Role and Therapeutic Potential of Melatonin in the Central Nervous System and Cancers
Abstract
1. Introduction
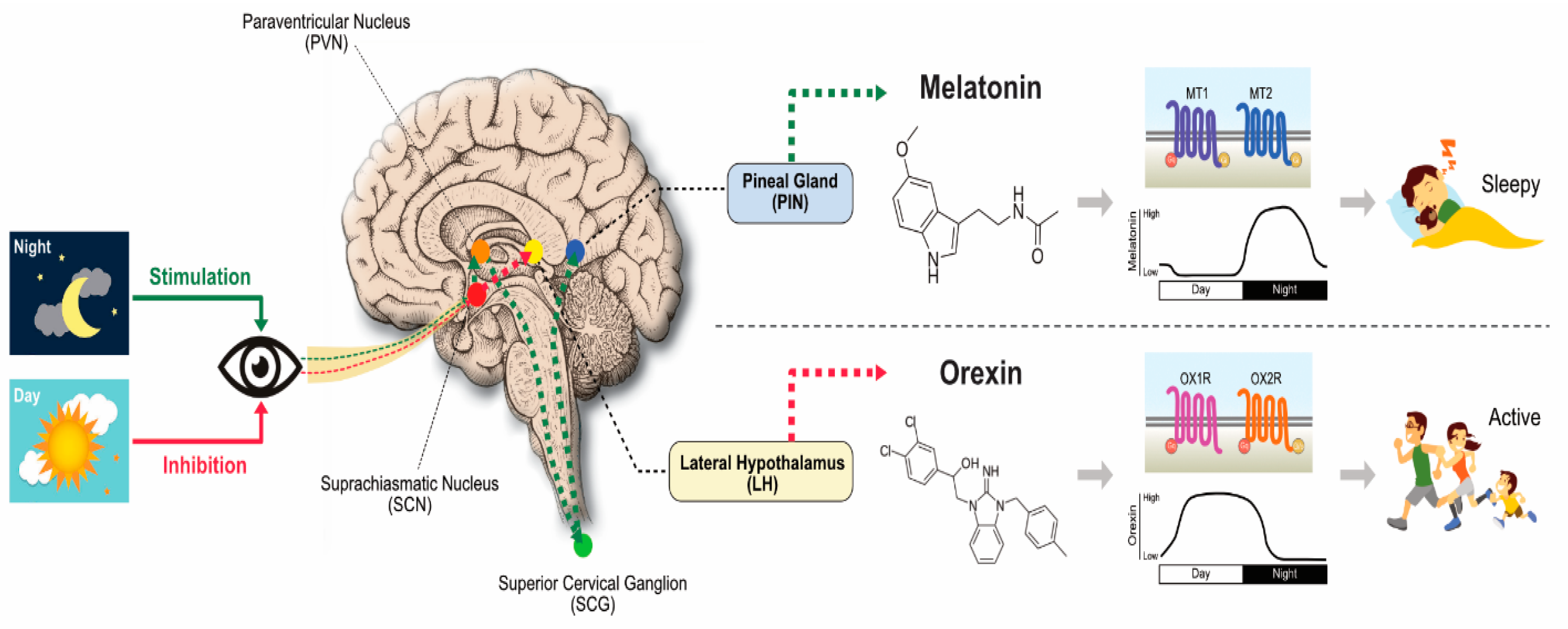
2. Synthesis, Biology, and Functions of MLT
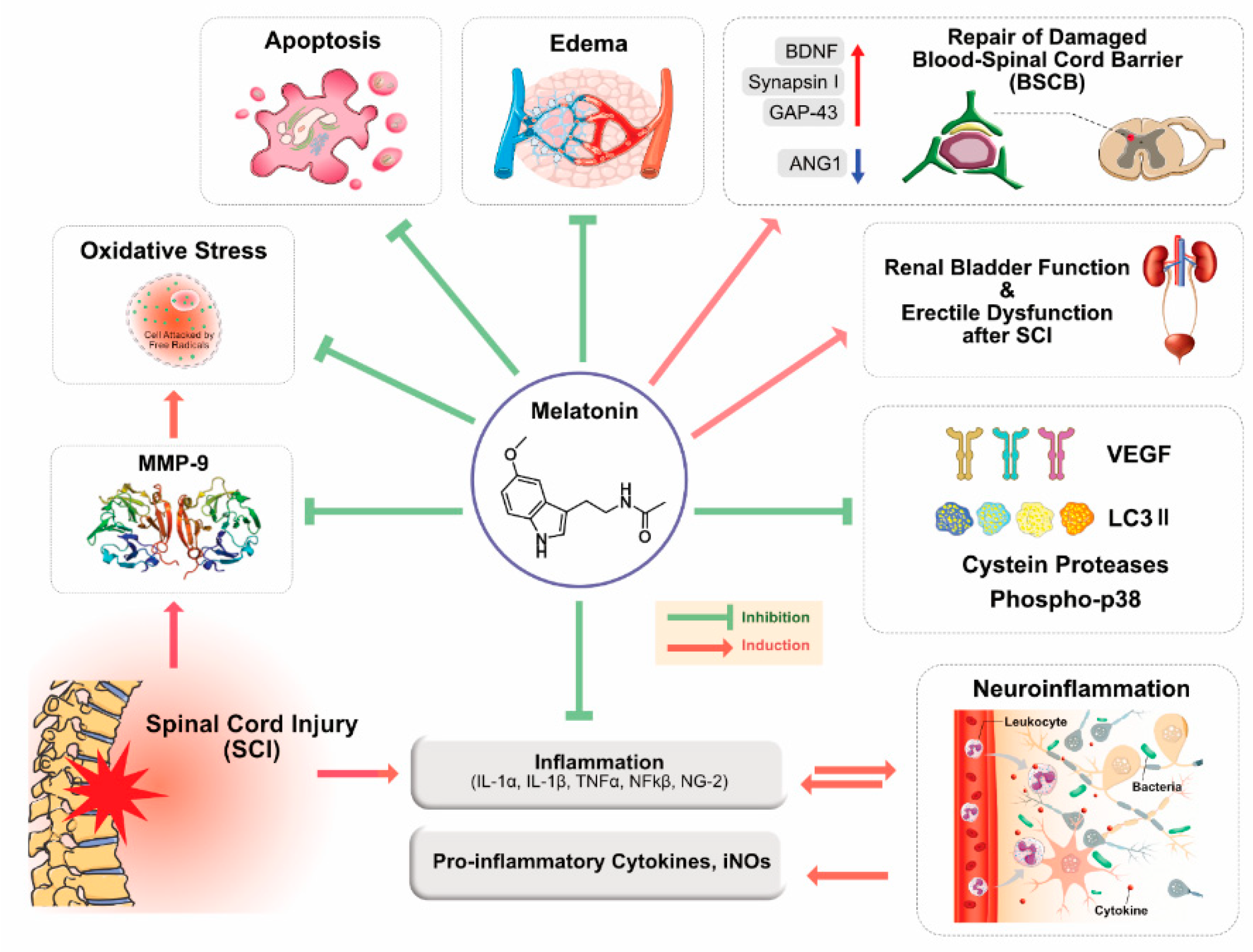
3. Role of MLT in Brain and Spinal Cord Injuries
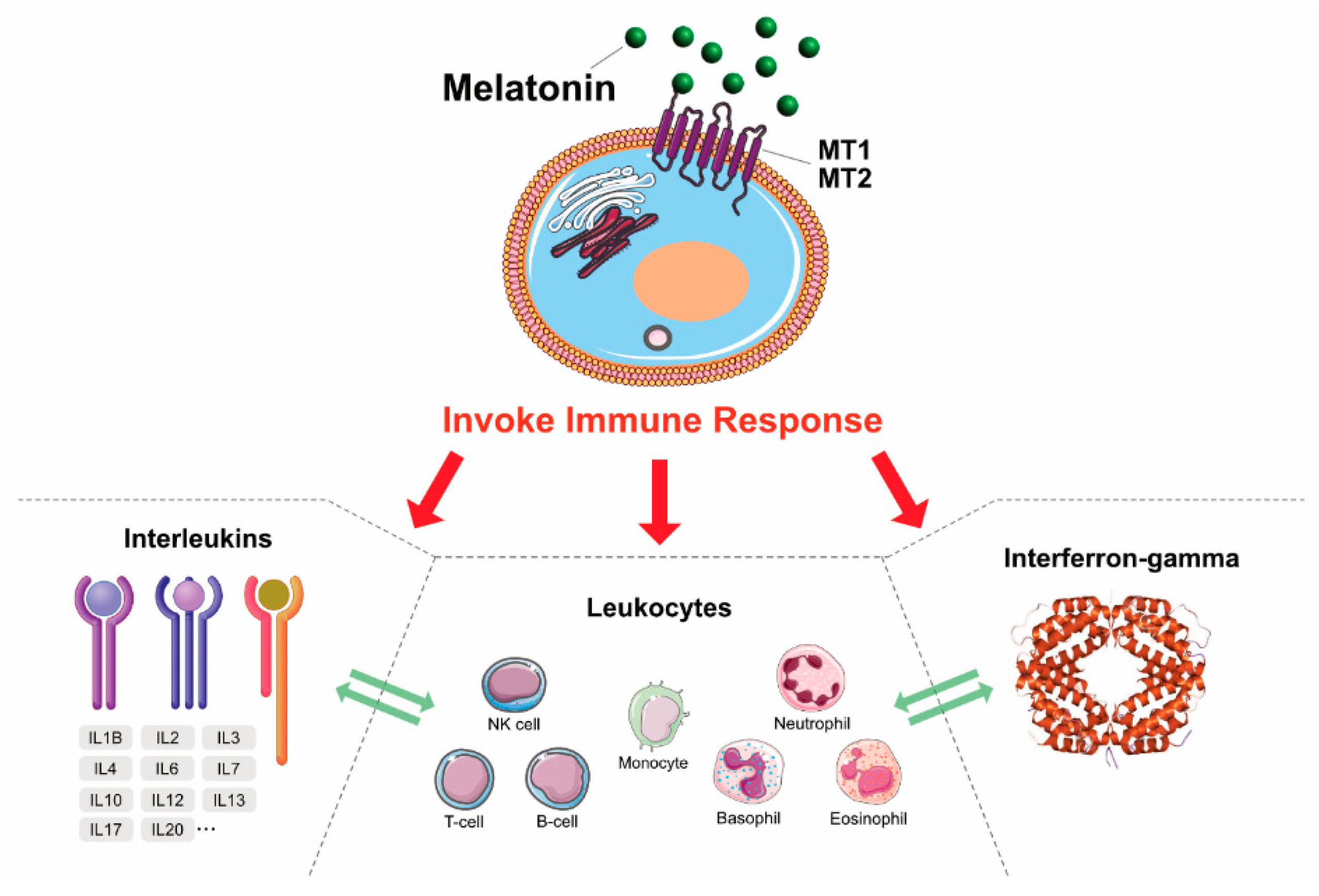
4. MLT: Antioxidant, Neuroprotectant, and Immunomodulatory Agent
5. MLT as an Anticancer Agent
6. CNS Cancers
7. MLT: A Novel Therapeutic Agent for the Treatment of CNS Disorders and Cancers
8. Conclusion and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samantaray, S.; Thakore, N.P.; Matzelle, D.D.; Varma, A.; Ray, S.K.; Banik, N.L. Neuroprotective drugs in traumatic CNS injury. Open Drug Discov. J. 2010, 2, 174–180. [Google Scholar]
- Samantaray, S.; Das, A.; Thakore, N.P.; Matzelle, D.D.; Reiter, R.J.; Ray, S.K.; Banik, N.L. Therapeutic potential of melatonin in traumatic central nervous system injury. J. Pineal Res. 2009, 47, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bhalala, O.G.; Srikanth, M.; Kessler, J.A. The emerging roles of microRNAs in CNS injuries. Nat. Rev. Neurol. 2013, 9, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cooke, M.J.; Shoichet, M.S. Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends Biotechnol. 2012, 30, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Khalatbary, A.R.; Tiraihi, T.; Boroujeni, M.B.; Ahmadvand, H.; Tavafi, M.; Tamjidipoor, A. Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Res. 2010, 1306, 168–175. [Google Scholar] [CrossRef]
- Bains, M.; Hall, E.D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta Bioenerg. 2011, 1822, 675–684. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Gaist, D.; A García-Rodríguez, L.; Soerensen, H.T.; Hallas, J.; Friis, S. Use of low-dose aspirin and non-aspirin nonsteroidal anti-inflammatory drugs and risk of glioma: A case–control study. Br. J. Cancer 2013, 108, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, G.J. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 2000, 28, 193–202. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in Aging and Disease—Multiple Consequences of Reduced Secretion, Options and Limits of Treatment. Aging Dis. 2011, 3, 194–225. [Google Scholar] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Casado, M.E.D.; Cabello, M.E.L.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Fraschini, F.; Descarries, L.; Gobbi, G. Anatomical and cellular localization of melatonin MT1and MT2receptors in the adult rat brain. J. Pineal Res. 2015, 58, 397–417. [Google Scholar] [CrossRef]
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin receptors: Distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 2017, 222, 2921–2939. [Google Scholar] [CrossRef]
- Pinato, L.; Ramos, D.; Hataka, A.; Rossignoli, P.S.; Granado, M.D.; Mazzetto, M.C.; Campos, L.; Junior, M.D.G. Day/night expression of MT 1 and MT 2 receptors in hypothalamic nuclei of the primate Sapajus apella. J. Chem. Neuroanat. 2017, 81, 10–17. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127, 46–63. [Google Scholar] [CrossRef]
- Watson, N.; Diamandis, T.; Gonzales-Portillo, C.; Reyes, S.; Borlongan, C.V. Melatonin as an antioxidant for stroke neuroprotection. Cell Transplant. 2016, 25, 883–891. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; LeÓn, J.; Carazo, A.; Khaldy, H. Mitochondrial regulation by melatonin and its metabolites. In Developments in Tryptophan and Serotonin Metabolism; Springer: Berlin/Heidelberg, Germany, 2003; pp. 549–557. [Google Scholar]
- Acuña-Castroviejo, D. Melatonin role in the mitochondrial function. Front. Biosci. 2007, 12, 947. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin: Signaling mechanisms of a pleiotropic agent. BioFactors 2009, 35, 183–192. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2016, 62, e12377. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Tan, D.-X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin′s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Rahim, I.; Acuña-Fernández, C.; Ortiz, F.; Solera-Marín, J.; Sayed, R.; Casado, M.E.D.; Rusanova, I.; López, L.C.; Escames, G. Melatonin, clock genes and mitochondria in sepsis. Cell. Mol. Life Sci. 2017, 74, 3965–3987. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Corral, S.A.; Acuña-Castroviejo, D.; Coto-Montes, A.; Boga, J.A.; Manchester, L.C.; Fuentes-Broto, L.; Korkmaz, A.; Ma, S.; Tan, D.-X.; Reiter, R.J. Alzheimer’s disease: Pathological mechanisms and the beneficial role of melatonin. J. Pineal Res. 2011, 52, 167–202. [Google Scholar] [CrossRef]
- Reiter, R.; Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 52. [Google Scholar]
- Zawilska, J.B.; Skene, D.J.; Arendt, J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep. 2009, 61, 383–410. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.-X.; Reiter, R. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R. Melatonin: Clinical relevance. Best Pr. Res. Clin. Endocrinol. Metab. 2003, 17, 273–285. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 Melatonin Receptors in Mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Melatonin: The chemical expression of darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Acarin, L.; González, B.; Castellano, B. Neuronal, astroglial and microglial cytokine expression after an excitotoxic lesion in the immature rat brain. Eur. J. Neurosci. 2000, 12, 3505–3520. [Google Scholar] [CrossRef]
