Abstract
Epithelial–mesenchymal transitions (EMTs) generate hybrid phenotypes with an enhanced ability to adapt to diverse microenvironments encountered during the metastatic spread. Accordingly, EMTs play a crucial role in the biology of circulating tumor cells (CTCs) and contribute to their heterogeneity. Here, we review major EMT-driven properties that may help hybrid Epithelial/Mesenchymal CTCs to survive in the bloodstream and accomplish early phases of metastatic colonization. We then discuss how interrogating EMT in CTCs as a companion biomarker could help refine cancer patient management, further supporting the relevance of CTCs in personalized medicine.
1. General Background
Circulating tumor cells (CTCs) contain the physical entities that cause metastases and therefore hold a special place in the era of liquid biopsies [1,2,3,4]. Although tumor biopsy is still the gold standard for cancer diagnosis of solid tumors, it is an invasive act both at the primary and metastatic sites, and it represents a snapshot during the progression of the disease. Analyzing CTCs through successive liquid biopsies may thus provide important additional clinical information.
The first observation of CTCs actually dates back to 1869, when Thomas Ashworth reported the presence of cells “with similar characteristics than those of the primary tumor” in the blood of a cancer patient [5]. Enumeration and characterization of CTCs may improve precision oncology through predicting metastases, monitoring recurrence, guiding treatment decisions and patient stratification, and assessing therapeutic efficacy [6,7].
Progressively understanding that CTCs represent a very heterogeneous population has urged researchers to examine epithelial–mesenchymal transitions (EMTs) and to characterize metastatic founders within the CTC population.
Nevertheless, although the clinical validity of analyzing CTCs as prognostic and predictive biomarkers is currently supported by many studies, they have still not been examined in clinical practice [8]. The technical challenge behind the isolation of these extremely rare cells may contribute to hampering their exploitation in the clinic [9,10,11,12].
2. CTC Enrichment, Identification, and Isolation Techniques
CTC enrichment/detection/isolation methods have been reviewed elsewhere [9,10,11,12]. We here recapitulate the general principles behind these techniques (Figure 1). Very schematically, one may distinguish enrichment systems based on biological characteristics of CTCs and those based on their physical properties. Methods combining both approaches are also frequently used.
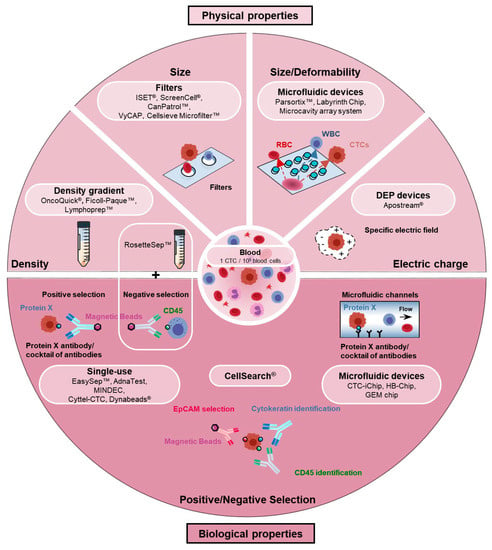
Figure 1.
Circulating tumor cell (CTC) enrichment techniques. Current devices/methods used to enrich and isolate CTCs exploit biological or biophysical properties to differentiate CTCs from blood cells. CTC enrichment methods based on biological properties take advantage of biological markers differentially expressed in CTCs and blood cells. Positive selection of CTCs and/or depletion (negative selection) of blood cells may thus be achieved using a specific antibody (such as EpCAM) or cocktails of antibodies. Immunomagnetic separation is used in many systems and kits (CellSearch®, EpCAM PlusCellectTM Kit, EasySepTM human EpCAM positive kit, EpCAM positive CELLectionTM beads or AdnaTest) but an abundance of microfluidic devices (CTC-Chip, CTC-iChip, HB-Chip or GEM Chip) has also been developed. CTC enrichment methods based on physical characteristics use the following criteria to separate tumor cells from blood cells: Size (filter-based methods: ISET®, ScreenCell®, VyCap, CanPatrolTM), deformability/size (microfluidic devices: ParsortixTM, Labyrinth chip, microcavity array system), density (ficoll-type density gradients: OncoQuick®, Ficoll-PaqueTM, LymphoprepTM or RosetteSepTM that combines an immune-depletion of white blood cells), and electric charge (Apostream®).
Enrichment techniques based on CTC biological properties assume that CTCs express or do not express specific markers that can be used to separate them from normal cells. This is achieved either by positively selecting cells expressing a specific marker or a combination of markers, or/and depleting populations of blood cells (negative selection). The CellSearch® is the only system that has been approved by FDA for CTC enumeration in metastatic breast, prostate, and colorectal cancer patients [13,14,15,16]. Based on an EpCAM immunomagnetic enrichment and a keratin+/CD45− identification, it is still considered a gold standard in CTC research. Aside the CellSearch®, other EpCAM-based immunomagnetic enrichment kits are also commonly used. It was nevertheless rapidly appraised that EpCAM is not a universal CTC marker, and that EpCAM-negative CTC populations may encompass metastatic precursors that will not be detected by such methods, particularly those derived from EMTs [17,18]. Several studies have indeed reported that EMTs decrease EpCAM levels in many, although not all, examined cellular backgrounds [17]. Systems using cocktails of antibodies have thus been developed to enrich more CTC populations. Conversely, negative selection approaches have also been developed. If many of these systems use immunomagnetic sorting, microfluidic-based enrichment technologies have also emerged, in which different supports coated with specific antibodies are precisely disposed in the flow so as to favor cell–antibody interactions [19,20,21,22,23,24,25,26,27,28,29,30]. Interestingly, aptamers are gaining major interest as an alternative to antibodies in positive selection-based CTC enrichment, and have for instance been exploited in magnetic bead separation assays or in microfluidic devices [31,32]. Aptamers are short DNA/RNA molecules with unique tertiary structures that bind specific targets, including proteins, with high specificity and affinity, and that may additionally be easily removed from their targets. Aptamers against EpCAM, EGFR, or MUC1 have for example been successfully generated.
As mentioned above, a general drawback of these techniques based on biological characteristics is their inability to enrich CTC subsets that do not express the examined biological markers. To circumvent this problem and improve the capture of EMT+ CTCs, and to enable the isolation of label-free CTCs that may facilitate downstream applications, an abundance of enrichment devices using biophysical parameters have been engineered. Size, density, deformability, and electric charge are most commonly at the basis of these assays. Thus density-based approaches often involve a centrifuged Ficoll-type density gradient [33,34,35,36,37]. Several filter-based assays exploit the knowledge that most hematopoietic cells are smaller (<10 µm) than CTCs (>10 µm) [38,39,40,41,42,43,44,45]. A profusion of recently conceived microfluidic devices also uses the size and/or the deformability parameters [46,47,48,49,50,51,52,53,54,55,56,57,58]. Finally, CTC isolation devices, many also being microfluidic-based [59,60,61,62,63], use dielectrophoresis (DEP) to differentiate tumor cells from normal cells by their electric charges [64].
The prominent place progressively taken by microfluidics in the CTC field within the last decade is worth highlighting [19,20,21,22,23]. Microfluidics has indeed emerged as an innovative approach to directly process whole blood, to use small amounts of reagents, and to reduce cost. By controlling the flow rate and the design of the chip in a coordinated manner, the capture efficiency and purity may eventually be improved. Microfluidic chips may also be optimized to particularly favor the isolation of viable label-free CTCs and to facilitate various specific downstream applications. An impressive number of microfluidic devices have been/are being designed to dispose obstacles (channels, pillars, labyrinths, spirals, weirs, layers, filters, ratchets) in flow chambers so as to efficiently separate CTCs from normal cells contained in the blood [28,50,56,57,58,65,66,67].
Thus far, spiking experiments, the gold standard for evaluating the efficiency of CTC isolation devices, have revealed important differences in capture efficiency among all these biological or biophysical property-based devices. If these discrepancies are certainly in part inherent to the technology used in each device, it also appears that the phenotype and the specific characteristics of tumor cells influence the recovery rate. CTC enrichment systems are thus still being optimized to increase isolation efficiency and purity but also to broaden the capture to different CTC phenotypes.
The engineering of a single CTC isolation device that addresses all the technical challenges currently seems unlikely. It may be more realistic to select an appropriate CTC isolation device with respect to the downstream application envisaged and the clinical question asked.
3. Epithelial–Mesenchymal Transitions: Impact on CTC Phenotypic Heterogeneity
EMTs have long been known as crucial actors in metastasis. The examination of EMT actors in CTCs has thus logically gained rapidly growing interest in the past decade [68,69,70,71,72,73,74].
The generally accepted view [75,76,77,78,79,80,81] is that EMTs generate various hybrid phenotypes along the epithelial (E) to mesenchymal (M) differentiation axis, thereby contributing importantly to tumor heterogeneity. If most epithelial and mesenchymal states are believed to harbor limited metastatic potential, certain E/M hybrid phenotypes are considered to harbor high degree of epithelial–mesenchymal plasticity (EMP), enabling them to undergo timely and spatially regulated dynamic and reversible interconversions within a “plasticity window”. These phenotypical adaptations are crucial for tumor cells to survive/develop in the different microenvironments encountered during the metastatic spread. After an eventual period of dormancy, a switch towards more epithelial proliferative states (mesenchymal–epithelial transitions, METs) is further suspected to occur during metastatic outgrowth. EMTs would therefore rather be involved in the initial steps of the metastatic spread: entry in the circulation, survival in the bloodstream, arrest on the vasculature, and early phases of metastatic niching [68,75,76,77,78,79,80,81,82,83]. Whether the same hybrid tumor cell is able to overcome all obstacles of the metastatic cascade through phenotypic adaptations or whether further genetic alterations occur during the metastatic cascade that empower some tumor cells to form metastases is still a subject of debate. Cooperative processes between different phenotypes may also occur, by which EMT-shifted cells would help more epithelial phenotypes (with higher competence for metastatic outgrowth) to gain and survive in the circulation, and niche in secondary organs [84,85].
Adding to the heterogeneity generated by this phenotypic plasticity occurring throughout the metastatic spread, EMTs are molecularly complex, diverse, and context-dependent.
Several EMT-associated genes have nevertheless been commonly examined in CTC studies. Among EMT target genes frequently examined in CTCs is certainly vimentin. This mesenchymal type III intermediate filament is considered a canonical marker of EMT and has been extensively examined both in tumors and CTCs over the years. More than being a marker, vimentin has also been functionally implicated in pro-metastatic functions including tumor cell migration or CTC survival [68]. In addition, EMTs are known to modulate the expression of several epithelial adhesion molecules, consequently altering cell–cell interactions. A cadherin switch characterized by a decrease of E-cadherin expression and an enhanced expression of N-cadherin has accordingly been associated with EMTs [86], and both molecules are frequently analyzed in CTCs. The adhesion molecule EpCAM, which, as discussed underneath, has been used in pioneer studies to enrich CTCs, has also been identified as an EMT target gene examined in many CTC studies [17]. EMT core transcription factors which finely regulate EMT target genes [87,88,89] are also commonly assessed in CTCs, particularly those of the ZEB (ZEB1 and ZEB2) and Snail (Snail and Slug) families, and Twist. Several membrane receptor and Receptor Tyrosine Kinase (RTK) signaling pathways, transmitting signals from the microenvironment, are key regulators of EMTs [90] and have been analyzed in many CTC studies. These include TGF-β, EGFR, c-Met, Notch, and Wnt pathways. The availability of targeted drugs against specific RTKs or their signaling molecules has also stimulated this axis of investigations. A growing interest in the EMT–associated RTK Axl, for which commercial inhibitors are being assessed in clinical trials [91,92,93], may be underlined. Although herein we will not debate the exact nature of the relationships linking EMT and Cancer-Stem Cells (CSCs) [94,95], it is important to note that EMT induces the expression of stem cell attributes [96,97] and that certain EMT and CSC markers are often coexpressed in tumor cells. Stem cell markers including CD44 (considered a marker of both EMT and CSC [98,99]), ALDH1 or CD133 (promin 1) are thus frequently appraised in CTCs, often in association with canonical EMT markers. As we detail underneath, many of these EMT-associated molecules analyzed in CTCs functionally impact CTC survival and metastatic potential.
As shown in Table 1 (for studies published before 2016, please refer to Table 1 in [68]), a profusion of studies has revealed EMT-associated heterogeneity in the CTC population, and the presence of CTCs encompassing hybrid E/M phenotypes in most types of epithelial cancers.

Table 1.
Detection of epithelial–mesenchymal transition (EMT) and stem-cell markers in CTCs from cancer patients.
4. Epithelial–Mesenchymal Transitions: Impact on Metastatic Competence
EMTs thus provide tumor cells with numerous dynamic/reversible properties that help them overcome environmental selective constraints of the metastatic translocation. Here, we will detail crucial mechanisms by which EMTs may impact the fate and metastatic competence of CTCs (Figure 2).
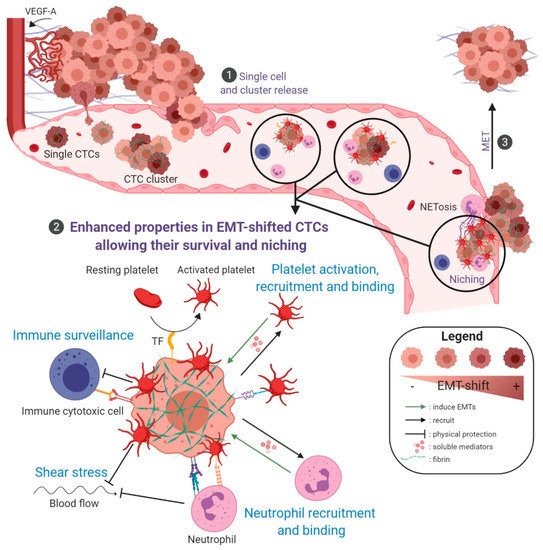
Figure 2.
Schematic representation of EMT-associated mechanisms supporting CTC survival and early metastasis. ❶ CTCs are liberated in the bloodstream through EMT-associated mechanisms (single cell or collective migration/intravasation) or passive processes (detachment of isolated tumor cells or clumps through corrupted vessels). ❷ Some single or clustered CTCs will eventually survive in the bloodstream and niche in secondary organs. A zoom on properties, enhanced in EMT-shifted cells, that favor CTC survival in the bloodstream and metastatic niching is depicted. Platelet activation and binding, activation of coagulation: CTCs activate and bind platelets either directly or through molecular intermediates such as fibrin. The liberation of soluble mediators, such as TGF-β from activated platelets, may in return induce/enhance/sustain EMT. In addition, EMT-shifted CTCs express Tissue Factor (TF) that also largely contributes to activate platelets. These coagulation-dependent mechanisms initiate the formation of a fibrin/platelet-rich cocoon around tumor cells that is considered to protect CTCs against shear stress, anoikis, immune attack, and which is determinant for CTC seeding and early niching. Neutrophil recruitment and binding, NETosis: tumor cells secrete soluble factors attracting neutrophils, among other immune cells, and neutrophils in turn secrete EMT-promoting soluble mediators. In addition, neutrophils physically interact with tumor cells and platelets, promoting tumor cell survival and proliferation, and helping CTC arrest on the vascular wall. Furthermore, through their ability to entrap tumor cells in Neutrophil Extracellular Traps, structures also known to favor coagulation events, neutrophils participate to the formation of a protective/anchoring scaffold that supports CTC survival, and facilitate CTC arrest in capillaries and early phases of metastatic niche formation. Immune surveillance: these coagulation/neutrophils-dependent mechanisms of shielding protect CTCs from immune destruction. In addition, CTCs, and more particularly EMT-shifted CTCs, have been reported to harbor increased ability to evade immune surveillance. Among mechanisms involved, the expression of immune checkpoint proteins, such as PD-L1, is likely to enhance their resistance to cytotoxic immune cells. ❸ After an eventual period of dormancy, it is considered that MET processes intervene, favoring metastatic outgrowth. Figure created with BioRender.com.
