Beneficial Molecular Adaptations in BRCA-Mutation Carriers by Combined HIT/HIRT Intervention: Results from a Pilot Study
Abstract
1. Introduction
2. Results
2.1. Questionnaires
2.2. Performance Parameters and Anthropometry
2.3. BRCA1 Protein and BRCA1 Gene Expression
2.4. Immunology and Growth Factors
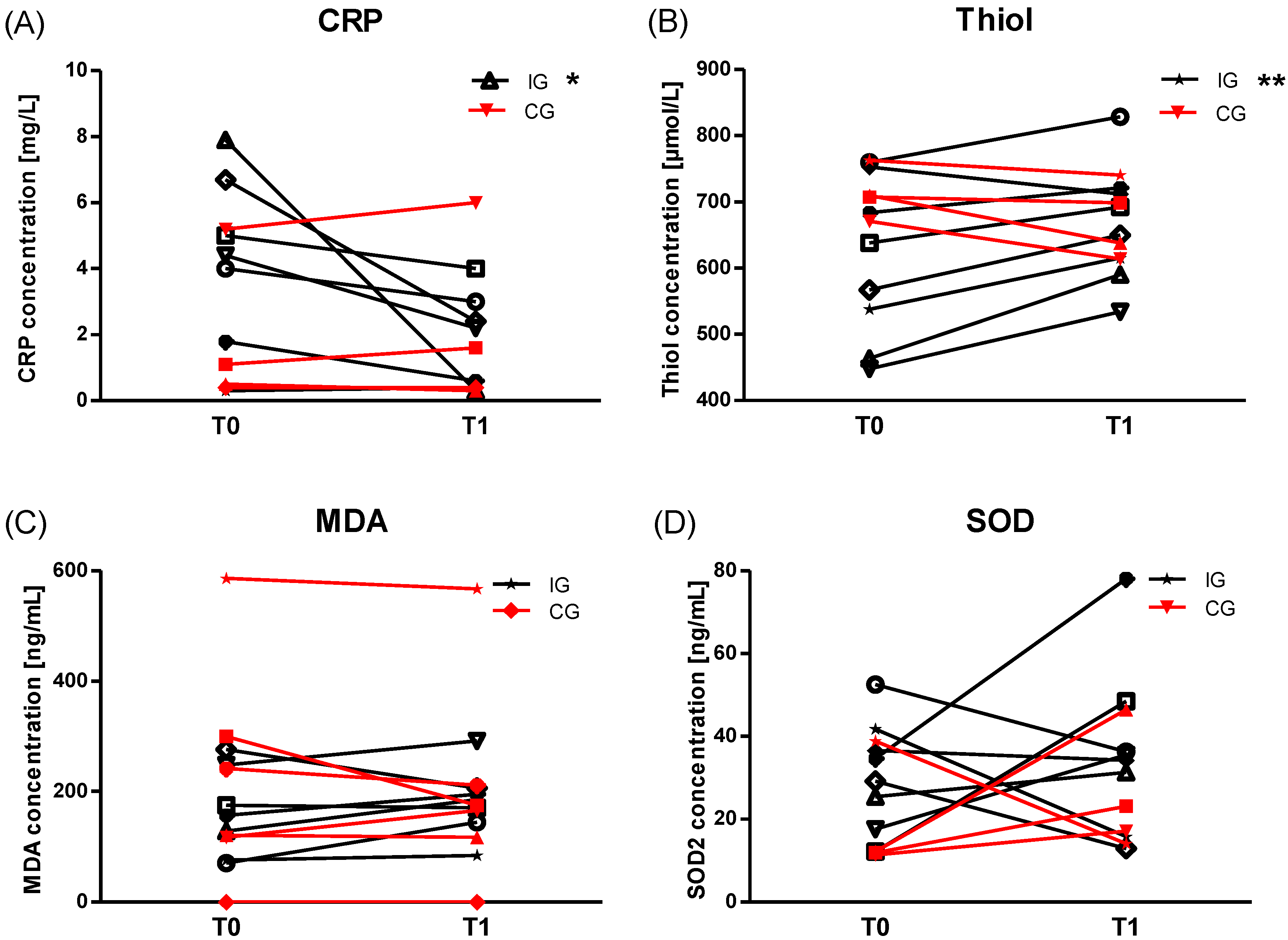
2.5. Anti-Oxidative Status
3. Discussion
Limitations
4. Materials and Methods
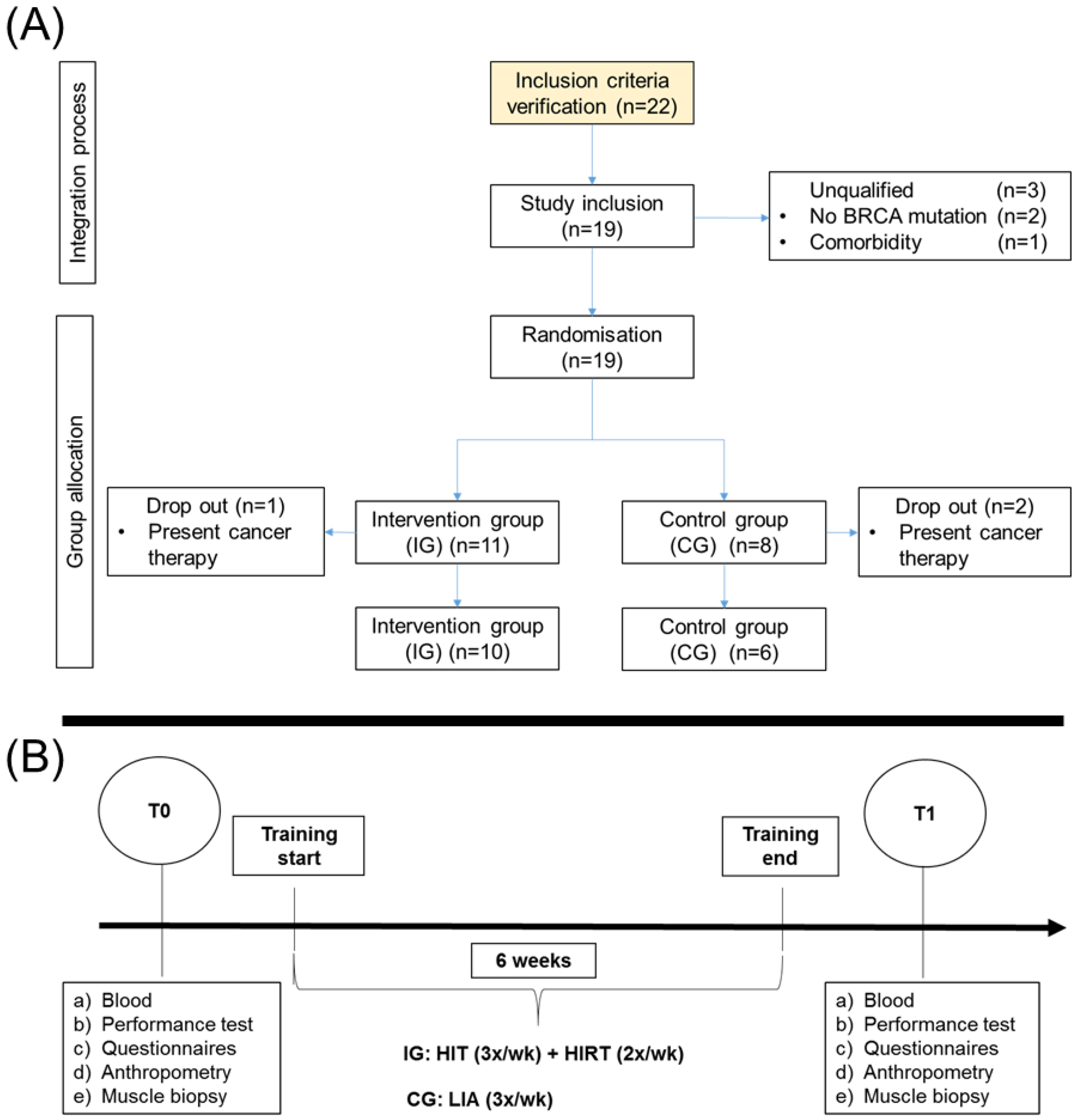
4.1. Study Group
4.2. Assignment of Study Group and Training
4.3. Psychological Parameters/ Questionnaires
4.4. Muscle Biopsy and Analysis
4.5. RNA Isolation and BRCA1 Analysis
BRCA1 Protein Determination
4.6. Growth Factors and Immunology
4.7. Oxidative Stress Markers and Anti-Oxidative Capacity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lux, M.; Fasching, P.; Beckmann, M. Hereditary breast and ovarian cancer: Review and future perspectives. J. Mol. Med. (Berl. Ger.) 2006, 84, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Peock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Evans, D.; Izatt, L.; Eeles, R.; Adlard, J.; et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. JNCI J. Natl. Cancer Inst. 2013, 105, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Pharoah, P.; Michailidou, K.; Tyrer, J.; Brook, M.; Bolla, M.; Wang, Q.s.; Dennis, J.; Dunning, A.; Shah, M.; et al. Prediction of Breast Cancer Risk Based on Profiling With Common Genetic Variants. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- King, M.C.; Marks, J.H.; Mandell, J.B. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Friebel, T.; Domchek, S.; Neuhausen, S.; Wagner, T.M.U.; Evans, D.; Isaacs, C.; Garber, J.; Daly, M.; Eeles, R.; Matloff, E.; et al. Bilateral Prophylactic Oophorectomy and Bilateral Prophylactic Mastectomy in a Prospective Cohort of Unaffected BRCA1 and BRCA2 Mutation Carriers. Clin. Breast Cancer 2007, 7, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.C.; Gidlund, E.-K.; Norrbom, J.; Valencia, A.P.; Thomson, D.M.; Schuh, R.A.; Neufer, P.D.; Spangenburg, E.E. BRCA1 is a novel regulator of metabolic function in skeletal muscle. J. Lipid Res. 2014, 55, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.W.; Kang, H.J.; Bae, I. BRCA1 and Oxidative Stress. Cancers (Basel) 2014, 6, 771–795. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.; Chen, W.; Feskanich, D.; Kroenke, C.; Colditz, G. Physical Activity and Survival after Breast Cancer Diagnosis. JAMA J. Am. Med. Assoc. 2005, 293, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Lemanne, D.; Cassileth, B.; Gubili, J. The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology 2013, 27, 580–585. [Google Scholar] [PubMed]
