Src Inhibitors Pyrazolo[3,4-d]pyrimidines, Si306 and Pro-Si306, Inhibit Focal Adhesion Kinase and Suppress Human Glioblastoma Invasion In Vitro and In Vivo
Abstract
1. Introduction
2. Results
2.1. Sensitivity of GBM Cells to Si306 and Pro-Si306 Corresponds to Src Expression
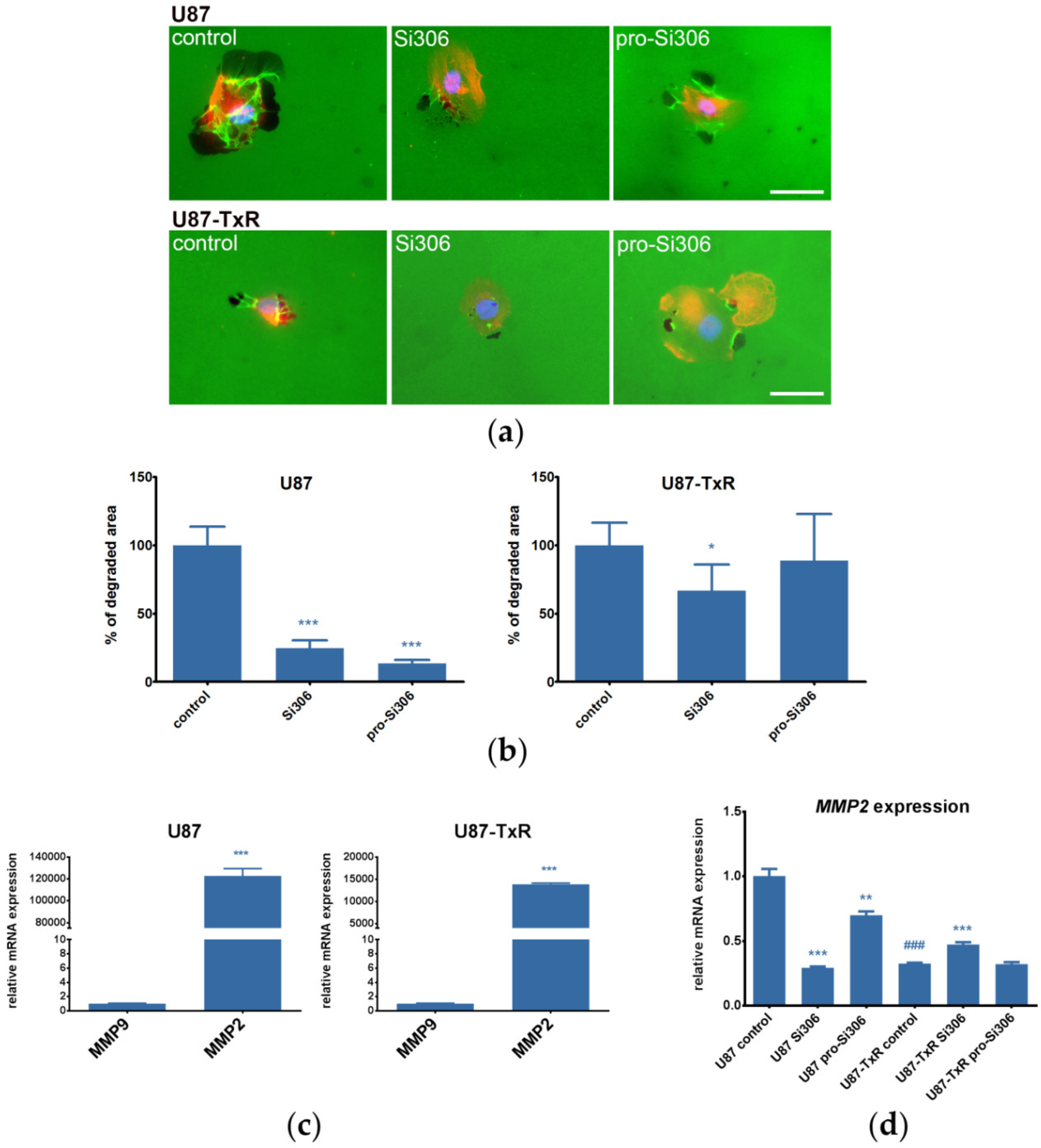
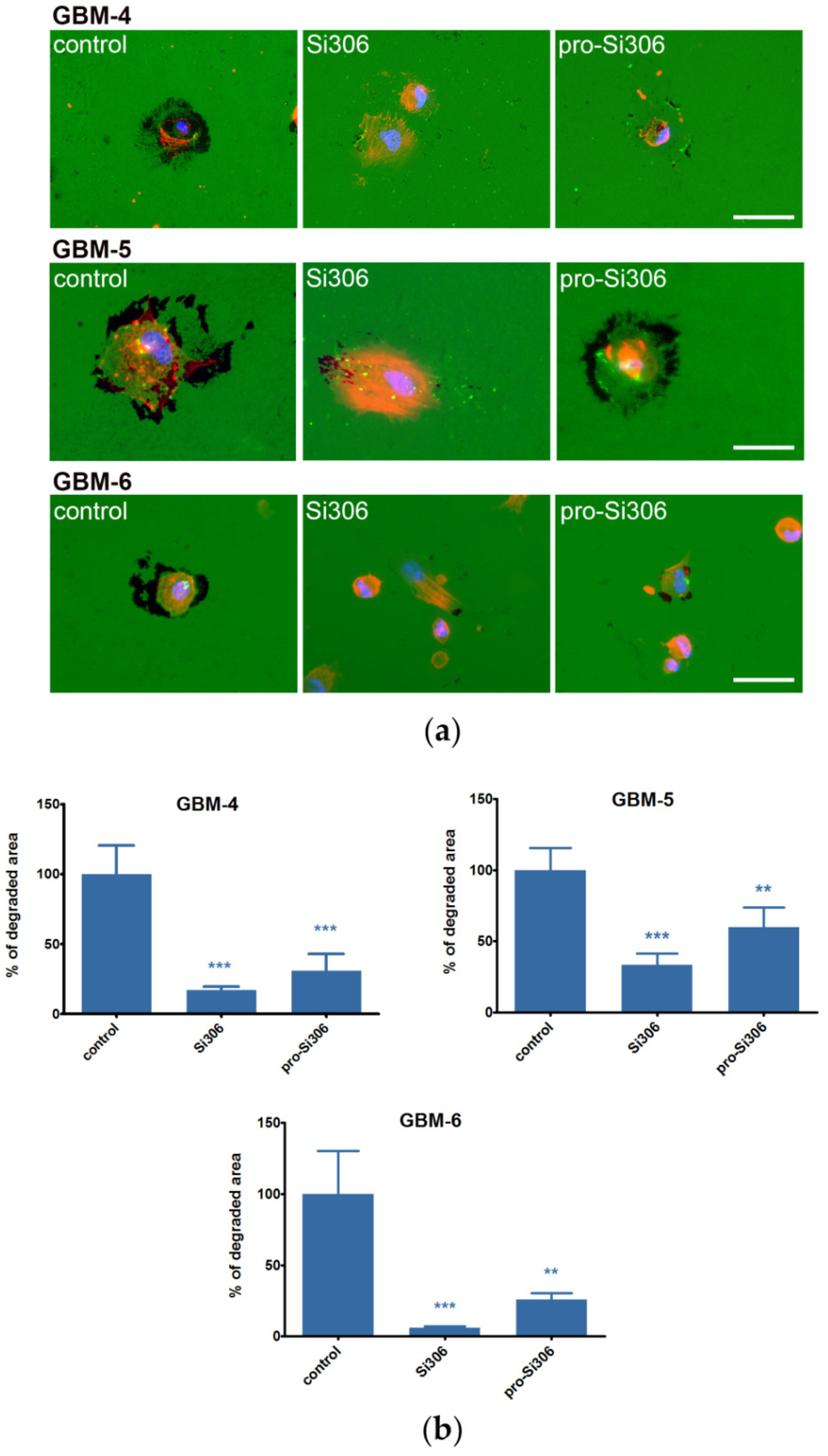
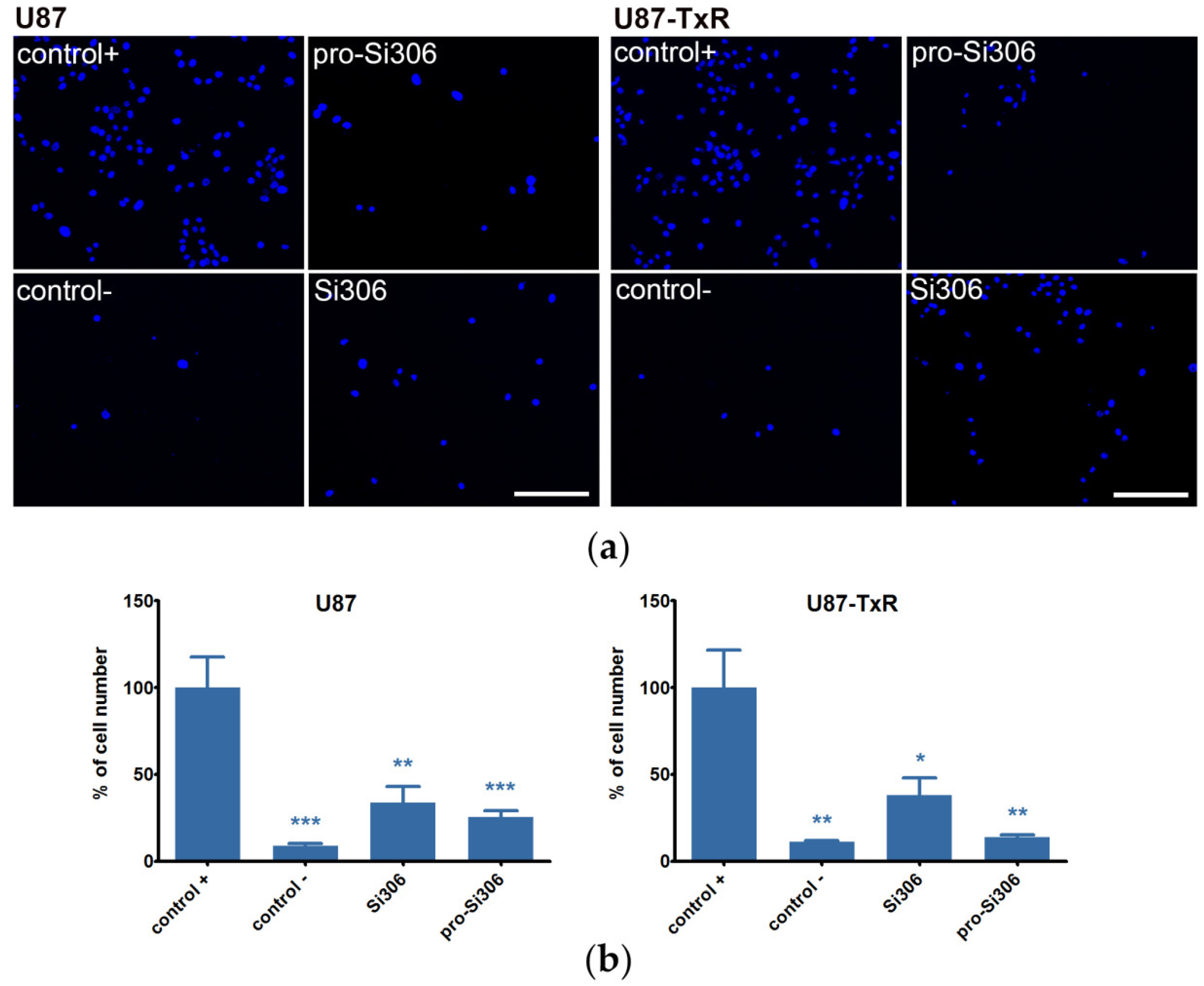
2.2. Si306 and Pro-Si306 Decrease Migration and Invasion in GBM Cells
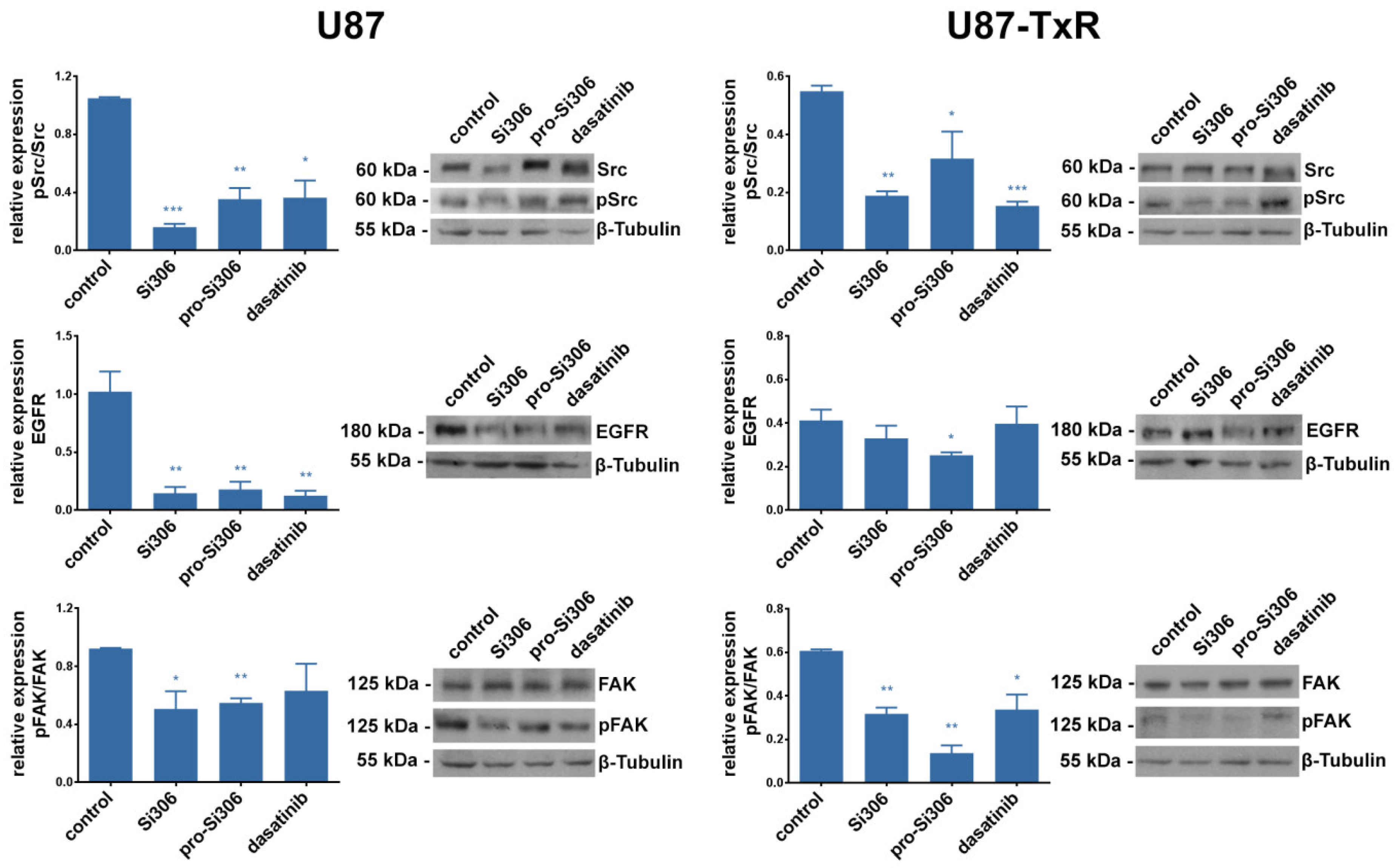
2.3. Si306 and Pro-Si306 Inhibit the Activity of Src and its Upstream Signaling Pathway Components FAK and EGFR
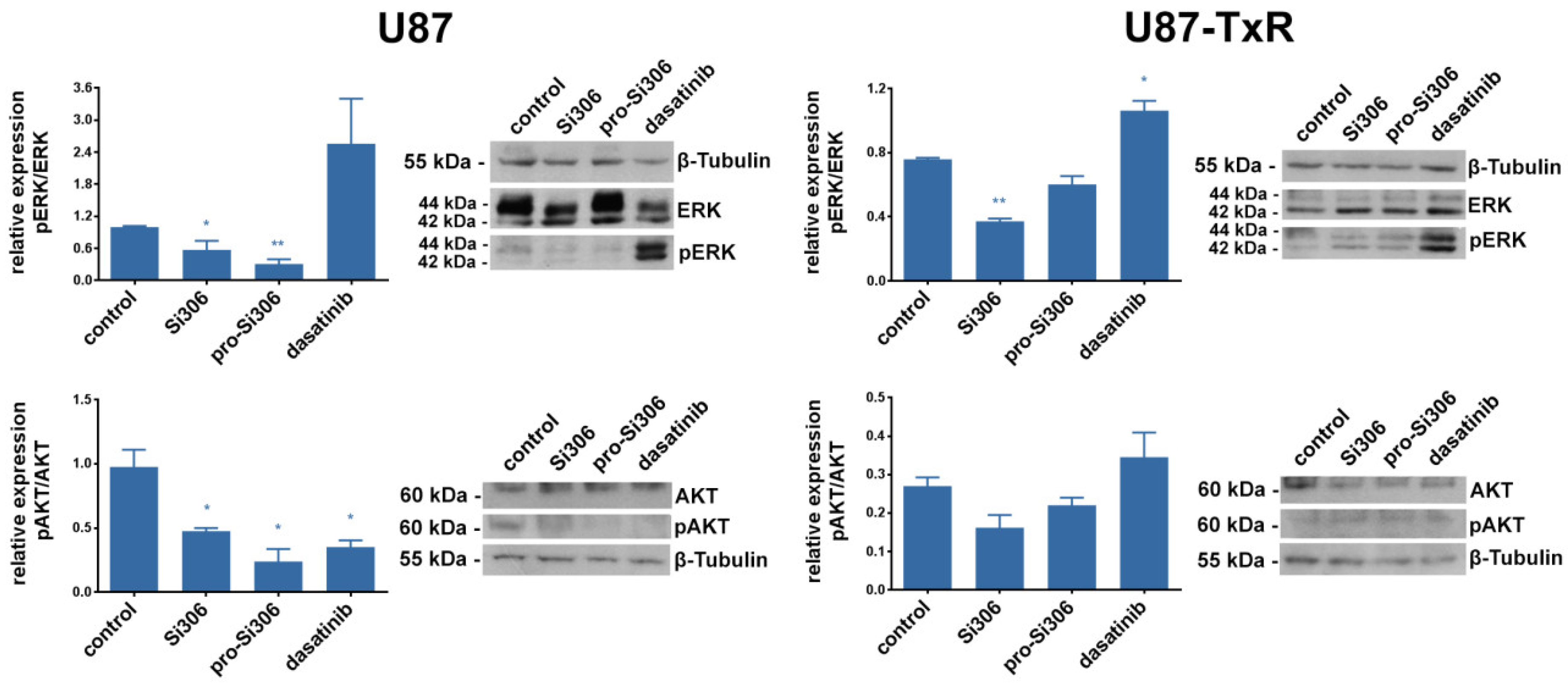
2.4. Si306 and Pro-Si306 Inhibit the Activity of Src Downstream Signaling Pathway Components AKT and ERK
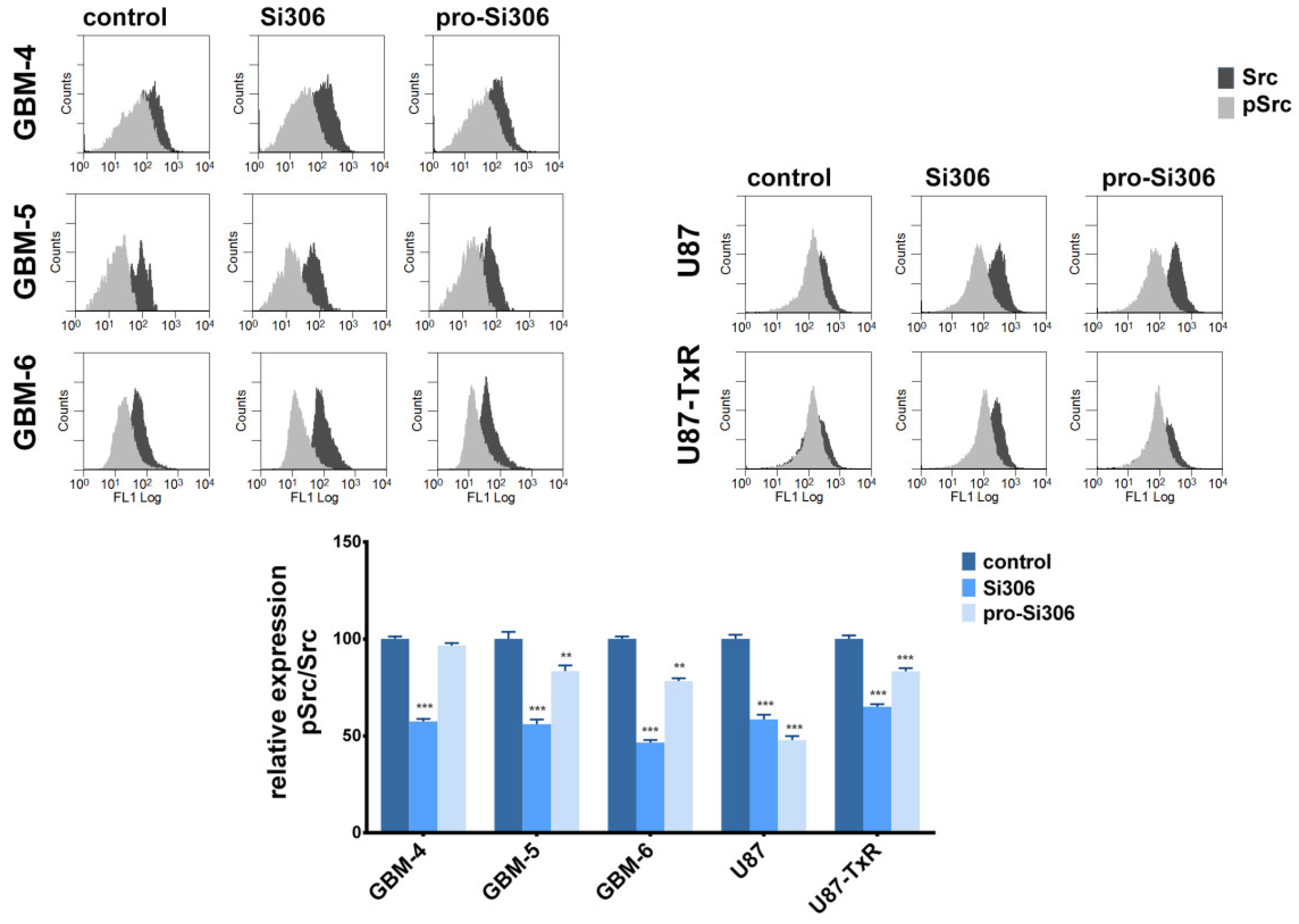
2.5. Si306 and Pro-Si306 Inhibit Src Activity in Primary GBM Cells
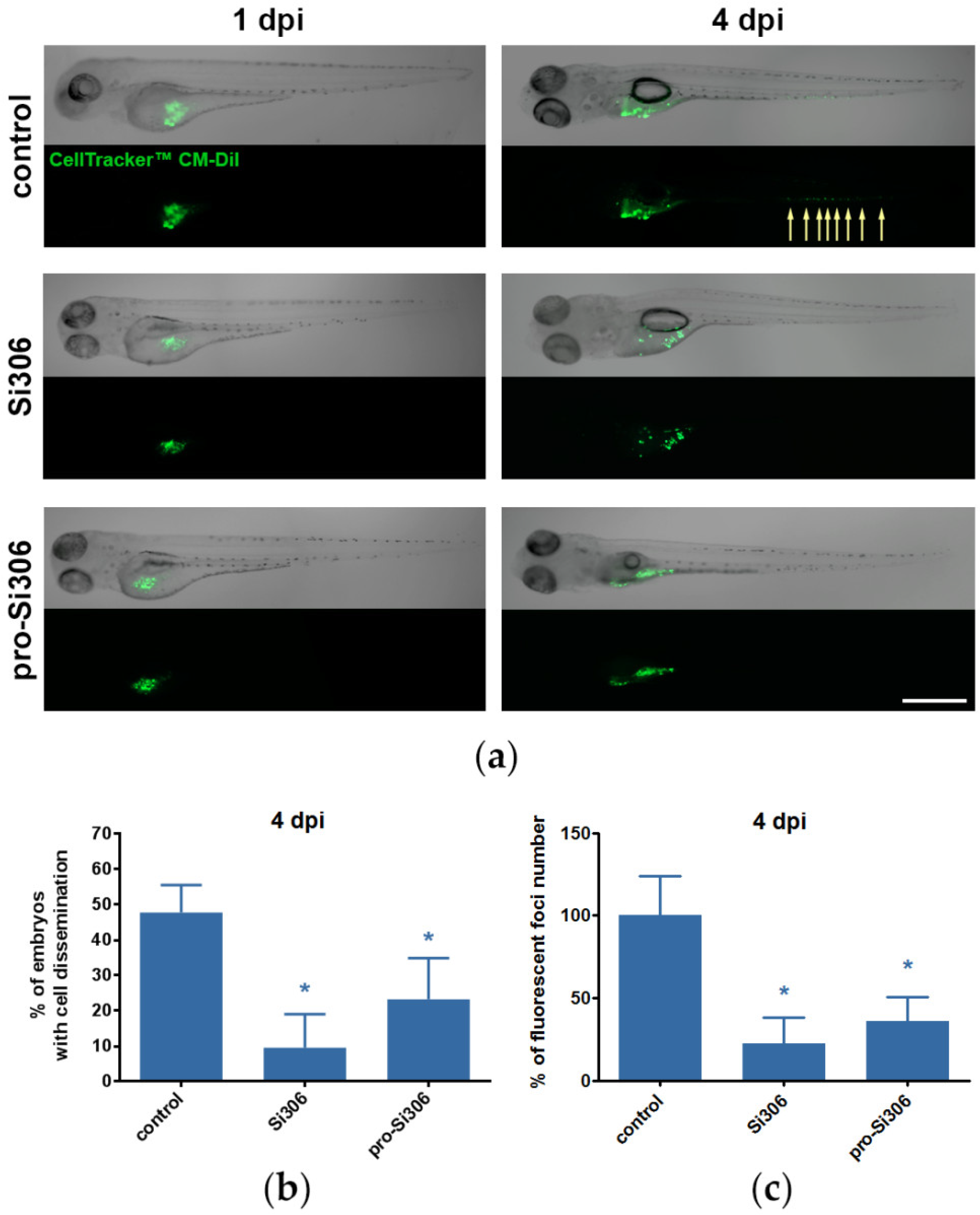
2.6. Si306 and Pro-Si306 Suppress Invasion of GBM Cells in Vivo
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Cell Lines
4.3. Primary Glioblastoma Cultures
4.4. Immunocytochemistry of Primary Glioblastoma Cultures
4.5. Flow Cytometry
4.6. Western Blot Analysis
4.7. MTT Assay
4.8. Wound Healing Assay
4.9. Gelatin Degradation Assay
4.10. Transwell Invasion Assay
4.11. RNA Extraction and Reverse Transcription Reaction
4.12. Quantitative Real-Time PCR
4.13. Zebrafish Husbandry
4.14. Zebrafish Xenograft Assay
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anjum, K.; Shagufta, B.I.; Abbas, S.Q.; Patel, S.; Khan, I.; Shah, S.A.A.; Akhter, N.; Hassan, S.S.U. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed. Pharmacother. 2017, 92, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Alifieris, K.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Tuffin, L.J.; Feathers, R.; Hari, P.; Durand, N.; Li, Z.; Rodriguez, F.J.; Bakken, K.; Carlson, B.; Schroeder, M.; Sarkaria, J.N.; et al. Src family kinases differentially influence glioma growth and motility. Mol. Oncol. 2015, 9, 1783–1798. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Weller, M.; Weiler, M.; Batchelor, T.; Yung, A.W.; Platten, M. Pathway inhibition: Emerging molecular targets for treating glioblastoma. J. Neurooncol. 2011, 13, 566–579. [Google Scholar] [CrossRef]
- Huveldt, D.; Lewis-Tuffin, L.J.; Carlson, B.L.; Schroeder, M.A.; Rodríguez, F.; Giannini, C.; Galanis, E.; Sarkaria, J.N.; Anastasiadis, P.Z. Targeting Src Family Kinases Inhibits Bevacizumab-Induced Glioma Cell Invasion. PLoS ONE 2013, 8, e56505. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; De Groot, J.F.; Liu, W.M.; Gladson, C.L. Targeting SRC in glioblastoma tumors and brain metastases: Rationale and preclinical studies. Cancer Lett. 2010, 298, 139–149. [Google Scholar] [CrossRef]