- Liu, W.-C.; Wang, X.; Zhang, X.; Chen, X.; Jin, X. Melatonin Supplementation, a Strategy to Prevent Neurological Diseases through Maintaining Integrity of Blood Brain Barrier in Old People. Front. Aging Neurosci. 2017, 9, 165. [Google Scholar] [CrossRef]
- Muxel, S.M.; Lapa, M.; Monteiro, A.W.A.; Cecon, E.; Tamura, E.K.; Winter, L.M.F.; Markus, R.P. NF-κB Drives the Synthesis of Melatonin in RAW 264.7 Macrophages by Inducing the Transcription of the Arylalkylamine-N-Acetyltransferase (AA-NAT) Gene. PLoS ONE 2012, 7, e52010. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Meng, F.-T.; Wu, L.; Zhou, J.-N. Serotoninergic and melatoninergic systems are expressed in mouse embryonic fibroblasts NIH3T3 cells. Neuro Endocrinol. Lett. 2013, 34, 236–240. [Google Scholar]
- Tan, D.-X.; Manchester, L.C.; Sánchez-Barceló, E.; Mediavilla, M.D.; Reiter, R. Significance of High Levels of Endogenous Melatonin in Mammalian Cerebrospinal Fluid and in the Central Nervous System. Curr. Neuropharmacol. 2010, 8, 162–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, D.-X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.-T.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. Antiinflammatory Activity of Melatonin in Central Nervous System. Curr. Neuropharmacol. 2010, 8, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Alvarez-Idaboy, J.R. Glutathione: Mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011, 1, 1763. [Google Scholar] [CrossRef]
- Brown, G.M.; Cardinali, D.P.; Adrien, J.; Agargun, M.Y.; Ahmadi, N.; Ahmed, I.; Arnedt, J.T.; Barbera, J.; Beaulieu-Bonneau, S.; Beitinger, M.E.; et al. Melatonin and mental illness. Sleep Ment. Illn. 2011, 119–129. [Google Scholar] [CrossRef]
- Mccarty, M. Minimizing the cancer-promotional activity of cox-2 as a central strategy in cancer prevention. Med. Hypotheses 2012, 78, 45–57. [Google Scholar] [CrossRef]
- Santoro, R.; Marani, M.; Blandino, G.; Muti, P.; Strano, S. Melatonin triggers p53Ser phosphorylation and prevents DNA damage accumulation. Oncogene 2011, 31, 2931–2942. [Google Scholar] [CrossRef]
- Mehta, A.; Kaur, G. Potential role of melatonin in prevention and treatment of oral carcinoma. Indian J. Dent. 2014, 5, 56–61. [Google Scholar] [CrossRef]
- Jardim, B.V.; Ferreira, L.C.; Borin, T.F.; Moschetta, M.G.; Gelaleti, G.B.; Lopes, J.R.; Maschio, L.B.; Leonel, C.; Gonçalves, N.N.; Martins, G.R. Evaluation of the anti-angiogenic action of melatonin in breast cancer. BMC Proc. 2013, 7, 11. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2015, 56, 361–383. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J.M. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C.; Campbell, A. Mechanisms Underlying Tumor Suppressive Properties of Melatonin. Int. J. Mol. Sci. 2018, 19, 2205. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Pariente, R.; Bejarano, I.; Espino, J.; Rodríguez, A.B.; Pariente, J.A. Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother. Pharmacol. 2017, 80, 985–998. [Google Scholar] [CrossRef]
- Naseem, M.; Parvez, S. Role of Melatonin in Traumatic Brain Injury and Spinal Cord Injury. Sci. World J. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Fibroblast growth factors: From molecular evolution to roles in development, metabolism and disease. J. Biochem. 2010, 149, 121–130. [Google Scholar] [CrossRef]
- Itoh, N. Hormone-like (endocrine) Fgfs: Their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. 2010, 342, 1–11. [Google Scholar] [CrossRef]
- Wang, X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009, 15, 345–557. [Google Scholar] [CrossRef]
- Bars, D.; Thivolle, P.; Vitte, P.; Bojkowski, C.; Chazot, G.; Arendt, J.; Frackowiak, R.; Claustrat, B. PET and plasma pharmacokinetic studies after bolus intravenous administration of [11C]melatonin in humans. Int. J. Radiat. Appl. Instrum. Part B Nucl. Med. Boil. 1991, 18, 357–362. [Google Scholar] [CrossRef]
- Shekleton, J.A.; Parcell, D.L.; Redman, J.R.; Phipps-Nelson, J.; Ponsford, J.L.; Rajaratnam, S.M. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology 2010, 74, 1732–1738. [Google Scholar] [CrossRef]
- Reiter, R.; Tan, D.-X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Reiter, R.J. Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur. J. Pharmacol. 2001, 426, 1–10. [Google Scholar] [CrossRef]
- Reiter, R.; Manchester, L.; Tan, D.-X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Oxidative processes and antioxidative defense mechanisms in the aging brain 1. FASEB J. 1995, 9, 526–533. [Google Scholar] [CrossRef]
- Lee, E.-J.; Lee, M.-Y.; Chen, H.-Y.; Hsu, Y.-S.; Wu, T.-S.; Chen, S.-T.; Chang, G.-L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005, 38, 42–52. [Google Scholar] [CrossRef]
- Lee, S.-H.; Chun, W.; Kong, P.-J.; Han, J.A.; Cho, B.P.; Kwon, O.-Y.; Lee, H.J.; Kim, S.-S. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J. Pineal Res. 2006, 40, 79–85. [Google Scholar] [CrossRef]
- Paredes, S.D.; Rancan, L.; Kireev, R.; Gonzalez, A.; Louzao, P.; Gonzalez, P.; Rodríguez-Bobada, C.; García, C.; Vara, E.; Tresguerres, J.A. Melatonin Counteracts at a Transcriptional Level the Inflammatory and Apoptotic Response Secondary to Ischemic Brain Injury Induced by Middle Cerebral Artery Blockade in Aging Rats. BioResearch Open Access 2015, 4, 407–416. [Google Scholar] [CrossRef]
