Assessment of Periprostatic and Subcutaneous Adipose Tissue Lipolysis and Adipocyte Size from Men with Localized Prostate Cancer
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
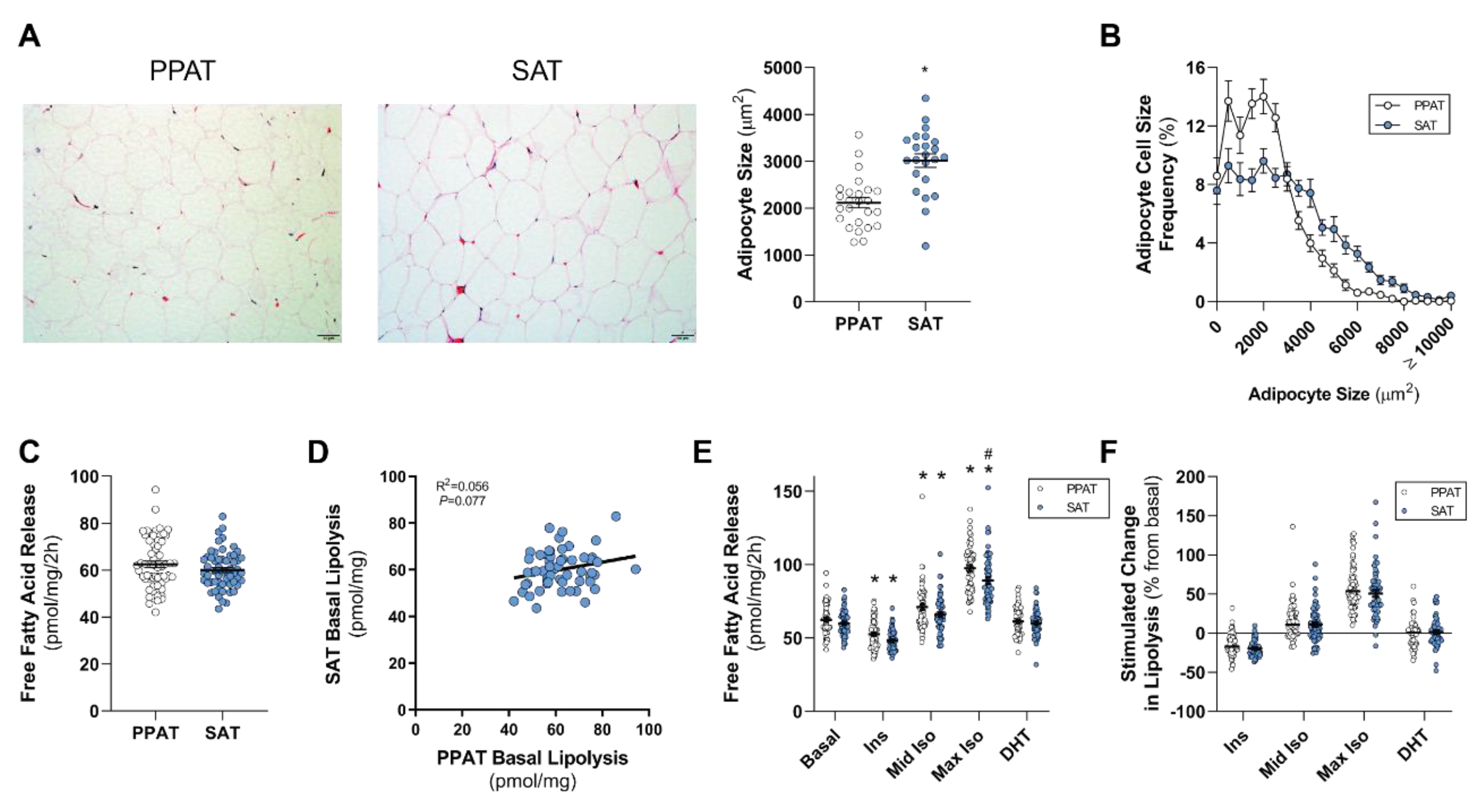
2.2. PPAT Adipocytes are Smaller than Subcutaneous Adipose Tissue Adipocytes and Secrete Less Polyunsaturated Fatty Acids but Basal and Stimulated Lipolysis are Essentially the Same
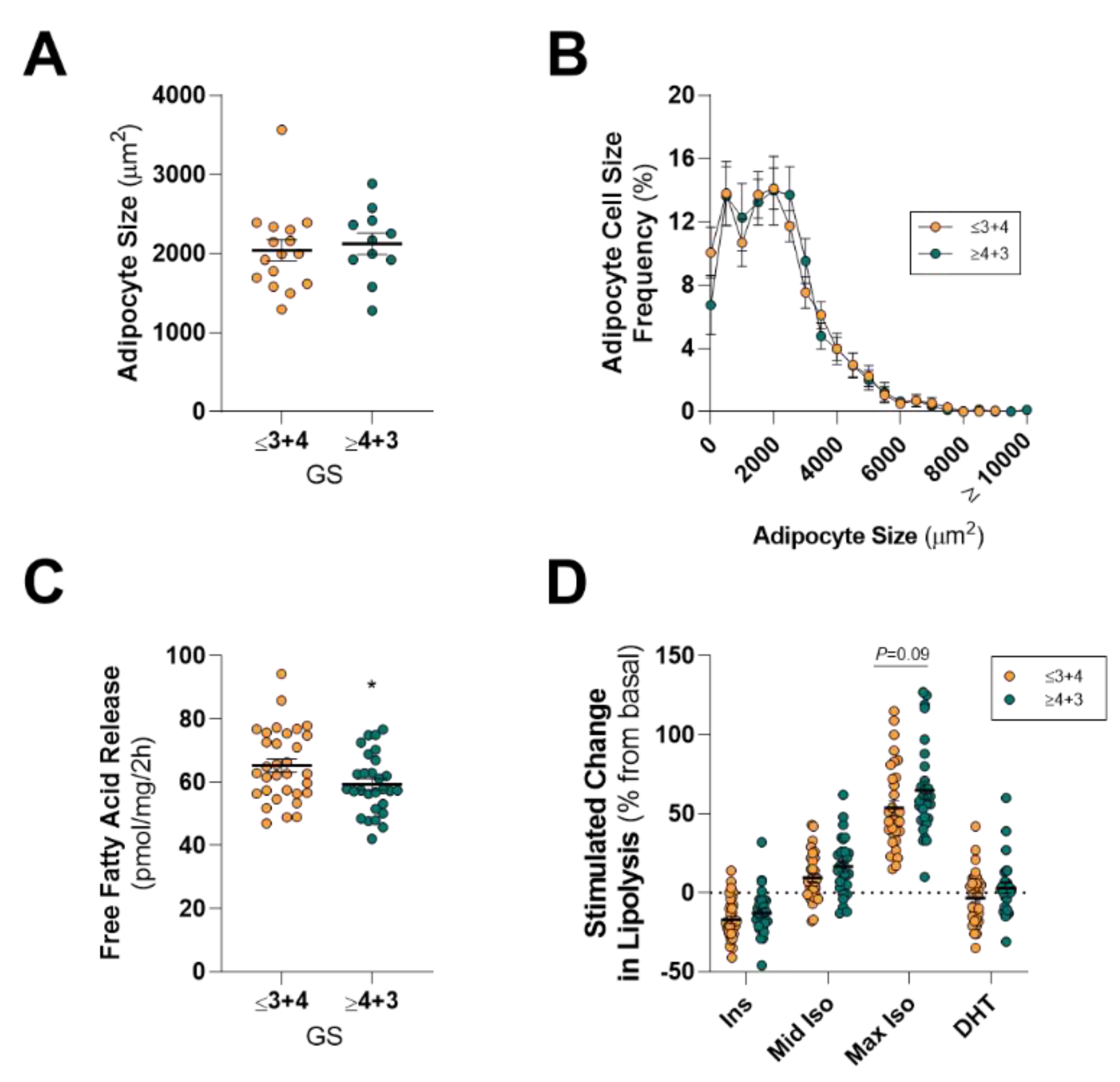
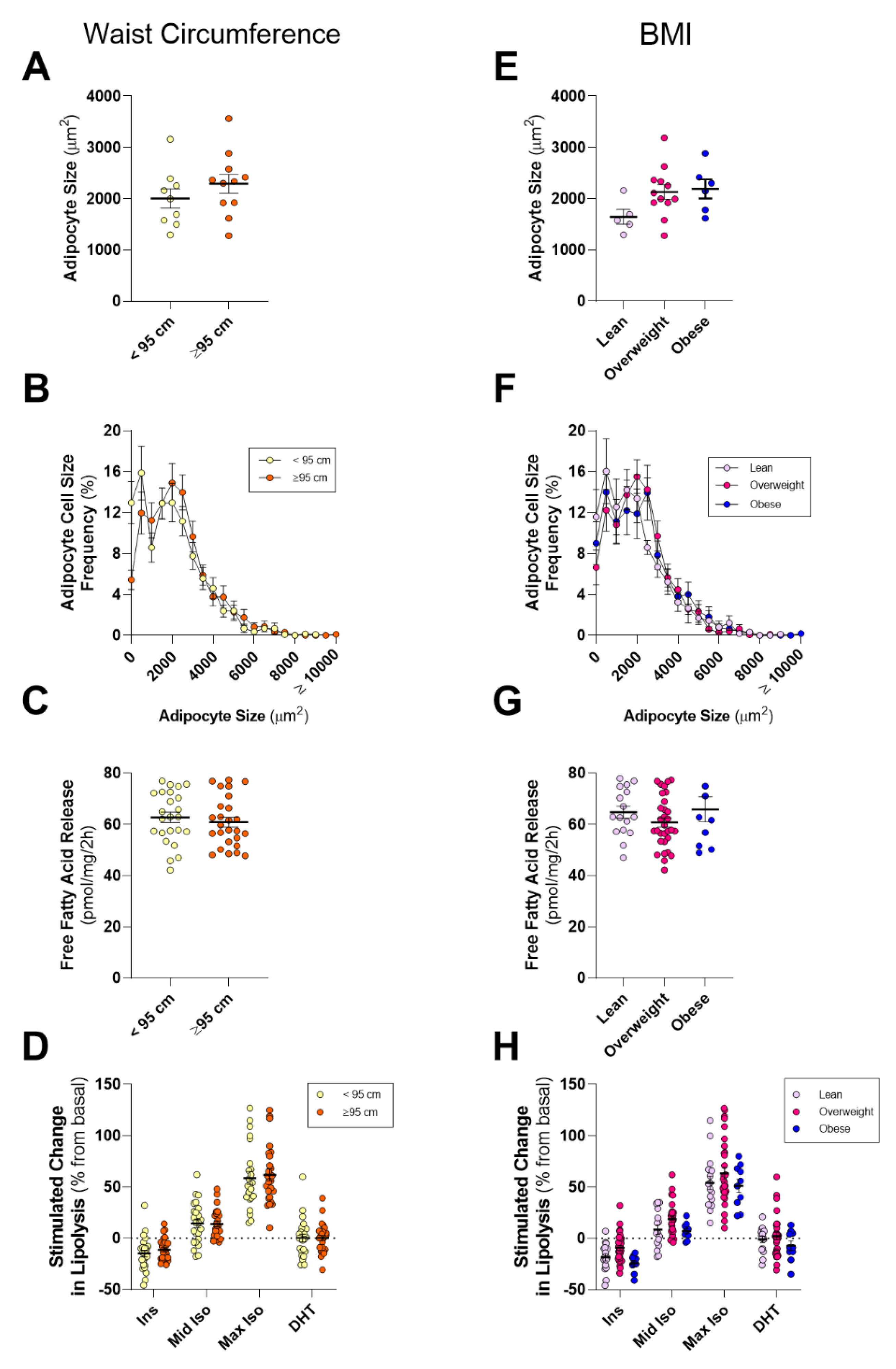
2.3. PPAT Adipocyte Size and Lipolysis Does not Differ between Low and High Aggressive Disease
3. Discussion
4. Materials and Methods
4.1. Tissue Collection and Clinical Data
4.2. Adipose Lipolysis
4.3. Fatty Acid Profile
4.4. Adipocyte Size
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, C.K.; Check, D.P.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Ferlay, J.; Bray, F.; Cook, M.B.; Devesa, S.S. Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int. J. Cancer 2016, 138, 1388–1400. [Google Scholar] [CrossRef]
- Parikesit, D.; Mochtar, C.A.; Umbas, R.; Hamid, A.R. The impact of obesity towards prostate diseases. Prostate Int. 2016, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, G.; Sun, B.; Zhao, G.; Liu, D.; Sun, J.; Liu, C.; Guo, H. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol. Lett. 2015, 9, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.C.; Howard, L.E.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L., Jr.; Freedland, S.J. Obesity increases the risk for high-grade prostate cancer: Results from the REDUCE study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2936–2942. [Google Scholar] [CrossRef] [PubMed]
- Boehm, K.; Sun, M.; Larcher, A.; Blanc-Lapierre, A.; Schiffmann, J.; Graefen, M.; Sosa, J.; Saad, F.; Parent, M.-E.; Karakiewicz, P.I. Waist circumference, waist-hip ratio, body mass index, and prostate cancer risk: Results from the North-American case-control study Prostate Cancer & Environment Study. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 494.e1–494.e7. [Google Scholar] [CrossRef]
- Perez-Cornago, A.; Appleby, P.N.; Pischon, T.; Tsilidis, K.K.; Tjonneland, A.; Olsen, A.; Overvad, K.; Kaaks, R.; Kuhn, T.; Boeing, H.; et al. Tall height and obesity are associated with an increased risk of aggressive prostate cancer: Results from the EPIC cohort study. BMC Med. 2017, 15, 115. [Google Scholar] [CrossRef]
- Zhong, S.; Yan, X.; Wu, Y.; Zhang, X.; Chen, L.; Tang, J.; Zhao, J. Body mass index and mortality in prostate cancer patients: A dose-response meta-analysis. Prostate Cancer Prostatic Dis. 2016, 19, 122–131. [Google Scholar] [CrossRef]
- Hu, M.B.; Liu, S.H.; Jiang, H.W.; Bai, P.D.; Ding, Q. Obesity affects the biopsy-mediated detection of prostate cancer, particularly high-grade prostate cancer: A dose-response meta-analysis of 29,464 patients. PLoS ONE 2014, 9, e106677. [Google Scholar] [CrossRef]
- Duarte, M.F.; Luis, C.; Baylina, P.; Faria, M.I.; Fernandes, R.; La Fuente, J.M. Clinical and metabolic implications of obesity in prostate cancer: Is testosterone a missing link? Aging Male 2019, 22, 228–240. [Google Scholar] [CrossRef]
- Zadra, G.; Photopoulos, C.; Loda, M. The fat side of prostate cancer. Biochim. Biophys. Acta 2013, 1831, 1518–1532. [Google Scholar] [CrossRef]
- Grossmann, M.; Wittert, G. Androgens, diabetes and prostate cancer. Endocr. Relat. Cancer 2012, 19, F47–F62. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Hursting, S.D. Obesity and cancer: Mechanistic insights from transdisciplinary studies. Endocr. Relat. Cancer 2015, 22, R365–R386. [Google Scholar] [CrossRef] [PubMed]