4.1. EMT and CTC Release
One of the longest-known properties driven by EMTs is certainly invasiveness. Enhanced invasive ability may be acquired within the primary tumors and contribute to invasion and intravasation, thereby facilitating the release of CTCs in the bloodstream [135,136,137]. EMT-induced proteolytic enzymes, such as matrix metalloproteinases (MMPs), are key players in tumor invasion [138,139], remodeling the extracellular matrix, activating growth factors and weakening intercellular contacts. Although they did not examine EMT markers, Dhar and coworkers supportively reported high MMP activity in CTCs isolated from prostate cancer patients [140]. The ability of EMT to stimulate angiogenesis is also certainly a promoting factor of CTC release. Thus, the expression of the potent angiogenic factor VEGF-A, known as an EMT target gene, has been reported in CTCs of breast cancer patients [141].
Nonetheless, despite the contribution of EMT to CTC release, it is clear today that not all CTCs express canonical EMT markers. Whether EMT characteristics of all EMT+ CTC subsets are acquired in the primary tumor or whether some are gained within the circulation are two possibilities that are not mutually exclusive, which may also account for the CTC heterogeneity [68]. Environmental factors present in the bloodstream may indeed also induce/sustain EMT, as detailed in following paragraphs [111,142]. Supportively, single-cell characterization of CTCs taken from different vascular sites in hepatocellular carcinoma (HCC) patients suggests that an EMT-activated phenotype is gained during hematogenous transit [111]. Additionally, a passive mode of entry of tumor cells in the circulation has been suggested and is well in line with the observation of corrupted blood vessels in most tumors [143,144,145]. Such a passive mode of entry may concern single tumor cells but may also liberate clumps/clusters of CTCs in the blood of cancer patients [146,147]. The presence of CTC clumps with metastatic abilities was in fact already recognized in the early 1950s by Watanabe and later by several pioneers in metastasis research [148,149,150]. The mechanisms of cluster formation and their importance in metastasis formation have since then been analyzed more in details using xenografts and experimental metastasis assays performed using a mixed population of tumor cells labeled with different fluorescent reporters [151,152,153]. As an alternative to the passive liberation of clusters in the bloodstream, active collective migration processes of intravasation have also been reported [146,147,152]. Alternatively, an intravascular aggregation of CTCs, particularly at the vicinity of the endothelium, has been identified [153,154], a process that may also involve various hybrid states [155,156].
Overall, whether they are passive or active, EMT-dependent or EMT-independent, these different mechanisms liberate various CTC phenotypes in the bloodstream, and thereby importantly contribute to the heterogeneity of the CTC population.
4.2. EMT in CTC Survival and Metastatic Seeding
Once in the circulation, CTCs are subjected to a harsh selective pressure imposed by shear stress, loss of anchorage triggering anoikis, and immune attack [68,157,158]. Studies in mice have accordingly shown that CTCs circulate in the bloodstream for a very short time and that the vast majority of CTCs are rapidly eliminated [159]. Several mechanisms will nevertheless eventually be deployed, enabling few CTCs to survive in the bloodstream and form a niche at secondary sites (Figure 2). Among these mechanisms, those regulated by EMTs are numerous as detailed below.
4.2.1. Activation of Survival Pathways
Some CTCs may activate autonomous survival mechanisms. In this context, an abundant literature shows that EMTs activate many survival pathways (e.g., activation of Akt, PI3K or EGFR pathways, induction of Bcl-2 antagonizing p53 activity), enabling EMT-shifted cells to better resist apoptosis or anoikis [160,161]. This enhanced survival ability of EMT-shifted cells certainly plays crucial role in their now-well recognized resistance to chemo- or targeted therapies [81,162,163,164,165,166]. Supportively, molecular actors of survival pathways (e.g., EGFR, Akt, PI3K) have been detected in CTCs isolated from several types of cancer, sometimes in association with canonical EMT markers such as EMT transcription factors or stem cell markers [101,104,110,167,168,169,170,171,172,173,174] (Table 1).
4.2.2. Traveling in Clusters
Regardless of the mechanisms implicated in the generation of clusters discussed above, traveling as clusters is currently considered to enhance CTC survival and niching at secondary sites. Although much less prevalent than isolated CTCs, experimental data in mouse models show that CTC clusters have higher metastatic potential [146,147,175]. Clusters of CTCs have been detected in a variety of epithelial cancers and, in some studies, their abundance has been associated with poor prognosis as shown in breast, lung, pancreatic, prostate, or kidney cancers [29,109,151,176,177,178,179,180,181,182,183,184]. If their bigger size certainly facilitates their arrest within capillaries, it appears that traveling as clusters may help CTCs survive in the bloodstream [185,186].
If the prevalence of EMT phenotypes in clusters is so far largely uncovered, it is nevertheless recognized that CTC clusters contain hybrid E/M phenotypes. Thus, single-cell RNA sequencing of clustered CTCs versus isolated CTCs from breast cancer patients has revealed that several cell–cell contact molecules are overexpressed/maintained in clusters, such as plakoglobin, which has been further shown to be functionally involved in cluster metastatic potential [151]. Complicating the theory and blurring the border between E and M states even further, EMT/CSC markers may also be implicated in cell–cell interactions in clusters. Accordingly, CD44, which has been consistently reported on CTCs in most types of cancer [50,100,187,188], was recently shown to contribute to intravascular aggregation of tumor cells through the formation of homophilic intercellular interactions [153]. CD44+ cell aggregates were also found to be more resistant to apoptosis than single cells. Other recognized markers of mesenchymal or CSC phenotypes have also been detected in CTC clusters in vitro or in mouse models such as Tenascin C or Jagged1 [152,189]. A recent study of CTCs isolated from breast cancer patients and mouse models, reported that the binding sites for CSC transcription factors such as OCT4, SOX2, or NANOG, are hypomethylated in CTC clusters compared with isolated CTCs [49]. Other data collected from human samples also emphasize the presence of hybrid E/M phenotypes in heterogeneous CTC clusters, particularly in lung cancers [51,52,109,190]. Very elegantly, a study by Yu and coworkers identified cells expressing both epithelial (such as EpCAM or cytokeratins) and mesenchymal markers (including fibronectin, N-cadherin or PAI-1) in isolated CTCs but also in CTC clusters from breast cancer patients [179].
Therefore, the presence of EMT-hybrid CTCs in clusters may combine the ability to establish cell–cell interactions, contributing to a better resistance to anoikis/apoptosis, with known pro-metastatic EMT-driven properties (stemness, activation of survival pathways, resistance to apoptosis, enhanced niching properties). This phenomenon may thus contribute to an overall better survival and higher seeding efficiency of clustered tumor cells [191].
Though clusters may be homotypic, they are often heterotypic, containing normal host cells and components, either from the primary tumors [192] or incorporated during their metastatic translocation, as we discuss below.
4.2.3. Interactions with Host Cells and Host Systems
During their metastatic translocation, CTCs continuously and reciprocally interact with host components and cells in all microenvironments encountered. Many of these interactions facilitate both CTC intravascular survival and metastatic niching.
Activation of Coagulation
The activation of the coagulation system is today recognized as a crucial process facilitating early metastasis. Hypercoagulability is actually a long-known correlate of malignancy (Trousseau’s syndrome), and venous thromboembolism (VTE) has been associated with worse prognosis [193,194,195]. Accordingly, the CTC count has been associated with hypercoagulability, increased risk of venous thrombosis and dismal prognosis [196,197,198,199,200]. Abundant experimental studies have supportively demonstrated the beneficial effects of anticoagulant strategies in inhibiting metastasis [201]. Many clinical trials examining the impact of the new generation of anticoagulant strategies in cancer are ongoing [202].
If the mechanisms linking cancer and the coagulation system are numerous and multifactorial, platelets are certainly central players [203,204,205,206,207]. Abundant literature indeed demonstrates that tumor cells bind to and activate platelets [203]. Tumor cell/platelet interactions have been shown to engage several receptors including P-selectin or αIIbβ3 on platelets and ανβ3 integrin, PSGL-1 or CD97 on tumor cells [208,209,210,211,212]. Fibrin, the end product of the coagulation cascade, may also bridge tumor cells to platelets. A mechanism by which fibrin connects ICAM-1 on tumor cells to integrin αIIbβ3 on platelets has been particularly highlighted [213]. Interestingly, CD44 has also been described as a receptor that binds P-selectin on platelets, as well as a fibrin receptor [214]. Platelet activation also liberates abundant soluble mediators that may favor the recruitment/activation of host cells such as neutrophils, and may also impact the tumor cell phenotype. All these interactions may thus favor the formation of fibrin/platelets aggregates trapping CTCs and host cells that have also been referred to as circulating tumor microemboli (CTM) [203,204,205,206,207].
The formation of a fibrin/platelets network around tumor cells is considered to favor CTC survival in the bloodstream [203,204,205,206]. It is recognized as an important mechanism shielding and protecting CTCs against shear stress [215] and natural killer (NK) elimination [216,217]. In addition to their contribution to a physical shielding, platelets have also been shown to decrease NK cell antitumor activity through a TGF-β-mediated decrease in NKG2D [218]. A platelet coat around CTCs may also expose platelet MHC-class I molecules and thus “hide” CTCs from NK cells [219]. Biggerstaff and coworkers have precisely shown that soluble fibrin augments platelet/tumor cell interactions in vitro and in vivo [213], hinders cellular cytotoxicity against tumor cells and increases experimental metastasis [220]. In addition, platelet/fibrin-dependent mechanisms also facilitate tumor cell arrest on the vascular wall and their niching at secondary sites [221,222]. If tumor cells possess receptors (CD44, P-selectin, integrins,...) enabling their adherence to the endothelium [223], fibrin or platelets may indeed also mediate and strengthen such interactions [224,225].
A mechanism by which tumor cells may induce coagulation and platelet activation is through their ability to express factors of the coagulation cascade, among which tissue factor (TF) holds a particular place, linking the processes described above to EMT. If TF displays coagulation-independent pro-tumoral signaling functions, it is essentially known as the major cell-associated activator of the coagulation cascade. Its expression by tumor cells, triggering coagulant properties, has been demonstrated to be determinant for CTC survival and seeding [194,195,206,226]. This has been exemplified in numerous animal studies using tumor cells modified for TF expression or TF blocking antibodies, demonstrating that tumor-cell expressed TF is associated with increased abilities to form micrometastases [227,228,229,230]. Further connecting TF-coagulant functions to metastasis, Palumbo and coworkers performed metastasis assays in mice deficient for fibrinogen and prothrombin, and they demonstrated that TF could support metastasis through mechanisms dependent on these distal hemostatic factors [231,232]. Importantly, few studies, including ours, have identified TF as a target gene of EMTs [233,234]. Interestingly, a relationship between TF expression and CSC phenotypes has also been reported [235,236]. The EMT core transcription factors ZEB1 and Snail were delineated to regulate TF expression [234], and recently, vimentin was reported to stabilize TF mRNA [237]. Interestingly, TGF-β, liberated from activated platelets, has been described to trigger EMTs [142], suggesting a regulatory loop between EMT and platelet activation. In addition to these experimental data, the TF/EMT relationship was also evidenced in cancer patients with a correlated expression of vimentin and TF in triple-negative breast cancers (TNBC). A subpopulation of CTC expressing TF and vimentin has also been observed in the blood of metastatic breast cancer patients [234]. Through their ability to express TF, EMT-shifted CTCs would thus be more efficient in activating coagulation and building a protective cocoon.
Interaction with Neutrophils
CTCs have been shown to communicate with various cells of the immune system, and interactions with neutrophils seem to be essential. In agreement with data reporting that CTCs are surrounded by white blood cells in Pancreatic Ductal AdenoCarcinoma (PDAC) [238], Szczerba and coworkers recently described that CTCs were found in clusters with neutrophils in invasive breast cancer patients [239]. The abundance of these CTC-neutrophil clusters was further associated with shorter PFS. Corroborating this finding, a higher metastatic potential of these CTC-neutrophil clusters has been demonstrated in experimental metastasis assays.
The mechanisms by which neutrophils may support CTC metastasis are various. Neutrophils have been proposed to escort CTCs and enable cell cycle progression [239]. Along with their ability to secrete soluble factors attracting neutrophils (such as G-CSF, CXCL1, CXCL8, or CXCL5) [240], CTCs indeed directly interact with neutrophils through different receptors including VCAM1 [239], ICAM-1 [241], and β1 integrin [242].
Neutrophils may also interact with CTCs through the coagulation system. Thus, platelets that, as discussed above are inseparable travel companions of CTCs, may bridge neutrophils to tumor cells. The release of soluble mediators (such as CXCL5 and CXCL7) by activated platelets accordingly contributes to neutrophil recruitment [221]. In addition to platelets, fibrin also mediates tumor cell/neutrophil interactions. A sequential binding of αvβ3 and ICAM-1 has been shown to determine fibrin-mediated melanoma adhesion of CD11b/CD18 (Mac-1) to neutrophils [243].
A role for neutrophils in facilitating CTC arrest on the vascular wall has also been evidenced [244,245]. Using an elegant in vitro model consisting of a microfluidic chip covered by HUVEC endothelial cells to mimic the vascular compartment, Chen et al. revealed interactions implicating CD11b on neutrophils and ICAM-1 on cancer and endothelial cells, which favored the formation and arrest on the endothelium wall of tumor cell/neutrophil complexes [244].
Neutrophils may also facilitate CTC survival and niching competencies given their ability to form neutrophil extracellular traps (NETs). These web structures have been identified to capture CTCs, to contribute to a physical protection against shear stress and to facilitate early phases of metastasis (arrest and adhesion on the vascular wall, extravasation and niching) in different mouse cancer models [246,247,248,249,250,251,252]. Conversely, tumor cells may activate neutrophils, thereby stimulating NETosis as shown in vitro and in mouse tumor models [248,253]. Another mechanism by which NETs favor CTC survival and colonization is through their ability to activate coagulation and thrombosis [254,255,256,257,258]. NETs have indeed been shown to activate platelets. In turn, activated platelets promote NETosis [259] and, through the liberation of soluble mediators, further recruit neutrophils to the site. Linking CTCs, neutrophils, platelets and coagulation, NETs may thus contribute to the creation of a protective/anchoring scaffold, helping CTCs to survive in the circulation, arrest in capillaries and niche in secondary sites. Supporting these experimental data, NETs have been associated with a poor prognosis in several cancer types and with cancer-associated VTE [260,261]. Recapitulating these relationships in renal cell carcinoma patients, Wen et al. reported that a high CTC count correlates with elevated levels of fibrinogen and RNA expression of NET’s markers in blood leukocytes [262].
Overall, narrow relationships may thus be established between CTCs, neutrophils, and the coagulation system, with many activation/induction loops existing between the different molecular and cellular actors involved [263]. Although this remains largely uncovered, numerous studies support the idea that EMTs could be central players in such loops and, at least partly, influence or be influenced by most of the above-discussed mechanisms.
Adding to their capacity to induce TF expression, EMTs are indeed known to induce several receptors mediating interactions between neutrophils and CTCs, or between CTCs and platelets/fibrin including CD44 [96,97,99], ICAM-1 [264], αvβ3 [265,266,267], or VCAM1 [268].
Additionally, EMTs importantly modulate the secretome of tumor cells notably by inducing the expression of soluble factors that may act as chemoattractants, or activate the pro-tumoral activities of many inflammatory cells including neutrophils [269,270,271]. Reciprocally, neutrophils have also been shown to induce EMT in several cellular systems [269,270], mostly through the release of soluble factors including CXCL-1 [272], IL-17 [273] or neutrophil elastase [274].
Together, these data suggest that EMT-shifted CTCs would be particularly efficient in deploying coagulation-dependent and stimulating neutrophil-mediated strategies that favor survival, resistance to shear stress and initiation of the metastatic niche.