- Preusser, M.; De Ribaupierre, S.; Woehrer, A.; Erridge, S.C.; Hegi, M.E.; Weller, M.; Stupp, R. Current concepts and management of glioblastoma. Ann. Neurol. 2011, 70, 9–21. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Bent, M.J.V.D.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.A.; Brandes, A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Tejada, S.; Aldave, G.; Marigil, M.; Pérez-Larraya, J.G.; De Gallego, J.; Dominguez, P.D.; Díez-Valle, R. Factors associated with a higher rate of distant failure after primary treatment for glioblastoma. J. Neurooncol. 2013, 116, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jaklitsch, M.T.; Bueno, R. Neoadjuvant Therapy in Non–Small Cell Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N. Molecular pathology of malignant gliomas. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 97–117. [Google Scholar] [CrossRef]
- Calgani, A.; Vignaroli, G.; Zamperini, C.; Coniglio, F.; Festuccia, C.; Di Cesare, E.; Gravina, G.L.; Mattei, C.; Vitale, F.; Schenone, S.; et al. Suppression of SRC signaling is effective in reducing synergy between glioblastoma and stromal cells. Mol. Cancer Ther. 2016, 15, 1535–1544. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: Turning promise into triumph. Trends Pharmacol. Sci. 2011, 33, 122–128. [Google Scholar] [CrossRef]
- Yeatman, T.J. A renaissance for SRC. Nat. Rev. Cancer 2004, 4, 470–480. [Google Scholar] [CrossRef]
- Saadeh, F.S.; Mahfouz, R.; Assi, H.I. EGFR as a clinical marker in glioblastomas and other gliomas. Int. J. Biol. Markers 2017, 33, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.N.; Bigner, S.H. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat. Res. Genet. Toxicol. 1992, 276, 299–306. [Google Scholar] [CrossRef]
- Frederick, L.; Wang, X.Y.; Eley, G.; James, C.D. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000, 60, 1383–1387. [Google Scholar]
- Hauck, C.R.; Hsia, D.A.; Schlaepfer, D.D. The Focal Adhesion Kinase–A Regulator of Cell Migration and Invasion. IUBMB Life 2002, 53, 115–119. [Google Scholar] [CrossRef]
- Ben-Baruch, A. Site-specific metastasis formation. Cell Adhes. Migr. 2009, 3, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Sabbineni, H.; Clarke, A.; Somanath, P.R. Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016, 157, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Kaye, A.H.; Hovens, C.M. Targeting malignant glioma survival signalling to improve clinical outcomes. J. Clin. Neurosci. 2007, 14, 301–308. [Google Scholar] [CrossRef]
- Chen, Y.; Agarwal, S.; Shaik, N.M.; Chen, C.; Yang, Z.; Elmquist, W.F. P-glycoprotein and Breast Cancer Resistance Protein Influence Brain Distribution of Dasatinib. J. Pharmacol. Exp. Ther. 2009, 330, 956–963. [Google Scholar] [CrossRef]