- Wang, F.-W.; Wang, Z.; Zhang, Y.; Du, Z.-X.; Zhang, X.-L.; Liu, Q.; Guo, Y.-J.; Li, X.; Hao, A. Protective effect of melatonin on bone marrow mesenchymal stem cells against hydrogen peroxide-induced apoptosis in vitro. J. Cell. Biochem. 2013, 114, 2346–2355. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2014, 58, 61–70. [Google Scholar] [CrossRef]
- Balduini, W.; Carloni, S.; Perrone, S.; Bertrando, S.; Tataranno, M.; Negro, S.; Proietti, F.; Longini, M.; Buonocore, G. The use of melatonin in hypoxic-ischemic brain damage: An experimental study. J. Matern. Neonatal Med. 2012, 25, 119–124. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Lee, M.-Y.; Chen, H.-Y.; Kuo, Y.-L.; Lin, S.-C.; Wu, T.-S.; Lee, E.-J. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J. Pineal Res. 2006, 40, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Khalatbary, A.R.; Ahmadvand, H. Anti-Inflammatory Effect of the Epigallocatechin Gallate Following Spinal Cord Trauma in Rat. Iran. Biomed. J. 2011, 15, 31–37. [Google Scholar] [PubMed]
- Zhang, Y.-K.; Liu, J.-T.; Peng, Z.-W.; Fan, H.; Yao, A.-H.; Cheng, P.; Liu, L.; Ju, G.; Kuang, F. Different TLR4 expression and microglia/macrophage activation induced by hemorrhage in the rat spinal cord after compressive injury. J. Neuroinflammation 2013, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Chen, X.; Zhang, Y.; Zhang, W.-X.; Liu, Y.-D.; Liu, Z.-J.; Wu, Q.-C.; Zhang, Y.-J. Melatonin for the treatment of spinal cord injury. Neural Regen. Res. 2018, 13, 1685–1692. [Google Scholar] [CrossRef]
- Feeser, V.R.; Loria, R.M. Modulation of traumatic brain injury using progesterone and the role of glial cells on its neuroprotective actions. J. Neuroimmunol. 2011, 237, 4–12. [Google Scholar] [CrossRef]
- Breunig, J.; Guillot-Sestier, M.-V.; Town, T. Brain injury, neuroinflammation and Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 5. [Google Scholar] [CrossRef]
- Dehghan, F.; Hadad, M.K.; Asadikaram, G.; Najafipour, H.; Shahrokhi, N. Effect of Melatonin on Intracranial Pressure and Brain Edema Following Traumatic Brain Injury: Role of Oxidative Stresses. Arch. Med. Res. 2013, 44, 251–258. [Google Scholar] [CrossRef]
- Kabadi, S.V.; Maher, T.J. Posttreatment with uridine and melatonin following traumatic brain injury reduces edema in various brain regions in rats. Ann. NY Acad. Sci. 2010, 1199, 105–113. [Google Scholar] [CrossRef]
- Sanz, O.; Acarin, L.; Gonzalez, B.; Castellano, B. NF-kappaB and IkappaBalpha expression following traumatic brain injury to the immature rat brain. J. Neurosci. Res. 2002, 67, 772–780. [Google Scholar] [CrossRef]
- Beni, S.M.; Kohen, R.; Reiter, R.J.; Tan, D.-X.; Shohami, E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-κB and AP-1. FASEB J. 2004, 18, 149–151. [Google Scholar] [CrossRef]
- Readnower, R.D.; Chavko, M.; Adeeb, S.; Conroy, M.D.; Pauly, J.R.; McCarron, R.M.; Sullivan, P.G. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 2010, 88, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.; Deniz, M.; Yüksel, M.; Keyer-Uysal, M.; Şener, G. The protective effect of melatonin and amlodipine against cerebral ischemia/reperfusion-induced oxidative brain injury in rats. Marmara Med. J. 2009, 22, 43–44. [Google Scholar]
- Hausmann, O.N. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003, 41, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, H.; Qiao, L.; Wang, X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediat. Inflamm. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Paterniti, I.; Genovese, T.; Crisafulli, C.; Mazzon, E.; Di Paola, R.; Galuppo, M.; Bramanti, P.; Cuzzocrea, S. Treatment with green tea extract attenuates secondary inflammatory response in an experimental model of spinal cord trauma. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 179–192. [Google Scholar] [CrossRef]
- Szczepanik, M. Melatonin and its influence on immune system. J. Physiol. Pharmacol. 2007, 58, 115–124. [Google Scholar]
- Schiaveto-de-Souza, A.; Da-Silva, C.; Defino, H.L.A.; Del Bel, E.A. Effect of melatonin on the functional recovery from experimental traumatic compression of the spinal cord. Braz. J. Med. Biol. Res. 2013, 46, 348–358. [Google Scholar] [CrossRef]
- Fang, X.-Q.; Mei, F.; Fan, S.-W.; Gu, C. Protection of erythropoietin on experimental spinal cord injury by reducing the expression of thrombospondin-1 and transforming growth factor-β. Chin. Med. J. 2009, 122, 1631–1635. [Google Scholar]
- Genovese, T.; Mazzon, E.; Muià, C.; Bramanti, P.; De Sarro, A.; Cuzzocrea, S. Attenuation in the evolution of experimental spinal cord trauma by treatment with melatonin. J. Pineal Res. 2005, 38, 198–208. [Google Scholar] [CrossRef]
- Park, K.; Lee, Y.; Park, S.; Lee, H.J.; Hong, Y.; Kil Lee, S.; Hong, Y. Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J. Pineal. Res. 2010, 48, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Genovese, T.; Caminiti, R.; Bramanti, P.; Meli, R.; Cuzzocrea, S. Melatonin reduces stress-activated/mitogen-activated protein kinases in spinal cord injury. J. Pineal. Res. 2009, 46, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Mazzon, E.; Crisafulli, C.; Esposito, E.; Di Paola, R.; Muià, C.; Di Bella, P.; Bramanti, P.; Cuzzocrea, S. Effects of combination of melatonin and dexamethasone on secondary injury in an experimental mice model of spinal cord trauma. J. Pineal. Res. 2007, 43, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Asayama, K.; Yamadera, H.; Ito, T.; Suzuki, H.; Kudo, Y.; Endo, S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J. Nippon. Med. Sch. 2003, 70, 334–341. [Google Scholar] [CrossRef]
