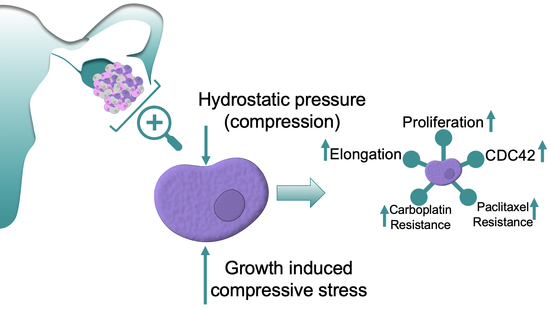
Compressive Stimulation Enhances Ovarian Cancer Proliferation, Invasion, Chemoresistance, and Mechanotransduction via CDC42 in a 3D Bioreactor
Abstract
1. Introduction
2. Results
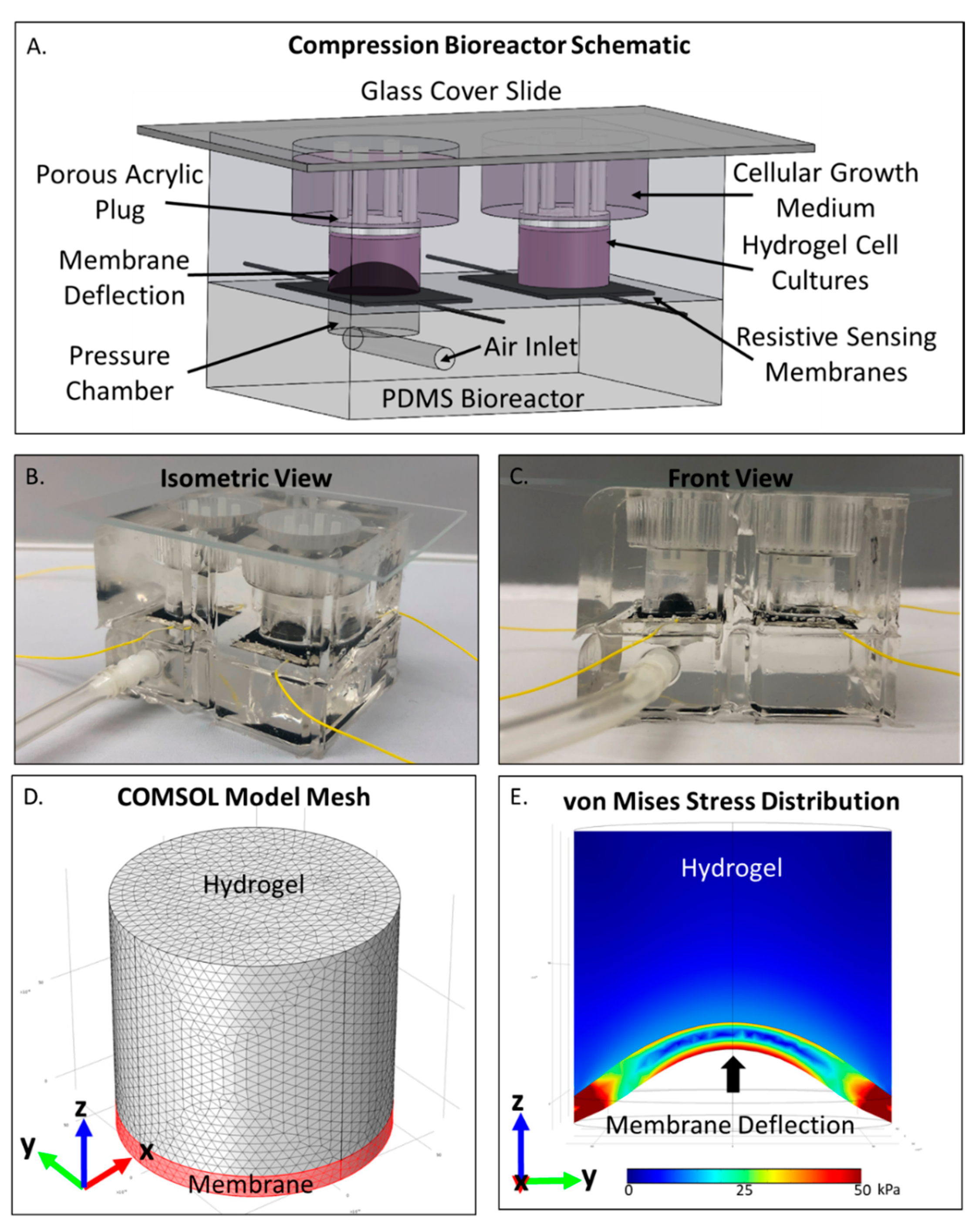
2.1. COMSOL Compression Bioreactor Model Shows Pressure Distribution Within Cell Laden Interpenetrating Hydrogel
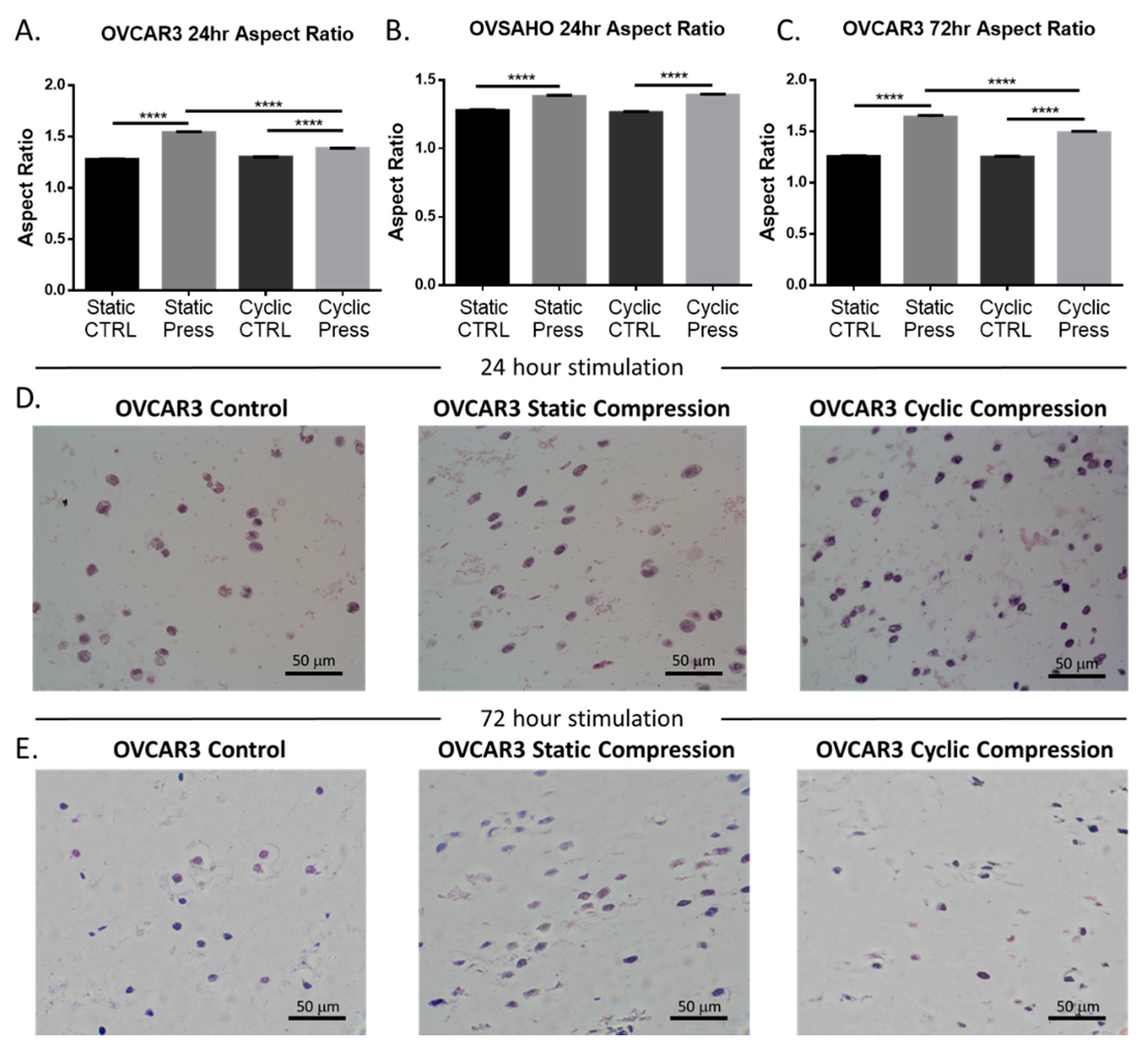
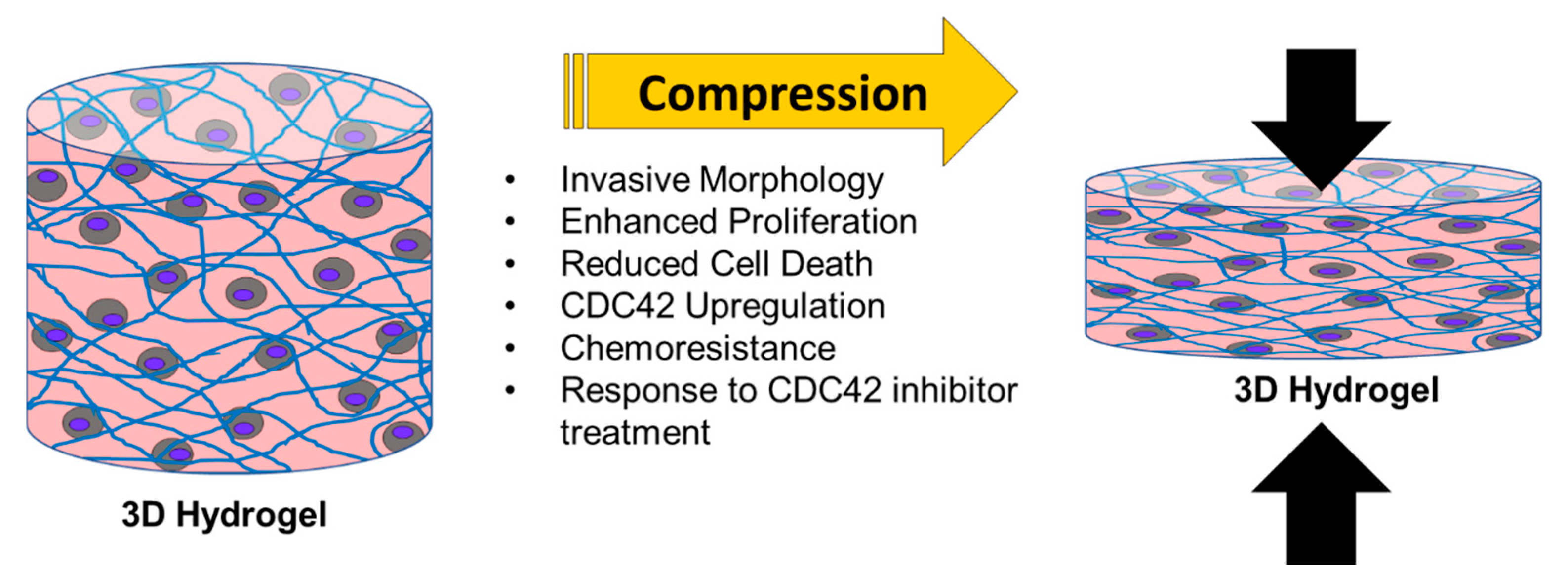
2.2. Compressive Stimulation Induces Invasive Morphology in High Grade Serous Ovarian Cancer Cells
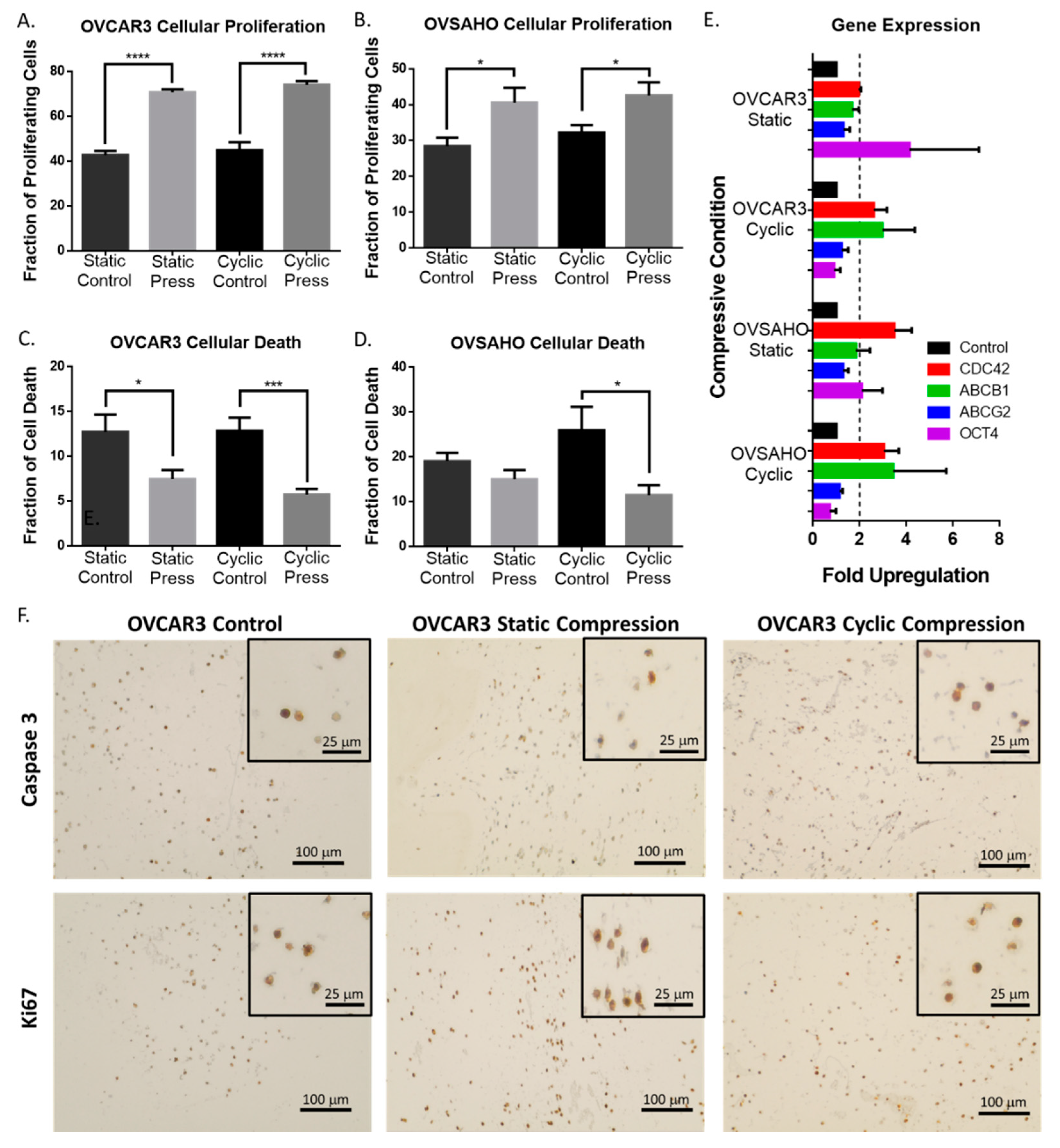
2.3. Compression Enhances High Grade Serous Ovarian Cancer Cell Proliferation and Reduces Cell Death
