Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights
Abstract
1. Introduction
2. The Epidemiology of Obesity and CRC
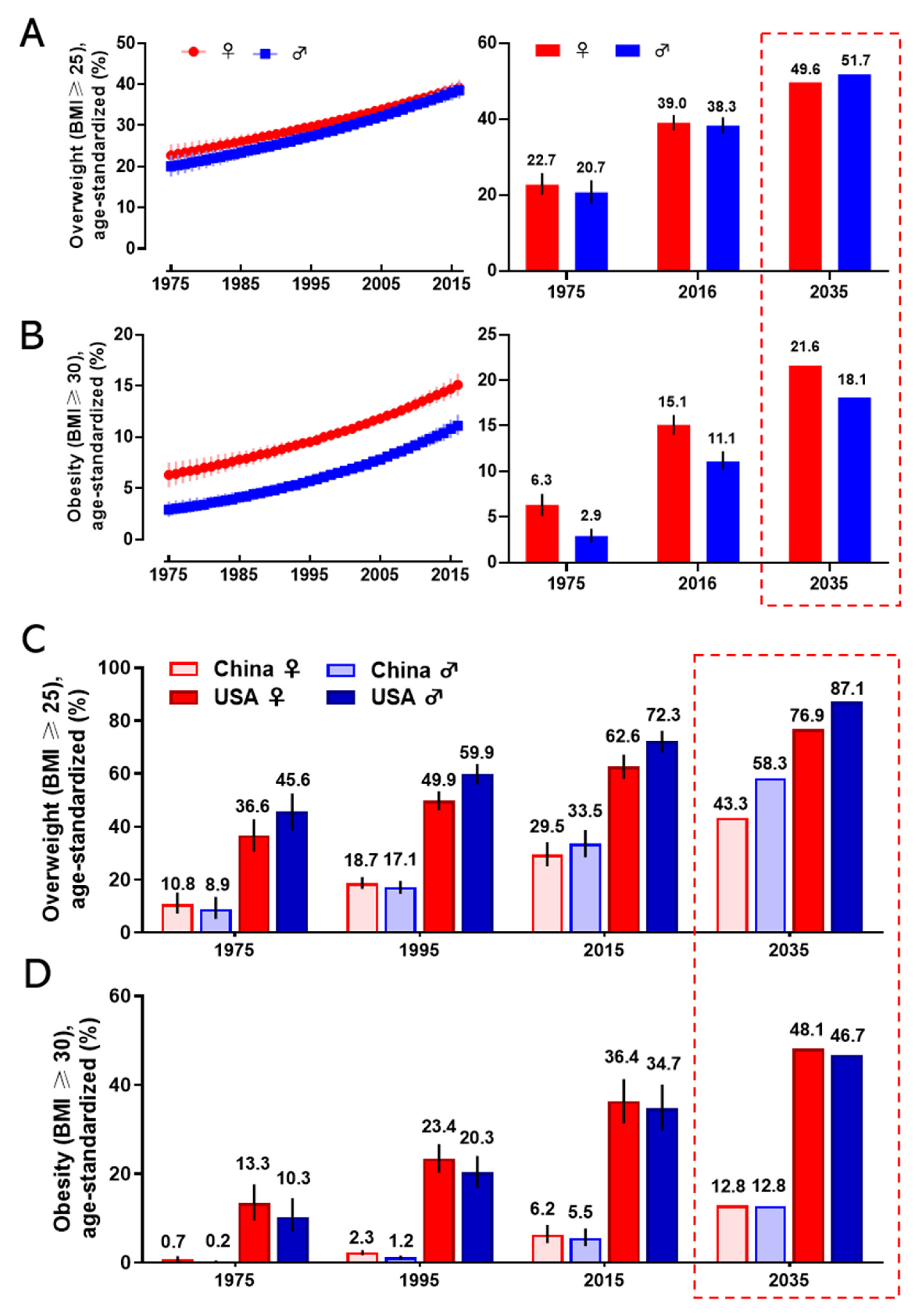
2.1. The Epidemiology of Obesity
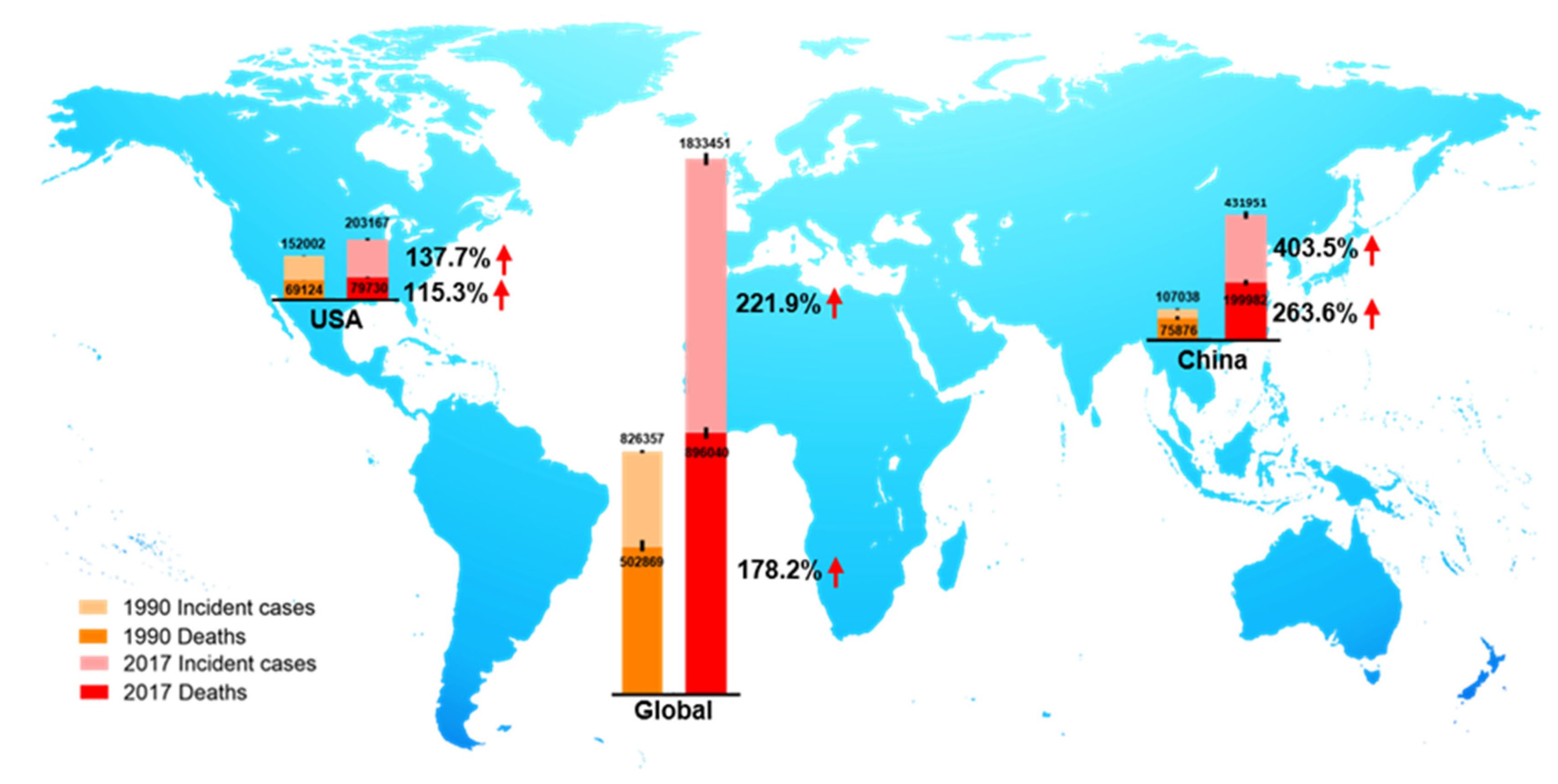
2.2. The Epidemiology of Colorectal Cancer
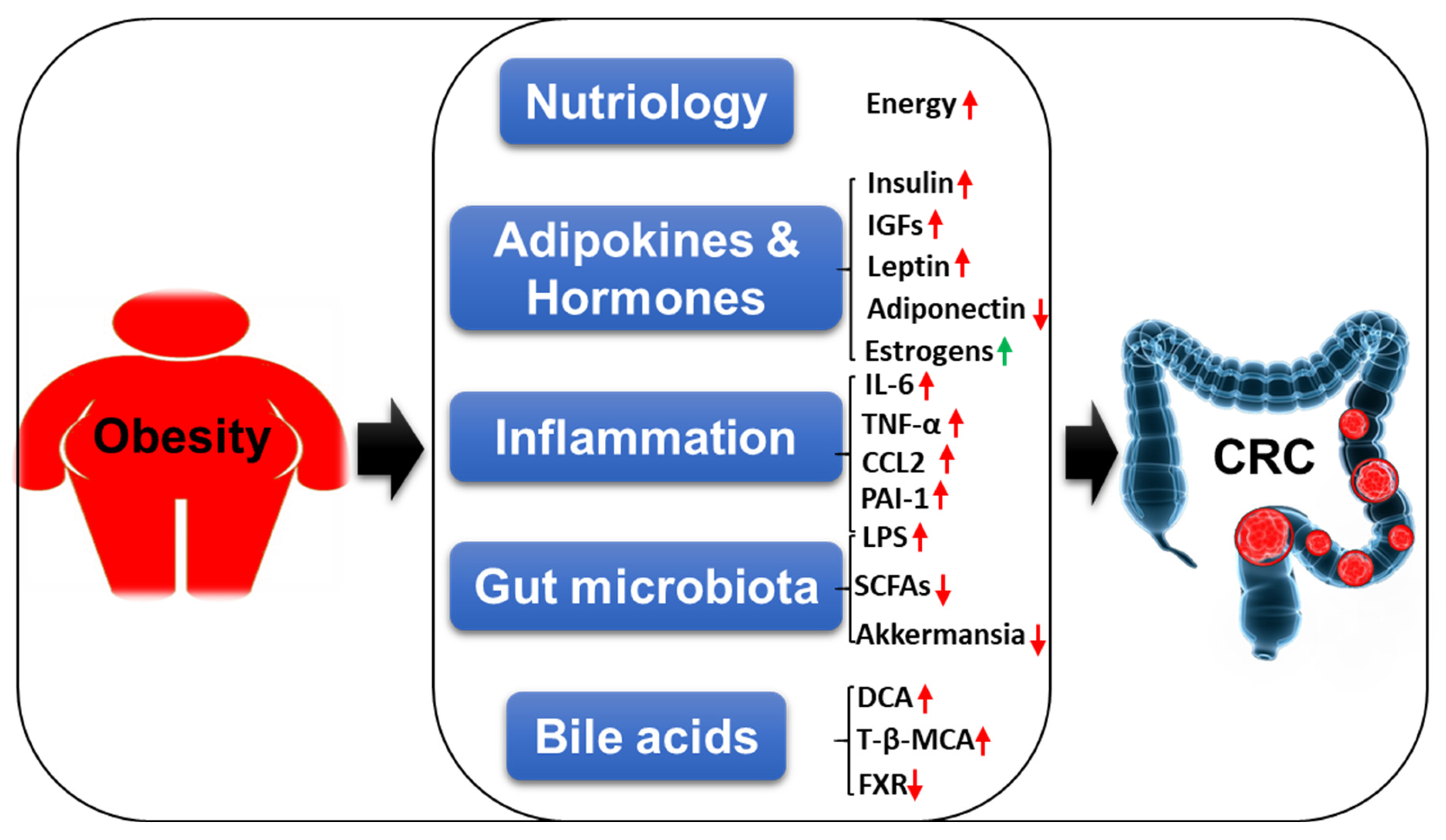
3. The Mechanistic Insights Linking Obesity with CRC
3.1. Nutriology
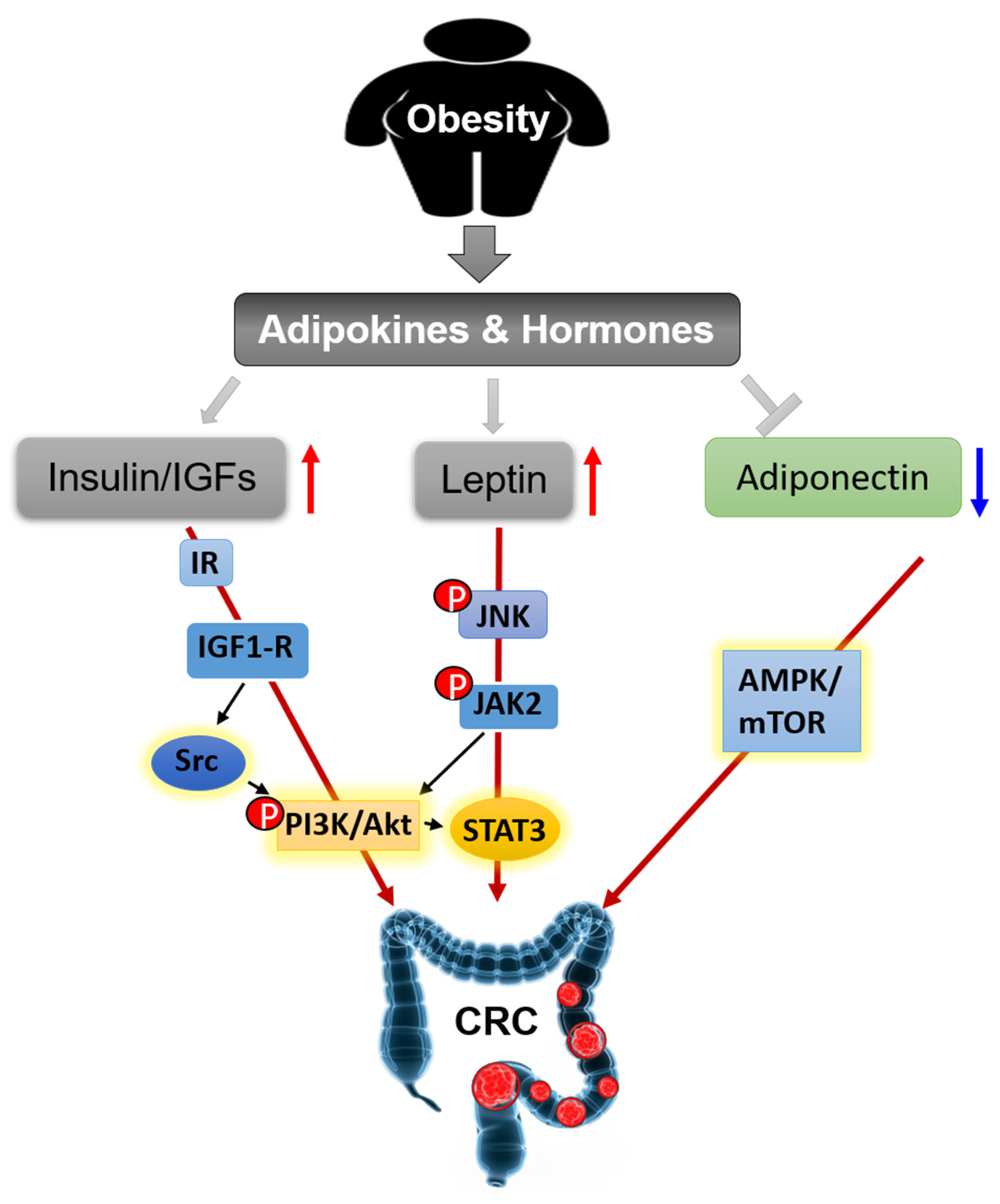
3.2. Adipokines and Hormones
3.2.1. Insulin and IGFs
3.2.2. Leptin and Adiponectin
3.2.3. Estrogens
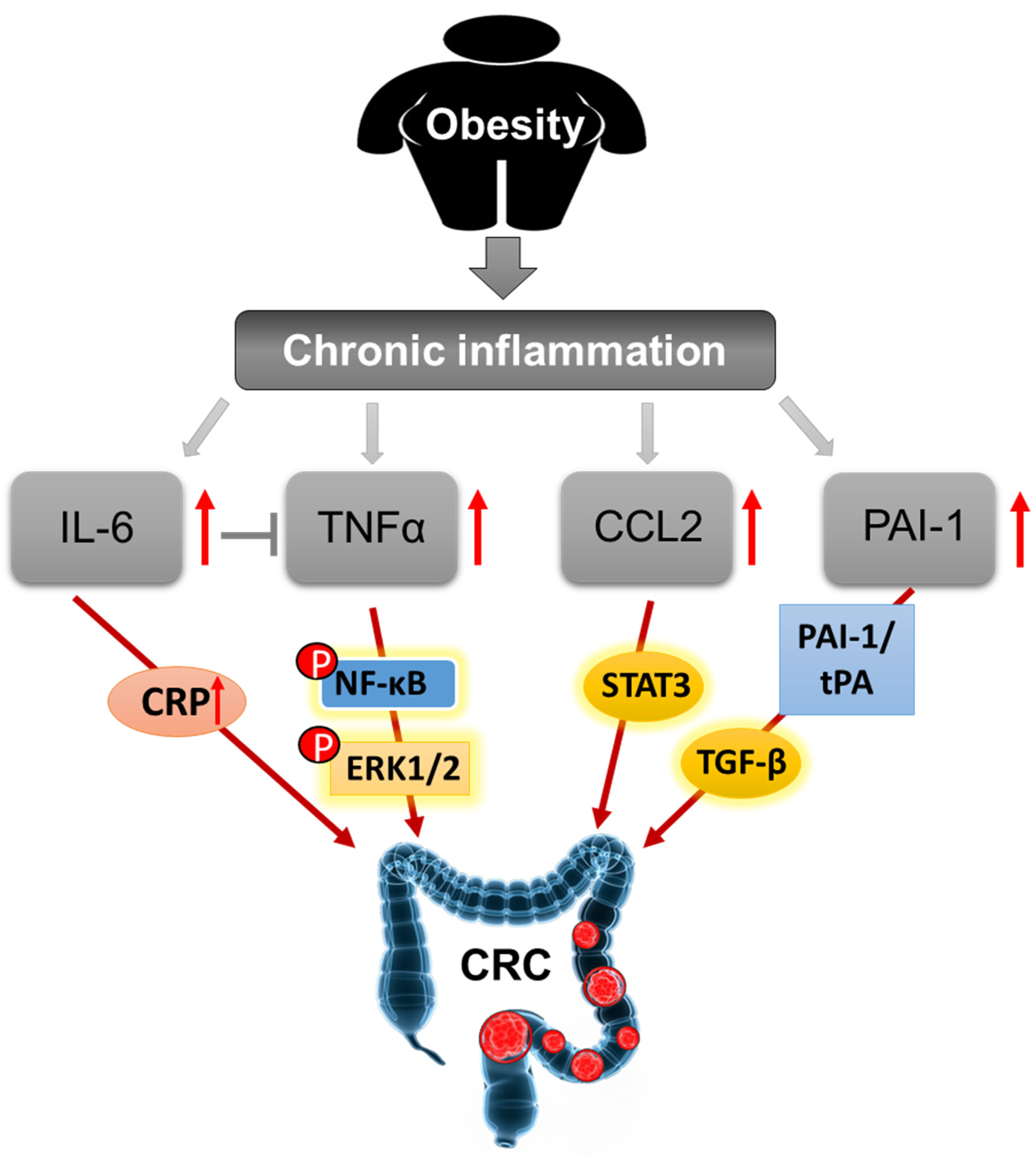
3.3. Inflammation
3.4. Gut Microbiota
3.5. Bile Acids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cho, E.J.; Kim, S.M. Explantation of Adjustable Gastric Bands: An Observation Study of 10 Years of Experience at a Tertiary Center. Yonsei Med. J. 2019, 60, 782–790. [Google Scholar]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, P.A.G.; Stevens, G.A.; Ezzati, P.M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Xu, Y.X.Z.; Mishra, S. Obesity-Linked Cancers: Current Knowledge, Challenges and Limitations in Mechanistic Studies and Rodent Models. Cancers 2018, 10, 523. [Google Scholar]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Li, S.; Peppelenbosch, M.P.; Smits, R. Bacterial biofilms as a potential contributor to mucinous colorectal cancer formation. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 74–79. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Collaborators, G.B.D.C.C. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933. [Google Scholar]
- Garcia, H.; Song, M. Early-life obesity and adulthood colorectal cancer risk: A meta-analysis. Rev. Panam. Salud Publica 2019, 43, e3. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, J.; Zhu, Y.; Luo, L.; He, T.; Hu, H.; Liu, H.; Zhang, Y.; Luo, D.; Xu, S.; et al. Abdominal obesity and colorectal cancer risk: Systematic review and meta-analysis of prospective studies. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Lee, J.; Meyerhardt, J.A.; Giovannucci, E.; Jeon, J.Y. Association between body mass index and prognosis of colorectal cancer: A meta-analysis of prospective cohort studies. PLoS ONE 2015, 10, e0120706. [Google Scholar] [CrossRef]
- Jochem, C.; Leitzmann, M. Obesity and Colorectal Cancer. Recent Results Cancer Res. 2016, 208, 17–41. [Google Scholar]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar]
- Schlesinger, S.; Aleksandrova, K.; Abar, L.; Vieria, A.R.; Vingeliene, S.; Polemiti, E.; Stevens, C.A.T.; Greenwood, D.C.; Chan, D.S.M.; Aune, D.; et al. Adult weight gain and colorectal adenomas-a systematic review and meta-analysis. Ann. Oncol. 2017, 28, 1217–1229. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Yang, J.; Jin, Z.; Shi, W.; Qin, Y.; Yu, F.; He, J. Association between adult weight gain and colorectal cancer: A dose-response meta-analysis of observational studies. Int. J. Cancer 2015, 136, 2880–2889. [Google Scholar] [CrossRef]