Immune Escape
A crucial property favoring the metastatic potential of CTCs is the ability to escape cytotoxic immune cells. As discussed above, coagulation-dependent mechanisms have been implicated in improved resistance of CTCs to NK-mediated clearance but other mechanisms have also been identified. Although EMT has been reported to stimulate some antitumor immune cytotoxicity [275], literature generally supports an improved resistance of EMT hybrid phenotypes to immune cytotoxic cells. Mechanisms induced by EMT such as increased expression of immune checkpoint proteins, altered autophagy, immunoproteasome deficiency and dysfunction of immunological synapses have been implicated and reviewed elsewhere [276,277]. More particularly in the context of CTCs, a reduced expression of ULBP1 (a major ligand of NKG2D) has been reported in EMT-shifted CTCs isolated from gastric cancer patients (Table 1) and in TGF-β-induced cells in vitro, and a mechanism has been proposed by which EMT-shifted CTC resistance to NK cells is increased [134]. In contrast, López-Soto and coworkers reported an enhanced susceptibility to NK cells and an increased expression of different NKG2D ligands in colorectal cancer cells induced to EMT by several means (TGF-β stimulation, inhibition of glycogen synthase kinase-3β, or Snail overexpression) [278]. The impact of EMT in modulating NKG2D-mediated antitumor response may likely be context-dependent and thus remains to be clarified, particularly in CTCs. Additionally, and together with the advent of immunotherapy, the expression of immune checkpoint proteins such as PD-L1 has been examined and detected on CTCs in different types of cancer [279,280,281]. Supporting an enhanced ability of EMT-shifted tumor phenotypes to better resist immune cytotoxic cells, correlated expression of PD-L1 expression and EMT markers has been evidenced in tumors and CTCs, particularly in NSCLC (non-small cell lung cancer) and TNBC [130,280,282,283,284,285,286,287]. Accordingly, PD-L1 has been identified as a gene regulated by EMTs in various cell systems [284,288,289,290,291,292,293].
These mechanisms of resistance to cytotoxic immune cells could thus contribute to a better survival of EMT+ CTCs in the bloodstream but also in the metastatic niche [82].
4.3. EMT in the Initiation of the Metastatic Niche
The timing of metastatic niche formation, and the implicated molecular and cellular entities are still poorly understood. It is generally recognized that, after CTC arrest in the vasculature (thus becoming disseminated tumor cells—DTCs), the niche will provide support to help DTCs recover from the stress endured in the bloodstream [82]. In this initiation phase, the niche signals to control EMT/MET plasticity and maintain/accentuate stem cell properties, thereby preventing differentiation, ensuring survival, and facilitating a quiescent state that, if prolonged, may result in dormancy. Whether EMT and CSC features are intertwined or somehow uncouple in this context is still a subject of debate [294]. A single cell analysis study in a breast cancer PDX model thus showed that CTCs/DTCs seeding in secondary organs displayed increased expression of stem cell-, EMT-, prosurvival-, and dormancy-associated genes [295]. Another study in MMTV–Her2 mice reported that a majority of early DTCs express Twist and are in a dormant state [296]. Accumulating data support that EMT-shifted CTCs have an enhanced ability to stimulate niche formation. Accordingly, all mechanisms mentioned in the previous section (enhanced survival properties, coagulation/TF/platelet/fibrin/thrombin-related mechanisms, interactions with neutrophils/NETs) have not only been shown to protect CTCs in the bloodstream and facilitate arrest in the vasculature but have also been demonstrated to contribute to niche initiation. Thus, coagulation/TF [226,297,298,299], platelets and fibrin[ogen] [207,221,222,300,301,302] and NETs [248] are all processes/entities/molecules that have been shown to play crucial roles in early phases of metastatic niche formation. If the microthrombus scaffold engendered by these mechanisms certainly contribute to the formation of an adequate matrix for the DTCs to niche, it also constitutes an environment in which host cells are recruited that are determinant for the consolidation of the niche environment. For instance, Gil-Bernabé and coworkers emphasized that the recruitment of monocytes/macrophages by TF-mediated coagulation is a determinant for tumor cell survival and metastatic niche establishment in mouse models [303]. Platelet-dependent processes also strongly support the recruitment of inflammatory cells into the niche [207,221,222,300,301,302]. It is also plausible that EMT hybrids are particularly efficient at establishing the immunosuppressive environment observed during niche initiation [304]. Although this was not studied in the particular context of niche formation, EMT-shifted cells have thus been shown to recruit immunosuppressive populations of immune cells [305,306,307] through the secretion of immunosuppressive mediators (e.g., CCL2, CXCL8, thrombospondin). The ability of EMT hybrids to better resist cytotoxic immune cells is also certainly a property facilitating the establishment of the metastatic niche.
Additionally, once arrested in the vasculature of colonized organs, EMT+ DTCs may also establish privileged interactions with resident host cells to initiate niche activation. In a mouse metastasis model, del Pozo et al. thus showed that Axl expressed by mesenchymally-shifted metastatic initiating cells is involved in the niche activation through the regulation of thrombospondin 2 secretion and the education of resident fibroblasts [308]. Subsequently, these DTCs reverted to an Axl-negative, more epithelial phenotype to proliferate. The ability of mesenchymally-shifted CTCs to accomplish early niching and a subsequent reversion to a more epithelial phenotype associated with metastatic outgrowth has also been observed by other authors [309,310]. Thus, in later stages of metastasis, the niche signals to induce MET and favor proliferation leading to metastatic outgrowth.
All in all, these abundant experimental data, considered together with the molecular characterization of human CTCs, support that mesenchymally-shifted hybrid CTCs may represent subpopulations with enhanced competence to survive in the circulation and to initiate niche formation at distant sites. If many mechanisms detailed above (activation of coagulation, interactions with neutrophils) are likely to favor the formation/consolidation of heterotypic clusters/CTM, they may also help isolated CTCs to overcome the constraints of metastatic translocation and niching. Various hybrid phenotypes may therefore travel and niche as isolated CTCs while others, potentially more dependent on homo/heterotypic interactions for survival, may travel and nest as clusters, thereby gaining even increased metastatic competence. Considering experimental data and clinical observations showing that isolated CTCs are largely predominant entities, it is very likely that these two scenarios coexist.
5. Functional Assays for CTCs
Current CTC research invests great efforts in the development of ex vivo, in vitro and in vivo models allowing a functional characterization of CTCs [311]. An underlying aim is certainly to understand CTC biology and to identify/isolate, within a heterogeneous population of CTCs, those CTCs with a high potential to initiate metastasis (so-called MICs, metastasis initiating cells).
Because CTCs are so rare, many researchers have aimed at shortly expanding CTCs in culture and even at establishing CTC-derived cell lines before downstream in vitro and in vivo characterization [312,313], although this may certainly modify the heterogeneity of the initial CTC population and introduce a bias. Cell lines have thus been successfully established from different CTC subpopulations isolated from different types of cancer including breast [314,315], colon [316], and lung [126,317,318] cancers. To better mimic the in vivo contexts, 3D (tumor) spheroids and organoids culture models are being optimized [319,320,321,322,323,324,325,326,327]. Additionally, 3D co-culture systems are also under development [317]. Although this remains largely uncovered, several studies have examined EMT heterogeneity in such culture models aiming to gain knowledge about CTC biology and their metastasis-initiating potential. Hybrid E/M phenotypes have thus been reported in CTC-derived cell lines [126,316]. Dynamic differences in E and M composition have also been observed in a 3D polymer scaffold culture [323] and in 3D spheroid cultures [324]. Aiming at isolating and characterizing invasive CTCs, VitatexTM developed a platform system to capture and culture viable CTCs with an ability to adhere and remodel/ingest a labeled matrix (Cell Adhesion Matrix -CAM) [187,328,329]. Friedlander and coworkers were thus able to identify invasive CTCs expressing EMT/CSC markers (vimentin, CD44) in the blood of prostate cancer patients [187]. Most importantly, in vivo CTC xenografts (CTC-derived xenografts, CDX) are also being optimized as most representative models to evaluate the metastatic competence of different subpopulations of CTCs and understand the biology of MICs. Although some studies use CTCs without prior in vitro expansion, CTC-derived cultures or cell lines are also commonly assessed. MICs have thus been successfully identified in CTCs isolated from different types of cancer including breast, lung or colon cancer [315,316,330,331,332,333,334]. In some studies, intravenous or intra-femoral injections have been directly performed. Therefore, breast-cancer derived CTC cell lines expressing E/M hybrid phenotypes (cytokeratins 8/18, vimentin, CD44) have been shown to metastasize after tail-vein or intracardiac injection [314]. Similar findings have been reported with CTC cell lines established from an initial xenograft model of a breast cancer-derived DTC cell line [334]. Baccelli and coworkers also identified CD44+/c-Met+/CD47+ CTCs isolated from breast cancer patients, which generated bone, lung, and liver metastases after intrafemoral injection [335].
Another important scope behind the elaboration of these CTC-derived assays is to develop patient-matched preclinical models that could be established for longitudinal assessment of disease progression and drug sensitivity, thereby customizing and improving individual patient management [320,321]. Thus, CTC cell lines [315,336,337,338] and 3D CTC-derived spheroid and organoids models [317,322,323,324,325,339] have been examined in drug screening settings. In this sector, microfluidics is also making a breakthrough, allowing a better control of the culture conditions and easier settings for drug delivery [340]. CDX have also been evaluated in drug screening assays [341,342,343] with an ultimate goal of being analyzed in patient-matched settings [344]. Although this line of research is very promising, further developments are needed to improve efficiency and reproducibility and, ultimately, to select models that could be transferrable in co-clinical trials on patients and patient-matched “avatars”.
6. Clinical Relevance of EMT-Related CTC Heterogeneity
A profusion of clinical studies supports the validity of CTC count before or during treatment (chemotherapy or targeted therapy) as a prognostic biomarker particularly for breast, prostate, and colorectal cancer [7,8,345,346,347,348,349,350]. Nevertheless, CTCs are thus far not utilized in clinical routine. Large-scale multicentered trials using identical detection methods seem crucial to establish the utility of CTC enumeration and implement this parameter in clinical practice.
In addition to enumeration, it is also becoming clear that a deeper molecular characterization of CTCs may provide a goldmine of information for clinicians and holds promises to improve personalized cancer management.
6.1. EMT+ CTCs as A Prognostic Factor
Numerous studies to date have already suggested that certain EMT-shifted CTC subsets harbor prognostic information. In agreement with the well-documented and numerous pro-metastatic functions of EMTs, the detection of certain EMT actors in CTCs has thus been correlated with poor clinical parameters such as an aggressive cancer types and a shortened OS or PFS (Table 1). More particularly, CTCs harboring mesenchymal features have been reported to associate with the presence of metastases in numerous cancers including breast, lung, pancreatic, colorectal, prostate, or hepatocellular cancers [101,109,114,116,122,125,169,351,352,353,354]. Considering particular EMT/CSC molecular actors, studies, for instance, have detected CD44, often in conjunction with other canonical EMT markers, in CTCs isolated from many cancer types, which was associated with poor clinical outcomes in some studies [50,100,170,187,188,335,355,356]. EMT transcription factors (Twist, ZEB or Snail) have also been frequently detected in CTCs and associated with a poor prognosis in different cancer types [101,174,353,357,358]. Axl was found in CTCs isolated from lung cancers, particularly in those expressing vimentin [52], and it was similarly associated with a poor prognosis [132]. Interestingly, high expression of PD-L1 and EMT markers in CTCs was reported to be a sign of a grim prognosis in patients with complete surgically resected lung cancer [130]. The exploration of EMT on CTCs may thus help to predict a poor outcome and could thus guide towards an adaptation of individual patient management (reinforced surveillance, treatment adaptation).
6.2. EMT+ CTCs in Therapy Management
In addition to this important prognostic information, EMT detection in CTCs also harbors meaningful predictive information. Identifying molecules that may predict the response or a non-response to an existing treatment accordingly constitutes a promising perspective of CTC molecular characterization that may guide/refine patient stratification and management, particularly when tumor biopsy is not informative or no longer an option.
Therefore, a combined detection of known therapeutic targets (such as EGFR, PD-L1 or HER2) together with poor-prognostic EMT markers may refine patient management and point to potential combinatory therapies. Illustrating this, an association between PD-L1 expression and EMT markers has been evidenced in tumors and CTCs, particularly in NSCLC and TNBC [130,280,282,283,284,285]. Along these lines, tandem expression of vimentin and PD-L1 has been shown to constitute a prognostic factor in NSCLC [287]. Hence, EMT has been proposed as a candidate biomarker to be explored on tumors and CTCs to predict immunotherapy outcome and to design combination approaches with immunotherapy [276,359].
A pivotal aspect of CTC analysis resides on the possibility to perform successive liquid biopsies to monitor the progression of the disease and to assess treatment efficacy. Importantly, the emergence of EMT-shifted CTC phenotypes after one or several lines of treatment was found to correlate with drug resistance in several studies [52,104,112,121,124,179,360]. This finding is in agreement with a large amount of experimental data demonstrating an enhanced ability of EMT-shifted cells to resist most existing therapeutic options (chemo- radio-resistance, and resistance to existing targeted therapies) [81,162,163,164,165,166]. Analyzing EMT as a companion marker in the course of a treatment may thus help predict drug resistance and eventually help guide therapeutic strategies.
In addition, a deeper molecular characterization of these drug-resistant phenotypes may also point to the emergence of other targetable pathways, or identify new potential therapeutic targets. For instance, NSCLC patients treated with one or several lines of therapies, progressively presented significantly more vimentin-positive Axl-expressing CTCs [52]. Overexpression of Axl in CTCs isolated from lung cancer patients who developed resistance to EGFR inhibitor therapies has also been reported [361]. Examining Axl on CTCs, against which drugs are currently evaluated in clinical trials, has accordingly gained interest within the last 5 years [132,362,363].
As debated numerous times [75,364], the dynamic and reversible nature of EMTs makes it difficult to define a single EMT molecular signature that fits the variety of hybrid phenotypes, particularly across various types of cancer. Due to this molecular complexity, the identification of specific EMT/CSC molecular markers or signatures to be used as prognostic or predictive markers in specific cancer contexts will most likely constitute a necessary steppingstone towards a clinical use. Defining an “EMT index” to somehow quantify the extend of EMT may also facilitate the implementation of EMT analysis in clinical practice. Fici and coworkers, analyzing EMT-related splicing factors ESRP1/ESRP2/RBFOX2, provided rationale to use the ESRP1/RBFOX2 ratio as a prognostic biomarker for early prediction of metastasis in breast cancer, and further suggested this ratio could also be evaluated in CTCs [365]
Further development is thus ongoing to delineate clear parameters (selection of appropriate CTC isolation techniques, selection of specific EMT markers or EMT signatures or EMT indexes, selection of adequate preclinical models for drug screening) that may be transferable to clinical routine. With increasing experimental data identifying new EMT molecular mechanisms supporting metastatic competence, emerging therapeutic targets will also undoubtedly be identified. Thus, targeting mechanisms and specific molecules involved in coagulation, NET formation or neutrophil interactions are currently interesting paths of exploration.
7. Concluding Remarks
A tremendous amount of experimental data shows that EMTs endow epithelial tumor cells with properties that help them overcome hostile signals encountered as they travel as CTCs: an enhanced survival potential, an increased ability to activate coagulation, an augmented aptitude to hijack host cell pro-metastatic functions, and a raised capacity to establish an early metastatic niche.
Nevertheless, examining EMT in human CTCs remains complicated, partly because enrichment and isolation of rare human CTCs remain a technical challenge. Microfluidic-based approaches of CTC isolation are undergoing rapid development and currently represent the most promising innovative avenue to isolate label-free human CTCs, hopefully accelerating CTC research and subsequently CTC consideration in the clinic.
The clinical potential of CTCs is indeed enormous and multiple. CTCs hold a prominent place in personalized medicine. When tumor biopsy is not or no longer an option, CTCs represent a unique accessibility to tumor material, and, moreover, a material containing the physical entities responsible for metastasis. Additionally, as liquid biopsies are non-invasive procedures that can be repeated during the course of the treatment, a live assessment of disease progression and a monitoring of treatment efficacy may be possible.
Thus far, and beyond the clinical interest of CTC enumeration, data collected on human CTCs point to a clinical utility to longitudinally interrogate the EMT status in CTCs. An EMT signature in CTCs is indeed a marker of poor prognosis in most cancer contexts. In advanced and metastatic situations, EMT traits also clearly point to drug resistance. EMT detection may thus represent a companion marker of further assessment to facilitate individual cancer patient management and guide treatment decisions. Although the examination of specific EMT markers seems easier to implement in the clinic, analysis of EMT signatures may harbor more significant power and point to new potential therapeutic targets to consider.
Additionally, the elaboration of preclinical models that would allow a deeper functional and molecular characterization of MICs within the CTC population and that could be utilized as drug-screening platforms, certainly represent a crucial axis of CTC research that may pave a way towards personalized medicine.
Funding
This research received no external funding.
