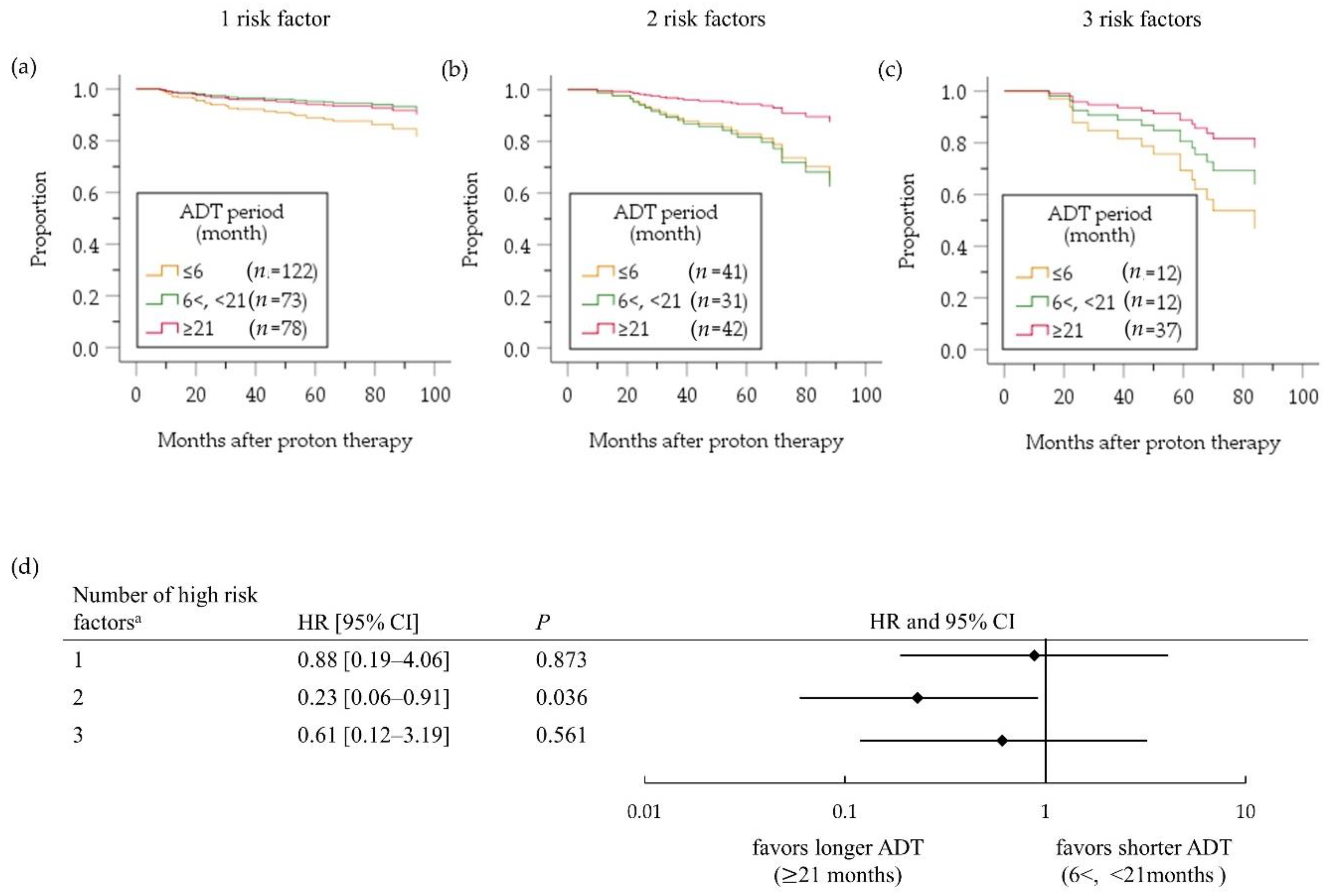
2.3. High-Risk PC Group
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cancer Information Service. Cancer Statistics in Japan. Available online: https://ganjoho.jp/reg_stat/statistics/dl/index.html (accessed on 31 December 2019).