- Schlesinger, S.; Lieb, W.; Koch, M.; Fedirko, V.; Dahm, C.C.; Pischon, T.; Nothlings, U.; Boeing, H.; Aleksandrova, K. Body weight gain and risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Obes. Rev. 2015, 16, 607–619. [Google Scholar] [CrossRef]
- Chang, S.H.; Stoll, C.R.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.; Kelly, S.B.; Seymour, K.; Lara, J.; Woodcock, S.; Mathers, J.C. The effects of bariatric surgery on colorectal cancer risk: Systematic review and meta-analysis. Obes. Surg. 2014, 24, 1793–1799. [Google Scholar]
- Xu, P.; Li, J.; Liu, J.; Wang, J.; Wu, Z.; Zhang, X.; Zhai, Y. Mature adipocytes observed to undergo reproliferation and polyploidy. FEBS Open Bio 2017, 7, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Dai, S.; Wang, J.; Zhang, J.; Liu, J.; Wang, F.; Zhai, Y. Preventive obesity agent montmorillonite adsorbs dietary lipids and enhances lipid excretion from the digestive tract. Sci. Rep. 2016, 6, 19659. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhai, Y.; Wang, J. The Role of PPAR and Its Cross-Talk with CAR and LXR in Obesity and Atherosclerosis. Int. J. Mol. Sci. 2018, 19, 1260. [Google Scholar]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498. [Google Scholar] [PubMed]
- Villanueva, M.T. Gynecological cancer: Home is where the fat is. Nat. Rev. Clin. Oncol. 2011, 9, 6. [Google Scholar] [CrossRef]
- Martin-Padura, I.; Gregato, G.; Marighetti, P.; Mancuso, P.; Calleri, A.; Corsini, C.; Pruneri, G.; Manzotti, M.; Lohsiriwat, V.; Rietjens, M.; et al. The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res. 2012, 72, 325–334. [Google Scholar]
- Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Power surge: Supporting cells “fuel” cancer cell mitochondria. Cell Metab. 2012, 15, 4–5. [Google Scholar]
- Xiao, L.; Wang, J.; Li, J.; Chen, X.; Xu, P.; Sun, S.; He, D.; Cong, Y.; Zhai, Y. RORalpha inhibits adipocyte-conditioned medium-induced colorectal cancer cell proliferation and migration and chick embryo chorioallantoic membrane angiopoiesis. Am. J. Physiol. Cell Physiol. 2015, 308, C385–C396. [Google Scholar]
- Amemori, S.; Ootani, A.; Aoki, S.; Fujise, T.; Shimoda, R.; Kakimoto, T.; Shiraishi, R.; Sakata, Y.; Tsunada, S.; Iwakiri, R.; et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G923–G929. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Lieffers, J.R.; Mourtzakis, M.; Hall, K.D.; McCargar, L.J.; Prado, C.M.; Baracos, V.E. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am. J. Clin. Nutr. 2009, 89, 1173–1179. [Google Scholar] [CrossRef]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Della-Morte, D.; Ferroni, P.; Guadagni, F. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Vigneri, P.G.; Tirro, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 2015, 5, 230. [Google Scholar] [CrossRef]
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol. Metab. 2006, 17, 328–336. [Google Scholar] [CrossRef]
- Chen, J.; Katsifis, A.; Hu, C.; Huang, X.F. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr. Drug Discov. Technol. 2011, 8, 119–125. [Google Scholar] [CrossRef]