- Nassar, Z.D.; Aref, A.T.; Miladinovic, D.; Mah, C.Y.; Raj, G.V.; Hoy, A.J.; Butler, L.M. Peri-prostatic adipose tissue: The metabolic microenvironment of prostate cancer. BJU Int. 2018, 121 (Suppl. 3), 9–21. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, K.; Fitchev, P.; Brendler, C.; Plunkett, B.; Dangle, P.; McGuire, M.; Shevrin, D.; Cornwell, M.; Quinn, M.; Kaul, K.; et al. 314 Active triacylglycerol metabolism and increased infiltrating adipocyte density in high grade prostate cancer. J. Urol. 2012, 187, e127. [Google Scholar] [CrossRef]
- Byar, D.P.; Mostofi, F.K. Carcinoma of the prostate: Prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer 1972, 30, 5–13. [Google Scholar] [CrossRef]
- Kapoor, J.; Namdarian, B.; Pedersen, J.; Hovens, C.; Moon, D.; Peters, J.; Costello, A.J.; Ruljancich, P.; Corcoran, N.M. Extraprostatic extension into periprostatic fat is a more important determinant of prostate cancer recurrence than an invasive phenotype. J. Urol. 2013, 190, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Darson, M.F.; Bergstralh, E.J.; Slezak, J.; Myers, R.P.; Bostwick, D.G. Correlation of margin status and extraprostatic extension with progression of prostate carcinoma. Cancer 1999, 86, 1775–1782. [Google Scholar] [CrossRef]
- Laurent, V.; Guerard, A.; Mazerolles, C.; Le Gonidec, S.; Toulet, A.; Nieto, L.; Zaidi, F.; Majed, B.; Garandeau, D.; Socrier, Y.; et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016, 7, 10230. [Google Scholar] [CrossRef]
- Laurent, V.; Toulet, A.; Attane, C.; Milhas, D.; Dauvillier, S.; Zaidi, F.; Clement, E.; Cinato, M.; Le Gonidec, S.; Guerard, A.; et al. Periprostatic Adipose Tissue Favors Prostate Cancer Cell Invasion in an Obesity-Dependent Manner: Role of Oxidative Stress. Mol. Cancer Res. 2019, 17, 821–835. [Google Scholar] [CrossRef]
- Ribeiro, R.; Monteiro, C.; Cunha, V.; Oliveira, M.J.; Freitas, M.; Fraga, A.; Principe, P.; Lobato, C.; Lobo, F.; Morais, A.; et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J. Exp. Clin. Cancer Res. 2012, 31, 32. [Google Scholar] [CrossRef]
- Finley, D.S.; Calvert, V.S.; Inokuchi, J.; Lau, A.; Narula, N.; Petricoin, E.F.; Zaldivar, F.; Santos, R.; Tyson, D.R.; Ornstein, D.K. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J. Urol. 2009, 182, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, A.; Satoh, Y.; Tokuda, Y.; Fujiyama, C.; Udo, K.; Uozumi, J. Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. Int. J. Urol. 2010, 17, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Estève, D.; Roumiguié, M.; Manceau, C.; Milhas, D.; Muller, C. Periprostatic adipose tissue: A heavy player in prostate cancer progression. Curr. Opin. Endocr. Metab. Res. 2020, 10, 29–35. [Google Scholar] [CrossRef]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; Van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjonneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Centenera, M.; Selth, L.A.; Ebrahimie, E.; Butler, L.M.; Tilley, W.D. New Opportunities for Targeting the Androgen Receptor in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030478. [Google Scholar] [CrossRef]
- O’Reilly, M.; House, P.; Tomlinson, J.W. Understanding androgen action in adipose tissue. J. Steroid Biochem. Mol. Boil. 2014, 143, 277–284. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef]
- Grogan, J.; Gupta, R.; Mahon, K.; Stricker, P.; Delprado, W.; Turner, J.; Horvath, L.G.; Kench, J.; Haynes, A.-M. Predictive value of the 2014 International Society of Urological Pathology grading system for prostate cancer in patients undergoing radical prostatectomy with long-term follow-up. BJU Int. 2017, 120, 651–658. [Google Scholar] [CrossRef]