2.4. Compressive Stimulation of High Grade Serous Ovarian Cancer Cells induces Overexpression of CDC42
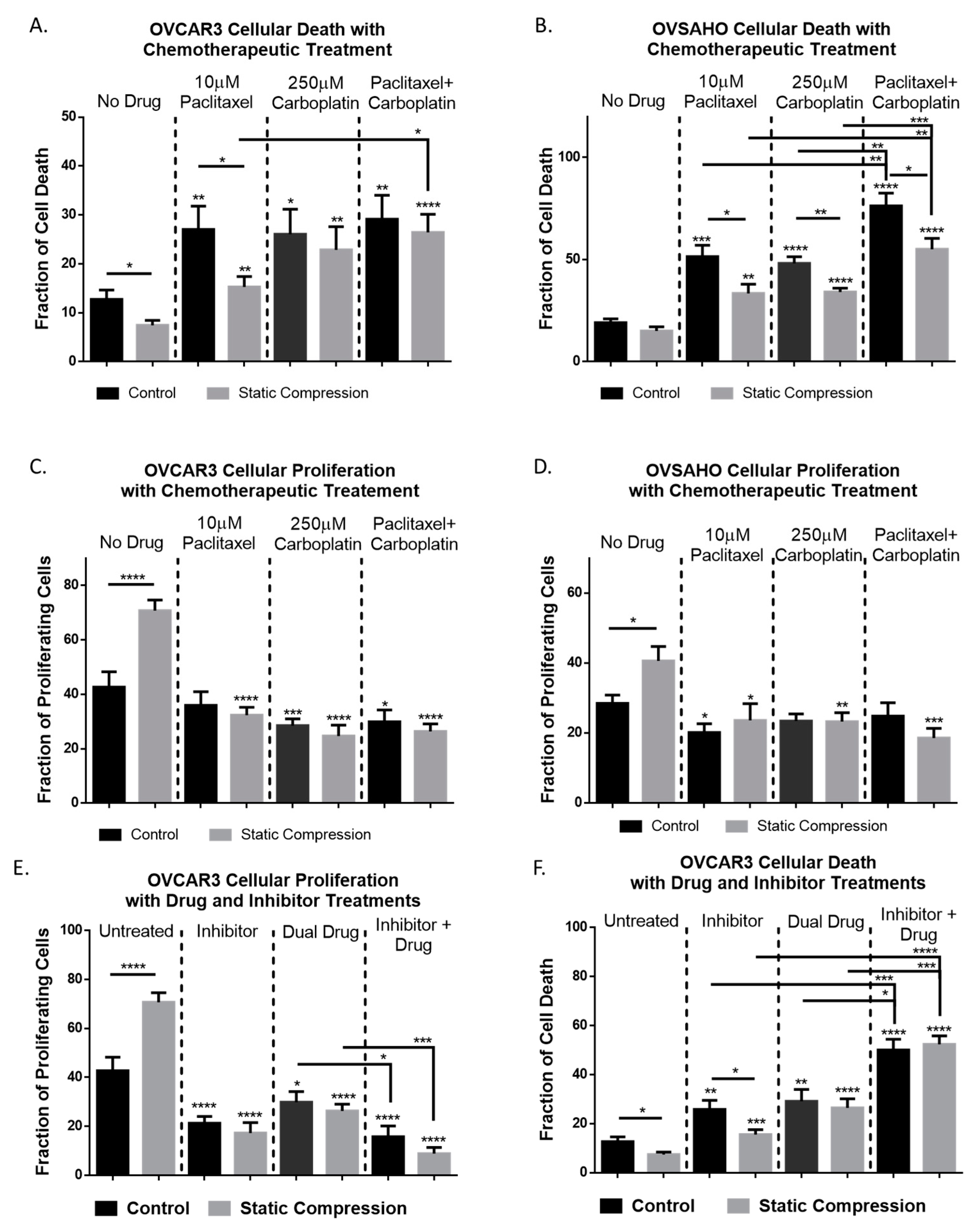
2.5. Chemoresistance in High Grade Serous Ovarian Cancer Cells Is Observed Under Compressive Stimulation
2.6. Inhibition of CDC42 in High Grade Serous Ovarian Cancer Cells Reduces Compression-Induced Proliferation, Cell Survival, and Chemoresistance
2.7. High Grade Serous Ovarian Cancer Cellular Area Is Significantly Increased With Inhibited CDC42 and Combination Chemotherapy
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Device Construction and Use
4.3. Electrical Hardware and Software Programming
4.4. COMSOL Computational Analysis
4.5. Morphological Cell Analysis and Immunohistochemistry
4.6. Gene Expression Analysis