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Lawton, C.A.F.; Lin, X.; Hanks, G.E.; Lepor, H.; Grignon, D.J.; Brereton, H.D.; Bedi, M.; Rosenthal, S.A.; Zeitzer, K.L.; Venkatesan, V.M.; et al. Duration of androgen deprivation in locally advanced prostate cancer: Long-term update of NRG oncology RTOG 9202. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; de Reijke, T.M.; Van Tienhoven, G.; Van den Bergh, A.C.; Oddens, J.; Poortmans, P.M.; Gez, E.; Kil, P.; Akdas, A.; Soete, G.; et al. Duration of androgen suppression in the treatment of prostate cancer. N. Engl. J. Med. 2009, 360, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Van Tienhoven, G.; Warde, P.; Dubois, J.B.; Mirimanoff, R.O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; Billiet, I.; et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010, 11, 1066–1073. [Google Scholar] [CrossRef]
- Roach, M.; Lu, J.; Pilepich, M.; Asbell, S.; Mohuidden, M.; Terry, R.; Grignon, D.; Lawton, C.; Shipley, W.; Cox, J. Predicting long-term survival, and the need for hormonal therapy: A meta-analysis of RTOG prostate cancer trials. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 617–627. [Google Scholar] [CrossRef]
- Ishikawa, H.; Tsuji, H.; Murayama, S.; Sugimoto, M.; Shinohara, N.; Maruyama, S.; Murakami, M.; Shirato, H.; Sakurai, H. Particle therapy for prostate cancer: The past, present and future. Int. J. Urol. 2019, 26, 971–979. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.F.; Freeman, J.L.; Goodwin, J.S. Risk of fracture after androgen deprivation for prostate cancer. N. Engl. J. Med. 2005, 352, 154–164. [Google Scholar] [CrossRef]
- Mitsuzuka, K.; Kyan, A.; Sato, T.; Orikasa, K.; Miyazato, M.; Aoki, H.; Kakoi, N.; Narita, S.; Koie, T.; Namima, T.; et al. Influence of 1 year of androgen deprivation therapy on lipid and glucose metabolism and fat accumulation in Japanese patients with prostate cancer. Prostate Cancer Prostatic Dis. 2016, 19, 57–62. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Duong-Hua, M.; Sutradhar, R.; Fleshner, N.E.; Warde, P.; Cheung, A.M.; Paszat, L.F. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J. Clin. Oncol. 2009, 27, 3452–3458. [Google Scholar] [CrossRef]
- Vargas, C.; Fryer, A.; Mahajan, C.; Indelicato, D.; Horne, D.; Chellini, A.; McKenzie, C.; Lawlor, P.; Henderson, R.; Li, Z.; et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, N.P.; Hoppe, B.S.; Nichols, R.C.; Mendenhall, W.M.; Morris, C.G.; Li, Z.; Su, Z.; Williams, C.R.; Costa, J.; Henderson, R.H. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Nihei, K.; Ogino, T.; Onozawa, M.; Murayama, S.; Fuji, H.; Murakami, M.; Hishikawa, Y. Multi-institutional phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Ishikawa, H.; Takagi, M.; Okimoto, T.; Murayama, S.; Akimoto, T.; Wada, H.; Arimura, T.; Sato, Y.; Araya, M.; et al. Long-term outcomes of proton therapy for prostate cancer in Japan: A multi-institutional survey of the Japanese Radiation Oncology Study Group. Cancer Med. 2018, 7, 677–689. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Spratt, D.E.; Pei, X.; Yamada, Y.; Kalikstein, A.; Kuk, D.; Zhang, Z.; Zelefsky, M.J. Short-term androgen-deprivation therapy improves prostate cancer-specific mortality in intermediate-risk prostate cancer patients undergoing dose-escalated external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1012–1017. [Google Scholar] [CrossRef]
- Pisansky, T.M.; Hunt, D.; Gomella, L.G.; Amin, M.B.; Balogh, A.G.; Chinn, D.M.; Seider, M.J.; Duclos, M.; Rosenthal, S.A.; Bauman, G.S.; et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: Radiation therapy oncology group randomized clinical trial 9910. J. Clin. Oncol. 2015, 33, 332–339. [Google Scholar] [CrossRef]
- Dubray, B.; Salleron, J.; Guerif, S.; Le Prise, E.; Reynaud-Bougnoux, A.; Hannoun-Levi, J.; Nguyen, T.; Hennequin, C.; Cretin, J.; Fayolle-Campana, M.; et al. Does short-term androgen depletion add to high dose radiotherapy (80 Gy) in localized intermediate risk prostate cancer? Final analysis of GETUG 14 randomized trial (EU-20503/NCT00104741). J. Clin. Oncol. 2016, 34, 5021. [Google Scholar] [CrossRef]
- Langenhuijsen, J.F.; van Lin, E.N.; Hoffmann, A.L.; Spitters-Post, I.; Alfred Witjes, J.; Kaanders, J.H.; Mulders, P.F. Neoadjuvant androgen deprivation for prostate volume reduction: The optimal duration in prostate cancer radiotherapy. Urol. Oncol. 2011, 29, 52–57. [Google Scholar] [CrossRef]