- Jackson, K.C.; Tarpey, M.D.; Valencia, A.P.; Iñigo, M.R.; Pratt, S.J.; Patteson, D.J.; McClung, J.M.; Lovering, R.M.; Thomson, D.M.; Spangenburg, E.E. Induced Cre-mediated knockdown of Brca1 in skeletal muscle reduces mitochondrial respiration and prevents glucose intolerance in adult mice on a high-fat diet. FASEB J. 2018, 32, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Welcsh, P.; Owens, K.; King, M.-C. Insights into the functions of BRCA1 and BRCA2. Trends Genet. 2000, 16, 69–74. [Google Scholar] [CrossRef]
- de Bruijn, K.; Arends, L.; Hansen, B.; Leeflang, S.; Ruiter, R.; Eijck, C. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br. J. Surg. 2013, 100, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Van Westerop, L.; Arts-deJong, M.; Hoogerbrugge, N.; Hullu, J.A.; Maas, A. Cardiovascular risk of BRCA1/2 mutation carriers: A review. Maturitas 2016, 91. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, L.; Lipscombe, L.; Lubinski, J.; Ghadirian, P.; Foulkes, W.D.; Neuhausen, S.; Ainsworth, P.; Pollak, M.; Sun, P.; Narod, S.A. Diabetes and breast cancer among women with BRCA1 and BRCA2 mutations. Cancer 2011, 117, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, M.; Dukatz, R.; Yahiaoui-Doktor, M.; Berling, A.; Basrai, M.; Staiger, V.; Niederberger, U.; Marter, N.; Lammert, J.; Grill, S.; et al. Feasibility of structured endurance training and Mediterranean diet in BRCA1 and BRCA2 mutation carriers—An interventional randomized controlled multicenter trial (LIBRE-1). BMC Cancer 2017, 17, 17–3732. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.V.W.; Laszlo, R.; Otto, S.; Prokopchuk, D.; Schumann, U.; Ebner, F.; Huober, J.; Steinacker, J.M. Feasibility and effects of a combined adjuvant high-intensity interval/strength training in breast cancer patients: A single-center pilot study. Disabil. Rehabil. 2018, 40, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Kushi, L.; Doyle, C.; McCullough, M.; Rock, C.; Demark-Wahnefried, W.; Bandera, E.; Gapstur, S.; Patel, A.; Andrews, K.; Gansler, T. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention Reducing the Risk of Cancer With Healthy Food Choices and Physical Activity. CA A Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef] [PubMed]