- Agarwal, S.; Mittapalli, R.K.; Zellmer, D.M.; Gallardo, J.L.; Donelson, R.; Seiler, C.; Decker, S.A.; Santacruz, K.S.; Pokorny, J.L.; Sarkaria, J.N.; et al. Active efflux of Dasatinib from the brain limits efficacy against murine glioblastoma: Broad implications for the clinical use of molecularly targeted agents. Mol. Cancer Ther. 2012, 11, 2183–2192. [Google Scholar] [CrossRef]
- Schenone, S.; Radi, M.; Musumeci, F.; Brullo, C.; Botta, M. Biologically Driven Synthesis of Pyrazolo[3,4-d]pyrimidines as Protein Kinase Inhibitors: An Old Scaffold As a New Tool for Medicinal Chemistry and Chemical Biology Studies. Chem. Rev. 2014, 114, 7189–7238. [Google Scholar] [CrossRef]
- Schenone, S.; Brullo, C.; Musumeci, F.; Botta, M. Novel dual Src/Abl inhibitors for hematologic and solid malignancies. Expert Opin. Investig. Drugs 2010, 19, 931–945. [Google Scholar] [CrossRef]
- Fallacara, A.L.; Zamperini, C.; Podolski-Renić, A.; Dinic, J.; Stanković, T.; Stepanović, M.; Mancini, A.; Rango, E.; Iovenitti, G.; Molinari, A.; et al. A New Strategy for Glioblastoma Treatment: In Vitro and In Vivo Preclinical Characterization of Si306, a Pyrazolo[3,4-d]Pyrimidine Dual Src/P-Glycoprotein Inhibitor. Cancers 2019, 11, 848. [Google Scholar] [CrossRef]
- Ceccherini, E.; Indovina, P.; Zamperini, C.; Dreassi, E.; Casini, N.; Cutaia, O.; Forte, I.M.; Pentimalli, F.; Esposito, L.; Polito, M.S.; et al. SRC Family Kinase Inhibition Through a New Pyrazolo[3,4-d]Pyrimidine Derivative as a Feasible Approach for Glioblastoma Treatment. J. Cell. Biochem. 2015, 116, 856–863. [Google Scholar] [CrossRef]
- Navarra, M.; Celano, M.; Maiuolo, J.; Schenone, S.; Botta, M.; Angelucci, A.; Bramanti, P.; Russo, D. Antiproliferative and pro-apoptotic effects afforded by novel Src-kinase inhibitors in human neuroblastoma cells. BMC Cancer 2010, 10, 602. [Google Scholar] [CrossRef]
- Casini, N.; Forte, I.M.; Mastrogiovanni, G.; Pentimalli, F.; Angelucci, A.; Festuccia, C.; Tomei, V.; Ceccherini, E.; Di Marzo, D.; Schenone, S.; et al. SRC family kinase (SFK) inhibition reduces rhabdomyosarcoma cell growth in vitro and in vivo and triggers p38 MAP kinase-mediated differentiation. Oncotarget 2015, 6, 12421–12435. [Google Scholar] [CrossRef][Green Version]
- Indovina, P.; Giorgi, F.; Rizzo, V.; Khadang, B.; Schenone, S.; Di Marzo, D.; Forte, I.M.; Tomei, V.; Mattioli, E.; D’Urso, V.; et al. New pyrazolo[3,4-d]pyrimidine SRC inhibitors induce apoptosis in mesothelioma cell lines through p27 nuclear stabilization. Oncogene 2011, 31, 929–938. [Google Scholar] [CrossRef]