Acknowledgments
A. Genna, A.M. Vanwynsberghe and A.V. Villard are supported by Télévie grants (Belgium). C. Gilles is a Senior Research Associates at the F.R.S-FNRS (Belgium).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kelley, S.O.; Pantel, K. A New Era in Liquid Biopsy: From Genotype to Phenotype. Clin. Chem. 2019. [Google Scholar] [CrossRef]
- Cortés-Hernández, L.E.; Eslami, S.Z.; Alix-Panabières, C. Circulating tumor cell as the functional aspect of liquid biopsy to understand the metastatic cascade in solid cancer. Mol. Aspects Med. 2020, 72, 100816. [Google Scholar] [CrossRef]
- Castro-Giner, F.; Aceto, N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef]
- Menyailo, M.E.; Tretyakova, M.S.; Denisov, E.V. Heterogeneity of Circulating Tumor Cells in Breast Cancer: Identifying Metastatic Seeds. Int. J. Mol. Sci. 2020, 21, 1696. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med. J. 1869, 14, 146–147. [Google Scholar]
- Rossi, E.; Fabbri, F. CTCs 2020: Great Expectations or Unreasonable Dreams. Cells 2019, 8, 989. [Google Scholar] [CrossRef] [PubMed]
- Abalde-Cela, S.; Piairo, P.; Dieguez, L. The Significance of Circulating Tumour Cells in the Clinic. Acta Cytol. 2019, 63, 466–478. [Google Scholar] [CrossRef]
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cells: Clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Boya, M.; Chu, C.H.; Liu, R.; Ozkaya-Ahmadov, T.; Sarioglu, A.F. Circulating Tumor Cell Enrichment Technologies. Recent Results Cancer Res. 2020, 215, 25–55. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef] [PubMed]
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for circulating tumor cell separation from whole blood. J. Hematol. Oncol. 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, A.; Kowalewska, M.; Gozdz, S. Current approaches for avoiding the limitations of circulating tumor cells detection methods-implications for diagnosis and treatment of patients with solid tumors. Transl. Res. 2017, 185, 58–84.e15. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Doyle, G.V.; Terstappen, L.W. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J. Oncol. 2010, 2010, 617421. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Keller, L.; Werner, S.; Pantel, K. Biology and clinical relevance of EpCAM. Cell Stress 2019, 3, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.K.; Cummins, A.G.; Price, T.J.; Roberts-Thomson, I.C.; Hardingham, J.E. Circulating tumour cells: The evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann. Oncol. 2014, 25, 1506–1516. [Google Scholar] [CrossRef]
- Khamenehfar, A.; Li, P.C. Microfluidic Devices for Circulating Tumor Cells Isolation and Subsequent Analysis. Curr. Pharm. Biotechnol. 2016, 17, 810–821. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, Z.; Wang, J.; Ren, Y.; Wu, A. Microfluidic applications on circulating tumor cell isolation and biomimicking of cancer metastasis. Electrophoresis 2020. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, G.; Du, W.; Kong, T.; Liu, C.; Liu, Z. Recent Advances in Microfluidic Platforms Applied in Cancer Metastasis: Circulating Tumor Cells’ (CTCs) Isolation and Tumor-On-A-Chip. Small (Weinheim an der Bergstrasse, Germany) 2020, 16, e1903899. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, J.; Song, H.; Sohn, K.Y.; Jeon, M.; Han, K.H. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018, 143, 2936–2970. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Z.; Wang, B. Size- and deformability-based isolation of circulating tumor cells with microfluidic chips and their applications in clinical studies. AIP Adv. 2018, 8, 120701. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Guo, S.S.; Zhang, Z.L.; Huang, W.H.; Baigl, D.; Xie, M.; Chen, Y.; Pang, D.W. A micropillar-integrated smart microfluidic device for specific capture and sorting of cells. Electrophoresis 2007, 28, 4713–4722. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Ogunwobi, O.O.; Chen, T.; Zhang, J.; George, T.J.; Liu, C.; Fan, Z.H. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab. Chip 2014, 14, 89–98. [Google Scholar] [CrossRef]
- Galletti, G.; Sung, M.S.; Vahdat, L.T.; Shah, M.A.; Santana, S.M.; Altavilla, G.; Kirby, B.J.; Giannakakou, P. Isolation of breast cancer and gastric cancer circulating tumor cells by use of an anti HER2-based microfluidic device. Lab. Chip 2014, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Varillas, J.I.; Zhang, J.; Chen, K.; Barnes, I.I.; Liu, C.; George, T.J.; Fan, Z.H. Microfluidic Isolation of Circulating Tumor Cells and Cancer Stem-Like Cells from Patients with Pancreatic Ductal Adenocarcinoma. Theranostics 2019, 9, 1417–1425. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef]
- Karabacak, N.M.; Spuhler, P.S.; Fachin, F.; Lim, E.J.; Pai, V.; Ozkumur, E.; Martel, J.M.; Kojic, N.; Smith, K.; Chen, P.-i.; et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014, 9, 694–710. [Google Scholar] [CrossRef]
- Zamay, A.S.; Zamay, G.S.; Kolovskaya, O.S.; Zamay, T.N.; Berezovski, M.V. Aptamer-Based Methods for Detection of Circulating Tumor Cells and Their Potential for Personalized Diagnostics. Adv. Exp. Med. Biol. 2017, 994, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhu, L.; Huang, M.; Song, J.; Zhang, H.; Song, Y.; Wang, W.; Yang, C. Aptamer-based microfluidics for isolation, release and analysis of circulating tumor cells. TrAC Trends Anal. Chem. 2019, 117, 69–77. [Google Scholar] [CrossRef]
- Konigsberg, R.; Obermayr, E.; Bises, G.; Pfeiler, G.; Gneist, M.; Wrba, F.; de Santis, M.; Zeillinger, R.; Hudec, M.; Dittrich, C. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 2011, 50, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Balic, M.; Dandachi, N.; Hofmann, G.; Samonigg, H.; Loibner, H.; Obwaller, A.; van der Kooi, A.; Tibbe, A.G.; Doyle, G.V.; Terstappen, L.W.; et al. Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytom. B Clin. Cytom. 2005, 68, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Maertens, Y.; Humberg, V.; Erlmeier, F.; Steffens, S.; Steinestel, J.; Bogemann, M.; Schrader, A.J.; Bernemann, C. Comparison of isolation platforms for detection of circulating renal cell carcinoma cells. Oncotarget 2017, 8, 87710–87717. [Google Scholar] [CrossRef]
- Boyer, M.; Cayrefourcq, L.; Garima, F.; Foulongne, V.; Dereure, O.; Alix-Panabieres, C. Circulating Tumor Cell Detection and Polyomavirus Status in Merkel Cell Carcinoma. Sci. Rep. 2020, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Naume, B.; Borgen, E.; Tossvik, S.; Pavlak, N.; Oates, D.; Nesland, J.M. Detection of isolated tumor cells in peripheral blood and in BM: Evaluation of a new enrichment method. Cytotherapy 2004, 6, 244–252. [Google Scholar] [CrossRef]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schutze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Farace, F.; Massard, C.; Vimond, N.; Drusch, F.; Jacques, N.; Billiot, F.; Laplanche, A.; Chauchereau, A.; Lacroix, L.; Planchard, D.; et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer 2011, 105, 847–853. [Google Scholar] [CrossRef]
- Pinzani, P.; Salvadori, B.; Simi, L.; Bianchi, S.; Distante, V.; Cataliotti, L.; Pazzagli, M.; Orlando, C. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: Correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum. Pathol. 2006, 37, 711–718. [Google Scholar] [CrossRef]
- Desitter, I.; Guerrouahen, B.S.; Benali-Furet, N.; Wechsler, J.; Janne, P.A.; Kuang, Y.; Yanagita, M.; Wang, L.; Berkowitz, J.A.; Distel, R.J.; et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011, 31, 427–441. [Google Scholar] [PubMed]
- Yanagita, M.; Luke, J.J.; Hodi, F.S.; Jänne, P.A.; Paweletz, C.P. Isolation and characterization of circulating melanoma cells by size filtration and fluorescent in-situ hybridization. Melanoma Res. 2018, 28, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Andree, K.C.; Mentink, A.; Zeune, L.L.; Terstappen, L.W.M.M.; Stoecklein, N.H.; Neves, R.P.; Driemel, C.; Lampignano, R.; Yang, L.; Neubauer, H.; et al. Toward a real liquid biopsy in metastatic breast and prostate cancer: Diagnostic LeukApheresis increases CTC yields in a European prospective multicenter study (CTCTrap). Int. J. Cancer 2018, 143, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- De Wit, S.; Van Dalum, G.; Lenferink, A.T.M.; Tibbe, A.G.J.; Hiltermann, T.J.N.; Groen, H.J.M.; Van Rijn, C.J.M.; Terstappen, L.W.M.M. The detection of EpCAM+ and EpCAM- circulating tumor cells. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Tsutsuyama, M.; Nakanishi, H.; Yoshimura, M.; Oshiro, T.; Kinoshita, T.; Komori, K.; Shimizu, Y.; Ichinosawa, Y.; Kinuta, S.; Wajima, K.; et al. Detection of circulating tumor cells in drainage venous blood from colorectal cancer patients using a new filtration and cytology-based automated platform. PLoS ONE 2019, 14, e0212221. [Google Scholar] [CrossRef]
- Obermayr, E.; Agreiter, C.; Schuster, E.; Fabikan, H.; Weinlinger, C.; Baluchova, K.; Hamilton, G.; Hochmair, M.; Zeillinger, R. Molecular Characterization of Circulating Tumor Cells Enriched by A Microfluidic Platform in Patients with Small-Cell Lung Cancer. Cells 2019, 8, 880. [Google Scholar] [CrossRef]
- Xu, L.; Mao, X.; Imrali, A.; Syed, F.; Mutsvangwa, K.; Berney, D.; Cathcart, P.; Hines, J.; Shamash, J.; Lu, Y.J. Optimization and evaluation of a novel size based circulating tumor cell isolation system. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef]
- Xu, L.; Mao, X.; Guo, T.; Chan, P.Y.; Shaw, G.; Hines, J.; Stankiewicz, E.; Wang, Y.; Oliver, R.T.D.; Ahmad, A.S.; et al. The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin. Cancer Res. 2017, 23, 5112–5122. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e114. [Google Scholar] [CrossRef]
- Wan, S.; Kim, T.H.; Smith, K.J.; Delaney, R.; Park, G.S.; Guo, H.; Lin, E.; Plegue, T.; Kuo, N.; Steffes, J.; et al. New Labyrinth Microfluidic Device Detects Circulating Tumor Cells Expressing Cancer Stem Cell Marker and Circulating Tumor Microemboli in Hepatocellular Carcinoma. Sci. Rep. 2019, 9, 18575. [Google Scholar] [CrossRef]
- Zeinali, M.; Lee, M.; Nadhan, A.; Mathur, A.; Hedman, C.; Lin, E.; Harouaka, R.; Wicha, M.S.; Zhao, L.; Palanisamy, N.; et al. High-Throughput Label-Free Isolation of Heterogeneous Circulating Tumor Cells and CTC Clusters from Non-Small-Cell Lung Cancer Patients. Cancers 2020, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Koh, Y.; Teraoka, S.; Sato, K.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, H.; Ozawa, Y.; Akamatsu, K.; et al. Detection of AXL expression in circulating tumor cells of lung cancer patients using an automated microcavity array system. Cancer Med. 2020, 9, 2122–2133. [Google Scholar] [CrossRef]
- Hosokawa, M.; Kenmotsu, H.; Koh, Y.; Yoshino, T.; Yoshikawa, T.; Naito, T.; Takahashi, T.; Murakami, H.; Nakamura, Y.; Tsuya, A.; et al. Size-Based Isolation of Circulating Tumor Cells in Lung Cancer Patients Using a Microcavity Array System. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Aghaamoo, M.; Zhang, Z.; Chen, X.; Xu, J. Deformability-based circulating tumor cell separation with conical-shaped microfilters: Concept, optimization, and design criteria. Biomicrofluidics 2015, 9, 034106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, J.; Hong, B.; Chen, X. The effects of 3D channel geometry on CTC passing pressure--towards deformability-based cancer cell separation. Lab. Chip 2014, 14, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- McFaul, S.M.; Lin, B.K.; Ma, H. Cell separation based on size and deformability using microfluidic funnel ratchets. Lab. Chip 2012, 12, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Duffy, S.P.; Ma, H. Microfluidic Separation of Circulating Tumor Cells Based on Size and Deformability. Methods Mol. Biol. 2017, 1634, 21–32. [Google Scholar] [CrossRef]
- Tan, S.J.; Yobas, L.; Lee, G.Y.; Ong, C.N.; Lim, C.T. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed. Microdevices 2009, 11, 883–892. [Google Scholar] [CrossRef]
- Gascoyne, P.R.; Noshari, J.; Anderson, T.J.; Becker, F.F. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis 2009, 30, 1388–1398. [Google Scholar] [CrossRef]
- Moon, H.S.; Kwon, K.; Kim, S.I.; Han, H.; Sohn, J.; Lee, S.; Jung, H.I. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab. Chip 2011, 11, 1118–1125. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chao, T.C.; Ariyasinghe, N.; Ruiz, Y.; Lake, D.; Ros, R.; Ros, A. Selective trapping of single mammalian breast cancer cells by insulator-based dielectrophoresis. Anal. Bioanal. Chem. 2014, 406, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Cortes, J.; Awada, A.; O’Shaughnessy, J.; Twelves, C.; Im, S.A.; Hannah, A.; Lu, L.; Sy, S.; Caygill, K.; et al. Change in Topoisomerase 1-Positive Circulating Tumor Cells Affects Overall Survival in Patients with Advanced Breast Cancer after Treatment with Etirinotecan Pegol. Clin. Cancer Res. 2018, 24, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Jafferji, I.; Garza, M.; Melnikova, V.O.; Hasegawa, D.K.; Pethig, R.; Davis, D.W. ApoStream(™), a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics 2012, 6, 24133. [Google Scholar] [CrossRef] [PubMed]
- S. Iliescu, F.; Sim, W.J.; Heidari, H.; P. Poenar, D.; Miao, J.; Taylor, H.K.; Iliescu, C. Highlighting the uniqueness in dielectrophoretic enrichment of circulating tumor cells. Electrophoresis 2019, 40, 1457–1477. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Samy, S.; Oliveira, M.I.; Pereira-Veiga, T.; Muinelo-Romay, L.; Carvalho, S.; Gaspar, J.; Freitas, P.P.; López-López, R.; Costa, C.; Diéguez, L. Fast and efficient microfluidic cell filter for isolation of circulating tumor cells from unprocessed whole blood of colorectal cancer patients. Sci. Rep. 2019, 9, 8032. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yu, V.; Garon, E.B.; Goldman, J.W.; Di Carlo, D. Biophysical isolation and identification of circulating tumor cells. Lab. Chip 2017, 17, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Lee, J.; Ra, M.; Gwon, H.; Lee, S.; Kim, M.Y.; Yoo, K.C.; Sul, O.; Kim, C.G.; Kim, W.Y.; et al. Continuous Separation of Circulating Tumor Cells from Whole Blood Using a Slanted Weir Microfluidic Device. Cancers 2019, 11, 200. [Google Scholar] [CrossRef]
- Francart, M.E.; Lambert, J.; Vanwynsberghe, A.M.; Thompson, E.W.; Bourcy, M.; Polette, M.; Gilles, C. Epithelial-mesenchymal plasticity and circulating tumor cells: Travel companions to metastases. Dev. Dyn. 2018, 247, 432–450. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Li, J.; Sun, B.; Qian, H.; Yin, Z. The biological and clinical importance of epithelial-mesenchymal transition in circulating tumor cells. J. Cancer Res. Clin. Oncol. 2015, 141, 189–201. [Google Scholar] [CrossRef]
- Markiewicz, A.; Zaczek, A.J. The Landscape of Circulating Tumor Cell Research in the Context of Epithelial-Mesenchymal Transition. Pathobiology 2017, 84, 264–283. [Google Scholar] [CrossRef]