- Lago, C.; Sung, H.J.; Ma, W.; Wang, P.-Y.; Hwang, P. p53, Aerobic Metabolism, and Cancer. Antioxid. Redox Signal. 2010, 15, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Lakoski, S.; Willis, B.; Barlow, C.; Leonard, D.; Gao, A.; Radford, N.; Farrell, S.; Douglas, P.; Berry, J.; Defina, L.; et al. Midlife Cardiorespiratory Fitness, Incident Cancer, and Survival After Cancer in Men: The Cooper Center Longitudinal Study. JAMA Oncol. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.; Howell, A.; Evans, D.G. Can diet and lifestyle prevent breast cancer: What is the evidence? Am. Soc. Clin. Oncol. Educ. Book 2015, 73, e66–e73. [Google Scholar] [CrossRef] [PubMed]
- Pettapiece-Phillips, R.; Narod, S.; Kotsopoulos, J. The role of body size and physical activity on the risk of breast cancer in BRCA mutation carriers. Cancer Causes Control 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Pijpe, A.; Manders, P.; Brohet, R.; Collée, J.; Verhoef, S.; Vasen, H.; Hoogerbrugge, N.; Asperen, C.; Dommering, C.; Ausems, M.; et al. Physical activity and the risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res. Treat. 2010, 120, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Nkondjock, A.; Robidoux, A.; Paredes, Y.; Narod, S.A.; Ghadirian, P. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res. Treat. 2006, 98, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Grill, S.; Yahiaoui-Doktor, M.; Dukatz, R.; Lammert, J.; Ullrich, M.; Engel, C.; Pfeifer, K.; Basrai, M.; Siniatchkin, M.; Schmidt, T.; et al. Smoking and physical inactivity increase cancer prevalence in BRCA-1 and BRCA-2 mutation carriers: Results from a retrospective observational analysis. Arch. Gynecol. Obstet. 2017, 296, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, B.; Westerlind, K.; Strange, R.; Khan, G.; Patil, D.; Boeneman, K.; Hilakivi-Clarke, L. Prepubertal physical activity up-regulates estrogen receptor β, BRCA1 and p53 mRNA expression in the rat mammary gland. Breast Cancer Res. Treat. 2008, 115, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lammert, J.; Lubinski, J.; Gronwald, J.; Huzarski, T.; Armel, S.; Eisen, A.; Meschino, W.S.; Lynch, H.T.; Snyder, C.; Eng, C.; et al. Physical activity during adolescence and young adulthood and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2018, 169, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Batacan, R.B., Jr.