- Iordanescu, G.; Brendler, C.; Crawford, S.E.; Wyrwicz, A.M.; Venkatasubramanian, P.N.; Doll, J.A. MRS measured fatty acid composition of periprostatic adipose tissue correlates with pathological measures of prostate cancer aggressiveness. J. Magn. Reson. Imaging 2014, 42, 651–657. [Google Scholar] [CrossRef]
- Mamalakis, G.; Kafatos, A.; Kalogeropoulos, N.; Andrikopoulos, N.; Daskalopulos, G.; Kranidis, A. Prostate cancer vs hyperplasia: Relationships with prostatic and adipose tissue fatty acid composition. Prostaglandins Leukot Essent Fat. Acids 2002, 66, 467–477. [Google Scholar] [CrossRef]
- Jacobs, E.J.; Newton, C.C.; Wang, Y.; Patel, A.V.; McCullough, M.L.; Campbell, P.T.; Thun, M.J.; Gapstur, S.M. Waist circumference and all-cause mortality in a large US cohort. Arch. Intern. Med. 2010, 170, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- De Hollander, E.L.; Bemelmans, W.J.; Boshuizen, H.C.; Friedrich, N.; Wallaschofski, H.; Guallar-Castillon, P.; Walter, S.; Zillikens, M.C.; Rosengren, A.; Lissner, L.; et al. The association between waist circumference and risk of mortality considering body mass index in 65- to 74-year-olds: A meta-analysis of 29 cohorts involving more than 58 000 elderly persons. Int. J. Epidemiol. 2012, 41, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.; Chittleborough, C.; Taylor, A.; Ruffin, R.; Wilson, D.; Phillips, P. Body mass index, waist hip ratio, and waist circumference: Which measure to classify obesity? Soz. Und Prav. 2003, 48, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, P.N.; Crawford, S.E.; Fitchev, P.S.; Brendler, C.B.; Plunkett, B.A.; Abroe, B.; Mafi, M.; Morgan, G.; Cornwell, M.L.; O’Leary, J.; et al. Abstract 1499: Periprostatic fat from obese patients promotes prostate cancer growth. Cancer Res. 2012, 72, 1499. [Google Scholar] [CrossRef]
- Venkatasubramanian, P.N.; Brendler, C.B.; Plunkett, B.A.; Crawford, S.E.; Fitchev, P.S.; Morgan, G.; Cornwell, M.L.; McGuire, M.S.; Wyrwicz, A.M.; Doll, J.A. Periprostatic adipose tissue from obese prostate cancer patients promotes tumor and endothelial cell proliferation: A functional and MR imaging pilot study. Prostate 2014, 74, 326–335. [Google Scholar] [CrossRef]
- Arner, P.; Langin, D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol. Metab. 2014, 25, 255–262. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar]
- Xu, J.; Wang, R.; Xie, Z.H.; Odero-Marah, V.; Pathak, S.; Multani, A.; Chung, L.W.; Zhau, H.E. Prostate cancer metastasis: Role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate 2006, 66, 1664–1673. [Google Scholar] [CrossRef]
- Park, Y.M.; White, A.J.; Nichols, H.B.; O’Brien, K.M.; Weinberg, C.R.; Sandler, D.P. The association between metabolic health, obesity phenotype and the risk of breast cancer. Int. J. Cancer 2017, 140, 2657–2666. [Google Scholar] [CrossRef]
- Andersson, S.O.; Wolk, A.; Bergstrom, R.; Adami, H.O.; Engholm, G.; Englund, A.; Nyren, O. Body size and prostate cancer: A 20-year follow-up study among 135006 Swedish construction workers. J. Natl. Cancer Inst. 1997, 89, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Cho, J.Y.; Kim, S.Y.; Kim, S.H. Periprostatic fat thickness on MRI: Correlation with Gleason score in prostate cancer. Ajr. Am. J. Roentgenol. 2015, 204, W43–W47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, L.J.; Qi, J.; Yang, Z.G.; Huang, T.; Huo, R.C. Periprostatic adiposity measured on magnetic resonance imaging correlates with prostate cancer aggressiveness. Urol. J. 2014, 11, 1793–1799. [Google Scholar] [PubMed]