- Huang, X.F.; Chen, J.Z. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 2009, 10, 610–616. [Google Scholar] [CrossRef]
- Watkins, L.F.; Lewis, L.R.; Levine, A.E. Characterization of the synergistic effect of insulin and transferrin and the regulation of their receptors on a human colon carcinoma cell line. Int. J. Cancer 1990, 45, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Echeverria, C.; Sellers, W.R. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 2008, 27, 5511–5526. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Is Src the key to understanding metastasis and developing new treatments for colon cancer? Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Zhu, S.; Bjorge, J.D.; Fujita, D.J. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 2007, 67, 10129–10137. [Google Scholar] [CrossRef]
- Nam, J.S.; Ino, Y.; Sakamoto, M.; Hirohashi, S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin. Cancer Res. 2002, 8, 2430–2436. [Google Scholar]
- Abbruzzese, C.; Diodoro, M.G.; Sperduti, I.; Mileo, A.M.; Pattaro, G.; De Salvo, L.; Cosimelli, M.; Perrotti, N.; Paggi, M.G. Detection of phosphorylated insulin receptor in colorectal adenoma and adenocarcinoma: Implications for prognosis and clinical outcome. J. Cell Physiol. 2015, 230, 562–567. [Google Scholar] [CrossRef]
- Sekharam, M.; Nasir, A.; Kaiser, H.E.; Coppola, D. Insulin-like growth factor 1 receptor activates c-SRC and modifies transformation and motility of colon cancer in vitro. Anticancer Res. 2003, 23, 1517–1524. [Google Scholar]
- Corrales, P.; Vidal-Puig, A.; Medina-Gomez, G. PPARs and Metab.olic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells 2020, 9, 352. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metab.olism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Diet-Induced Obesity and the Mechanism of Leptin Resistance. Adv. Exp. Med. Biol. 2017, 960, 381–397. [Google Scholar] [PubMed]
- Modzelewska, P.; Chludzinska, S.; Lewko, J.; Reszec, J. The influence of leptin on the process of carcinogenesis. Contemp. Oncol. 2019, 23, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Hosono, K.; Uchiyama, T.; Sakai, E.; Sugiyama, M.; Takahashi, H.; Nakajima, N.; Wada, K.; Takeda, K.; Nakagama, H. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 2011, 60, 1363–1371. [Google Scholar] [CrossRef]
- Koda, M.; Sulkowska, M.; Kanczuga-Koda, L.; Surmacz, E.; Sulkowski, S. Overexpression of the obesity hormone leptin in human colorectal cancer. J. Clin. Pathol. 2007, 60, 902–906. [Google Scholar]
- Aleksandrova, K.; Schlesinger, S.; Fedirko, V.; Jenab, M.; Bueno-de-Mesquita, B.; Freisling, H.; Romieu, I.; Pischon, T.; Kaaks, R.; Gunter, M.J.; et al. Metab.olic Mediators of the Association Between Adult Weight Gain and Colorectal Cancer: Data From the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Am. J. Epidemiol. 2017, 185, 751–764. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Boeing, H.; Jenab, M.; Bueno-de-Mesquita, H.B.; Jansen, E.; van Duijnhoven, F.J.; Rinaldi, S.; Fedirko, V.; Romieu, I.; Riboli, E.; et al. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res. 2012, 72, 5328–5337. [Google Scholar]