- Kawamura, H.; Kubo, N.; Sato, H.; Mizukami, T.; Katoh, H.; Ishikawa, H.; Ohno, T.; Matsui, H.; Ito, K.; Suzuki, K.; et al. Moderately hypofractionated carbon ion radiotherapy for prostate cancer; a prospective observational study “GUNMA0702”. BMC Cancer 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Serrano, N.A.; Anscher, M.S. Favorable vs unfavorable intermediate-risk prostate cancer: A review of the new classification system and its impact on treatment recommendations. Oncology 2016, 30, 229–236. [Google Scholar]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; Gonzalez San Segundo, C.; Cabeza Rodriguez, M.A.; Macias, V.; Pedro Olive, A.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 320–327. [Google Scholar] [CrossRef]
- Nabid, A.; Carrier, N.; Martin, A.G.; Bahary, J.P.; Lemaire, C.; Vass, S.; Bahoric, B.; Archambault, R.; Vincent, F.; Bettahar, R.; et al. Duration of androgen deprivation therapy in high-risk prostate cancer: A randomized phase III trial. Eur. Urol. 2018, 74, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, G.; Ishikawa, H.; Tsuji, H.; Nomiya, T.; Makishima, H.; Kamada, T.; Akakura, K.; Suzuki, H.; Shimazaki, J.; Haruyama, Y.; et al. Significant impact of biochemical recurrence on overall mortality in patients with high-risk prostate cancer after carbon-ion radiotherapy combined with androgen deprivation therapy. Cancer 2016, 122, 3225–3231. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, M.C.; Li, T.; Buyyounouski, M.K.; Ross, E.; Uzzo, R.G.; Pollack, A.; Horwitz, E.M. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer 2008, 112, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.; Lemeshow, S.; May, S. Regression Models for Survival Data. In Applied Survival Analysis: Regression Modeling of Time-to-Event Data, 2nd ed.; Balding, D., Cressie, N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 67–91. [Google Scholar]


| Variable | Level | Intermediate-Risk PC Group | High-Risk PC Group |
|---|---|---|---|
| Number | n | 520 | 555 |
| Age | Mean ± SD | 67 ± 7 | 69 ± 7 |
| T stage | 1c–2a | 378 (72.7%) | 233 (42.2%) |
| 2b–2c | 142 (27.3%) | 123 (22.6%) | |
| 3a–4 | 0 | 195 (35.2%) | |
| PSA level (ng/mL) | <10 | 319 (61.6%) | 228 (41.1%) |
| 10–20 | 199 (38.4%) | 112 (20.2%) | |
| >20 | 0 | 215 (38.7%) | |
| Gleason score | 5–6 | 112 (21.5%) | 27 (4.9%) |
| 7 | 408 (78.5%) | 119 (21.6%) | |
| 8–10 | 0 | 406 (73.5%) | |
| Number of intermediate-risk factors a | 1 | 318 (61.2%) | |
| 2 | 170 (32.8%) | ||
| 3 | 31 (6.0%) | ||
| Number of | 1 | 360 (64.9%) | |
| high-risk factors b | 2 | 129 (23.2%) | |
| 3 | 66 (11.9%) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, M.; Ishikawa, H.; Shimizu, S.; Iwata, H.; Okimoto, T.; Takagi, M.; Murayama, S.; Akimoto, T.; Wada, H.; Arimura, T.; et al. Optimal Androgen Deprivation Therapy Combined with Proton Beam Therapy for Prostate Cancer: Results from a Multi-Institutional Study of the Japanese Radiation Oncology Study Group. Cancers 2020, 12, 1690. https://doi.org/10.3390/cancers12061690
Murakami M, Ishikawa H, Shimizu S, Iwata H, Okimoto T, Takagi M, Murayama S, Akimoto T, Wada H, Arimura T, et al. Optimal Androgen Deprivation Therapy Combined with Proton Beam Therapy for Prostate Cancer: Results from a Multi-Institutional Study of the Japanese Radiation Oncology Study Group. Cancers. 2020; 12(6):1690. https://doi.org/10.3390/cancers12061690
Chicago/Turabian StyleMurakami, Motohiro, Hitoshi Ishikawa, Shosei Shimizu, Hiromitsu Iwata, Tomoaki Okimoto, Masaru Takagi, Shigeyuki Murayama, Tetsuo Akimoto, Hitoshi Wada, Takeshi Arimura, and et al. 2020. "Optimal Androgen Deprivation Therapy Combined with Proton Beam Therapy for Prostate Cancer: Results from a Multi-Institutional Study of the Japanese Radiation Oncology Study Group" Cancers 12, no. 6: 1690. https://doi.org/10.3390/cancers12061690
APA StyleMurakami, M., Ishikawa, H., Shimizu, S., Iwata, H., Okimoto, T., Takagi, M., Murayama, S., Akimoto, T., Wada, H., Arimura, T., Sato, Y., Gosho, M., Nakamura, K., & Sakurai, H. (2020). Optimal Androgen Deprivation Therapy Combined with Proton Beam Therapy for Prostate Cancer: Results from a Multi-Institutional Study of the Japanese Radiation Oncology Study Group. Cancers, 12(6), 1690. https://doi.org/10.3390/cancers12061690