- Cerhan, J.R.; Moore, S.C.; Jacobs, E.J.; Kitahara, C.M.; Rosenberg, P.S.; Adami, H.O.; Ebbert, J.O.; English, D.R.; Gapstur, S.M.; Giles, G.G.; et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin. Proc. 2014, 89, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Van Roermund, J.G.; Hinnen, K.A.; Tolman, C.J.; Bol, G.H.; Witjes, J.A.; Bosch, J.L.; Kiemeney, L.A.; Van Vulpen, M. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int. 2011, 107, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Lo, J.; Ascui, N.; Watt, M.J. Linking obesogenic dysregulation to prostate cancer progression. Endocr. Connect. 2015, 4, R68–R80. [Google Scholar] [CrossRef]
- Sacca, P.A.; Creydt, V.P.; Choi, H.; Mazza, O.N.; Fletcher, S.; Vallone, V.B.F.; Scorticati, C.; Chasseing, N.A.; Calvo, J.C. Human Periprostatic Adipose Tissue: Its Influence on Prostate Cancer Cells. Cell. Physiol. Biochem. 2012, 30, 113–122. [Google Scholar] [CrossRef]
- Ribeiro, R.J.; Monteiro, C.P.; Cunha, V.; Azevedo, A.S.; Oliveira, M.J.; Monteiro, R.; Fraga, A.M.; Príncipe, P.; Lobato, C.; Lobo, F.; et al. Tumor Cell-educated Periprostatic Adipose Tissue Acquires an Aggressive Cancer-promoting Secretory Profile. Cell. Physiol. Biochem. 2012, 29, 233–240. [Google Scholar] [CrossRef]
- Fang, L.; Guo, F.; Zhou, L.; Stahl, R.; Grams, J. The cell size and distribution of adipocytes from subcutaneous and visceral fat is associated with type 2 diabetes mellitus in humans. Adipocyte 2015, 4, 273–279. [Google Scholar] [CrossRef]
- Baglioni, S.; Cantini, G.; Poli, G.; Francalanci, M.; Squecco, R.; Di Franco, A.; Borgogni, E.; Frontera, S.; Nesi, G.; Liotta, F.; et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS ONE 2012, 7, e36569. [Google Scholar] [CrossRef]
- Arner, P. Differences in Lipolysis between Human Subcutaneous and Omental Adipose Tissues. Ann. Med. 1995, 27, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Bolinder, J.; Kager, L.; Ostman, J.; Arner, P. Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis. Diabetes 1983, 32, 117–123. [Google Scholar] [CrossRef]
- Reynisdottir, S.; Dauzats, M.; Thorne, A.; Langin, D. Comparison of hormone-sensitive lipase activity in visceral and subcutaneous human adipose tissue. J. Clin. Endocrinol. Metab. 1997, 82, 4162–4166. [Google Scholar] [CrossRef] [PubMed]
- Careaga, V.; Sacca, P.; Mazza, O.N.; Scorticati, C.; Vitagliano, G.; Fletcher, S.; Maier, M.; Calvo, J.C. Fatty Acid Composition of Human Periprostatic Adipose Tissue from Argentine Patients and Its Relationship to Prostate Cancer and Benign Prostatic Hyperplasia. Res. Cancer Tumor 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Hellmuth, C.; Demmelmair, H.; Schmitt, I.; Peissner, W.; Bluher, M.; Koletzko, B. Association between plasma nonesterified fatty acids species and adipose tissue fatty acid composition. PLoS ONE 2013, 8, e74927. [Google Scholar] [CrossRef] [PubMed]
- Figiel, S.; Pinault, M.; Domingo, I.; Guimaraes, C.; Guibon, R.; Besson, P.; Tavernier, E.; Blanchet, P.; Multigner, L.; Bruyere, F.; et al. Fatty acid profile in peri-prostatic adipose tissue and prostate cancer aggressiveness in African-Caribbean and Caucasian patients. Eur. J. Cancer 2018, 91, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef]
- Farzaneh-Far, R.; Lin, J.; Epel, E.S.; Harris, W.S.; Blackburn, E.H.; Whooley, M.A. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010, 303, 250–257. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef]