- Waldbaum, S.; Patel, M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2009, 88, 23–45. [Google Scholar] [CrossRef]
- Bai, S.-K.; Lee, S.-J.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Lee, H.; Kwon, Y.-G.; Chung, C.-K.; Kim, Y.-M. β-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-κB activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free. Radic. Boil. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Ates, O.; Cayli, S.R.; Gurses, I.; Turkoz, Y.; Tarim, O.; Cakir, C.O.; Kocak, A. Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J. Clin. Neurosci. 2007, 14, 658–665. [Google Scholar] [CrossRef]
- Tsai, H.-L.; Chang, C.-N.; Chang, S.-J. The effects of pilocarpine-induced status epilepticus on oxidative stress/damage in developing animals. Brain Dev. 2010, 32, 25–31. [Google Scholar] [CrossRef]
- Scannevin, R.H.; Chollate, S.; Jung, M.Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Ianăş, O.; Olinescu, R.; Bădescu, I. Melatonin involvement in oxidative processes. Endocrinologie 1991, 29, 147–153. [Google Scholar] [PubMed]
- Poeggeler, B.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J. Pineal Res. 1993, 14, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Reiter, R.; Manchester, L.; Yan, M.-T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardelan, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Pieri, C.; Marra, M.; Moroni, F.; Recchioni, R.; Marcheselli, F. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994, 55, PL271–PL276. [Google Scholar] [CrossRef]
- Srinivasan, V. Melatonin oxidative stress and neurodegenerative diseases. Indian J. Exp. Boil. 2002, 40, 668–679. [Google Scholar]
- Lin, A.M.Y.; Fang, S.F.; Chao, P.L.; Yang, C.H. Melatonin attenuates arsenite-induced apoptosis in rat brain: Involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of α-synuclein. J. Pineal Res. 2007, 43, 163–171. [Google Scholar] [CrossRef]
- Lin, A.M.Y.; Feng, S.F.; Chao, P.L.; Yang, C.H. Melatonin inhibits arsenite-induced peripheral neurotoxicity. J. Pineal Res. 2009, 46, 64–70. [Google Scholar] [CrossRef]
- Uygur, R.; Aktas, C.; Caglar, V.; Uygur, E.; Erdogan, H.; Ozen, O.A. Protective effects of melatonin against arsenic-induced apoptosis and oxidative stress in rat testes. Toxicol. Ind. Heal. 2013, 32, 848–859. [Google Scholar] [CrossRef]
- Fan, L.-L.; Sun, G.-P.; Wei, W.; Wang, Z.-G.; Ge, L.; Fu, W.-Z.; Wang, H. Melatonin and Doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J. Gastroenterol. 2010, 16, 1473–1481. [Google Scholar] [CrossRef]
- Pant, H.H.; Rao, M.V. Evaluation of in vitro anti-genotoxic potential of melatonin against arsenic and fluoride in human blood cultures. Ecotoxicol. Environ. Saf. 2010, 73, 1333–1337. [Google Scholar] [CrossRef]
- Akbulut, K.G.; Gönül, B.; Akbulut, H. The role of melatonin on gastric mucosal cell proliferation and telomerase activity in ageing. J. Pineal Res. 2009, 47, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Swarnakar, S. Induction of matrix metalloproteinase-9 and -3 in nonsteroidal anti-inflammatory drug-induced acute gastric ulcers in mice: Regulation by melatonin. J. Pineal Res. 2009, 47, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Tajes Orduna, M.; Pelegrí Gabalda, C.; Vilaplana Hortensi, J.; Pallàs LLiberia, M.; Camins Espuny, A. An evaluation of the neuroprotective effects of melatonin in an in vitro experimental model of age-induced neuronal apoptosis. J. Pineal Res. 2009, 46, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Macias, M.; Escames, G.; Reiter, R.J.; Agapito, M.; Ortiz, G.; Acuña-Castroviejo, D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 2000, 28, 242–248. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress. A review. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Martín, M.; Macías, M.; Escames, G.; León, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 2001, 30, 65–74. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Costantino, G.; Mazzon, E.; Micali, A.; De Sarro, A.; Caputi, A.P. Beneficial effects of melatonin in a rat model of splanchnic artery occlusion and reperfusion. J. Pineal Res. 2000, 28, 52–63. [Google Scholar] [CrossRef]
- Paskaloğlu, K.; Sener, T.E.; Kapucu, C.; Ayanoğlu-Dülger, G. Melatonin treatment protects against sepsis-induced functional and biochemical changes in rat ileum and urinary bladder. Life Sci. 2004, 74, 1093–1104. [Google Scholar] [CrossRef]
- Pei, Z.; Cheung, R.T.F. Pretreatment with melatonin exerts anti-inflammatory effects against ischemia/reperfusion injury in a rat middle cerebral artery occlusion stroke model. J. Pineal Res. 2004, 37, 85–91. [Google Scholar] [CrossRef]