- Ogunwobi, O.O.; Beales, I.L. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int. J. Colorectal. Dis. 2007, 22, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, T.; Schwartz, B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int. J. Cancer 2008, 123, 2543–2556. [Google Scholar] [CrossRef]
- Yehuda-Shnaidman, E.; Nimri, L.; Tarnovscki, T.; Kirshtein, B.; Rudich, A.; Schwartz, B. Secreted human adipose leptin decreases mitochondrial respiration in HCT116 colon cancer cells. PLoS ONE 2013, 8, e74843. [Google Scholar] [CrossRef]
- Ristic, B.; Bhutia, Y.D.; Ganapathy, V. Cell-surface G-protein-coupled receptors for tumor-associated Metab.olites: A direct link to mitochondrial dysfunction in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar]
- Polito, R.; Nigro, E.; Elce, A.; Monaco, M.L.; Iacotucci, P.; Carnovale, V.; Comegna, M.; Gelzo, M.; Zarrilli, F.; Corso, G.; et al. Adiponectin Expression Is Modulated by Long-Term Physical Activity in Adult Patients Affected by Cystic Fibrosis. Mediators Inflamm. 2019, 2019, 2153934. [Google Scholar] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A.; Monaco, M.L.; Capasso, M.; Forestieri, P.; Pilone, V.; Nardelli, C.; Buono, P.; Daniele, A. Adiponectin oligomers as potential indicators of adipose tissue improvement in obese subjects. Eur. J. Endocrinol. 2013, 169, 37–43. [Google Scholar]
- Wei, E.K.; Giovannucci, E.; Fuchs, C.S.; Willett, W.C.; Mantzoros, C.S. Low plasma adiponectin levels and risk of colorectal cancer in men: A prospective study. J. Natl. Cancer Inst. 2005, 97, 1688–1694. [Google Scholar] [CrossRef]
- Kelesidis, I.; Kelesidis, T.; Mantzoros, C.S. Adiponectin and cancer: A systematic review. Br. J. Cancer 2006, 94, 1221–1225. [Google Scholar] [CrossRef]
- Saxena, A.; Baliga, M.S.; Ponemone, V.; Kaur, K.; Larsen, B.; Fletcher, E.; Greene, J.; Fayad, R. Mucus and adiponectin deficiency: Role in chronic inflammation-induced colon cancer. Int. J. Colorectal. Dis. 2013, 28, 1267–1279. [Google Scholar]
- Moon, H.S.; Liu, X.; Nagel, J.M.; Chamberland, J.P.; Diakopoulos, K.N.; Brinkoetter, M.T.; Hatziapostolou, M.; Wu, Y.; Robson, S.C.; Iliopoulos, D.; et al. Salutary effects of adiponectin on colon cancer: In vivo and in vitro studies in mice. Gut 2013, 62, 561–570. [Google Scholar]
- La Cava, A. Adiponectin: A relevant player in obesity-related colorectal cancer? Gut 2013, 62, 483–484. [Google Scholar]
- Sugiyama, M.; Takahashi, H.; Hosono, K.; Endo, H.; Kato, S.; Yoneda, K.; Nozaki, Y.; Fujita, K.; Yoneda, M.; Wada, K.; et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J. Oncol. 2009, 34, 339–344. [Google Scholar] [PubMed]
- Kim, A.Y.; Lee, Y.S.; Kim, K.H.; Lee, J.H.; Lee, H.K.; Jang, S.H.; Kim, S.E.; Lee, G.Y.; Lee, J.W.; Jung, S.A.; et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol. Endocrinol. 2010, 24, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.P.; Grossmann, M.E. Minireview: Obesity and breast cancer: The estrogen connection. Endocrinology 2009, 150, 2537–2542. [Google Scholar] [CrossRef]