- Aucoin, M.; Cooley, K.; Knee, C.; Fritz, H.; Balneaves, L.G.; Breau, R.; Fergusson, D.; Skidmore, B.; Wong, R.; Seely, D. Fish-Derived Omega-3 Fatty Acids and Prostate Cancer: A Systematic Review. Integr. Cancer 2017, 16, 32–62. [Google Scholar] [CrossRef]
- Gucalp, A.; Iyengar, N.M.; Zhou, X.K.; Giri, D.D.; Falcone, D.J.; Wang, H.; Williams, S.; Krasne, M.D.; Yaghnam, I.; Kunzel, B.; et al. Periprostatic adipose inflammation is associated with high-grade prostate cancer. Prostate Cancer Prostatic Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Van Roermund, J.G.; Bol, G.H.; Witjes, J.A.; Ruud Bosch, J.L.; Kiemeney, L.A.; Van Vulpen, M. Periprostatic fat measured on computed tomography as a marker for prostate cancer aggressiveness. World J. Urol. 2010, 28, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Zincke, H.; Bergstralh, E.J.; Blute, M.L.; Myers, R.P.; Barrett, D.M.; Lieber, M.M.; Martin, S.K.; Oesterling, J.E. Radical prostatectomy for clinically localized prostate cancer: Long-term results of 1,143 patients from a single institution. J. Clin. Oncol. 1994, 12, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Partin, A.W.; Sauvageot, J.; Walsh, P.C. Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am. J. Surg. Pathol. 1996, 20, 286–292. [Google Scholar] [CrossRef] [PubMed]
- McNeal, J.E.; Villers, A.A.; Redwine, E.A.; Freiha, F.S.; Stamey, T.A. Capsular penetration in prostate cancer. Significance for natural history and treatment. Am. J. Surg. Pathol. 1990, 14, 240–247. [Google Scholar] [CrossRef]
- Zagars, G.K.; Geara, F.B.; Pollack, A.; Von Eschenbach, A.C. The T classification of clinically localized prostate cancer. An appraisal based on disease outcome after radiation therapy. Cancer 1994, 73, 1904–1912. [Google Scholar] [CrossRef]
- Arbones-Mainar, J.M.; Johnson, L.A.; Altenburg, M.K.; Maeda, N. Differential modulation of diet-induced obesity and adipocyte functionality by human apolipoprotein E3 and E4 in mice. Int. J. Obes. (2005) 2008, 32, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, B.L.; Zhang, X.; Xie, X.; Saarinen, A.; Lu, X.; Yang, X.; Liu, J. Defective Adipose Lipolysis and Altered Global Energy Metabolism in Mice with Adipose Overexpression of the Lipolytic Inhibitor G(0)/G(1) Switch Gene 2 (G0S2). J. Biol. Chem. 2014, 289, 1905–1916. [Google Scholar] [CrossRef]
- Arimochi, H.; Sasaki, Y.; Kitamura, A.; Yasutomo, K. Differentiation of preadipocytes and mature adipocytes requires PSMB8. Sci. Rep. 2016, 6, 26791. [Google Scholar] [CrossRef]
- Jaworski, K.; Sarkadi-Nagy, E.; Duncan, R.E.; Ahmadian, M.; Sul, H.S. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1–G4. [Google Scholar] [CrossRef]
- Hales, C.N.; Luzio, J.P.; Siddle, K. Hormonal control of adipose-tissue lipolysis. Biochem. Soc. Symp. 1978, 97–135. [Google Scholar]
- Balaban, S.; Lee, L.S.; Schreuder, M.; Hoy, A.J. Obesity and cancer progression: Is there a role of fatty acid metabolism? Biomed. Res. Int. 2015, 2015, 274585. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.E.; Blackburn, O.A.; Marchildon, F.; Cohen, P. Insights into the Link between Obesity and Cancer. Curr. Obes. Rep. 2017, 6, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Ribeiro, R.; Monteiro, C.; Catalan, V.; Hu, P.; Cunha, V.; Rodriguez, A.; Gomez-Ambrosi, J.; Fraga, A.; Principe, P.; Lobato, C.; et al. Obesity and prostate cancer: Gene expression signature of human periprostatic adipose tissue. BMC Med. 2012, 10, 108. [Google Scholar] [CrossRef]
- Zhang, T.; Tseng, C.; Zhang, Y.; Sirin, O.; Corn, P.G.; Li-Ning-Tapia, E.M.; Troncoso, P.; Davis, J.; Pettaway, C.; Ward, J.; et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun. 2016, 7, 11674. [Google Scholar] [CrossRef]