- Jesudason, E.P.; Baben, B.; Ashok, B.S.; Masilamoni, J.G.; Kirubagaran, R.; Jebaraj, W.C.E.; Jayakumar, R. Anti-inflammatory effect of melatonin on Aβ vaccination in mice. Mol. Cell. Biochem. 2006, 298, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; Acuña-Castroviejo, D.; Doerrier, C.; Dayoub, J.C.; López, L.C.; Venegas, C.; García, J.A.; López, A.; Volt, H.; Sánchez, M.L.; et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J. Pineal Res. 2014, 58, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gil, B.I.; Moneim, A.E.A.; Ortiz, F.; Shen, Y.-Q.; Soto-Mercado, V.; Mendivil-Perez, M.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Molina-Navarro, M.M.; Garcia-Verdugo, J.M.; et al. Melatonin protects rats from radiotherapy-induced small intestine toxicity. PLoS ONE 2017, 12, e0174474. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Fan, C.; Hu, W.; Jiang, S.; Ma, Z.; Yan, X.; Deng, C.; Di, S.; Xin, Z.; Wu, G.; et al. Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J. Pineal Res. 2016, 60, 253–262. [Google Scholar] [CrossRef]
- Hardeland, R.; Backhaus, C.; Fadavi, A. Reactions of the NO redox forms NO+, •NO and HNO (protonated NO–) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J. Pineal Res. 2007, 43, 382–388. [Google Scholar] [CrossRef]
- Gilad, E.; Pick, E.; Matzkin, H.; Zisapel, N. Melatonin receptors in benign prostate epithelial cells: Evidence for the involvement of cholera and pertussis toxins-sensitive G proteins in their signal transduction pathways. Prostate 1998, 35, 27–34. [Google Scholar] [CrossRef]
- Cao, S.; Shrestha, S.; Li, J.; Yu, X.; Chen, J.; Yan, F.; Ying, G.; Gu, C.; Wang, L.; Chen, G. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci. Rep. 2017, 7, 2417. [Google Scholar] [CrossRef]
- Permpoonputtana, K.; Govitrapong, P. The anti-inflammatory effect of melatonin on methamphetamine-induced pro-inflammatory mediators in human neuroblastoma dopamine SH-SY5Y cell lines. Neurotox. Res. 2013, 23, 189–199. [Google Scholar] [CrossRef]
- Krityakiarana, W.; Zhao, P.M.; Nguyen, K.; Gomez-Pinilla, F.; Kotchabhakdi, N.; De Vellis, J.; Espinosa-Jeffrey, A. Proof-of Concept that an Acute Trophic Factors Intervention After Spinal Cord Injury Provides an Adequate Niche for Neuroprotection, Recruitment of Nestin-Expressing Progenitors and Regeneration. Neurochem. Res. 2016, 41, 431–449. [Google Scholar] [CrossRef]
- Paterniti, I.; Campolo, M.; Cordaro, M.; Impellizzeri, D.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. PPAR-α modulates the anti-inflammatory effect of melatonin in the secondary events of spinal cord injury. Mol. Neurobiol. 2016, 54, 5973–5987. [Google Scholar] [CrossRef]
- Haddadi, G.H.; Fardid, R. Oral administration of melatonin modulates the expression of tumor necrosis factor-alpha (TNF-alpha) gene in irradiated rat cervical spinal cord. Rep. Pract. Oncol. Radiother. 2015, 20, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Meregalli, S.; Nosetto, L.; Barni, S.; Tancini, G.; Fossati, V.; Maestroni, G. Increased survival time in brain glioblastomas by a radioneuroendocrine strategy with radiotherapy plus melatonin compared to radiotherapy alone. Oncology 1996, 53, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Giani, L.; Zerbini, S.; Trabattoni, P.; Rovelli, F. Biotherapy with the pineal immunomodulating hormone melatonin versus melatonin plus aloe vera in untreatable advanced solid neoplasms. Nat. Immun. 1998, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, A.; Luboshitzky, R.; Tiosano, D.; Ben-Harush, M.; Goldsher, D.; Lavie, P. Melatonin replacement corrects sleep disturbances in a child with pineal tumor. Neurology 1996, 46, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Sos, M.; Omar, R.A.; Bick, R.J.; Hickson-Bick, D.L.; Reiter, R.J.; Efthimiopoulos, S.; Robakis, N.K. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 1997, 17, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.E.; Tai, J.; Hahn, G.; Rothstein, R.R. Melatonin replacement therapy in a child with a pineal tumor. J. Child Neurol. 2001, 16, 139–140. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Zavarzina, N.Y.; Zabezhinski, M.A.; Popovich, I.G.; Zimina, O.A.; Shtylick, A.V.; Arutjunyan, A.V.; Oparina, T.I.; Prokopenko, V.M.; Mikhalski, A.I. Melatonin increases both life span and tumor incidence in female CBA mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, B311–B323. [Google Scholar] [CrossRef]
- Granzotto, M.; Rapozzi, V.; Decorti, G.; Giraldi, T. Effects of melatonin on doxorubicin cytotoxicity in sensitive and pleiotropically resistant tumor cells. J. Pineal Res. 2001, 31, 206–213. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Xu, J.-N.; Xu, R.-K.; Pang, S.-F. Inhibitory effects of melatonin on the growth of pituitary prolactin-secreting tumor in rats. J. Pineal Res. 2006, 40, 230–235. [Google Scholar] [CrossRef]
- Martin, V.; Herrera, F.; Carrera-González, M.D.P.; García-Santos, G.; Antolín, I.; Blanco, J.R.; Rodriguez, C. Intracellular Signaling Pathways Involved in the Cell Growth Inhibition of Glioma Cells by Melatonin. Cancer Res. 2006, 66, 1081–1088. [Google Scholar] [CrossRef]
- García-Santos, G.; Antolín, I.; Herrera, F.; Martin, V.; Carrera-González, M.D.P.; Rodriguez, C.; Blanco, J.R. Melatonin induces apoptosis in human neuroblastoma cancer cells. J. Pineal Res. 2006, 41, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P. Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol. Boil. 2007, 55, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Brivio, F.; Fumagalli, L.; Messina, G.; Vigoré, L.; Parolini, D.; Colciago, M.; Rovelli, F. Neuroimmunomodulation in medical oncology: Application of psychoneuroimmunology with subcutaneous low-dose IL-2 and the pineal hormone melatonin in patients with untreatable metastatic solid tumors. Anticancer. Res. 2008, 28, 1377–1381. [Google Scholar]