- Rossi, A.; Schenone, S.; Angelucci, A.; Cozzi, M.; Caracciolo, V.; Pentimalli, F.; Puca, A.; Pucci, B.; La Montagna, R.; Bologna, M.; et al. New pyrazolo-[3,4-d]-pyrimidine derivative Src kinase inhibitors lead to cell cycle arrest and tumor growth reduction of human medulloblastoma cells. FASEB J. 2010, 24, 2881–2892. [Google Scholar] [CrossRef]
- Morisi, R.; Celano, M.; Tosi, E.; Schenone, S.; Navarra, M.; Ferretti, E.; Costante, G.; Durante, C.; Botta, G.; D’Agostino, M.; et al. Growth inhibition of medullary thyroid carcinoma cells by pyrazolo-pyrimidine derivates. J. Endocrinol. Investig. 2007, 30, RC31–RC34. [Google Scholar] [CrossRef]
- Radi, M.; Tintori, C.; Musumeci, F.; Brullo, C.; Zamperini, C.; Dreassi, E.; Fallacara, A.L.; Vignaroli, G.; Crespan, E.; Zanoli, S.; et al. Design, Synthesis, and Biological Evaluation of Pyrazolo[3,4-d]pyrimidines Active in Vivo on the Bcr-Abl T315I Mutant. J. Med. Chem. 2013, 56, 5382–5394. [Google Scholar] [CrossRef]
- Cozzi, M.; Giorgi, F.; Marcelli, E.; Pentimalli, F.; Forte, I.M.; Schenone, S.; D’Urso, V.; De Falco, G.; Botta, M.; Giordano, A.; et al. Antitumor activity of new pyrazolo[3,4-d]pyrimidine SRC kinase inhibitors in Burkitt lymphoma cell lines and its enhancement by WEE1 inhibition. Cell Cycle 2012, 11, 1029–1039. [Google Scholar] [CrossRef][Green Version]
- Spreafico, A.; Schenone, S.; Serchi, T.; Orlandini, M.; Angelucci, A.; Magrini, D.; Bernardini, G.; Collodel, G.; Di Stefano, A.; Tintori, C.; et al. Antiproliferative and proapoptotic activities of new pyrazolo[3,4-d]pyrimidine derivative Src kinase inhibitors in human osteosarcoma cells. FASEB J. 2008, 22, 1560–1571. [Google Scholar] [CrossRef]
- Angelucci, A.; Schenone, S.; Gravina, G.L.; Muzi, P.; Festuccia, C.; Vicentini, C.; Botta, M.; Bologna, M. Pyrazolo[3,4-d]pyrimidines c-Src inhibitors reduce epidermal growth factor-induced migration in prostate cancer cells. Eur. J. Cancer 2006, 42, 2838–2845. [Google Scholar] [CrossRef]
- Tintori, C.; Fallacara, A.L.; Radi, M.; Zamperini, C.; Dreassi, E.; Crespan, E.; Maga, G.; Schenone, S.; Musumeci, F.; Brullo, C.; et al. Combining X-ray Crystallography and Molecular Modeling toward the Optimization of Pyrazolo[3,4-d]pyrimidines as Potent c-Src Inhibitors Active in Vivo against Neuroblastoma. J. Med. Chem. 2014, 58, 347–361. [Google Scholar] [CrossRef]
- Vignaroli, G.; Iovenitti, G.; Zamperini, C.; Coniglio, F.; Calandro, P.; Molinari, A.; Fallacara, A.L.; Sartucci, A.; Calgani, A.; Colecchia, D.; et al. Prodrugs of Pyrazolo[3,4-d]pyrimidines: From Library Synthesis to Evaluation as Potential Anticancer Agents in an Orthotopic Glioblastoma Model. J. Med. Chem. 2017, 60, 6305–6320. [Google Scholar] [CrossRef]