- Lo, J.C.; Clark, A.K.; Ascui, N.; Frydenberg, M.; Risbridger, G.P.; Taylor, R.A.; Watt, M.J. Obesity does not promote tumorigenesis of localized patient-derived prostate cancer xenografts. Oncotarget 2016, 7, 47650–47662. [Google Scholar] [CrossRef]
- Taussky, D.; Barkati, M.; Campeau, S.; Zerouali, K.; Nadiri, A.; Saad, F.; Delouya, G. Changes in periprostatic adipose tissue induced by 5alpha-reductase inhibitors. Andrology 2017, 5, 511–515. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar]
- Björnheden, T.; Jakubowicz, B.; Levin, M.; Odén, B.; Edén, S.; Sjöström, L.; Lönn, M. Computerized determination of adipocyte size. Obes. Res. 2004, 12, 95–105. [Google Scholar] [CrossRef]
- Galarraga, M.; Campion, J.; Munoz-Barrutia, A.; Boque, N.; Moreno, H.; Martinez, J.A.; Milagro, F.; Ortiz-de-Solorzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E.; Watt, M.J. Adipocyte triglyceride lipase expression in human obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E958–E964. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Bernard, S.; Naslund, E.; Salehpour, M.; Possnert, G.; Appelsved, L.; Fu, K.Y.; Alkass, K.; Druid, H.; Thorell, A.; et al. Impact of fat mass and distribution on lipid turnover in human adipose tissue. Nat. Commun. 2017, 8, 15253. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | Range |
|---|---|---|
| Age, years | 62 ± 8 (60) | 42–78 |
| PSA, µg/L | 6.88 ± 5.50 (58) | 1.4–30 |
| BMI, kg/m2 | 26.9 ± 3.4 (57) | 21.3–35.6 |
| Waist Circumference, cm | 95.8 ± 8.8 (50) | 81–117 |
| Gleason Score | ||
| ≤3 + 3 | 3 | |
| 3 + 4 | 29 | |
| 4 + 3 | 13 | |
| ≥4 + 4 | 15 | |
| Pathological T Stage | ||
| T2 | 29 | |
| T3a | 27 | |
| T3b | 4 | |
| T4 | 0 | |
| Blood Serum, n | 60 | |
| Glucose, mmol/L | 5.3 ± 0.7 | 3.6–7.1 |
| Insulin, pmol/L | 31 ± 23 | 7–136 |
| Cholesterol, mmol/L | 4.5 ± 1.0 | 2.4–6.2 |
| Triacylglycerol, mmol/L | 1.2 ± 0.4 | 0.5–2.4 |
| HDL, mmol/L | 1.3 ± 0.4 | 0.76–2.63 |
| LDL, mmol/L | 2.6 ± 1.0 | 0.3–5.2 |
| Fatty Acid (mol %) | PPAT (n = 54) | SAT (n = 48) |
|---|---|---|
| SFA | ||
| 14:0 | 2.47 ± 0.08 | 2.26 ± 0.11 |
| 15:0 | 0.07 ± 0.03 | 0.04 ± 0.02 |
| 16:0 | 22.32 ± 0.18 | 21.54 ± 0.18 * |
| 17:0 | 0.84 ± 0.04 | 0.79 ± 0.04 |
| 18:0 | 15.89 ± 0.31 | 16.50 ± 0.46 |
| 20:0 | 0.45 ± 0.11 | 0.19 ± 0.04 * |
| 22:0 | 0.06 ± 0.02 | 0.04 ± 0.04 |
| 24:0 | 0.63 ± 0.04 | 0.70 ± 0.05 |
| MUFA | ||
| 14:1 n-9 | 0.04 ± 0.02 | 0.05 ± 0.02 |
| 15:1 n-10 | 0.11 ± 0.04 | 0.30 ± 0.06 * |
| 16:1 n-7 | 2.46 ± 0.10 | 2.63 ± 0.14 |
| 17:1 n-7 | 0.02 ± 0.02 | 0.04 ± 0.02 |
| 18:1 n-7 | 0.67 ± 0.05 | 0.62 ± 0.05 |
| 18:1 n-9 | 31.16 ± 0.46 | 30.65 ± 0.61 |
| 20:1 n-9 | 0.20 ± 0.04 | 0.13 ± 0.03 |
| 22:1 n-9 | 1.71 ± 0.31 | 0.62 ± 0.14 * |
| 24:1 n-9 | 0.07 ± 0.02 | 0.13 ± 0.04 |
| PUFA n-6 | ||
| 18:2 n-6 | 12.00 ± 0.23 | 12.20 ± 0.21 |
| 18:3 n-6 | 0.11 ± 0.04 | 0.32 ± 0.07 * |
| 20:3 n-6 | 1.28 ± 0.05 | 1.54 ± 0.08 * |
| 20:4 n-6 | 1.84 ± 0.06 | 2.21 ± 0.09 * |
| 22:2 n-6 | 0.04 ± 0.04 | 0.45 ± 0.04 |
| 22:4 n-6 | 0.04 ± 0.03 | 0.10 ± 0.05 |
| 22:5 n-6 | 0.04 ± 0.04 | 0.13 ± 0.06 |
| 22:2 n-6 | 0.36 ± 0.04 | 0.45 ± 0.04 |
| PUFA n-3 | ||
| 18:3 n-3 | 2.24 ± 0.06 | 2.31 ± 0.07 |
| 20:5 n-3 | 1.02 ± 0.04 | 1.10 ± 0.05 |
| 22:5 n-3 | 1.64 ± 0.05 | 1.83 ± 0.05 * |
| 22:6 n-3 | 0.27 ± 0.04 | 0.58 ± 0.10 * |
| Summary statistics | ||
| ∑ SFA | 42.71 ± 0.42 | 42.07 ± 0.50 |
| ∑ MUFA | 36.46 ± 0.52 | 35.16 ± 0.75 |
| ∑ PUFA | 20.83± 0.37 | 22.77 ± 0.5 * |
| ∑ n-6 | 15.66 ± 0.27 | 16.95 ± 0.34 * |
| ∑ n-3 | 5.17 ± 0.15 | 5.81 ±0.18 * |
| Characteristics | ≤3 + 4 (n = 32) | ≥4 + 3 (n = 28) | p |
|---|---|---|---|