- Kolbl, A.C.; Jeschke, U.; Andergassen, U. The Significance of Epithelial-to-Mesenchymal Transition for Circulating Tumor Cells. Int. J. Mol. Sci. 2016, 17, 1308. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Mader, S.; Pantel, K. Epithelial-mesenchymal plasticity in circulating tumor cells. J. Mol. Med. (Berl.) 2016. [Google Scholar] [CrossRef] [PubMed]
- Burr, R.; Gilles, C.; Thompson, E.W.; Maheswaran, S. Epithelial-Mesenchymal Plasticity in Circulating Tumor Cells, the Precursors of Metastasis. Adv. Exp. Med. Biol. 2020, 1220, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Haber, D.A.; Maheswaran, S. Cancer metastasis through the prism of epithelial-to-mesenchymal transition in circulating tumor cells. Mol. Oncol. 2017, 11, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Wang, P.; Toh, A.; Thompson, E. New Insights Into the Role of Phenotypic Plasticity and EMT in Driving Cancer Progression. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef]
- Williams, E.D.; Gao, D.; Redfern, A.; Thompson, E.W. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer 2019, 19, 716–732. [Google Scholar] [CrossRef]
- Jolly, M.K.; Somarelli, J.A.; Sheth, M.; Biddle, A.; Tripathi, S.C.; Armstrong, A.J.; Hanash, S.M.; Bapat, S.A.; Rangarajan, A.; Levine, H. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol. Ther. 2019, 194, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Celia-Terrassa, T.; Kang, Y. Metastatic niche functions and therapeutic opportunities. Nat. Cell Biol. 2018, 20, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Ombrato, L.; Malanchi, I. The EMT universe: Space between cancer cell dissemination and metastasis initiation. Crit. Rev. Oncog. 2014, 19, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Ibaragi, S.; Hu, G.F. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009, 69, 7135–7139. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, D.; Drasin, D.J.; Ford, H.L. Intratumoral heterogeneity: Clonal cooperation in epithelial-to-mesenchymal transition and metastasis. Cell Adhes. Migr. 2015, 9, 265–276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Goossens, S.; Vandamme, N.; Van Vlierberghe, P.; Berx, G. EMT transcription factors in cancer development re-evaluated: Beyond EMT and MET. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, M.P.; Eccles, R.L.; Brabletz, S.; Brabletz, T. Non-redundant functions of EMT transcription factors. Nat. Cell Biol. 2019, 21, 102–112. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Colavito, S.A. AXL as a Target in Breast Cancer Therapy. J. Oncol. 2020, 2020, 5291952. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; Huang, R.Y. AXL-Driven EMT State as a Targetable Conduit in Cancer. Cancer Res. 2017, 77, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.T.; Yang, M.H. Revisiting epithelial-mesenchymal transition in cancer metastasis: The connection between epithelial plasticity and stemness. Mol. Oncol. 2017, 11, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Jia, D.; Boareto, M.; Mani, S.A.; Pienta, K.J.; Ben-Jacob, E.; Levine, H. Coupling the modules of EMT and stemness: A tunable ‘stemness window’ model. Oncotarget 2015, 6, 25161–25174. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.P.; Lievre, M.; Thomas, C.; Hinkal, G.; Ansieau, S.; Puisieux, A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 2008, 3, e2888. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Xie, K.; Wei, D. The Role of CD44 and Cancer Stem Cells. Methods Mol. Biol. 2018, 1692, 31–42. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Lapin, M.; Tjensvoll, K.; Oltedal, S.; Javle, M.; Smaaland, R.; Gilje, B.; Nordgård, O. Single-cell mRNA profiling reveals transcriptional heterogeneity among pancreatic circulating tumour cells. BMC Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Zhao, R.; Cai, Z.; Li, S.; Cheng, Y.; Gao, H.; Liu, F.; Wu, S.; Liu, S.; Dong, Y.; Zheng, L.; et al. Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells from colorectal cancer. Oncotarget 2017, 8, 9293–9302. [Google Scholar] [CrossRef] [PubMed]
- Bystricky, B.; Jurisova, S.; Karaba, M.; Minarik, G.; Benca, J.; Sedlácková, T.; Tothova, L.; Vlkova, B.; Cierna, Z.; Janega, P.; et al. Relationship between circulating tumor cells and tissue plasminogen activator in patients with early breast cancer. Anticancer Res. 2017, 37, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Q.; Li, H.; Wu, Y.; Feng, J.; Yan, Y. Mesenchymal circulating tumor cells (CTCs) and OCT4 mRNA expression in CTCs for prognosis prediction in patients with non-small-cell lung cancer. Clin. Transl. Oncol. 2017, 19, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Chebouti, I.; Kasimir-Bauer, S.; Buderath, P.; Wimberger, P.; Hauch, S.; Kimmig, R.; Kuhlmann, J.D. EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget 2017, 8, 48820–48831. [Google Scholar] [CrossRef]
- Togo, S.; Katagiri, N.; Namba, Y.; Tulafu, M.; Nagahama, K.; Kadoya, K.; Takamochi, K.; Oh, S.; Suzuki, K.; Sakurai, F.; et al. Sensitive detection of viable circulating tumor cells using a novel conditionally telomerase-selective replicating adenovirus in non-small cell lung cancer patients. Oncotarget 2017, 8, 34884–34895. [Google Scholar] [CrossRef]
- Satelli, A.; Batth, I.; Brownlee, Z.; Mitra, A.; Zhou, S.; Noh, H.; Rojas, C.R.; Li, H.; Meng, Q.H.; Li, S. EMT circulating tumor cells detected by cell-surface vimentin are associated with prostate cancer progression. Oncotarget 2017, 8, 49329–49337. [Google Scholar] [CrossRef]
- Lindsay, C.R.; Faugeroux, V.; Michiels, S.; Pailler, E.; Facchinetti, F.; Ou, D.; Bluthgen, M.V.; Pannet, C.; Ngo-Camus, M.; Bescher, G.; et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann. Oncol. 2017, 28, 1523–1531. [Google Scholar] [CrossRef]
- Bredemeier, M.; Edimiris, P.; Mach, P.; Kubista, M.; Sjöback, R.; Rohlova, E.; Kolostova, K.; Hauch, S.; Aktas, B.; Tewes, M.; et al. Gene expression signatures in circulating tumor cells correlate with response to therapy in metastatic breast cancer. Clin. Chem. 2017, 63, 1585–1593. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, J.; Mao, Y.; Li, X.; Hu, B.; Zhang, D. Associations between the Epithelial-Mesenchymal Transition Phenotypes of Circulating Tumor Cells and the Clinicopathological Features of Patients with Colorectal Cancer. Dis. Markers 2017, 2017. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, W.; Hanna, D.L.; Yang, D.; Okazaki, S.; Berger, M.D.; Miyamoto, Y.; Suenaga, M.; Schirripa, M.; El-Khoueiry, A.; et al. Clinical relevance of EMT and stem-like gene expression in circulating tumor cells of metastatic colorectal cancer patients. Pharmacogenomics J. 2018, 18, 29–34. [Google Scholar] [CrossRef]
- Sun, Y.F.; Guo, W.; Xu, Y.; Shi, Y.H.; Gong, Z.J.; Ji, Y.; Du, M.; Zhang, X.; Hu, B.; Huang, A.; et al. Circulating tumor cells from different vascular sites exhibit spatial heterogeneity in epithelial and mesenchymal composition and distinct clinical significance in hepatocellular carcinoma. Clin. Cancer Res. 2018, 24, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Lazaridou, M.; Paraskevopoulos, P.; Chen, S.; Swierczewska, M.; Budna, J.; Kuske, A.; Gorges, T.M.; Joosse, S.A.; Kroneis, T.; et al. Multiplex Gene Expression Profiling of In Vivo Isolated Circulating Tumor Cells in High-Risk Prostate Cancer Patients. Clin. Chem. 2018, 64, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, L.; Cheng, Y.; He, G.; Peng, B.; Gao, Y.; Jiang, Z.s.; Pan, M.X. Correlation Between Postoperative Early Recurrence of Hepatocellular Carcinoma and Mesenchymal Circulating Tumor Cells in Peripheral Blood. J. Gastrointest. Surg. 2018, 22, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.N.; Xiang, B.D.; Wu, F.X.; Ye, J.Z.; Zhong, J.H.; Wang, Y.Y.; Chen, Y.Y.; Chen, Z.S.; Ma, L.; Chen, J.; et al. Circulating tumor cells undergoing emt provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res. 2018, 78, 4731–4744. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Huang, Y.; Xiang, L.; Chen, Z.; Fang, Y.; Lin, Y.; Cui, Z.; Yu, S.; Li, X.; Yang, D. Circulating Tumor Cell Phenotype Indicates Poor Survival and Recurrence After Surgery for Hepatocellular Carcinoma. Dig. Dis. Sci. 2018, 63, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wan, L.; Wu, S.; Yang, J.; Zhou, Y.; Liu, F.; Wu, Z.; Cheng, Y. Mesenchymal marker and LGR5 expression levels in circulating tumor cells correlate with colorectal cancer prognosis. Cell. Oncol. 2018, 41, 495–504. [Google Scholar] [CrossRef]
- Yin, L.C.; Luo, Z.C.; Gao, Y.X.; Li, Y.; Peng, Q.; Gao, Y. Twist expression in circulating hepatocellular carcinoma cells predicts metastasis and prognoses. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Han, D.; Chen, K.; Che, J.; Hang, J.; Li, H. Detection of Epithelial-Mesenchymal Transition Status of Circulating Tumor Cells in Patients with Esophageal Squamous Carcinoma. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- De Wit, S.; Manicone, M.; Rossi, E.; Lampignano, R.; Yang, L.; Zill, B.; Rengel-Puertas, A.; Ouhlen, M.; Crespo, M.; Berghuis, A.M.S.; et al. EpCAMhigh and EpCAMlow circulating tumor cells in metastatic prostate and breast cancer patients. Oncotarget 2018, 9, 35705–35716. [Google Scholar] [CrossRef]
- Pereira-Veiga, T.; Martínez-Fernández, M.; Abuin, C.; Piñeir, R.; Cebey, V.; Cueva, J.; Palacios, P.; Blanco, C.; Muinelo-Romay, L.; Abalo, A.; et al. CTCs expression profiling for advanced breast cancer monitoring. Cancers 2019, 11, 1941. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, L.; Li, J.; Zheng, J.; Zhang, S.; Zhou, J. Epithelial-mesenchymal transition phenotype of circulating tumor cells is associated with distant metastasis in patients with NSCLC. Mol. Med. Rep. 2019, 19, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Kong, Y.Y.; Li, G.X.; Wang, Y.; Ye, D.W.; Dai, B. Phenotypes of circulating tumour cells predict time to castration resistance in metastatic castration-sensitive prostate cancer. BJU Int. 2019, 124, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Ma, F.; Li, C.; Wu, S.; Hu, S.; Huang, J.; Sun, X.; Wang, J.; Luo, Y.; Cai, R.; et al. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. Cancer Commun. (Lond.) 2019, 39, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Wang, Z.R.; Chen, C.L.; Di, L.; Bi, Z.F.; Li, Z.H.; Liu, Y.M. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J. Gastroenterol. 2019, 25, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.; Luo, B.; Zhou, Z.; Dong, C.; Jiang, Y.; Wang, L.; Shi, Q.; Tian, J. Establishment and characterization of a patient-derived circulating lung tumor cell line in vitro and in vivo. Cancer Cell Int. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zmetakova, I.; Kalinkova, L.; Smolkova, B.; Horvathova Kajabova, V.; Cierna, Z.; Danihel, L.; Bohac, M.; Sedlackova, T.; Minarik, G.; Karaba, M.; et al. A disintegrin and metalloprotease 23 hypermethylation predicts decreased disease-free survival in low-risk breast cancer patients. Cancer Sci. 2019, 110, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Gasparini-Junior, J.L.; Fanelli, M.F.; Abdallah, E.A.; Chinen, L.T.D. Evaluating mmp-2 and tgfß-ri expression in circulating tumor cells of pancreatic cancer patients and their correlation with clinical evolution. Arquivos Brasileiros de Cirurgia Digestiva 2019, 32, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.J.; Hsieh, C.H.; Hung, F.C.; Wang, H.M.; Chou, W.P.; Wu, M.H. The Integration of a Three-Dimensional Spheroid Cell Culture Operation in a Circulating Tumor Cell (CTC) Isolation and Purification Process: A Preliminary Study of the Clinical Significance and Prognostic Role of the CTCs Isolated from the Blood Samples of Head and Neck Cancer Patients. Cancers 2019, 11, 783. [Google Scholar] [CrossRef]
- Manjunath, Y.; Upparahalli, S.V.; Avella, D.M.; Deroche, C.B.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Smith, C.J.; Li, G.; Kaifi, J.T. PD-L1 Expression with Epithelial Mesenchymal Transition of Circulating Tumor Cells Is Associated with Poor Survival in Curatively Resected Non-Small Cell Lung Cancer. Cancers 2019, 11, 806. [Google Scholar] [CrossRef]
- Pan, L.; Yan, G.; Chen, W.; Sun, L.; Wang, J.; Yang, J. Distribution of circulating tumor cell phenotype in early cervical cancer. Cancer Manag. Res. 2019, 11, 5531–5536. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Perez, D.; Bayarri-Lara, C.I.; Ortega, F.G.; Russo, A.; Moyano Rodriguez, M.J.; Alvarez-Cubero, M.J.; Maza Serrano, E.; Lorente, J.A.; Rolfo, C.; Serrano, M.J. Post-Surgery Circulating Tumor Cells and AXL Overexpression as New Poor Prognostic Biomarkers in Resected Lung Adenocarcinoma. Cancers 2019, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhu, D.; Tang, X.; Qiu, X.; Lu, D.; Li, B.; Lin, D.; Zhou, Q. Detection of circulating tumor cell molecular subtype in pulmonary vein predicting prognosis of stage i–iii non-small cell lung cancer patients. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Tian, X.; Li, Y.; Liu, Y.; Yang, T.; Han, Z.; An, J.; Kong, L.; Li, Y. Epithelial-mesenchymal transition may be involved in the immune evasion of circulating gastric tumor cells via downregulation of ULBP1. Cancer Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jie, X.X.; Zhang, X.Y.; Xu, C.J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558–81571. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef]
- Pearson, G.W. Control of Invasion by Epithelial-to-Mesenchymal Transition Programs during Metastasis. J. Clin. Med. 2019, 8, 646. [Google Scholar] [CrossRef]
- Gilles, C.; Newgreen, D.; Sato, H.; Thompson, E.W. Matrix Metalloproteases and Epithelia-to-mesenchymal transition: Implications for carcinoma metastasis. In Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition; Savagner, P., Ed.; Springer: Boston, MA, USA, 2005; pp. 1–19. [Google Scholar]
- Ota, I.; Li, X.Y.; Hu, Y.; Weiss, S.J. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc. Natl. Acad. Sci. USA 2009, 106, 20318–20323. [Google Scholar] [CrossRef]
- Dhar, M.; Lam, J.N.; Walser, T.; Dubinett, S.M.; Rettig, M.B.; Di Carlo, D. Functional profiling of circulating tumor cells with an integrated vortex capture and single-cell protease activity assay. Proc. Natl. Acad. Sci. USA 2018, 115, 9986–9991. [Google Scholar] [CrossRef]
- Kallergi, G.; Markomanolaki, H.; Giannoukaraki, V.; Papadaki, M.A.; Strati, A.; Lianidou, E.S.; Georgoulias, V.; Mavroudis, D.; Agelaki, S. Hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2009, 11, R84. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Jain, R.K.; Munn, L.L. Active versus passive mechanisms in metastasis: Do cancer cells crawl into vessels, or are they pushed? Lancet. Oncol. 2007, 8, 444–448. [Google Scholar] [CrossRef]
- Alpaugh, M.L.; Tomlinson, J.S.; Kasraeian, S.; Barsky, S.H. Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene 2002, 21, 3631–3643. [Google Scholar] [CrossRef] [PubMed]
- Bednarz-Knoll, N.; Alix-Panabieres, C.; Pantel, K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metastasis Rev. 2012, 31, 673–687. [Google Scholar] [CrossRef]