- Greco, C.; Taresco, V.; Pearce, A.K.; Vasey, C.E.; Smith, S.; Rahman, R.; Alexander, C.; Cavanagh, R.J.; Musumeci, F.; Schenone, S. Development of Pyrazolo[3,4-d]pyrimidine Kinase Inhibitors as Potential Clinical Candidates for Glioblastoma Multiforme. ACS Med. Chem. Lett. 2020, 11, 657–663. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Wojcieszak, J.; Olejniczak, A.B. Prodrugs: A challenge for the drug development. Pharmacol. Rep. 2013, 65, 1–14. [Google Scholar] [CrossRef]
- Wirsching, H.-G.; Galanis, E.; Weller, M. Glioblastoma: Parkinson’s Disease and Related Disorders, Part. I. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Mahringer, A.; Fricker, G. ABC transporters at the blood–brain barrier. Expert Opin. Drug Metab. Toxicol. 2016, 12, 499–508. [Google Scholar] [CrossRef]
- Giese, A.; Bjerkvig, R.; Berens, M.E.; Westphal, M. Cost of Migration: Invasion of Malignant Gliomas and Implications for Treatment. J. Clin. Oncol. 2003, 21, 1624–1636. [Google Scholar] [CrossRef]
- Taylor, J.W.; Dietrich, J.; Gerstner, E.R.; Norden, A.D.; Rinne, M.L.; Cahill, D.P.; Stemmer-Rachamimov, A.; Wen, P.Y.; Betensky, R.A.; Giorgio, D.H.; et al. Phase 2 study of bosutinib, a Src inhibitor, in adults with recurrent glioblastoma. J. Neurooncol. 2014, 121, 557–563. [Google Scholar] [CrossRef]
- Jubran, M.R.; Rubinstein, A.M.; Cojocari, I.; Adejumobi, I.A.; Mogilevsky, M.; Tibi, S.; Sionov, R.V.; Verreault, M.; Idbaih, A.; Karni, R.; et al. Dissecting the role of crosstalk between glioblastoma subpopulations in tumor cell spreading. Oncogenesis 2020, 9, 11–15. [Google Scholar] [CrossRef]
- Smolinski, M.P.; Bu, Y.; Clements, J.; Gelman, I.; Hegab, T.; Cutler, D.L.; Fang, J.W.S.; Fetterly, G.; Kwan, R.; Barnett, A.; et al. Discovery of Novel Dual Mechanism of Action Src Signaling and Tubulin Polymerization Inhibitors (KX2-391 and KX2-361). J. Med. Chem. 2018, 61, 4704–4719. [Google Scholar] [CrossRef]
- Stamenkovic, I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef]
- Aimes, R.T.; Quigley, J.P. Matrix Metalloproteinase-2 is an Interstitial Collagenase. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Desimone, N.; Hahn-Dantona, E.; Sipley, J.; Nagase, H.; French, D.L.; Quigley, J.P. Activation of Matrix Metalloproteinase-9 (MMP-9) via a Converging Plasmin/Stromelysin-1 Cascade Enhances Tumor Cell Invasion. J. Biol. Chem. 1999, 274, 13066–13076. [Google Scholar] [CrossRef] [PubMed]
- Uhm, J.; Dooley, N.; Villemure, J.-G.; Yong, V. Mechanisms of Glioma Invasion: Role of Matrix-Metalloproteinases. Can. J. Neurol. Sci. 1997, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, R.E.; Yamamoto, M.; Gokaslan, Z.L.; Wang, S.W.; Mohanam, S.; Fuller, G.N.; McCutcheon, I.E.; Stetler-Stevenson, W.G.; Nicolson, G.L.; Rao, J.S. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin. Exp. Metastasis 1996, 14, 35–42. [Google Scholar] [CrossRef]