- Casado-Zapico, S.; Rodriguez-Blanco, J.; García-Santos, G.; Martín, V.; Sánchez-Sánchez, A.M.; Antolín, I.; Rodriguez, C. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: Potentiation of the extrinsic apoptotic pathway. J. Pineal Res. 2010, 48, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Sanchez-Sanchez, A.M.; Herrera, F.; Gomez-Manzano, C.; Fueyo, J.; Alvarez-Vega, M.A.; Antolín, I.; Rodriguez, C. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br. J. Cancer 2013, 108, 2005–2012. [Google Scholar] [CrossRef]
- Blask, D.E.; Brainard, G.C.; Dauchy, R.T.; Hanifin, J.P.; Davidson, L.K.; Krause, J.A.; Sauer, L.A.; Rivera-Bermudez, M.A.; Dubocovich, M.L.; Jasser, S.A. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005, 65, 11174–11184. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Dauchy, R.T.; Dauchy, E.M.; Mao, L.; Hill, S.M.; Greene, M.W.; Belancio, V.P.; Sauer, L.A.; Davidson, L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE 2014, 9, e102776. [Google Scholar] [CrossRef]
- Martin, V.; Sanchez, A.M.S.; Puente-Moncada, N.; Gomez-Lobo, M.; Álvarez-Vega, M.A.; Antolín, I.; Rodriguez, C. Involvement of autophagy in melatonin-induced cytotoxicity in glioma-initiating cells. J. Pineal Res. 2014, 57, 308–316. [Google Scholar] [CrossRef]
- Jumnongprakhon, P.; Govitrapong, P.; Tocharus, C.; Pinkaew, D.; Tocharus, J. Melatonin Protects Methamphetamine-Induced Neuroinflammation Through NF-κB and Nrf2 Pathways in Glioma Cell Line. Neurochem. Res. 2015, 40, 1448–1456. [Google Scholar] [CrossRef]
- Xu, S.; Pi, H.; Zhang, L.; Zhang, N.; Li, Y.; Zhang, H.; Tang, J.; Li, H.; Feng, M.; Deng, P.; et al. Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J. Pineal Res. 2016, 60, 291–302. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Ma, H.; Zhang, S.; Yang, H.; Wang, H.; Fang, Z. Melatonin attenuates hypoxia-induced epithelial-mesenchymal transition and cell aggressive via Smad7/CCL20 in glioma. Oncotarget 2017, 8, 93580. [Google Scholar] [CrossRef] [PubMed]
- González, A.; González-González, A.; Alonso-González, C.; Menéndez-Menéndez, J.; Martínez-Campa, C.; Cos, S. Melatonin inhibits angiogenesis in SH-SY5Y human neuroblastoma cells by downregulation of VEGF. Oncol. Rep. 2017, 37, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.J.; Jung, J.H.; Shin, E.A.; Kim, S.H. Melatonin disturbs SUMO ylation-mediated crosstalk between c-Myc and nestin via MT 1 activation and promotes the sensitivity of paclitaxel in brain cancer stem cells. J. Pineal Res. 2018, 65, e12496. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.J.; Kim, S.H.; Kwak, S.; Park, S.H.; Song, J.H.; Jung, J.H.; Kim, H.; Choi, K.C. Inhibition of TFEB oligomerization by co-treatment of melatonin with vorinostat promotes the therapeutic sensitivity in glioblastoma and glioma stem cells. J. Pineal Res. 2019, 66, e12556. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Shakiba, S.; Mehrzadi, S.; Afshari, K.; Rahimnia, A.H.; Dehpour, A.R. Anticonvulsant effect of melatonin through ATP-sensitive channels in mice. Fundam. Clin. Pharmacol. 2019, 34, 148–155. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Z.; Hu, L.; Zhang, S.; Zhao, C.; Yang, H.; Wang, H.; Fang, Z.; Wu, L.; Chen, X. The melatonin-MT1 receptor axis modulates tumor growth in PTEN-mutated gliomas. Biochem. Biophys. Res. Commun. 2018, 496, 1322–1330. [Google Scholar] [CrossRef]
- Hall, W.A.; Djalilian, H.R.; Nussbaum, E.S.; Cho, K.H. Long-term survival with metastatic cancer to the brain. Med. Oncol. 2000, 17, 279–286. [Google Scholar] [CrossRef]
- Gavrilovic, I.; Posner, J.B. Brain metastases: Epidemiology and pathophysiology. J. Neuro Oncol. 2005, 75, 5–14. [Google Scholar] [CrossRef]
- Davis, F.G.; Dolecek, T.A.; McCarthy, B.J.; Villano, J.L. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012, 14, 1171–1177. [Google Scholar] [CrossRef]
- Tabouret, E.; Chinot, O.; Metellus, P.; Tallet, A.; Viens, P.; Gonçalves, A. Recent trends in epidemiology of brain metastases: An overview. Anticancer. Res. 2012, 32, 4655–4662. [Google Scholar]
- Arvold, N.D.; Lee, E.Q.; Mehta, M.P.; Margolin, K.; Alexander, B.M.; Lin, N.U.; Anders, C.K.; Soffietti, R.; Camidge, D.R.; Vogelbaum, M.A.; et al. Updates in the management of brain metastases. Neuro Oncol. 2016, 18, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.E.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.J.; Buter, J.; Honkoop, A.H.; Boerman, D.; De Vos, F.Y.F.; et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014, 15, 943–953. [Google Scholar] [CrossRef]
- Stafford, J.H.; Hirai, T.; Deng, L.; Chernikova, S.B.; Urata, K.; West, B.; Brown, J.M. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016, 18, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Yong, Z. Upconverting nanoparticles as nanotransducers for photodynamic therapy in cancer cells. Nanomedince 2008, 3, 73–82. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Tanaka, S.; Louis, D.N.; Curry, W.T.; Batchelor, T.T.; Dietrich, J. Diagnostic and therapeutic avenues for glioblastoma: No longer a dead end? Nat. Rev. Clin. Oncol. 2013, 10, 14–26. [Google Scholar] [CrossRef]