4.7. G-Lisa Assay (CDC42 Activation and Inhibition)
4.8. Chemotherapeutic Treatment
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novak, C.; Horst, E.; Mehta, G. Review: Mechanotransduction in ovarian cancer: Shearing into the unknown. APL Bioeng. 2018, 2, 031701. [Google Scholar] [CrossRef] [PubMed]
- Mukuda, N.; Fujii, S.; Inoue, C.; Fukunaga, T.; Oishi, T.; Harada, T.; Ogawa, T. Bilateral Ovarian Tumors on MRI: How Should We Differentiate the Lesions? Yonago Acta Med. 2018, 61, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Bae, J.-H.; Lee, A.-W.; Tong, S.-Y.; Park, Y.G.; Park, J.S. Clinical Characteristics of Metastatic Tumors to the Ovaries. J. Korean Med. Sci. 2009, 24, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Stylianopoulos, T. Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, Y.; Wates, R.B.; Weiss-Bilka, H.; Lombard, R.; Liu, Y.; Campbell, L.; Kim, O.; Wagner, D.; Ravosa, M.J.; Stack, M.S. Modeling the effect of ascites-Induced compression on ovarian cancer multicellular aggregates. Dis. Model. Mech. 2018, 11, dmm034199. [Google Scholar] [CrossRef] [PubMed]
- Bregenzer, M.E.; Horst, E.N.; Mehta, G.; Novak, C.M.; Repetto, T. The Role of Cancer Stem Cells and Mechanical Forces in Ovarian Cancer Metastasis. Cancers 2019, 11, 1008. [Google Scholar] [CrossRef] [PubMed]
- Delarue, M.; Montel, F.; Vignjevic, D.; Prost, J.; Joanny, J.-F.; Cappello, G. Compressive Stress Inhibits Proliferation in Tumor Spheroids through a Volume Limitation. Biophys. J. 2014, 107, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Lu, H.; Xu, L.; Xu, X.; Xiao, W. Mechanical loading promotes Lewis lung cancer cell growth through periostin. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 467–472. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Kieu, Q.M.N.; Dawson, M.R. The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho-ROCK pathway. J. Cell Sci. 2014, 127, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Horst, E.N.; Taylor, C.C.; Liu, C.Z.; Mehta, G. Fluid shear stress stimulates breast cancer cells to display invasive and chemoresistant phenotypes while upregulating PLAU in a 3D bioreactor. Biotechnol. Bioeng. 2019, 116, 3084–3097. [Google Scholar] [CrossRef] [PubMed]
- Bregenzer, M.E.; Horst, E.N.; Mehta, P.; Novak, C.M.; Raghavan, S.; Snyder, C.S.; Mehta, G. Integrated cancer tissue engineering models for precision medicine. PLoS ONE 2019, 14, e0216564. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.M.; Cheng, G.; Tyrrell, J.A.; Wilcox-Adelman, S.A.; Boucher, Y.; Jain, R.K.; Munn, L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. USA 2011, 109, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Voutouri, C.; Minia, A.; Pliaka, V.; Fotis, C.; Alexopoulos, L.G.; Stylianopoulos, T. Mechanical Compression Regulates Brain Cancer Cell Migration through MEK1/Erk1 Pathway Activation and GDF15 Expression. Available online: https://link.galegroup.com/apps/doc/A602440408/AONE?sid=lms (accessed on 5 November 2019).