- Aceto, N. Bring along your friends: Homotypic and heterotypic circulating tumor cell clustering to accelerate metastasis. Biomed. J. 2020, 43, 18–23. [Google Scholar] [CrossRef]
- Fabisiewicz, A.; Grzybowska, E. CTC clusters in cancer progression and metastasis. Med. Oncol. 2017, 34, 1–10. [Google Scholar] [CrossRef]
- Watanabe, S. The metastasizability of tumor cells. Cancer 1954, 7, 215–223. [Google Scholar] [CrossRef]
- Fidler, I.J. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur. J. Cancer 1973, 9, 223–227. [Google Scholar] [CrossRef]
- Liotta, L.a.; Kleinerman, J.; Saidel, G.M. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976, 36, 889–894. [Google Scholar]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Cheung, K.J.; Padmanaban, V.; Silvestri, V.; Schipper, K.; Cohen, J.D.; Fairchild, A.N.; Gorin, M.A.; Verdone, J.E.; Pienta, K.J.; Bader, J.S.; et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. USA 2016, 113, E854–E863. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B.; et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 2019, 9, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Glinsky, G.V.; Glinskii, O.V.; Huxley, V.H.; Turk, J.R.; Mossine, V.V.; Deutscher, S.L.; Pienta, K.J.; Quinn, T.P. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003, 63, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Ishikawa, T.; Minami, Y.; Nishita, M. Tactics of cancer invasion: Solitary and collective invasion. J. Biochem. 2020, 167, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Casanova, J. A common framework for EMT and collective cell migration. Development 2016, 143, 4291–4300. [Google Scholar] [CrossRef]
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Heeke, S.; Mograbi, B.; Alix-Panabieres, C.; Hofman, P. Never Travel Alone: The Crosstalk of Circulating Tumor Cells and the Blood Microenvironment. Cells 2019, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Cao, Z.; Livas, T.; Kyprianou, N. Anoikis and EMT: Lethal “Liaisons” during Cancer Progression. Crit. Rev. Oncog. 2016, 21, 155–168. [Google Scholar] [CrossRef]
- Gupta, S.; Maitra, A. EMT: Matter of Life or Death? Cell 2016, 164, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Mishra, L.; Li, S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015, 6, 10697–10711. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P.G.; Moreno-Bueno, G.; Cano, A. Contribution of Epithelial Plasticity to Therapy Resistance. J. Clin. Med. 2019, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Song, K.A.; Faber, A.C. Epithelial-to-mesenchymal transition and drug resistance: Transitioning away from death. J. Thorac. Dis. 2019, 11, E82–E85. [Google Scholar] [CrossRef] [PubMed]
- Staalduinen, J.v.; Baker, D.; Dijke, P.t.; Dam, H.v. Epithelial–mesenchymal-transition-inducing transcription factors: New targets for tackling chemoresistance in cancer? Oncogene 2018, 37, 6195–6211. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Agelaki, S.; Kalykaki, A.; Stournaras, C.; Mavroudis, D.; Georgoulias, V. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2008, 10, R80. [Google Scholar] [CrossRef]
- Aktas, B.; Tewes, M.; Fehm, T.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009, 11, R46. [Google Scholar] [CrossRef]
- Kallergi, G.; Papadaki, M.A.; Politaki, E.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011, 13, R59. [Google Scholar] [CrossRef] [PubMed]
- Barriere, G.; Riouallon, A.; Renaudie, J.; Tartary, M.; Rigaud, M. Mesenchymal and stemness circulating tumor cells in early breast cancer diagnosis. BMC Cancer 2012, 12, 114. [Google Scholar] [CrossRef]
- Barriere, G.; Riouallon, A.; Renaudie, J.; Tartary, M.; Rigaud, M. Mesenchymal characterization: Alternative to simple CTC detection in two clinical trials. Anticancer Res. 2012, 32, 3363–3369. [Google Scholar]
- Kasimir-Bauer, S.; Hoffmann, O.; Wallwiener, D.; Kimmig, R.; Fehm, T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.; Wagner, J.; Gorges, T.M.; Taenzer, A.; Uzunoglu, F.G.; Driemel, C.; Stoecklein, N.H.; Knoefel, W.T.; Angenendt, S.; Hauch, S.; et al. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci. Rep. 2016, 6, 28010. [Google Scholar] [CrossRef] [PubMed]
- Todenhofer, T.; Hennenlotter, J.; Dorner, N.; Kuhs, U.; Aufderklamm, S.; Rausch, S.; Bier, S.; Mischinger, J.; Schellbach, D.; Hauch, S.; et al. Transcripts of circulating tumor cells detected by a breast cancer-specific platform correlate with clinical stage in bladder cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Fang, F.; Zhang, Q. Circulating tumor cell clusters: What we know and what we expect (Review). Int. J. Oncol. 2016, 49, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Molnar, B.; Ladanyi, A.; Tanko, L.; Sreter, L.; Tulassay, Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin. Cancer Res. 2001, 7, 4080–4085. [Google Scholar] [PubMed]
- Cho, E.H.; Wendel, M.; Luttgen, M.; Yoshioka, C.; Marrinucci, D.; Lazar, D.; Schram, E.; Nieva, J.; Bazhenova, L.; Morgan, A.; et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys. Biol. 2012, 9, 016001. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Hou, J.M.; Sloane, R.; Lancashire, L.; Priest, L.; Nonaka, D.; Ward, T.H.; Backen, A.; Clack, G.; Hughes, A.; et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 2012, 7, 306–315. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Wang, C.; Ye, Z.; Austin, L.; Civan, J.; Hyslop, T.; Palazzo, J.P.; Jaslow, R.; Li, B.; Myers, R.E.; et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res. Treat. 2015, 154, 563–571. [Google Scholar] [CrossRef]
- Paoletti, C.; Li, Y.; Muniz, M.C.; Kidwell, K.M.; Aung, K.; Thomas, D.G.; Brown, M.E.; Abramson, V.G.; Irvin, W.J., Jr.; Lin, N.U.; et al. Significance of Circulating Tumor Cells in Metastatic Triple-Negative Breast Cancer Patients within a Randomized, Phase II Trial: TBCRC 019. Clin. Cancer Res. 2015, 21, 2771–2779. [Google Scholar] [CrossRef]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef]
- Costa, C.; Muinelo-Romay, L.; Cebey-Lopez, V.; Pereira-Veiga, T.; Martinez-Pena, I.; Abreu, M.; Abalo, A.; Lago-Leston, R.M.; Abuin, C.; Palacios, P.; et al. Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-clusters) as Predictors of Patient Outcomes. Cancers 2020, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Al Habyan, S.; Kalos, C.; Szymborski, J.; McCaffrey, L. Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer. Oncogene 2018, 37, 5127–5135. [Google Scholar] [CrossRef] [PubMed]
- Jansson, S.; Bendahl, P.O.; Larsson, A.M.; Aaltonen, K.E.; Rydén, L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 2016, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, T.W.; Ngo, V.T.; Dong, H.; Premasekharan, G.; Weinberg, V.; Doty, S.; Zhao, Q.; Gilbert, E.G.; Ryan, C.J.; Chen, W.T.; et al. Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration-resistant prostate cancer. Int. J. Cancer 2014, 134, 2284–2293. [Google Scholar] [CrossRef]
- Bulfoni, M.; Gerratana, L.; Del Ben, F.; Marzinotto, S.; Sorrentino, M.; Turetta, M.; Scoles, G.; Toffoletto, B.; Isola, M.; Beltrami, C.A.; et al. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. 2016, 18, 30. [Google Scholar] [CrossRef]
- Boareto, M.; Jolly, M.K.; Goldman, A.; Pietila, M.; Mani, S.A.; Sengupta, S.; Ben-Jacob, E.; Levine, H.; Onuchic, J.N. Notch-Jagged signalling can give rise to clusters of cells exhibiting a hybrid epithelial/mesenchymal phenotype. J. R Soc. Interface 2016, 13. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.; Ward, T.; Sloane, R.; Priest, L.; Hughes, A.; Clack, G.; Ranson, M.; Blackhall, F.; Dive, C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am. J. Pathol. 2011, 178, 989–996. [Google Scholar] [CrossRef]
- Aceto, N.; Toner, M.; Maheswaran, S.; Haber, D.A. En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition. Trends in cancer 2015, 1, 44–52. [Google Scholar] [CrossRef]
- Duda, D.G.; Duyverman, A.M.; Kohno, M.; Snuderl, M.; Steller, E.J.; Fukumura, D.; Jain, R.K. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl. Acad. Sci. USA 2010, 107, 21677–21682. [Google Scholar] [CrossRef] [PubMed]
- Ruf, W.; Rothmeier, A.S.; Graf, C. Targeting clotting proteins in cancer therapy—Progress and challenges. Thromb. Res. 2016, 140 (Suppl. 1), S1–S7. [Google Scholar] [CrossRef]
- Unlu, B.; Versteeg, H.H. Cancer-associated thrombosis: The search for the holy grail continues. Res. Pract Thromb Haemost 2018, 2, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin. Thromb. Hemost. 2019, 45, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Bystricky, B.; Reuben, J.M.; Mego, M. Circulating tumor cells and coagulation-Minireview. Crit. Rev. Oncol. Hematol. 2017, 114, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mitrugno, A.; Tormoen, G.W.; Kuhn, P.; McCarty, O.J. The prothrombotic activity of cancer cells in the circulation. Blood Rev. 2016, 30, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Beinse, G.; Berger, F.; Cottu, P.; Dujaric, M.E.; Kriegel, I.; Guilhaume, M.N.; Dieras, V.; Cabel, L.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cell count and thrombosis in metastatic breast cancer. J. Thromb. Haemost. 2017, 15, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; De Giorgi, U.; Broglio, K.; Dawood, S.; Valero, V.; Andreopoulou, E.; Handy, B.; Reuben, J.M.; Cristofanilli, M. Circulating tumour cells are associated with increased risk of venous thromboembolism in metastatic breast cancer patients. Br. J. Cancer 2009, 101, 1813–1816. [Google Scholar] [CrossRef]
- Kirwan, C.C.; Descamps, T.; Castle, J. Circulating tumour cells and hypercoagulability: A lethal relationship in metastatic breast cancer. Clin. Transl. Oncol. 2019. [Google Scholar] [CrossRef]
- Najidh, S.; Versteeg, H.H.; Buijs, J.T. A systematic review on the effects of direct oral anticoagulants on cancer growth and metastasis in animal models. Thromb. Res. 2020, 187, 18–27. [Google Scholar] [CrossRef]
- Mosarla, R.C.; Vaduganathan, M.; Qamar, A.; Moslehi, J.; Piazza, G.; Giugliano, R.P. Anticoagulation Strategies in Patients With Cancer: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Li, N. Platelets in cancer metastasis: To help the “villain” to do evil. Int. J. Cancer 2016, 138, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Remiker, A.S.; Palumbo, J.S. Mechanisms coupling thrombin to metastasis and tumorigenesis. Thromb. Res. 2018, 164 (Suppl. 1), S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B. Involvement of platelets in tumor cell metastasis. Pharmacol. Ther. 2016, 157, 112–119. [Google Scholar] [CrossRef]
- Gong, L.; Cai, Y.; Zhou, X.; Yang, H. Activated platelets interact with lung cancer cells through P-selectin glycoprotein ligand-1. Pathol. Oncol. Res. 2012, 18, 989–996. [Google Scholar] [CrossRef]
- Lonsdorf, A.S.; Krämer, B.F.; Fahrleitner, M.; Schönberger, T.; Gnerlich, S.; Ring, S.; Gehring, S.; Schneider, S.W.; Kruhlak, M.J.; Meuth, S.G.; et al. Engagement of αIIbβ3 (GPIIb/IIIa) with ανβ3 integrin mediates interaction of melanoma cells with platelets: A connection to hematogenous metastasis. J. Biol. Chem. 2012, 287, 2168–2178. [Google Scholar] [CrossRef]
- Ward, Y.; Lake, R.; Faraji, F.; Sperger, J.; Martin, P.; Gilliard, C.; Ku, K.P.; Rodems, T.; Niles, D.; Tillman, H.; et al. Platelets Promote Metastasis via Binding Tumor CD97 Leading to Bidirectional Signaling that Coordinates Transendothelial Migration. Cell Rep. 2018, 23, 808–822. [Google Scholar] [CrossRef]
- Chen, M.; Geng, J.-G. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch. Immunol. Ther. Exp. (Warsz.) 2006, 54, 75–84. [Google Scholar] [CrossRef]
- Qi, C.L.; Wei, B.; Ye, J.; Yang, Y.; Li, B.; Zhang, Q.Q.; Li, J.C.; He, X.D.; Lan, T.; Wang, L.J. P-selectin-mediated platelet adhesion promotes the metastasis of murine melanoma cells. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Biggerstaff, J.P.; Seth, N.; Amirkhosravi, A.; Amaya, M.; Fogarty, S.; Meyer, T.V.; Siddiqui, F.; Francis, J.L. Soluble fibrin augments platelet/tumor cell adherence in vitro and in vivo, and enhances experimental metastasis. Clin. Exp. Metastasis 1999, 17, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.S.; Burdick, M.M.; Thomas, S.N.; Pawar, P.; Konstantopoulos, K. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am. J. Physiol. Cell Physiol. 2008, 294, C907–C916. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.; Cooke, N.; Kenny, D. Living in shear: Platelets protect cancer cells from shear induced damage. Clin. Exp. Metastasis 2014, 31, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Männel, D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar]
- Palumbo, J.S.; Talmage, K.E.; Massari, J.V.; La Jeunesse, C.M.; Flick, M.J.; Kombrinck, K.W.; Jirouskova, M.; Degen, J.L. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005, 105, 178–185. [Google Scholar] [CrossRef]
- Kopp, H.G.; Placke, T.; Salih, H.R. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009, 69, 7775–7783. [Google Scholar] [CrossRef]
- Placke, T.; Orgel, M.; Schaller, M.; Jung, G.; Rammensee, H.G.; Kopp, H.G.; Salih, H.R. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012, 72, 440–448. [Google Scholar] [CrossRef]
- Biggerstaff, J.P.; Weidow, B.; Dexheimer, J.; Warnes, G.; Vidosh, J.; Patel, S.; Newman, M.; Patel, P. Soluble fibrin inhibits lymphocyte adherence and cytotoxicity against tumor cells: Implications for cancer metastasis and immunotherapy. Clin. Appl. Thromb. Hemost. 2008, 14, 193–202. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets guide the formation of early metastatic niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–E3061. [Google Scholar] [CrossRef]
- Im, J.H.; Fu, W.; Wang, H.; Bhatia, S.K.; Hammer, D.A.; Kowalska, M.A.; Muschel, R.J. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004, 64, 8613–8619. [Google Scholar] [CrossRef] [PubMed]
- Osmani, N.; Follain, G.; García León, M.J.; Lefebvre, O.; Busnelli, I.; Larnicol, A.; Harlepp, S.; Goetz, J.G. Metastatic Tumor Cells Exploit Their Adhesion Repertoire to Counteract Shear Forces during Intravascular Arrest. Cell Rep. 2019, 28, 2491–2500.e2495. [Google Scholar] [CrossRef] [PubMed]
- Gaddes, E.R.; Lee, D.; Gydush, G.; Wang, Y.; Dong, C. Regulation of fibrin-mediated tumor cell adhesion to the endothelium using anti-thrombin aptamer. Exp. Cell Res. 2015, 339, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G.; Borsig, L. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int. J. Cell Biol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bernabé, A.M.; Lucotti, S.; Muschel, R.J. Coagulation And Metastasis: What Does The Experimental Literature Tell Us? Br. J. Haematol. 2013, 162, 433–441. [Google Scholar] [CrossRef]
- Mueller, B.M.; Reisfeld, R.A.; Edgington, T.S.; Ruf, W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc. Natl. Acad. Sci. USA 1992, 89, 11832–11836. [Google Scholar] [CrossRef]