| Age, years | 58 ± 8 | 65 ± 7 | 0.006 |
| PSA, µg/L | 6.04 ± 5.21 (30) | 7.79 ± 5.65 (28) | 0.01 |
| BMI, kg/m2 | 26.8 ± 3.5 (32) | 27.0 ± 3.3 (25) | 0.70 |
| Waist Circumference, cm | 93.7 ± 8.9 (26) | 98.1 ± 8.1 (24) | 0.054 |
| Pathological T Stage | |||
| T2 | 19 | 10 | |
| T3a | 13 | 14 | |
| T3b | 1 | 3 | |
| T4 | 0 | 0 | |
| Blood Serum | |||
| Glucose, mmol/L | 5.2 ± 0.6 | 5.4 ± 0.8 | 0.34 |
| Insulin, pmol/L | 29 ± 19 | 33 ± 26 | 0.72 |
| Cholesterol, mmol/L | 4.6 ± 1.0 | 4.5 ± 0.9 | 0.61 |
| Triacylglycerols, mmol/L | 1.3 ± 0.5 | 1.2 ± 0.3 | 0.82 |
| HDL, mmol/L | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.56 |
| LDL, mmol/L | 2.6 ± 1.0 | 2.6 ± 0.8 | 0.81 |
| Fatty Acid (mol %) | ≤3 + 4 (n = 29) | ≥4 + 3 (n = 25) |
|---|---|---|
| SFA | ||
| 14:0 | 2.58 ± 0.10 | 2.35 ± 0.12 |
| 15:0 | 0.11 ± 0.04 | 0.04 ± 0.02 |
| 16:0 | 22.09 ± 0.28 | 22.58 ± 0.19 |
| 17:0 | 0.83 ± 0.06 | 0.85 ± 0.06 |
| 18:0 | 15.76 ± 0.45 | 16.04 ± 0.43 |
| 20:0 | 0.63 ± 0.18 | 0.23 ± 0.11 |
| 22:0 | 0.07 ± 0.03 | 0.04 ± 0.03 |
| 24:0 | 0.60 ± 0.06 | 0.66 ± 0.06 |
| MUFA | ||
| 14:1 n-9 | 0.02 ± 0.02 | 0.06 ± 0.04 |
| 15:1 n-10 | 0.07 ± 0.05 | 0.17 ± 0.07 |
| 16:1 n-7 | 2.39 ± 0.12 | 2.54 ± 0.18 |
| 17:1 n-7 | 0.03 ± 0.03 | 0.01 ± 0.01 |
| 18:1 n-7 | 0.66 ± 0.07 | 0.69 ± 0.06 |
| 18:1 n-9 | 31.52 ± 0.62 | 30.75 ± 0.68 |
| 20:1 n-9 | 0.17 ± 0.05 | 0.24 ± 0.06 |
| 22:1 n-9 | 1.84 ± 0.43 | 1.55 ± 0.47 |
| 24:1 n-9 | 0.11 ± 0.03 | 0.03 ± 0.02 * |
| PUFA n-6 | ||
| 18:2 n-6 | 11.80 ± 0.34 | 12.24 ± 0.30 |
| 18:3 n-6 | 0.14 ± 0.06 | 0.06 ± 0.03 |
| 20:3 n-6 | 1.29 ± 0.08 | 1.28 ± 0.07 |
| 20:4 n-6 | 1.77 ± 0.11 | 1.92 ± 0.06 |
| 22:2 n-6 | 0.36 ± 0.05 | 0.35 ± 0.05 |
| 22:4 n-6 | 0.07 ± 0.05 | ND |
| 22:5 n-6 | 0.08 ± 0.08 | ND |
| PUFA n-3 | ||
| 18:3 n-3 | 2.17 ± 0.10 | 2.32 ± 0.06 |
| 20:5 n-3 | 1.01 ± 0.05 | 1.05 ± 0.06 |
| 22:5 n-3 | 1.56 ± 0.08 | 1.73 ± 0.05 |
| 22:6 n-3 | 0.27 ± 0.05 | 0.26 ± 0.06 |
| Summary statistics | ||
| ∑ SFA | 42.67 ± 0.64 | 42.77 ± 0.55 |
| ∑ MUFA | 36.83 ± 0.73 | 36.03 ± 0.73 |
| ∑ PUFA | 20.50 ± 0.59 | 21.21 ± 0.41 |
| ∑ n-6 | 14.98 ± 0.65 | 15.85 ± 0.32 |
| ∑ n-3 | 4.84 ± 0.29 | 5.36 ± 0.15 |
| Characteristics | Normal (<95 cm; n = 24) | High (≥95 cm; n = 26) | p * |
|---|---|---|---|
| Age, years | 61 ± 9 | 64 ± 6 | 0.46 |
| PSA, µg/L | 6.98 ± 6.85 (23) | 7.24 ± 4.93 (26) | 0.74 |
| BMI, kg/m2 | 25.12 ± 1.98 (24) | 28.37 ± 3.53 (26) | 0.0005 |
| Waist Circumference, cm | 88.65 ± 4.37 (24) | 102.46 ± 6.23 (26) | <0.0001 |
| Blood Serum | |||
| Glucose, mmol/L | 5.3 ± 0.7 | 5.2 ± 0.8 | 0.97 |
| Insulin, pmol/L | 24 ± 11 | 37 ± 27 | 0.13 |
| Cholesterol, mmol/L | 4.3 ± 0.8 | 4.7 ± 0.9 | 0.04 |
| Triacylglycerols, mmol/L | 1.2 ± 0.4 | 1.3 ± 0.4 | 0.80 |
| HDL, mmol/L | 1.3 ± 0.4 | 1.4 ± 0.4 | 0.25 |
| LDL, mmol/L | 2.4 ± 0.8 | 2.7 ± 0.9 | 0.07 |
| Fatty Acid (mol %) | WC | BMI | |||
|---|---|---|---|---|---|
| Normal (<95 cm) (n = 23) | High (≥95 cm) (n = 22) | Lean (n = 15) | Overweight (n = 28) | Obese (n = 9) | |
| SFA | |||||
| 14:0 | 2.62 ± 0.10 | 2.37 ± 0.08 | 2.46 ± 0.13 | 2.49 ± 0.08 | 2.65 ± 0.17 |
| 15:0 | 0.11 ± 0.05 | 0.05 ± 0.04 | 0.10 ± 0.05 | 0.08 ± 0.04 | ND |
| 16:0 | 22.2 ± 0.34 | 22.35 ± 0.19 | 21.65 ± 0.44 | 22.44 ± 0.20 | 22.89 ± 0.33 |
| 17:0 | 0.94 ± 0.04 | 0.83 ± 0.06 | 0.86 ± 0.08 | 0.88 ± 0.04 | 0.74 ± 0.15 |
| 18:0 | 15.77 ± 0.51 | 15.94 ± 0.46 | 15.54 ± 0.56 | 15.72 ± 0.41 | 16.43 ± 0.95 |
| 20:0 | 0.35 ± 0.09 | 0.36 ± 0.14 | 0.27 ± 0.09 | 0.45 ± 0.12 | 0.83 ± 0.54 |
| 22:0 | 0.05 ± 0.02 | 0.08 ± 0.04 | 0.06 ± 0.03 | 0.08 ± 0.03 | ND |
| 24:0 | 0.65 ± 0.04 | 0.64 ± 0.06 | 0.61 ± 0.07 | 0.66 ± 0.04 | 0.54 ± 0.11 |