- Del Paggio, J.C. Immunotherapy: Cancer immunotherapy and the value of cure. Nat. Rev. Clin. Oncol. 2018, 15, 268–270. [Google Scholar] [CrossRef]
- Boussiotis, V.A.; Charest, A. Immunotherapies for malignant glioma. Oncogene 2018, 37, 1121–1141. [Google Scholar] [CrossRef]
- Jackson, C.; Lim, M. Glioma special issue introduction. Glioma 2019, 2, 1. [Google Scholar] [CrossRef]
- Liu, L.; Ni, F.; Zhang, J.; Wang, C.; Lu, X.; Guo, Z.; Yao, S.; Shu, Y.; Xu, R. Thermal analysis in the rat glioma model during directly multipoint injection hyperthermia incorporating magnetic nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 10333–10338. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shi, Z.; Liu, R.; Wu, Y.; Zhang, X. Combined-therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology. Theranostics 2020, 10, 3223–3239. [Google Scholar] [CrossRef] [PubMed]
- Candolfi, M.; Yagiz, K.; Foulad, D.; Alzadeh, G.E.; Tesarfreund, M.; Muhammad, A.G.; Puntel, M.; Kroeger, K.M.; Liu, C.; Lee, S.; et al. Release of HMGB1 in Response to Proapoptotic Glioma Killing Strategies: Efficacy and Neurotoxicity. Clin. Cancer Res. 2009, 15, 4401–4414. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Delgado, E.L.; Grafals-Ruiz, N.; Vivas-Mejía, P.E. RNA interference for glioblastoma therapy: Innovation ladder from the bench to clinical trials. Life Sci. 2017, 188, 26–36. [Google Scholar] [CrossRef]
- Xie, F.; Xu, M.; Lu, J.; Mao, L.; Wang, S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 2019, 18, 146. [Google Scholar] [CrossRef]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective Effects of PD-1 on Akt and Ras Pathways Regulate Molecular Components of the Cell Cycle and Inhibit T Cell Proliferation. Sci. Signal. 2012, 5, 46. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, J.; Liu, Q.; Liu, Y.; Fan, L.; Wang, F.; Yu, H.; Li, Y.; Bu, L.; Li, X.; et al. Exosomes from Melatonin Treated Hepatocellularcarcinoma Cells Alter the Immunosupression Status through STAT3 Pathway in Macrophages. Int. J. Boil. Sci. 2017, 13, 723–734. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Sileni, V.C.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. New Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.K.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; González, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Moretti, E.; Favero, G.; Rodella, L.F.; Rezzani, R. Melatonin’s Antineoplastic Potential Against Glioblastoma. Cells 2020, 9, 599. [Google Scholar] [CrossRef]
- Zheng, X.; Pang, B.; Gu, G.; Gao, T.; Zhang, R.; Pang, Q.; Liu, Q. Melatonin Inhibits Glioblastoma Stem-like cells through Suppression of EZH2-NOTCH1 Signaling Axis. Int. J. Boil. Sci. 2017, 13, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Osanai, K.; Kobayashi, Y.; Otsu, M.; Izawa, T.; Sakai, K.; Iwashita, M. Ramelteon, a selective MT1/MT2 receptor agonist, suppresses the proliferation and invasiveness of endometrial cancer cells. Hum. Cell 2017, 30, 397. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-M.; Lin, C.-W.; Yang, J.-S.; Yang, W.-E.; Su, S.-C.; Yang, S.-F. Melatonin inhibits TPA-induced oral cancer cell migration by suppressing matrix metalloproteinase-9 activation through the histone acetylation. Oncotarget 2016, 7, 21952–21967. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-Y.; Lin, C.-W.; Chien, M.; Reiter, R.J.; Su, S.-C.; Hsieh, Y.-H.; Yang, S.-F. Melatonin suppresses TPA-induced metastasis by downregulating matrix metalloproteinase-9 expression through JNK/SP-1 signaling in nasopharyngeal carcinoma. J. Pineal Res. 2016, 61, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-L.; Liu, Y.-F.; Su, C.-W.; Su, S.-C.; Chen, M.-K.; Yang, S.-F.; Lin, C.-W. Functional genetic variant in the Kozak sequence of WW domain-containing oxidoreductase (WWOX) gene is associated with oral cancer risk. Oncotarget 2016, 7, 69384–69396. [Google Scholar] [CrossRef]
- Bouatia-Naji, N.; Bonnefond, A.; Cavalcanti-Proença, C.; Sparsø, T.; Holmkvist, J.; Marchand, M.; Delplanque, J.; Lobbens, S.; Rocheleau, G.; Durand, E.; et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 2009, 41, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef]
- Ortiz, G.G.; A Benítez-King, G.; A Rosales-Corral, S.; Pacheco-Moisés, F.P.; E Velázquez-Brizuela, I. Cellular and Biochemical Actions of Melatonin which Protect Against Free Radicals: Role in Neurodegenerative Disorders. Curr. Neuropharmacol. 2008, 6, 203–214. [Google Scholar] [CrossRef]