- Chiang, S.P.H.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.K.; Jung, H.J.; Park, J.-Y.; Kang, J.-H.; Lee, B.I.; Shin, D.Y.; Nho, C.W.; Cho, S.-Y.; Seong, J.K.; Oh, S.H. Daurinol blocks breast and lung cancer metastasis and development by inhibition of focal adhesion kinase (FAK). Oncotarget 2017, 8, 57058–57071. [Google Scholar] [CrossRef]
- Hu, B.; Jarzynka, M.J.; Guo, P.; Imanishi, Y.; Schlaepfer, D.D.; Cheng, S.Y. Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the alphavbeta1 integrin and focal adhesion kinase signaling pathway. Cancer Res. 2006, 66, 775–783. [Google Scholar] [CrossRef]
- Lu, W.; Zhou, X.; Hong, B.; Liu, J.; Yue, Z. Suppression of invasion in human U87 glioma cells by adenovirus-mediated co-transfer of TIMP-2 and PTEN gene. Cancer Lett. 2004, 214, 205–213. [Google Scholar] [CrossRef]
- Kolli-Bouhafs, K.; Boukhari, A.; Abusnina, A.; Velot, É.; Gies, J.-P.; Lugnier, C.; Rondé, P. Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Investig. New Drugs 2011, 30, 2121–2131. [Google Scholar] [CrossRef]
- Cho, H.-J.; Park, J.-H.; Nam, J.H.; Chang, Y.-C.; Park, B.; Hoe, H.-S. Ascochlorin Suppresses MMP-2-Mediated Migration and Invasion by Targeting FAK and JAK-STAT Signaling Cascades. J. Cell. Biochem. 2017, 119, 300–313. [Google Scholar] [CrossRef]
- Edwin, F.; Wiepz, G.J.; Singh, R.; Peet, C.R.; Chaturvedi, D.; Bertics, P.J.; Patel, T.B.; Tarun, P.B.; Paul, B.J.; Tarun, B.P. A Historical Perspective of the EGF Receptor and Related Systems. Methods Mol. Biol. 2006, 327, 1–24. [Google Scholar] [CrossRef]
- Carpenter, G.; Purcell, K.; Artavanis-Tsakonas, S. Employment of the Epidermal Growth Factor Receptor in Growth Factor–Independent Signaling Pathways. J. Cell Biol. 1999, 146, 697–702. [Google Scholar] [CrossRef]
- Osherov, N.; Levitzki, A. Epidermal-Growth-Factor-Dependent Activation of the Src-Family Kinases. JBIC J. Biol. Inorg. Chem. 1994, 225, 1047–1053. [Google Scholar] [CrossRef]
- Bao, J.; Gur, G.; Yarden, Y. Src Promotes Destruction of c-Cbl: Implications for Oncogenic Synergy between Src and Growth Factor Receptors. Proc. Natl. Acad Sci. USA 2003, 100, 2438–2443. [Google Scholar] [CrossRef]
- Kopetz, S. Targeting Src and Epidermal Growth Factor Receptor in Colorectal Cancer: Rationale and Progress Into the Clinic. Gastrointest Cancer Res. 2007, 1, S37–S41. [Google Scholar]
- Li, Y.-J.; He, Y.-F.; Han, X.-H.; Hu, B. Dasatinib suppresses invasion and induces apoptosis in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 7818–7824. [Google Scholar]
- Buettner, R.; Mesa, T.; Vultur, A.; Lee, F.; Jove, R. Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol. Cancer Res. 2008, 6, 1766–1774. [Google Scholar] [CrossRef]