- Hunter, C.J.; Imler, S.M.; Malaviya, P.; Nerem, R.M.; Levenston, M.E. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials 2002, 23, 1249–1259. [Google Scholar] [CrossRef]
- Frieden, B.R.; Gatenby, R.A. Cancer Suppression by Compression. Bull. Math. Biol. 2014, 77, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Hirata, H.; Samsonov, M.; Sokabe, M. Planar compression of extracellular substrates induces S phase arrest via ATM-Independent CHK2 activation. Biochem. Biophys. Res. Commun. 2018, 506, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Tse, J.; Jain, R.K.; Munn, L. Micro-Environmental Mechanical Stress Controls Tumor Spheroid Size and Morphology by Suppressing Proliferation and Inducing Apoptosis in Cancer Cells. PLoS ONE 2009, 4, e4632. [Google Scholar] [CrossRef] [PubMed]
- Takao, S.; Taya, M.; Chiew, C. Mechanical stress-Induced cell death in breast cancer cells. Biol. Open 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Minia, A.; Pliaka, V.; Fotis, C.; Alexopoulos, L.G.; Stylianopoulos, T. Solid stress-induced migration is mediated by GDF15 through Akt pathway activation in pancreatic cancer cells. Sci. Rep. 2019, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Papageorgis, P.; Gkretsi, V.; Stylianopoulos, T. Solid Stress Facilitates Fibroblasts Activation to Promote Pancreatic Cancer Cell Migration. Ann. Biomed. Eng. 2018, 46, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F. Compression-Induced changes in the shape and volume of the chondrocyte nucleus. J. Biomech. 1995, 28, 1529–1541. [Google Scholar] [CrossRef]
- Ma, D.; Kou, X.; Jin, J.; Xu, T.; Wu, M.; Deng, L.; Fu, L.; Liu, Y.; Wu, G.; Lu, H. Hydrostatic Compress Force Enhances the Viability and Decreases the Apoptosis of Condylar Chondrocytes through Integrin-FAK-ERK/PI3K Pathway. Int. J. Mol. Sci. 2016, 17, 1847. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H.A.; Spring, K. Removal of the MDCK Cell Primary Cilium Abolishes Flow Sensing. J. Membr. Biol. 2003, 191, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, J.T.; Daneshmand, M.; Bizios, R.; Rizzo, V. Depletion of plasma membrane cholesterol dampens hydrostatic pressure and shear stress-Induced mechanotransduction pathways in osteoblast cultures. Am. J. Physiol. Physiol. 2004, 286, C831–C839. [Google Scholar] [CrossRef] [PubMed]
- Heasman, S.J.; Ridley, A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008, 9, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Rho GTPases and the Actin Cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Arias-Romero, L.E.; Chernoff, J. Targeting Cdc42 in cancer. Expert Opin. Ther. Targets 2013, 17, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-Kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Liang, X.; Yin, X.; Zhang, L.; Chen, D.; Jin, X.; Fiskesund, R.; Tang, K.; Ma, J.; et al. Fibrin Stiffness Mediates Dormancy of Tumor-Repopulating Cells via a Cdc42-Driven Tet2 Epigenetic Program. Cancer Res. 2018, 78, 3926–3937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, H.; Jiang, K.; Wang, Y.; Zhang, W.; Chu, Q.; Li, J.; Huang, H.; Cai, T.; Ji, H.; et al. MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration. Cell Rep. 2016, 16, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Gilad, E.; Rothman, K.; Peyrollier, K. Hyaluronan-CD44 Interaction with IQGAP1 Promotes Cdc42 and ERK Signaling, Leading to Actin Binding, Elk-1/Estrogen Receptor Transcriptional Activation, and Ovarian Cancer Progression. J. Biol. Chem. 2005, 280, 11961–11972. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kenney, S.R.; Muller, C.Y.; Adams, S.; Rutledge, T.; Romero, E.; Murray-Krezan, C.; Prekeris, R.; Sklar, L.A.; Hudson, L.G.; et al. R-Ketorolac Targets Cdc42 and Rac1 and Alters Ovarian Cancer Cell Behaviors Critical for Invasion and Metastasis. Mol. Cancer Ther. 2015, 14, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.K.M.; Li, S.-S.; Tang, M.Y.H.; Sy, K.H.S.; Ren, Y.; Shum, H.C.; Wong, A.S.T. Stemness and chemoresistance in epithelial ovarian carcinoma cells under shear stress. Sci. Rep. 2016, 6, 26788. [Google Scholar] [CrossRef] [PubMed]