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017, 51, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, A.; Cerrillo-Urbina, A.J.; Herrera-Valenzuela, T.; Cristi-Montero, C.; Saavedra, J.M.; Martinez-Vizcaino, V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? A meta-analysis. Obes. Rev. 2016, 17, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Coletta, A.M.; Brewster, A.M.; Chen, M.; Li, Y.; Bevers, T.B.; Basen-Engquist, K.; Gilchrist, S.C. High-Intensity Interval Training Is Feasible in Women at High Risk for Breast Cancer. Med. Sci. Sports Exerc. 2019, 51, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Zob, D.; Vasilescu, M.; Gruia, M.; Anghel, R. Breast cancer. Screening criteria. Chirurgia 2013, 108, 557–562. [Google Scholar] [PubMed]
- Banne, A.F.; Amiri, A.; Pero, R.W. Reduced level of serum thiols in patients with a diagnosis of active disease. J Anti Aging Med. 2003, 6, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Greilberger, J.F.; Schwaberger, G.; Hofmann, P.; Oettl, K. Single bouts of exercise affect albumin redox state and carbonyl groups on plasma protein of trained men in a workload-dependent manner. J. Appl. Physiol. 1985, 104, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.T.; Cho, S.Y.; So, W.Y. Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise. J. Sport Health Sci. 2017, 6, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Stavrinou, P.; Fatouros, I.G.; Philippou, A.; Chatzinikolaou, A.; Draganidis, D.; Ermidis, G.; Maridaki, M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. 2013, 61, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Pugliese, L.; Marzorati, M.; Serpiello, F.R.; La Torre, A.; Porcelli, S. Time-course changes of oxidative stress response to high-intensity discontinuous training versus moderate-intensity continuous training in masters runners. PLoS ONE 2014, 9, e87506. [Google Scholar] [CrossRef] [PubMed]
- Asegaonkar, S.B.; Asegaonkar, B.N.; Takalkar, U.V.; Advani, S.; Thorat, A.P. C-Reactive Protein and Breast Cancer: New Insights from Old Molecule. Int. J. Breast Cancer 2015, 2015, 145647. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.A.M.; Dantas, P.M.S.; Dos Santos, I.K.; Dantas, M.; da Silva, D.C.P.; de Cabral, B.G.A.T.; Guerra, R.O.; Júnior, G.B.C. Effect of Acute and Chronic Aerobic Exercise on Immunological Markers: A Systematic Review. Front. Physiol. 2020, 10, 1602. [Google Scholar] [CrossRef] [PubMed]