- Ngo, C.V.; Picha, K.; McCabe, F.; Millar, H.; Tawadros, R.; Tam, S.H.; Nakada, M.T.; Anderson, G.M. CNTO 859, a humanized anti-tissue factor monoclonal antibody, is a potent inhibitor of breast cancer metastasis and tumor growth in xenograft models. Int. J. Cancer 2007, 120, 1261–1267. [Google Scholar] [CrossRef]
- Saito, Y.; Hashimoto, Y.; Kuroda, J.; Yasunaga, M.; Koga, Y.; Takahashi, A.; Matsumura, Y. The inhibition of pancreatic cancer invasion-metastasis cascade in both cellular signal and blood coagulation cascade of tissue factor by its neutralisation antibody. Eur. J. Cancer 2011, 47, 2230–2239. [Google Scholar] [CrossRef]
- Amarzguioui, M.; Peng, Q.; Wiiger, M.T.; Vasovic, V.; Babaie, E.; Holen, T.; Nesland, J.M.; Prydz, H. Ex vivo and in vivo delivery of anti-tissue factor short interfering RNA inhibits mouse pulmonary metastasis of B16 melanoma cells. Clin. Cancer Res. 2006, 12, 4055–4061. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Talmage, K.E.; Massari, J.V.; La Jeunesse, C.M.; Flick, M.J.; Kombrinck, K.W.; Hu, Z.; Barney, K.A.; Degen, J.L. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood 2007, 110, 133–141. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Kombrinck, K.W.; Drew, A.F.; Grimes, T.S.; Kiser, J.H.; Degen, J.L.; Bugge, T.H. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000, 96, 3302–3309. [Google Scholar] [CrossRef]
- Milsom, C.C.; Yu, J.L.; Mackman, N.; Micallef, J.; Anderson, G.M.; Guha, A.; Rak, J.W. Tissue factor regulation by epidermal growth factor receptor and epithelial-to-mesenchymal transitions: Effect on tumor initiation and angiogenesis. Cancer Res. 2008, 68, 10068–10076. [Google Scholar] [CrossRef]
- Bourcy, M.; Suarez-Carmona, M.; Lambert, J.; Francart, M.E.; Schroeder, H.; Delierneux, C.; Skrypek, N.; Thompson, E.W.; Jerusalem, G.; Berx, G.; et al. Tissue Factor Induced by Epithelial-Mesenchymal Transition Triggers a Procoagulant State That Drives Metastasis of Circulating Tumor Cells. Cancer Res. 2016, 76, 4270–4282. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, J.; Cheng, J.; McMichael, E.; Yu, L.; Carson, W.E., 3rd. Targeting tissue factor as a novel therapeutic oncotarget for eradication of cancer stem cells isolated from tumor cell lines, tumor xenografts and patients of breast, lung and ovarian cancer. Oncotarget 2017, 8, 1481–1494. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, R.; Campbell, A.; McMichael, E.; Yu, L.; Ramaswamy, B.; London, C.A.; Xu, T.; Carson, W.E., 3rd. Targeting Tissue Factor for Immunotherapy of Triple-Negative Breast Cancer Using a Second-Generation ICON. Cancer Immunol. Res. 2018, 6, 671–684. [Google Scholar] [CrossRef]
- Francart, M.E.; Vanwynsberghe, A.M.; Lambert, J.; Bourcy, M.; Genna, A.; Ancel, J.; Perez-Boza, J.; Noel, A.; Birembaut, P.; Struman, I.; et al. Vimentin prevents a miR-dependent negative regulation of tissue factor mRNA during epithelial-mesenchymal transitions and facilitates early metastasis. Oncogene 2020, 39, 3680–3692. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, L.; Peng, Y.; Tao, M.; Li, L.; Xiu, D.; Yuan, C.; Ma, Z.; Jiang, B. Neutrophils assist the metastasis of circulating tumor cells in pancreatic ductal adenocarcinoma: A new hypothesis and a new predictor for distant metastasis. Medicine 2016, 95, e4932. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Cancer-related circulating and tumor-associated neutrophils – subtypes, sources and function. FEBS J. 2018, 285, 4316–4342. [Google Scholar] [CrossRef]
- Spicer, J.D.; McDonald, B.; Cools-Lartigue, J.J.; Chow, S.C.; Giannias, B.; Kubes, P.; Ferri, L.E. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012, 72, 3919–3927. [Google Scholar] [CrossRef]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1-integrin mediated interactions. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ozdemir, T.; Chung, C.-Y.; Robertson, G.P.; Dong, C. Sequential binding of αVβ3 and ICAM-1 determines fibrin-mediated melanoma capture and stable adhesion to CD11b/CD18 on neutrophils. J. Immunol. (Baltimore, Md.: 1950) 2011, 186, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Hajal, C.; Benjamin, D.C.; Yu, C.; Azizgolshani, H.; Hynes, R.O.; Kamm, R.D. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc. Natl. Acad. Sci. USA 2018, 115, 7022–7027. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, T.; Zhang, P.; Fu, C.; Dong, C. Fibrin serves as a divalent ligand that regulates neutrophil-mediated melanoma cells adhesion to endothelium under shear conditions. Am. J. Physiol. Cell Physiol. 2012, 302, C1189–C1201. [Google Scholar] [CrossRef]
- Al-Haidari, A.A.; Algethami, N.; Lepsenyi, M.; Rahman, M.; Syk, I.; Thorlacius, H. Neutrophil extracellular traps promote peritoneal metastasis of colon cancer cells. Oncotarget 2019, 10, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013. [Google Scholar] [CrossRef]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef]
- Rocks, N.; Vanwinge, C.; Radermecker, C.; Blacher, S.; Gilles, C.; Maree, R.; Gillard, A.; Evrard, B.; Pequeux, C.; Marichal, T.; et al. Ozone-primed neutrophils promote early steps of tumour cell metastasis to lungs by enhancing their NET production. Thorax 2019, 74, 768–779. [Google Scholar] [CrossRef]
- Huh, S.J.; Liang, S.; Sharma, A.; Dong, C.; Robertson, G.P. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010, 70, 6071–6082. [Google Scholar] [CrossRef]
- Cedervall, J.; Zhang, Y.; Olsson, A.K. Tumor-Induced NETosis as a Risk Factor for Metastasis and Organ Failure. Cancer Res. 2016, 76, 4311–4315. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Wagner, D.D. NETosis: A new factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 2014, 40, 277–283. [Google Scholar] [CrossRef]
- Savchenko, A.S.; Martinod, K.; Seidman, M.A.; Wong, S.L.; Borissoff, J.I.; Piazza, G.; Libby, P.; Goldhaber, S.Z.; Mitchell, R.N.; Wagner, D.D. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J. Thromb. Haemost. 2014, 12, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.C.; Mizurini, D.M.; Gomes, T.; Rochael, N.C.; Saraiva, E.M.; Dias, M.S.; Werneck, C.C.; Sielski, M.S.; Vicente, C.P.; Monteiro, R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017, 7, 6438. [Google Scholar] [CrossRef]
- Noubouossie, D.F.; Reeves, B.N.; Strahl, B.D.; Key, N.S. Neutrophils: Back in the thrombosis spotlight. Blood 2019, 133, 2186–2197. [Google Scholar] [CrossRef]
- Hisada, Y.; Grover, S.P.; Maqsood, A.; Houston, R.; Ay, C.; Noubouossie, D.F.; Cooley, B.C.; Wallén, H.; Key, N.S.; Thålin, C.; et al. Neutrophils and neutrophil extracellular traps enhance venous thrombosis in mice bearing human pancreatic tumors. Haematologica 2020, 105, 218. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef]
- Snoderly, H.T.; Boone, B.A.; Bennewitz, M.F. Neutrophil extracellular traps in breast cancer and beyond: Current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019, 21, 145. [Google Scholar] [CrossRef]
- Berger-Achituv, S.; Brinkmann, V.; Abed, U.A.; Kuhn, L.I.; Ben-Ezra, J.; Elhasid, R.; Zychlinsky, A. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front. Immunol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Wen, L.; Guo, L.; Zhang, W.; Li, Y.; Jiang, W.; Di, X.; Ma, J.; Feng, L.; Zhang, K.; Shou, J. Cooperation Between the Inflammation and Coagulation Systems Promotes the Survival of Circulating Tumor Cells in Renal Cell Carcinoma Patients. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating With the Enemy. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Choi, M.Y.; Cui, Y.H.; Kaushik, N.; Uddin, N.; Yoo, K.C.; Kim, M.J.; Lee, S.J. Regulation of FBXO4-mediated ICAM-1 protein stability in metastatic breast cancer. Oncotarget 2017, 8, 83100–83113. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, C.; Jenudi, Y.; Tshuva, R.Y.; Moskovich, D.; Alfandari, A.; Hercbergs, A.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. The Interplay Between Epithelial-Mesenchymal Transition (EMT) and the Thyroid Hormones-αvβ3 Axis in Ovarian Cancer. Horm. Cancer 2018, 9, 22–32. [Google Scholar] [CrossRef]
- Mori, S.; Kodaira, M.; Ito, A.; Okazaki, M.; Kawaguchi, N.; Hamada, Y.; Takada, Y.; Matsuura, N. Enhanced expression of integrin ανβ3 induced by TGF-β is required for the enhancing effect of fibroblast growth factor 1 (FGF1) in TGF-β-induced epithelial-mesenchymal transition (EMT) in mammary epithelial cells. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Shah, P.P.; Fong, M.Y.; Kakar, S.S. PTTG induces EMT through integrin α v Β 3-focal adhesion kinase signaling in lung cancer cells. Oncogene 2012, 31, 3124–3135. [Google Scholar] [CrossRef]
- Wang, P.C.; Weng, C.C.; Hou, Y.S.; Jian, S.F.; Fang, K.T.; Hou, M.F.; Cheng, K.H. Activation of VCAM-1 and its associated molecule CD44 leads to increased malignant potential of breast cancer cells. Int. J. Mol. Sci. 2014, 15, 3560–3579. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Chockley, P.J.; Keshamouni, V.G. Immunological Consequences of Epithelial-Mesenchymal Transition in Tumor Progression. J. Immunol. 2016, 197, 691–698. [Google Scholar] [CrossRef]
- Dominguez, C.; David, J.M.; Palena, C. Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin. Cancer Biol. 2017, 47, 177–184. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, B.; Xu, F.; Xu, Y.; Pan, J.; Song, J.; Zhang, J.; Huang, Y.; Xue, W. CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell Commun. Signal. 2019, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Steffen, T.; Giese, T.; Giese, N.; Longerich, T.; Schirmacher, P.; Hansch, G.M.; Gaida, M.M. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: The role of neutrophils and neutrophil-derived elastase. Clin. Dev. Immunol. 2012, 2012, 720768. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Savagner, P.; Ortiz-Cuaran, S.; Mahjoubi, L.; Saintigny, P.; Thiery, J.P.; Chouaib, S. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 2017, 11, 824–846. [Google Scholar] [CrossRef]
- Romeo, E.; Caserta, C.A.; Rumio, C.; Marcucci, F. The Vicious Cross-Talk between Tumor Cells with an EMT Phenotype and Cells of the Immune System. Cells 2019, 8, 460. [Google Scholar] [CrossRef]
- López-Soto, A.; Huergo-Zapico, L.; Galván, J.A.; Rodrigo, L.; de Herreros, A.G.; Astudillo, A.; Gonzalez, S. Epithelial-mesenchymal transition induces an antitumor immune response mediated by NKG2D receptor. J. Immunol. 2013, 190, 4408–4419. [Google Scholar] [CrossRef]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabieres, C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 2015, 9, 1773–1782. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; Del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Anantharaman, A.; Friedlander, T.; Lu, D.; Krupa, R.; Premasekharan, G.; Hough, J.; Edwards, M.; Paz, R.; Lindquist, K.; Graf, R.; et al. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer 2016, 16, 744. [Google Scholar] [CrossRef]
- Satelli, A.; Batth, I.S.; Brownlee, Z.; Rojas, C.; Meng, Q.H.; Kopetz, S.; Li, S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci. Rep. 2016, 6, 28910. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, C.; Carpino, G.; Nicolazzo, C.; Gradilone, A.; Gianni, W.; Gelibter, A.; Gaudio, E.; Cortesi, E.; Gazzaniga, P. PD-L1 and epithelial-mesenchymal transition in circulating tumor cells from non-small cell lung cancer patients: A molecular shield to evade immune system? Oncoimmunology 2017, 6, e1315488. [Google Scholar] [CrossRef] [PubMed]
- Alsuliman, A.; Colak, D.; Al-Harazi, O.; Fitwi, H.; Tulbah, A.; Al-Tweigeri, T.; Al-Alwan, M.; Ghebeh, H. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: Significance in claudin-low breast cancer cells. Mol. Cancer 2015, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Asgarova, A.; Asgarov, K.; Godet, Y.; Peixoto, P.; Nadaradjane, A.; Boyer-Guittaut, M.; Galaine, J.; Guenat, D.; Mougey, V.; Perrard, J.; et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology 2018, 7, e1423170. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.Y.; Kim, S.; Keam, B.; Kim, M.; Kim, T.M.; Kim, J.H.; Jeon, Y.K.; Lee, J.S.; Kwon, S.K.; Hah, J.H.; et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 15901–15914. [Google Scholar] [CrossRef]
- Ancel, J.; Birembaut, P.; Dewolf, M.; Durlach, A.; Nawrocki-Raby, B.; Dalstein, V.; Delepine, G.; Blacher, S.; Deslee, G.; Gilles, C.; et al. Programmed Death-Ligand 1 and Vimentin: A Tandem Marker as Prognostic Factor in NSCLC. Cancers 2019, 11, 1411. [Google Scholar] [CrossRef]
- Dongre, A.; Rashidian, M.; Reinhardt, F.; Bagnato, A.; Keckesova, Z.; Ploegh, H.L.; Weinberg, R.A. Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas. Cancer Res. 2017, 77, 3982–3989. [Google Scholar] [CrossRef]
- Noman, M.Z.; Janji, B.; Abdou, A.; Hasmim, M.; Terry, S.; Tan, T.Z.; Mami-Chouaib, F.; Thiery, J.P.; Chouaib, S. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology 2017, 6, e1263412. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhan, H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett. 2020, 468, 72–81. [Google Scholar] [CrossRef]
- Hamilton, D.H.; Huang, B.; Fernando, R.I.; Tsang, K.Y.; Palena, C. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res. 2014, 74, 2510–2519. [Google Scholar] [CrossRef]
- Kong, T.; Ahn, R.; Yang, K.; Zhu, X.; Fu, Z.; Morin, G.; Bramley, R.; Cliffe, N.C.; Xue, Y.; Kuasne, H.; et al. CD44 Promotes PD-L1 Expression and Its Tumor-Intrinsic Function in Breast and Lung Cancers. Cancer Res. 2020, 80, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W.; et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef] [PubMed]
- Weidenfeld, K.; Barkan, D. EMT and Stemness in Tumor Dormancy and Outgrowth: Are They Intertwined Processes? Front. Oncol. 2018, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.A.; Bhakta, N.R.; Kessenbrock, K.; Prummel, K.D.; Yu, Y.; Takai, K.; Zhou, A.; Eyob, H.; Balakrishnan, S.; Wang, C.Y.; et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015, 526, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.L.; Sosa, M.S.; Entenberg, D.; Hosseini, H.; Cheung, J.F.; Nobre, R.; Avivar-Valderas, A.; Nagi, C.; Girnius, N.; Davis, R.J.; et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 2016. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.S.; Degen, J.L. Mechanisms linking tumor cell-associated procoagulant function to tumor metastasis. Thromb. Res. 2007, 120 (Suppl. 2), S22–S28. [Google Scholar] [CrossRef]
- Palumbo, J.S. Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin. Thrombosis Hemost. 2008, 34, 154–160. [Google Scholar] [CrossRef]
- Degen, J.L.; Palumbo, J.S. Hemostatic factors, innate immunity and malignancy. Thromb. Res. 2012, 129 (Suppl. 1), S1–S5. [Google Scholar] [CrossRef]
- Labelle, M.; Hynes, R.O. The initial hours of metastasis: The importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012, 2, 1091–1099. [Google Scholar] [CrossRef]
- Lou, X.L.; Sun, J.; Gong, S.Q.; Yu, X.F.; Gong, R.; Deng, H. Interaction between circulating cancer cells and platelets: Clinical implication. Chin. J. Cancer Res. 2015, 27, 450–460. [Google Scholar] [CrossRef]