- Hermida-Prado, F.; Villaronga, M.Á.; Granda-Díaz, R.; Del-Río-Ibisate, N.; Santos, L.; Hermosilla, M.A.; Oro, P.; Allonca, E.; Agorreta, J.; Garmendia, I.; et al. The SRC Inhibitor Dasatinib Induces Stem Cell-Like Properties in Head and Neck Cancer Cells that are Effectively Counteracted by the Mithralog EC-8042. J. Clin. Med. 2019, 8, 1157. [Google Scholar] [CrossRef]
- Chen, T.; Wang, C.; Liu, Q.; Meng, Q.; Sun, H.; Huo, X.; Sun, P.; Peng, J.; Liu, Z.; Yang, X.; et al. Dasatinib reverses the multidrug resistance of breast cancer MCF-7 cells to doxorubicin by downregulating P-gp expression via inhibiting the activation of ERK signaling pathway. Cancer Biol. Ther. 2014, 16, 106–114. [Google Scholar] [CrossRef]
- Beadnell, T.C.; Mishall, K.M.; Zhou, Q.; Riffert, S.M.; Wuensch, K.E.; Kessler, B.E.; Corpuz, M.L.; Jing, X.; Kim, J.; Wang, G.; et al. The Mitogen-Activated Protein Kinase Pathway Facilitates Resistance to the Src Inhibitor Dasatinib in Thyroid Cancer. Mol. Cancer Ther. 2016, 15, 1952–1963. [Google Scholar] [CrossRef]
- Wu, J.; Liao, X.; Yu, B.; Su, B. Dasatinib inhibits primary melanoma cell proliferation through morphology-dependent disruption of Src-ERK signaling. Oncol. Lett. 2012, 5, 527–532. [Google Scholar] [CrossRef]
- Agarwal, S.; Hartz, A.M.; Elmquist, W.F.; Bauer, B. Breast cancer resistance protein and P-glycoprotein in brain cancer: Two gatekeepers team up. Curr. Pharm. Des. 2011, 17, 2793–2802. [Google Scholar] [CrossRef]
- Yang, X.-J.; Cui, W.; Gu, A.; Xu, C.; Yu, S.-C.; Li, T.-T.; Cui, Y.-H.; Zhang, X.; Bian, X.-W. A Novel Zebrafish Xenotransplantation Model for Study of Glioma Stem Cell Invasion. PLoS ONE 2013, 8, e61801. [Google Scholar] [CrossRef]
- Jung, D.-W.; Oh, E.-S.; Park, S.-H.; Chang, Y.-T.; Kim, C.-H.; Choi, S.-Y.; Williams, D.R. A novel zebrafish human tumor xenograft model validated for anti-cancer drug screening. Mol. BioSyst. 2012, 8, 1930. [Google Scholar] [CrossRef]
- Podolski-Renić, A.; Stanković, T.; Bancovik, J.; Tanić, N.; Ruzdijic, S.; Pesic, M. The role of paclitaxel in the development and treatment of multidrug resistant cancer cell lines. Biomed. Pharmacother. 2011, 65, 345–353. [Google Scholar] [CrossRef]
- Vogel, T.W.; Zhuang, Z.; Li, J.; Okamoto, H.; Furuta, M.; Lee, Y.-S.; Zeng, W.; Oldfield, E.H.O.; Vortmeyer, A.; Weil, R.J. Proteins and Protein Pattern Differences between Glioma Cell Lines and Glioblastoma Multiforme. Clin. Cancer Res. 2005, 11, 3624–3632. [Google Scholar] [CrossRef]
- Ledur, P.F.; Onzi, G.R.; Zong, H.; Lenz, G. Culture conditions defining glioblastoma cells behavior: What is the impact for novel discoveries? Oncotarget 2017, 8, 69185–69197. [Google Scholar] [CrossRef]