- Schuit, A.J.; Schouten, E.G.; Kluft, C.; de Maat, M.; Menheere, P.P.; Kok, F.J. Effect of strenuous exercise on fibrinogen and fibrinolysis in healthy elderly men and women. Thromb. Haemost. 1997, 78, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L. Effect of exercise training on C reactive protein: A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 2017, 51, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Kilic, N.; Yavuz Taslipinar, M.; Guney, Y.; Tekin, E.; Onuk, E. An investigation into the serum thioredoxin, superoxide dismutase, malondialdehyde, and advanced oxidation protein products in patients with breast cancer. Ann. Surg. Oncol. 2014, 21, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
- Ookawara, T.; Haga, S.; Ha, S.; Oh-Ishi, S.; Toshinai, K.; Kizaki, T.; Ji, L.L.; Suzuki, K.; Ohno, H. Effects of endurance training on three superoxide dismutase isoenzymes in human plasma. Free Radic. Res. 2003, 37, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Songstad, N.T.; Kaspersen, K.H.; Hafstad, A.D.; Basnet, P.; Ytrehus, K.; Acharya, G. Effects of High Intensity Interval Training on Pregnant Rats, and the Placenta, Heart and Liver of Their Fetuses. PLoS ONE 2015, 10, e0143095. [Google Scholar] [CrossRef] [PubMed]
- Alessio, H.M.; Goldfarb, A.H.; Cutler, R.G. MDA content increases in fast- and slow-twitch skeletal muscle with intensity of exercise in a rat. Am. J. Physiol. 1988, 255. [Google Scholar] [CrossRef] [PubMed]
- Fix, A.J.; Daughton, D. Human Activity Profile: Professional Manual; Psychological Assessment Resources Inc.: Odessa, FL, USA, 1988. [Google Scholar]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998; ISBN 0-88011-623-4. [Google Scholar]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Scharhag-Rosenberger, F.; Becker, T.; Streckmann, F.; Schmidt, K.; Berling, A.; Bernardi, A.; Engeroff, T.; Exner, A.K.; Gutekunst, K.; Hofmeister, D.; et al. Studien zu körperlichem Training bei onkologischen Patienten: Empfehlungen zu den Erhebungsmethoden. Dtsch. Z. Sportmed. 2014, 65, 304–313. [Google Scholar] [CrossRef]