- Ando, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef]
- Chen, J.; Iverson, D. Estrogen in obesity-associated colon cancer: Friend or foe? Protecting postmenopausal women but promoting late-stage colon cancer. Cancer Causes Control. 2012, 23, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.D.; Bostick, R.M.; Grandits, G.A.; Fosdick, L.; Elmer, P.; Wood, J.; Grambsch, P.; Louis, T.A. Hormone replacement therapy is associated with lower risk of adenomatous polyps of the large bowel: The Minnesota Cancer Prevention Research Unit Case-Control Study. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 779–784. [Google Scholar] [PubMed]
- Al-Azzawi, F.; Wahab, M. Estrogen and colon cancer: Current issues. Climacteric 2002, 5, 3–14. [Google Scholar] [CrossRef]
- Percik, R.; Stumvoll, M. Obesity and cancer. Exp. Clin. Endocrinol. Diabetes 2009, 117, 563–566. [Google Scholar] [CrossRef]
- Terry, P.D.; Miller, A.B.; Rohan, T.E. Obesity and colorectal cancer risk in women. Gut 2002, 51, 191–194. [Google Scholar] [CrossRef]
- Liu, P.H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.H.; Song, M.; He, X.; Fuchs, C.S.; Ogino, S.; Willett, W.C.; et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019, 5, 37–44. [Google Scholar] [CrossRef]
- Bernstein, L.; Ross, R.K. Endogenous hormones and breast cancer risk. Epidemiol. Rev. 1993, 15, 48–65. [Google Scholar] [CrossRef]
- Terry, P.; Giovannucci, E.; Bergkvist, L.; Holmberg, L.; Wolk, A. Body weight and colorectal cancer risk in a cohort of Swedish women: Relation varies by age and cancer site. Br. J. Cancer 2001, 85, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Ballard-Barbash, R.; Edwards, S.; Caan, B.J.; Potter, J.D. Body mass index and colon cancer: An evaluation of the modifying effects of estrogen (United States). Cancer Causes Control. 2003, 14, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Barzi, A.; Lenz, A.M.; Labonte, M.J.; Lenz, H.J. Molecular pathways: Estrogen pathway in colorectal cancer. Clin. Cancer Res. 2013, 19, 5842–5848. [Google Scholar] [CrossRef] [PubMed]
- Papaxoinis, K.; Triantafyllou, K.; Sasco, A.J.; Nicolopoulou-Stamati, P.; Ladas, S.D. Subsite-specific differences of estrogen receptor beta expression in the normal colonic epithelium: Implications for carcinogenesis and colorectal cancer epidemiology. Eur. J. Gastroenterol. Hepatol. 2010, 22, 614–619. [Google Scholar] [CrossRef]
- Castiglione, F.; Taddei, A.; Rossi Degl’Innocenti, D.; Buccoliero, A.M.; Bechi, P.; Garbini, F.; Chiara, F.G.; Moncini, D.; Cavallina, G.; Marascio, L.; et al. Expression of estrogen receptor beta in colon cancer progression. Diagn. Mol. Pathol. 2008, 17, 231–236. [Google Scholar] [CrossRef]
- Hartman, J.; Edvardsson, K.; Lindberg, K.; Zhao, C.; Williams, C.; Strom, A.; Gustafsson, J.A. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009, 69, 6100–6106. [Google Scholar] [CrossRef]