- Avraham-Chakim, L.; Elad, D.; Zaretsky, U.; Kloog, Y.; Jaffa, A.; Grisaru, D. Fluid-Flow Induced Wall Shear Stress and Epithelial Ovarian Cancer Peritoneal Spreading. PLoS ONE 2013, 8, e60965. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Tensegrity-Based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008, 97, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumour-Cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.C.; Zhang, W.; Li, Y.Y.; Chan, B. Mechanoresponsive, omni-Directional and local matrix-Degrading actin protrusions in human mesenchymal stem cells microencapsulated in a 3D collagen matrix. Biomaterials 2015, 53, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, N.F.; Hsu, S. Mechanotransduction in endothelial cell migration. J. Cell. Biochem. 2005, 96, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62. [Google Scholar] [CrossRef]
- Wan, Q.; Cho, E.; Yokota, H.; Na, S. Rac1 and Cdc42 GTPases regulate shear stress-Driven β-Catenin signaling in osteoblasts. Biochem. Biophys. Res. Commun. 2013, 433, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Shyy, J.Y. Mechanotransduction in endothelial responses to shear stress: Review of work in Dr. Chien’s laboratory. Biorheology 2001, 38, 109–117. [Google Scholar] [PubMed]
- Li, G.; Song, Y.; Shi, M.; Du, Y.; Wang, W.; Zhang, Y. Mechanisms of Cdc42-mediated rat MSC differentiation on micro/nano-Textured topography. Acta Biomater. 2017, 49, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.M.; Dharmawardhane, S. Targeting Rac and Cdc42 GTPases in Cancer. Cancer Res. 2018, 78, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, L.; Chebotarev, O.; Simmons, C.A.; Sun, Y. Miniaturized platform with on-Chip strain sensors for compression testing of arrayed materials. Lab. Chip 2012, 12, 4178. [Google Scholar] [CrossRef] [PubMed]
- Cell Counter. Available online: https://imagej.net/Cell_Counter (accessed on 21 April 2020).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-Time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Surviladze, Z.; Waller, A.; Strouse, J.J.; Bologa, C.; Ursu, O.; Salas, V.; Parkinson, J.F.; Phillips, G.K.; Romero, E.; Wandinger-Ness, A.; et al. A Potent and Selective Inhibitor of Cdc42 GTPase. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar]
- Lee, C.S.; Kim, Y.J.; Jang, E.-R.; Myung, S.C.; Kim, W. Akt inhibitor enhances apoptotic effect of carboplatin on human epithelial ovarian carcinoma cell lines. Eur. J. Pharmacol. 2010, 632, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Munkarah, A.R.; Genhai, Z.; Morris, R.; Baker, V.V.; Deppe, G.; Diamond, M.P.; Saed, G.M. Inhibition of paclitaxel-Induced apoptosis by the specific COX-2 inhibitor, NS398, in epithelial ovarian cancer cells. Gynecol. Oncol. 2003, 88, 429–433. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novak, C.M.; Horst, E.N.; Lin, E.; Mehta, G. Compressive Stimulation Enhances Ovarian Cancer Proliferation, Invasion, Chemoresistance, and Mechanotransduction via CDC42 in a 3D Bioreactor. Cancers 2020, 12, 1521. https://doi.org/10.3390/cancers12061521
Novak CM, Horst EN, Lin E, Mehta G. Compressive Stimulation Enhances Ovarian Cancer Proliferation, Invasion, Chemoresistance, and Mechanotransduction via CDC42 in a 3D Bioreactor. Cancers. 2020; 12(6):1521. https://doi.org/10.3390/cancers12061521
Chicago/Turabian StyleNovak, Caymen M., Eric N. Horst, Emily Lin, and Geeta Mehta. 2020. "Compressive Stimulation Enhances Ovarian Cancer Proliferation, Invasion, Chemoresistance, and Mechanotransduction via CDC42 in a 3D Bioreactor" Cancers 12, no. 6: 1521. https://doi.org/10.3390/cancers12061521
APA StyleNovak, C. M., Horst, E. N., Lin, E., & Mehta, G. (2020). Compressive Stimulation Enhances Ovarian Cancer Proliferation, Invasion, Chemoresistance, and Mechanotransduction via CDC42 in a 3D Bioreactor. Cancers, 12(6), 1521. https://doi.org/10.3390/cancers12061521