| MUFA | |||||
| 14:1 n-9 | 0.07 ± 0.03 | 0.03 ± 0.03 | 0.05 ± 0.05 | 0.05 ± 0.04 | ND |
| 15:1 n-10 | 0.08 ± 0.06 | 0.14 ± 0.06 | 0.12 ± 0.09 | 0.14 ± 0.09 | 0.05 ± 0.05 |
| 16:1 n-7 | 2.46 ± 0.14 | 2.52 ± 0.20 | 2.39 ± 0.17 | 2.55 ± 0.17 | 2.40 ± 0.23 |
| 17:1 n-7 | 0.06 ±0.04 | ND | 0.02 ± 0.02 | 0.03 ± 0.03 | ND |
| 18:1 n-7 | 0.77 ± 0.06 | 0.67 ± 0.06 | 0.68 ± 0.10 | 0.73 ± 0.04 | 0.54 ± 0.14 |
| 18:1 n-9 | 31.73 ± 0.65 | 30.58 ± 0.66 | 32.26 ± 0.85 | 30.88 ± 0.56 | 30.65 ± 1.41 |
| 20:1 n-9 | 0.18 ± 0.06 | 0.27 ± 0.06 | 0.17 ± 0.07 | 0.28 ± 0.07 | 0.06 ± 0.06 |
| 22:1 n-9 | 1.13 ± 0.25 | 2.08 ± 0.59 | 1.04 ± 0.33 | 1.84 ± 0.50 | 2.23 ± 0.90 |
| 24:1 n-9 | 0.10 ± 0.04 | 0.06 ± 0.03 | 0.10 ± 0.04 | 0.09 ± 0.3 | ND |
| PUFA n-6 | |||||
| 18:2 n-6 | 11.82 ± 0.35 | 11.85 ± 0.31 | 12.66 ± 0.50 | 11.68 ± 0.28 | 11.63 ± 0.47 |
| 18:3 n-6 | 0.17 ± 0.08 | 0.07 ± 0.07 | 0.14 ± 0.05 | 0.13 ± 0.07 | ND |
| 20:3 n-6 | 1.32 ± 0.08 | 1.35 ± 0.05 | 1.32 ± 0.06 | 1.31 ± 0.07 | 1.26 ± 0.18 |
| 20:4 n-6 | 1.73 ± 0.08 | 2.02 ± 0.08 * | 1.84 ± 0.08 | 1.80 ± 0.07 | 1.92 ± 0.29 |
| 22:2 n-6 | 0.37 ± 0.05 | 0.40 ± 0.05 | 0.35 ± 0.07 | 0.40 ± 0.05 | 0.32 ± 0.11 |
| 22:4 n-6 | 0.06 ± 0.06 | 0.03 ± 0.03 | ND | 0.07 ± 0.05 | ND |
| 22:5 n-6 | 0.10 ± 0.04 | ND | ND | 0.08 ± 0.08 | ND |
| PUFA n-3 | |||||
| 18:3 n-3 | 2.23 ± 0.07 | 2.25 ± 0.07 | 2.32 ± 0.07 | 2.21 ± 0.06 | 2.07 ± 0.28 |
| 20:5 n-3 | 1.02 ± 0.04 | 1.09 ± 0.04 | 1.05 ± 0.05 | 1.03 ± 0.04 | 1.03 ± 0.15 |
| 22:5 n-3 | 1.59 ± 0.06 | 1.71 ± 0.06 | 1.65 ± 0.07 | 1.61 ± 0.05 | 1.60 ± 0.24 |
| 22:6 n-3 | 0.32 ± 0.06 | 0.26 ± 0.06 | 0.29 ± 0.07 | 0.31 ± 0.05 | 0.16 ± 0.11 |
| Summary statistics | |||||
| ∑ SFA | 42.68 ± 0.79 | 42.62 ± 0.45 | 41.55 ± 0.89 | 42.80 ± 0.58 | 44.08 ± 0.50 |
| ∑ MUFA | 36.57 ± 0.78 | 36.35 ± 0.81 | 36.83 ± 1.03 | 36.58 ± 0.66 | 35.93 ± 1.54 |
| ∑ PUFA | 20.74 ± 0.47 | 21.01 ± 0.55 | 21.62 ± 0.59 | 20.62 ± 0.47 | 19.98 ± 1.36 |
| ∑ n-6 | 14.90 ± 0.77 | 15.70 ± 0.40 | 16.30 ± 0.51 | 15.46 ± 0.34 | 15.13 ± 0.79 |
| ∑ n-3 | 4.94 ± 0.28 | 5.32 ± 0.18 | 5.32 ± 00.18 | 5.16 ± 0.34 | 4.86 ± 0.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miladinovic, D.; Cusick, T.; Mahon, K.L.; Haynes, A.-M.; Cortie, C.H.; Meyer, B.J.; Stricker, P.D.; Wittert, G.A.; Butler, L.M.; Horvath, L.G.; et al. Assessment of Periprostatic and Subcutaneous Adipose Tissue Lipolysis and Adipocyte Size from Men with Localized Prostate Cancer. Cancers 2020, 12, 1385. https://doi.org/10.3390/cancers12061385
Miladinovic D, Cusick T, Mahon KL, Haynes A-M, Cortie CH, Meyer BJ, Stricker PD, Wittert GA, Butler LM, Horvath LG, et al. Assessment of Periprostatic and Subcutaneous Adipose Tissue Lipolysis and Adipocyte Size from Men with Localized Prostate Cancer. Cancers. 2020; 12(6):1385. https://doi.org/10.3390/cancers12061385
Chicago/Turabian StyleMiladinovic, Dushan, Thomas Cusick, Kate L. Mahon, Anne-Maree Haynes, Colin H. Cortie, Barbara J. Meyer, Phillip D. Stricker, Gary A. Wittert, Lisa M. Butler, Lisa G. Horvath, and et al. 2020. "Assessment of Periprostatic and Subcutaneous Adipose Tissue Lipolysis and Adipocyte Size from Men with Localized Prostate Cancer" Cancers 12, no. 6: 1385. https://doi.org/10.3390/cancers12061385
APA StyleMiladinovic, D., Cusick, T., Mahon, K. L., Haynes, A.-M., Cortie, C. H., Meyer, B. J., Stricker, P. D., Wittert, G. A., Butler, L. M., Horvath, L. G., & Hoy, A. J. (2020). Assessment of Periprostatic and Subcutaneous Adipose Tissue Lipolysis and Adipocyte Size from Men with Localized Prostate Cancer. Cancers, 12(6), 1385. https://doi.org/10.3390/cancers12061385