- Weyant, M.J.; Carothers, A.M.; Mahmoud, N.N.; Bradlow, H.L.; Remotti, H.; Bilinski, R.T.; Bertagnolli, M.M. Reciprocal expression of ERalpha and ERbeta is associated with estrogen-mediated modulation of intestinal tumorigenesis. Cancer Res. 2001, 61, 2547–2551. [Google Scholar]
- Giroux, V.; Lemay, F.; Bernatchez, G.; Robitaille, Y.; Carrier, J.C. Estrogen receptor beta deficiency enhances small intestinal tumorigenesis in ApcMin/+ mice. Int. J. Cancer 2008, 123, 303–311. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, T.; Hara, M.; Nakanishi, H.; Takahashi, H.; Sato, M.; Takeyama, H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: Anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br. J. Cancer 2014, 110, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, N.; Rose-John, S. ADAM17 Activity and IL-6 Trans-Signaling in Inflammation and Cancer. Cancers 2019, 11, 62. [Google Scholar]
- Holm, M.; Saraswat, M.; Joenvaara, S.; Ristimaki, A.; Haglund, C.; Renkonen, R. Colorectal cancer patients with different C-reactive protein levels and 5-year survival times can be differentiated with quantitative serum proteomics. PLoS ONE 2018, 13, e0195354. [Google Scholar] [CrossRef]
- Rasic, I.; Rebic, V.; Rasic, A.; Aksamija, G.; Radovic, S. The Association of Simultaneous Increase in Interleukin-6, C Reactive Protein, and Matrix Metalloproteinase-9 Serum Levels with Increasing Stages of Colorectal Cancer. J. Oncol. 2018, 2018, 2830503. [Google Scholar] [CrossRef]
- Himbert, C.; Ose, J.; Lin, T.; Warby, C.A.; Gigic, B.; Steindorf, K.; Schrotz-King, P.; Abbenhardt-Martin, C.; Zielske, L.; Boehm, J.; et al. Inflammation- and angiogenesis-related biomarkers are correlated with cancer-related fatigue in colorectal cancer patients: Results from the ColoCare Study. Eur. J. Cancer Care 2019, 28, e13055. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Pietrani, N.T.; Bosco, A.A.; Campos, F.M.F.; Sandrim, V.C.; Gomes, K.B. IL-6, TNF-alpha, and IL-10 levels/polymorphisms and their association with type 2 diabetes mellitus and obesity in Brazilian individuals. Arch. Endocrinol. Metab. 2017, 61, 438–446. [Google Scholar] [CrossRef]
- Hong, F.; Xu, P.; Zhai, Y. The Opportunities and Challenges of Peroxisome Proliferator-Activated Receptors Ligands in Clinical Drug Discovery and Development. Int. J. Mol. Sci. 2018, 19, 2189. [Google Scholar]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar]
- Wei, X.; Li, X.; Kong, F.; Ma, L.; Sui, Y.; Chen, D.; Xu, F. TNF-alpha activates Wnt signaling pathway to promote the invasion of human colon cancer stem cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2018, 34, 982–988. [Google Scholar] [PubMed]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, C.; Banz-Jansen, C.; Benhidjeb, T.; Beshay, M.; Forster, C.; Greiner, J.; Hamelmann, E.; Jorch, N.; Mertzlufft, F.; Pfitzenmaier, J.; et al. A Role for NF-kappaB in Organ Specific Cancer and Cancer Stem Cells. Cancers 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, Z. TNF-alpha promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol. Lett. 2018, 15, 3820–3827. [Google Scholar] [PubMed]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Molnar, I. Interactions among thyroid hormone (FT4), chemokine (MCP-1) and neurotrophin (NGF-beta) levels studied in Hungarian postmenopausal and obese women. Cytokine 2020, 127, 154948. [Google Scholar] [CrossRef]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef]