- Bjarnason-Wehrens, B.; Mayer-Berger, W.; Meister, E.R.; Baum, K.; Hambrecht, R.; Gielen, S. Recommendations for resistance exercise in cardiac rehabilitation. Recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Scheier, M.; Carver, C.; Bridges, M. Distinguishing Optimism From Neuroticism (and Trait Anxiety, Self-Mastery, and Self-Esteem): A Reevaluation of the Life Orientation Test. J. Personal. Soc. Psychol. 1995, 67, 1063–1078. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety And Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Schwarz, R.; Herrmann, C.; Buss, U.; Snaith, R. Hospital Anxiety and Depression Scale—Deutsche Version(HADS-D). Diagnostica 2002, 48, 112–113. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zwetsloot, K.A.; Triplett, N.T.; Meaney, M.P.; Farris, G.E.; Nieman, D.C. Human skeletal muscle biopsy procedures using the modified Bergström technique. J. Vis. Exp. 2014, 51812. [Google Scholar] [CrossRef]
- Mahoney, D.; Carey, K.; Fu, H.-H.; Snow, R.; Cameron-Smith, D.; Parise, G.; Tarnopolsky, M. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol. Genom. 2004, 18, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Jemiolo, B.; Trappe, S. Single muscle fiber gene expression in human skeletal muscle: Validation of internal control with exercise. Biochem. Biophys. Res. Commun. 2004, 320, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; No. 0805802835; Lawrence Earlbam Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Glaesmer, H.; Hoyer, J.; Klotsche, J.; Herzberg, P. Die Deutsche Version des Life-Orientation-Tests (LOT-R) zum dispositionellen Optimismus und Pessimismus. Zeitschrift Gesundheitspsychologie 2008, 16, 26–31. [Google Scholar] [CrossRef]
- Herrmann, C.; Buss, U. Vorstellung und Validierung einer deutschen Version der “Hospital Anxiety and Depression Scale” (HAD-Skala). Diagnostica 1994, 40, 143–154. [Google Scholar]


| Parameter | Group | T0 | T1 | p-Value |
|---|---|---|---|---|
| IGF-1 [μg/L] | CG (n = 6) | 121.5 ± 71.7 | 104.3 ± 38.3 | 0.379 |
| IG (n = 9) | 181.4 ± 72.7 | 207.4 ± 51.3 | 0.450 | |
| IGFBP-3 [μg/dL] | CG (n = 6) | 93.5 ± 28.5 | 89.2 ± 24.6 | 0.465 |
| IG (n = 10) | 70.7 ± 21.8 | 88.8 ± 17.6 | 0.063 | |
| IL-2R [U/mL] | CG (n = 5) | 414.2 ± 215.4 | 353.2 ± 98.3 | 0.540 |
| IG (n = 9) | 379.8 ± 145.9 | 471.1 ± 158.2 | 0.155 | |
| GDF-15 [pg/mL] | CG (n = 6) | 467.1 ± 187.0 | 511.3 ± 174.4 | 0.485 |
| IG (n = 10) | 391.2 ± 213.1 | 383.3 ± 161.3 | 0.872 | |
| TNF-α [pg/mL] | CG (n = 5) | 5.4 ± 1.6 | 6.3 ± 3.4 | 0.664 |
| IG (n = 9) | 5.9 ± 2.6 | 6.9 ± 2.7 | 0.191 | |
| IL-6 [pg/mL] | CG (n = 5) | 2.0 ± 1.1 | 1.9 ± 0.6 | 0.672 |
| IG (n = 9) | 1.6 ± 0.4 | 1.6 ± 0.3 | 0.351 | |
| IL-1β [pg/mL] | CG (n = 5) | 2.9 ± 1.9 | 2.9 ± 1.9 | 1.000 |