- Stojković, S.; Podolski-Renić, A.; Dinic, J.; Pavković, Ž.; Ayuso, J.M.; Fernández, L.J.; Ochoa, I.; Pérez-García, V.M.; Pešić, V.; Pesic, M. Resistance to DNA Damaging Agents Produced Invasive Phenotype of Rat Glioma Cells—Characterization of a New in Vivo Model. Molecules 2016, 21, 843. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yoganantharjah, P.; Gibert, Y. The Use of the Zebrafish Model to Aid in Drug Discovery and Target Validation. Curr. Top. Med. Chem. 2017, 17, 1. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Teng, Y.; Xie, X.; Walker, S.; White, D.T.; Mumm, J.S.; Cowell, J.K. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer 2013, 13, 453. [Google Scholar] [CrossRef]
- Drabsch, Y.; He, S.; Zhang, L.; Snaar-Jagalska, E.; Dijke, P.T. Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013, 15, R106. [Google Scholar] [CrossRef]
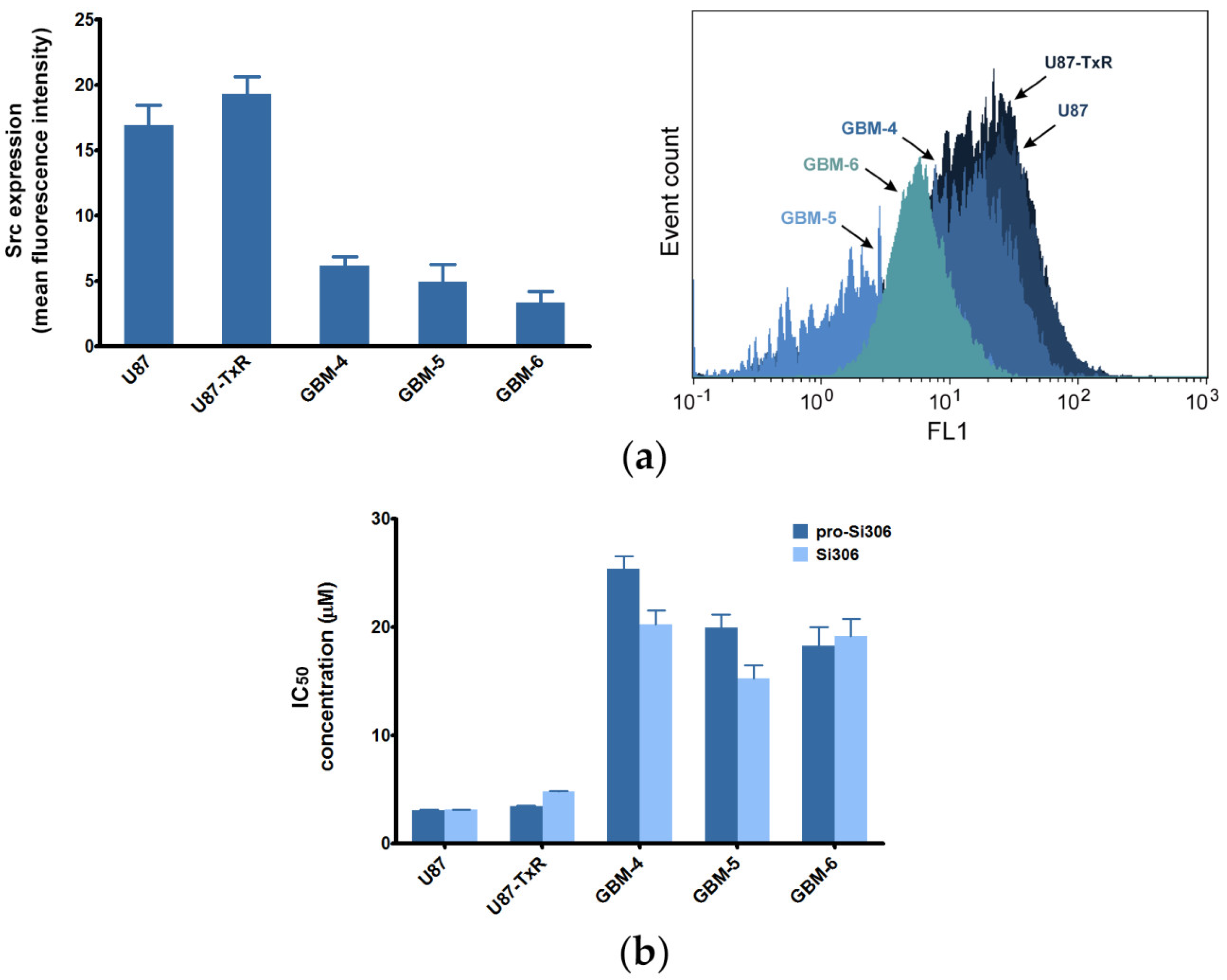
| Compounds | U87 1 | U87-TxR 1 | GBM-4 | GBM-5 | GBM-6 |
|---|---|---|---|---|---|
| Si306 | 3.081 ± 0.260 | 4.775 ± 0.322 | 20.270 ± 1.225 | 15.250 ± 1.207 | 19.120 ± 1.652 |
| Pro-Si306 | 3.045 ± 0.343 | 3.419 ± 0.359 | 25.390 ± 1.159 | 19.920 ± 1.196 | 18.280 ± 1.696 |
| Dasatinib | 6.143 ± 0.464 | 8.516 ± 0691 | 18.60 ± 1.259 | 25.610 ± 1.216 | 25.350 ± 1.199 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nešović, M.; Divac Rankov, A.; Podolski-Renić, A.; Nikolić, I.; Tasić, G.; Mancini, A.; Schenone, S.; Pešić, M.; Dinić, J. Src Inhibitors Pyrazolo[3,4-d]pyrimidines, Si306 and Pro-Si306, Inhibit Focal Adhesion Kinase and Suppress Human Glioblastoma Invasion In Vitro and In Vivo. Cancers 2020, 12, 1570. https://doi.org/10.3390/cancers12061570
Nešović M, Divac Rankov A, Podolski-Renić A, Nikolić I, Tasić G, Mancini A, Schenone S, Pešić M, Dinić J. Src Inhibitors Pyrazolo[3,4-d]pyrimidines, Si306 and Pro-Si306, Inhibit Focal Adhesion Kinase and Suppress Human Glioblastoma Invasion In Vitro and In Vivo. Cancers. 2020; 12(6):1570. https://doi.org/10.3390/cancers12061570
Chicago/Turabian StyleNešović, Marija, Aleksandra Divac Rankov, Ana Podolski-Renić, Igor Nikolić, Goran Tasić, Arianna Mancini, Silvia Schenone, Milica Pešić, and Jelena Dinić. 2020. "Src Inhibitors Pyrazolo[3,4-d]pyrimidines, Si306 and Pro-Si306, Inhibit Focal Adhesion Kinase and Suppress Human Glioblastoma Invasion In Vitro and In Vivo" Cancers 12, no. 6: 1570. https://doi.org/10.3390/cancers12061570
APA StyleNešović, M., Divac Rankov, A., Podolski-Renić, A., Nikolić, I., Tasić, G., Mancini, A., Schenone, S., Pešić, M., & Dinić, J. (2020). Src Inhibitors Pyrazolo[3,4-d]pyrimidines, Si306 and Pro-Si306, Inhibit Focal Adhesion Kinase and Suppress Human Glioblastoma Invasion In Vitro and In Vivo. Cancers, 12(6), 1570. https://doi.org/10.3390/cancers12061570