- Seifman, M.; Adamides, A.A.; Nguyen, P.N.; Vallance, A.S.; Cooper, D.J.; Kossmann, T.; Rosenfeld, J.V.; Morganti-Kossmann, C. Endogenous Melatonin Increases in Cerebrospinal Fluid of Patients after Severe Traumatic Brain Injury and Correlates with Oxidative Stress and Metabolic Disarray. Br. J. Pharmacol. 2008, 28, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Bumb, J.; Enning, F.; Mueller, J.; Van Der List, T.; Rohleder, C.; Findeisen, P.; Noelte, I.; Schwarz, E.; Leweke, F. Differential melatonin alterations in cerebrospinal fluid and serum of patients with major depressive disorder and bipolar disorder. Compr. Psychiatry 2016, 68, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Angeles-Castellanos, M.; Ramírez-Gonzalez, F.; Ubaldo-Reyes, L.; Rodríguez-Mayoral, O.; Escobar, C. Loss of melatonin daily rhythmicity is asociated with delirium development in hospitalized older adults. Sleep Sci. 2016, 9, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, J.; Liu, K.J.; Rosenberg, G.A.; Yang, Y.; Liu, W. Normobaric hyperoxia combined with minocycline provides greater neuroprotection than either alone in transient focal cerebral ischemia. Exp. Neurol. 2012, 240, 9–16. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Pan, S.; Zhang, H.; Fang, M.; Jiang, H.; Zhang, H.; Gao, Z.; Xu, K.; Li, Z.; et al. Melatonin protects against blood-brain barrier damage by inhibiting the TLR4/ NF-κB signaling pathway after LPS treatment in neonatal rats. Oncotarget 2017, 8, 31638–31654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; An, R.; Yang, Y.; Yang, X.; Liu, H.; Yue, L.; Li, X.; Lin, Y.; Reiter, R.; Qu, Y. Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: The role of SIRT1 signaling. J. Pineal Res. 2015, 59, 230–239. [Google Scholar] [CrossRef]
- López, L.C.; Escames, G.; Ortiz, F.; Ros, E.; Acuña-Castroviejo, D. Melatonin restores the mitochondrial production of ATP in septic mice. Neuro Endocrinol. Lett. 2006, 27, 623–630. [Google Scholar]
- Ortiz, F.; García, J.A.; Acuña-Castroviejo, D.; Doerrier, C.; López, A.; Venegas, C.; Volt, H.; Sánchez, M.L.; López, L.C.; Escames, G. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: Inhibition of iNOS and preservation of nNOS. J. Pineal Res. 2013, 56, 71–81. [Google Scholar] [CrossRef]
- An, R.; Zhao, L.; Xi, C.; Li, H.; Shen, G.; Liu, H.; Zhang, S.; Sun, L. Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res. Cardiol. 2015, 111, 8. [Google Scholar] [CrossRef]
- Lewy, A.J.; Bauer, V.K.; Cutler, N.L.; Sack, R.L. Melatonin treatment of winter depression: A pilot study. Psychiatry Res. Neuroimaging 1998, 77, 57–61. [Google Scholar] [CrossRef]
- Lewy, A.J.; Emens, J.S.; Lefler, B.J.; Yuhas, K.; Jackman, A. Melatonin Entrains Free-running Blind People According to a Physiological Dose-response Curve. Chrono Int. 2005, 22, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Harrold, J.A.; Cutler, D.J. The hypothalamus and the regulation of energy homeostasis: Lifting the lid on a black box. Proc. Nutr. Soc. 2000, 59, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Sainz, R.M.; Mayo, J.C.; León, J.; Reiter, R.J. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production. Endocrine 2005, 27, 149–157. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2012, 54, 245–257. [Google Scholar] [CrossRef]
- Barni, S.; Lissoni, P.; Cazzaniga, M.E.; Ardizzoia, A.; Meregalli, S.; Fossati, V.; Fumagalli, L.; Brivio, F.; Tancini, G. A Randomized Study of Low-Dose Subcutaneous lnterleukin-2 Plus Melatonin versus Supportive Care Alone in Metastatic Colorectal Cancer Patients Progressing under 5-Fluorouracil and Folates. Oncology 1995, 52, 243–245. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Meregalli, S.; Fossati, V.; Cazzaniga, M.E.; Esposti, D.; Tancini, G. Modulation of cancer endocrine therapy by melatonin: A phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br. J. Cancer 1995, 71, 854–856. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Mandala, M.; Ardizzoia, A.; Paolorossi, F.; Vaghi, M.; Longarini, R.; Malugani, F.; Tancini, G. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur. J. Cancer 1999, 35, 1688–1692. [Google Scholar] [CrossRef]
- Lissoni, P.; Chilelli, M.; Villa, S.; Cerizza, L.; Tancini, G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: A randomized trial. J. Pineal Res. 2003, 35, 12–15. [Google Scholar] [CrossRef]
- Cerea, G.; Vaghi, M.; Ardizzoia, A.; Villa, S.; Bucovec, R.; Mengo, S.; Gardani, G.; Tancini, G.; Lissoni, P. Biomodulation of cancer chemotherapy for metastatic colorectal cancer: A randomized study of weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal hormone melatonin in metastatic colorectal cancer patients progressing on 5-fluorouracil-containing combinations. Anticancer. Res. 2003, 23, 1951–1954. [Google Scholar]
- Seely, D.; Wu, P.; Fritz, H.; Kennedy, D.A.; Tsui, T.; Seely, A.J.; Mills, E. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integr. Cancer Ther. 2012, 11, 293–303. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Zheng, X.; Du, X. Therapeutic strategies of melatonin in cancer patients: A systematic review and meta-analysis. Onco Targets Ther. 2018, 11, 7895–7908. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurunathan, S.; Kang, M.-H.; Kim, J.-H. Role and Therapeutic Potential of Melatonin in the Central Nervous System and Cancers. Cancers 2020, 12, 1567. https://doi.org/10.3390/cancers12061567
Gurunathan S, Kang M-H, Kim J-H. Role and Therapeutic Potential of Melatonin in the Central Nervous System and Cancers. Cancers. 2020; 12(6):1567. https://doi.org/10.3390/cancers12061567
Chicago/Turabian StyleGurunathan, Sangiliyandi, Min-Hee Kang, and Jin-Hoi Kim. 2020. "Role and Therapeutic Potential of Melatonin in the Central Nervous System and Cancers" Cancers 12, no. 6: 1567. https://doi.org/10.3390/cancers12061567
APA StyleGurunathan, S., Kang, M.-H., & Kim, J.-H. (2020). Role and Therapeutic Potential of Melatonin in the Central Nervous System and Cancers. Cancers, 12(6), 1567. https://doi.org/10.3390/cancers12061567