| IG (n = 9) | 2.3 ± 1.5 | 2.5 ± 1.7 | 0.356 | |
| IL-10 [pg/mL] | CG (n = 5) | 3.6 ± 2.5 | 2.9 ± 2.0 | 0.374 |
| IG (n = 10) | 2.0 ± 1.7 | 1.9 ± 1.8 | 0.347 |
| Parameter | IG (n = 10) | CG (n = 6) | ||
|---|---|---|---|---|
| Mean (SD) | CI (5;95) | Mean (SD) | CI (5;95) | |
| Age (years) *1 | 35.5 (10.5) | (28.0; 43.0) | 46.3 (5.3) | (40.8; 51.9) |
| Body size (cm) *1 | 170 (0.1) | (165; 174) | 171 (0.1) | (161; 181) |
| Body mass (kg) *1 | 75.8 (14.1) | (65.7; 85.9) | 72.0 (16.0) | (55.3; 88.7) |
| Waist-to-height ratio *1 | 0.51 (0.04) | (0.48; 0.53) | 0.55 (0.13) | (0.41; 0.69) |
| Sex (men/women) *2 | 2/8 | 1/5 | ||
| BRCA (1/2) *2 | 5/5 | 3/3 | ||
| Menopausal status (pre/peri) | 6/2 | 3/2 | ||
| Medical treatment before study participation | ||||
| Mastectomy/adenectomy | 0/2 | 1/3 | ||
| Treated breast cancer (chemotherapy/irradiation) | 2 (2/1) | 2 (2/1) | ||
| Ovarian cancer | 0 | 0 | ||
| High daily physical activity *3 | 6 | 3 | ||
| Average of physically strenuous, sweat-inducing activities | 1 time per week | 2–4 times per week | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizjak, D.A.; Schulz, S.V.W.; Schumann, U.; Otto, S.; Kirsten, J.; Ebner, F.; Leinert, E.; Huober, J.; Janni, W.; Steinacker, J.M. Beneficial Molecular Adaptations in BRCA-Mutation Carriers by Combined HIT/HIRT Intervention: Results from a Pilot Study. Cancers 2020, 12, 1526. https://doi.org/10.3390/cancers12061526
Bizjak DA, Schulz SVW, Schumann U, Otto S, Kirsten J, Ebner F, Leinert E, Huober J, Janni W, Steinacker JM. Beneficial Molecular Adaptations in BRCA-Mutation Carriers by Combined HIT/HIRT Intervention: Results from a Pilot Study. Cancers. 2020; 12(6):1526. https://doi.org/10.3390/cancers12061526
Chicago/Turabian StyleBizjak, Daniel A., Sebastian V. W. Schulz, Uwe Schumann, Stephanie Otto, Johannes Kirsten, Florian Ebner, Elena Leinert, Jens Huober, Wolfgang Janni, and Jürgen Michael Steinacker. 2020. "Beneficial Molecular Adaptations in BRCA-Mutation Carriers by Combined HIT/HIRT Intervention: Results from a Pilot Study" Cancers 12, no. 6: 1526. https://doi.org/10.3390/cancers12061526
APA StyleBizjak, D. A., Schulz, S. V. W., Schumann, U., Otto, S., Kirsten, J., Ebner, F., Leinert, E., Huober, J., Janni, W., & Steinacker, J. M. (2020). Beneficial Molecular Adaptations in BRCA-Mutation Carriers by Combined HIT/HIRT Intervention: Results from a Pilot Study. Cancers, 12(6), 1526. https://doi.org/10.3390/cancers12061526