- Leblanc, R.; Peyruchaud, O. Metastasis: New functional implications of platelets and megakaryocytes. Blood 2016, 128, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bernabe, A.M.; Ferjancic, S.; Tlalka, M.; Zhao, L.; Allen, P.D.; Im, J.H.; Watson, K.; Hill, S.A.; Amirkhosravi, A.; Francis, J.L.; et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 2012, 119, 3164–3175. [Google Scholar] [CrossRef] [PubMed]
- Celia-Terrassa, T.; Meca-Cortes, O.; Mateo, F.; Martinez de Paz, A.; Rubio, N.; Arnal-Estape, A.; Ell, B.J.; Bermudo, R.; Diaz, A.; Guerra-Rebollo, M.; et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J. Clin. Investig. 2012, 122, 1849–1868. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Shirako, H.; Ohike, M.; Tsukamoto, N.; Kawakami, Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin. Exp. Metastasis 2013, 30, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Shirako, H.; Takeuchi, T.; Kawakami, Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 2009, 15, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Carmona, M.; Bourcy, M.; Lesage, J.; Leroi, N.; Syne, L.; Blacher, S.; Hubert, P.; Erpicum, C.; Foidart, J.M.; Delvenne, P.; et al. Soluble factors regulated by epithelial-mesenchymal transition mediate tumour angiogenesis and myeloid cell recruitment. J. Pathol. 2015, 236, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo Martin, Y.; Park, D.; Ramachandran, A.; Ombrato, L.; Calvo, F.; Chakravarty, P.; Spencer-Dene, B.; Derzsi, S.; Hill, C.S.; Sahai, E.; et al. Mesenchymal Cancer Cell-Stroma Crosstalk Promotes Niche Activation, Epithelial Reversion, and Metastatic Colonization. Cell Rep. 2015, 13, 2456–2469. [Google Scholar] [CrossRef]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef]
- Ocana, O.H.; Corcoles, R.; Fabra, A.; Moreno-Bueno, G.; Acloque, H.; Vega, S.; Barrallo-Gimeno, A.; Cano, A.; Nieto, M.A. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 2012, 22, 709–724. [Google Scholar] [CrossRef]
- Cortes-Hernandez, L.E.; Eslami, S.Z.; Pantel, K.; Alix-Panabieres, C. Molecular and Functional Characterization of Circulating Tumor Cells: From Discovery to Clinical Application. Clin. Chem. 2019. [Google Scholar] [CrossRef]
- Maheswaran, S.; Haber, D.A. Ex Vivo Culture of CTCs: An Emerging Resource to Guide Cancer Therapy. Cancer Res. 2015, 75, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Guo, T. Culture of Circulating Tumor Cells - Holy Grail and Big Challenge. Int. J. Cancer Clin. Res. 2016, 3. [Google Scholar] [CrossRef]
- Zhang, L.; Ridgway, L.D.; Wetzel, M.D.; Ngo, J.; Yin, W.; Kumar, D.; Goodman, J.C.; Groves, M.D.; Marchetti, D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013, 5, 180ra148. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014, 345, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Cayrefourcq, L.; Mazard, T.; Joosse, S.; Solassol, J.; Ramos, J.; Assenat, E.; Schumacher, U.; Costes, V.; Maudelonde, T.; Pantel, K.; et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015, 75, 892–901. [Google Scholar] [CrossRef]
- Zhang, Z.; Shiratsuchi, H.; Lin, J.; Chen, G.; Reddy, R.M.; Azizi, E.; Fouladdel, S.; Chang, A.C.; Lin, L.; Jiang, H.; et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget 2014, 5, 12383–12397. [Google Scholar] [CrossRef]
- Hamilton, G.; Burghuber, O.; Zeillinger, R. Circulating tumor cells in small cell lung cancer: Ex vivo expansion. Lung 2015, 193, 451–452. [Google Scholar] [CrossRef]
- Praharaj, P.P.; Bhutia, S.K.; Nagrath, S.; Bitting, R.L.; Deep, G. Circulating tumor cell-derived organoids: Current challenges and promises in medical research and precision medicine. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Tayoun, T.; Faugeroux, V.; Oulhen, M.; Aberlenc, A.; Pawlikowska, P.; Farace, F. CTC-Derived Models: A Window into the Seeding Capacity of Circulating Tumor Cells (CTCs). Cells 2019, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Gabriel, M.; Cochonneau, D.; Cadé, M.; Jubelin, C.; Heymann, M.F.; Heymann, D. Circulating tumor cell-derived pre-clinical models for personalized medicine. Cancers 2019, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef] [PubMed]
- De, T.; Goyal, S.; Balachander, G.; Chatterjee, K.; Kumar, P.; Babu, K.G.; Rangarajan, A. A Novel Ex Vivo System Using 3D Polymer Scaffold to Culture Circulating Tumor Cells from Breast Cancer Patients Exhibits Dynamic E-M Phenotypes. J. Clin. Med. 2019, 8, 1473. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.L.; Lee, S.C.; Kumar, P.; Tan, T.Z.; Warkiani, M.E.; Ow, S.G.; Nandi, S.; Lim, C.T.; Thiery, J.P. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget 2015, 6, 15578–15593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Zhang, J.; Sun, B.; Zheng, L.; Li, J.; Liu, S.; Sui, G.; Yin, Z. Microfluidic chip for isolation of viable circulating tumor cells of hepatocellular carcinoma for their culture and drug sensitivity assay. Cancer Biol. Ther. 2016, 17, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, B.R.; Jin, W.L.; Lou, G. Circulating tumor cells in precision oncology: Clinical applications in liquid biopsy and 3D organoid model. Cancer Cell Int. 2019, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, M.; Peddibhotla, S.; Yin, W.; A, T.S.; George, G.C.; Hong, D.S.; Marchetti, D. The isolation and characterization of CTC subsets related to breast cancer dormancy. Sci. Rep. 2015, 5, 17533. [Google Scholar] [CrossRef]
- Lu, J.; Fan, T.; Zhao, Q.; Zeng, W.; Zaslavsky, E.; Chen, J.J.; Frohman, M.A.; Golightly, M.G.; Madajewicz, S.; Chen, W.T. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. Int. J. Cancer 2010, 126, 669–683. [Google Scholar] [CrossRef]
- Pearl, M.L.; Zhao, Q.; Yang, J.; Dong, H.; Tulley, S.; Zhang, Q.; Golightly, M.; Zucker, S.; Chen, W.T. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 581–590. [Google Scholar] [CrossRef]
- Sullivan, J.P.; Nahed, B.V.; Madden, M.W.; Oliveira, S.M.; Springer, S.; Bhere, D.; Chi, A.S.; Wakimoto, H.; Rothenberg, S.M.; Sequist, L.V.; et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 2014, 4, 1299–1309. [Google Scholar] [CrossRef]
- Rossi, E.; Rugge, M.; Facchinetti, A.; Pizzi, M.; Nardo, G.; Barbieri, V.; Manicone, M.; De Faveri, S.; Chiara Scaini, M.; Basso, U.; et al. Retaining the long-survive capacity of Circulating Tumor Cells (CTCs) followed by xeno-transplantation: Not only from metastatic cancer of the breast but also of prostate cancer patients. Oncoscience 2014, 1, 49–56. [Google Scholar] [CrossRef]
- Hodgkinson, C.L.; Morrow, C.J.; Li, Y.; Metcalf, R.L.; Rothwell, D.G.; Trapani, F.; Polanski, R.; Burt, D.J.; Simpson, K.L.; Morris, K.; et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 2014, 20, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, M.; Liu, N.H.; Yin, W.; Boral, D.; Scamardo, A.; Hong, D.; Marchetti, D. The identification of a TNBC liver metastasis gene signature by sequential CTC-xenograft modeling. Mol. Oncol. 2019, 13, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Cadilha, B.L.; Markota, A.; Voigt, C.; Huang, Z.; Lin, P.P.; Wang, D.D.; Dai, J.; Kranz, G.; et al. Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.V.; Bardia, A.; Wittner, B.S.; Benes, C.; Ligorio, M.; Zheng, Y.; Yu, M.; Sundaresan, T.K.; Licausi, J.A.; Desai, R.; et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 2016, 537, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shiratsuchi, H.; Palanisamy, N.; Nagrath, S.; Ramnath, N. Expanded Circulating Tumor Cells from a Patient with ALK-Positive Lung Cancer Present with EML4-ALK Rearrangement Along with Resistance Mutation and Enable Drug Sensitivity Testing: A Case Study. J. Thorac. Oncol. 2017, 12, 397–402. [Google Scholar] [CrossRef]
- Khoo, B.L.; Grenci, G.; Jing, T.; Bena Lim, Y.; Lee, S.C.; Thiery, J.P.; Han, J.; Lim, C.T. Liquid biopsy and therapeutic response: Circulating tumor cell cultures for evaluation of anticancer treatment. Sci. Adv. 2016, 2, 1–15. [Google Scholar] [CrossRef]
- Klameth, L.; Rath, B.; Hochmaier, M.; Moser, D.; Redl, M.; Mungenast, F.; Gelles, K.; Ulsperger, E.; Zeillinger, R.; Hamilton, G. Small cell lung cancer: Model of circulating tumor cell tumorospheres in chemoresistance. Sci. Rep. 2017, 7, 5337. [Google Scholar] [CrossRef]
- Khoo, B.L.; Grenci, G.; Lim, Y.B.; Lee, S.C.; Han, J.; Lim, C.T. Expansion of patient-derived circulating tumor cells from liquid biopsies using a CTC microfluidic culture device. Nat. Protoc. 2018, 13, 34–58. [Google Scholar] [CrossRef]
- Lallo, A.; Schenk, M.W.; Frese, K.K.; Blackhall, F.; Dive, C. Circulating tumor cells and CDX models as a tool for preclinical drug development. Transl. Lung Cancer Res. 2017, 6, 397–408. [Google Scholar] [CrossRef]
- Lallo, A.; Gulati, S.; Schenk, M.W.; Khandelwal, G.; Berglund, U.W.; Pateras, I.S.; Chester, C.P.E.; Pham, T.M.; Kalderen, C.; Frese, K.K.; et al. Ex vivo culture of cells derived from circulating tumour cell xenograft to support small cell lung cancer research and experimental therapeutics. Br. J. Pharmacol. 2019, 176, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Drapkin, B.J.; George, J.; Christensen, C.L.; Mino-Kenudson, M.; Dries, R.; Sundaresan, T.; Phat, S.; Myers, D.T.; Zhong, J.; Igo, P.; et al. Genomic and Functional Fidelity of Small Cell Lung Cancer Patient-Derived Xenografts. Cancer Discov. 2018, 8, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.J.; Trapani, F.; Metcalf, R.L.; Bertolini, G.; Hodgkinson, C.L.; Khandelwal, G.; Kelly, P.; Galvin, M.; Carter, L.; Simpson, K.L.; et al. Tumourigenic non-small-cell lung cancer mesenchymal circulating tumour cells: A clinical case study. Ann. Oncol. 2016, 27, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Schochter, F.; Friedl, T.W.P.; deGregorio, A.; Krause, S.; Huober, J.; Rack, B.; Janni, W. Are Circulating Tumor Cells (CTCs) Ready for Clinical Use in Breast Cancer? An Overview of Completed and Ongoing Trials Using CTCs for Clinical Treatment Decisions. Cells 2019, 8, 1412. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.; O’Neill, A.; Alpaugh, K.; Wolff, A.C.; Northfelt, D.W.; Dang, C.T.; Sledge, G.W.; Miller, K.D. Association of Circulating Tumor Cells With Late Recurrence of Estrogen Receptor-Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1700–1706. [Google Scholar] [CrossRef]
- Pierga, J.Y.; Bidard, F.C.; Cropet, C.; Tresca, P.; Dalenc, F.; Romieu, G.; Campone, M.; Mahier Ait-Oukhatar, C.; Le Rhun, E.; Goncalves, A.; et al. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: The LANDSCAPE trial. Ann. Oncol. 2013, 24, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O’Rourke, M.A.; Lew, D.L.; et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 2014, 32, 3483–3489. [Google Scholar] [CrossRef]
- Scher, H.I.; Heller, G.; Molina, A.; Attard, G.; Danila, D.C.; Jia, X.; Peng, W.; Sandhu, S.K.; Olmos, D.; Riisnaes, R.; et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2015, 33, 1348–1355. [Google Scholar] [CrossRef]
- Sastre, J.; Vidaurreta, M.; Gomez, A.; Rivera, F.; Massuti, B.; Lopez, M.R.; Abad, A.; Gallen, M.; Benavides, M.; Aranda, E.; et al. Prognostic value of the combination of circulating tumor cells plus KRAS in patients with metastatic colorectal cancer treated with chemotherapy plus bevacizumab. Clin. Colorectal Cancer 2013, 12, 280–286. [Google Scholar] [CrossRef]
- Wu, S.; Liu, S.; Liu, Z.; Huang, J.; Pu, X.; Li, J.; Yang, D.; Deng, H.; Yang, N.; Xu, J. Classification of Circulating Tumor Cells by Epithelial-Mesenchymal Transition Markers. PLoS ONE 2015, 10, e0123976. [Google Scholar] [CrossRef]
- Markiewicz, A.; Ksiazkiewicz, M.; Welnicka-Jaskiewicz, M.; Seroczynska, B.; Skokowski, J.; Szade, J.; Zaczek, A.J. Mesenchymal phenotype of CTC-enriched blood fraction and lymph node metastasis formation potential. PLoS ONE 2014, 9, e93901. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.A.; Kallergi, G.; Zafeiriou, Z.; Manouras, L.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Co-expression of putative stemness and epithelial-to-mesenchymal transition markers on single circulating tumour cells from patients with early and metastatic breast cancer. BMC Cancer 2014, 14, 651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Hu, B.S.; Li, Z.L.; He, X.; Li, Y.; Lu, L.G. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol. Int. 2016, 10, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, P.A.; Polioudaki, H.; Agelaki, S.; Kallergi, G.; Saridaki, Z.; Mavroudis, D.; Georgoulias, V. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010, 288, 99–106. [Google Scholar] [CrossRef]
- Alonso-Alconada, L.; Muinelo-Romay, L.; Madissoo, K.; Diaz-Lopez, A.; Krakstad, C.; Trovik, J.; Wik, E.; Hapangama, D.; Coenegrachts, L.; Cano, A.; et al. Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer. Mol. Cancer 2014, 13, 223. [Google Scholar] [CrossRef]
- Mego, M.; Karaba, M.; Minarik, G.; Benca, J.; Silvia, J.; Sedlackova, T.; Manasova, D.; Kalavska, K.; Pindak, D.; Cristofanilli, M.; et al. Circulating Tumor Cells with Epithelial–to–mesenchymal Transition Phenotypes Associated with Inferior Outcomes in Primary Breast Cancer. Anticancer Res. 2019, 39, 1829–1837. [Google Scholar] [CrossRef]
- Cierna, Z.; Mego, M.; Janega, P.; Karaba, M.; Minarik, G.; Benca, J.; Sedlackova, T.; Cingelova, S.; Gronesova, P.; Manasova, D.; et al. Matrix metalloproteinase 1 and circulating tumor cells in early breast cancer. BMC Cancer 2014, 14, 472. [Google Scholar] [CrossRef]
- Chouaib, S.; Janji, B.; Tittarelli, A.; Eggermont, A.; Thiery, J.P. Tumor plasticity interferes with anti-tumor immunity. Crit. Rev. Immunol. 2014, 34, 91–102. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, H.; Li, F.P.; Hu, Y.F.; Mou, T.Y.; Lin, T.; Yu, J.; Zheng, L.; Li, G.X. Evaluation of epithelial-mesenchymal transitioned circulating tumor cells in patients with resectable gastric cancer: Relevance to therapy response. World J. Gastroenterol. 2015, 21, 13259–13267. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, M.S.; Lee, J.S.; Lee, H.K.; Lee, J.H.; Sohn, Y.W.; Tazaki, K.; Nakamaru, K.; Wakita, K.; Jeon, B.H.; et al. Abstract 1586: Evaluation of AXL expression on circulating tumor cells from EGFR mutated lung cancer patients who have relapsed after the EGFR TKI treatment. Cancer Res. 2018. [Google Scholar] [CrossRef]
- Hamilton, G.; Rath, B.; Klameth, L.; Hochmair, M. Receptor tyrosine kinase expression of circulating tumor cells in small cell lung cancer. Oncoscience 2015, 2, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Mahalingam, D.; Osmulski, P.; Jadhav, R.R.; Wang, C.M.; Leach, R.J.; Chang, T.C.; Weitman, S.D.; Kumar, A.P.; Sun, L.; et al. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate 2013, 73, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Fici, P.; Gallerani, G.; Morel, A.-P.; Mercatali, L.; Ibrahim, T.; Scarpi, E.; Amadori, D.; Puisieux, A.; Rigaud, M.; Fabbri, F. Splicing factor ratio as an index of epithelial-mesenchymal transition and tumor aggressiveness in breast cancer. Oncotarget 2017, 8, 2423–2436. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).