- McClellan, J.L.; Davis, J.M.; Steiner, J.L.; Enos, R.T.; Jung, S.H.; Carson, J.A.; Pena, M.M.; Carnevale, K.A.; Berger, F.G.; Murphy, E.A. Linking tumor-associated macrophages, inflammation, and intestinal tumorigenesis: Role of MCP-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1087–G1095. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Liu, Z.; Liu, Y.; Luo, M.; Chen, N.; Deng, X.; Luo, Y.; He, J.; Zhang, L.; et al. PAI-1 Exacerbates White Adipose Tissue Dysfunction and Metab.olic Dysregulation in High Fat Diet-Induced Obesity. Front. Pharmacol. 2018, 9, 1087. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.; He, J.; Tian, X.; Yuan, S.; Sun, L. Plasminogen activator inhibitor-1 in cancer research. Biomed. Pharmacother. 2018, 105, 83–94. [Google Scholar] [CrossRef]
- Kim, E.R.; Yang, M.H.; Lim, Y.J.; Lee, J.H.; Chang, D.K.; Kim, Y.H.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Kim, J.Y. Association between Plasma Levels of Plasminogen Activator Inhibitor-1 and Colorectal Neoplasms. Gut Liver 2013, 7, 519–523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaiko, G.E.; Chen, F.; Lai, C.W.; Chiang, I.L.; Perrigoue, J.; Stojmirovic, A.; Li, K.; Muegge, B.D.; Jain, U.; VanDussen, K.L.; et al. PAI-1 augments mucosal damage in colitis. Sci. Transl. Med. 2019, 11, eaat0852. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. Coagulation and IBD—PAI1 provides the missing link. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.; Wang, Y.J.; Schoen, R.E.; Finn, O.J.; Yu, J.; Zhang, L. Colorectal cancer prevention: Immune modulation taking the stage. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 138–148. [Google Scholar] [CrossRef]
- Li, X.; Larsson, P.; Ljuslinder, I.; Ohlund, D.; Myte, R.; Lofgren-Burstrom, A.; Zingmark, C.; Ling, A.; Edin, S.; Palmqvist, R. Ex Vivo Organoid Cultures Reveal the Importance of the Tumor Microenvironment for Maintenance of Colorectal Cancer Stem Cells. Cancers 2020, 12, 923. [Google Scholar] [CrossRef]
- Integrative, H.M.P.R.N.C. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar]
- Xu, P.; Hong, F.; Wang, J.; Wang, J.; Zhao, X.; Wang, S.; Xue, T.; Xu, J.; Zheng, X.; Zhai, Y. DBZ is a putative PPARgamma agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2690–2701. [Google Scholar] [CrossRef]
- Castillo, D.J.; Rifkin, R.F.; Cowan, D.A.; Potgieter, M. The Healthy Human Blood Microbiome: Fact or Fiction? Front. Cell Infect. Microbiol. 2019, 9, 148. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A.; on behalf of the Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [PubMed]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Musolino, C.; Tonacci, A.; Pioggia, G.; Gangemi, S. Interactions between the MicroRNAs and Microbiota in Cancer Development: Roles and Therapeutic Opportunities. Cancers 2020, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Kim, N. The Effect of Microbiota on Colon Carcinogenesis. J. Cancer Prev. 2018, 23, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Stepankova, R.; Kozakova, H.; Fiserova, A.; Rossmann, P.; Tlaskalova-Hogenova, H. Colorectal carcinogenesis in germ-free and conventionally reared rats: Different intestinal environments affect the systemic immunity. Int. J. Oncol. 2008, 32, 609–617. [Google Scholar] [PubMed]
- Kado, S.; Uchida, K.; Funabashi, H.; Iwata, S.; Nagata, Y.; Ando, M.; Onoue, M.; Matsuoka, Y.; Ohwaki, M.; Morotomi, M. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001, 61, 2395–2398. [Google Scholar]
- Tomkovich, S.; Yang, Y.; Winglee, K.; Gauthier, J.; Muhlbauer, M.; Sun, X.; Mohamadzadeh, M.; Liu, X.; Martin, P.; Wang, G.P.; et al. Locoregional Effects of Microbiota in a Preclinical Model of Colon Carcinogenesis. Cancer Res. 2017, 77, 2620–2632. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sa-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Xu, P.; Hong, F.; Wang, J.; Cong, Y.; Dai, S.; Wang, S.; Wang, J.; Jin, X.; Wang, F.; Liu, J. Microbiome Remodeling via the Montmorillonite Adsorption-Excretion Axis Prevents Obesity-related Metab.olic Disorders. EBioMedicine 2017, 16, 251–261. [Google Scholar] [CrossRef]
- Tai, N.; Wong, F.S.; Wen, L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev. Endocr. Metab. Disord. 2015, 16, 55–65. [Google Scholar] [CrossRef]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [PubMed]
- Killeen, S.D.; Wang, J.H.; Andrews, E.J.; Redmond, H.P. Bacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-kappaB-dependent activation of the urokinase plasminogen activator system. Br. J. Cancer 2009, 100, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Jing, Y.Y.; Yan, F.; Han, Z.P.; Lai, F.B.; Zeng, J.X.; Yu, G.F.; Fan, Q.M.; Li, R.; Zhao, Q.D.; et al. LPS-induced CXCR4-dependent migratory properties and a mesenchymal-like phenotype of colorectal cancer cells. Cell Adh. Migr. 2017, 11, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shimizu, Y.; Kimura, I. Gut microbial Metab.olite short-chain fatty acids and obesity. Biosci. Microbiota Food Health 2017, 36, 135–140. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Sun, J.; Kato, I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016, 3, 130–143. [Google Scholar] [CrossRef]
- Shuwen, H.; Miao, D.; Quan, Q.; Wei, W.; Zhongshan, Z.; Chun, Z.; Xi, Y. Protective effect of the “food-microorganism-SCFAs” axis on colorectal cancer: From basic research to practical application. J. Cancer Res. Clin. Oncol. 2019, 145, 2169–2197. [Google Scholar]
- Gomes, S.D.; Oliveira, C.S.; Azevedo-Silva, J.; Casanova, M.; Barreto, J.; Pereira, H.; Chaves, S.; Rodrigues, L.; Casal, M.; Corte-Real, M.; et al. The Role of Diet Related Short-Chain Fatty Acids in Colorectal Cancer Metab.olism and Survival: Prevention and Therapeutic Implications. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Naito, Y.; Uchiyama, K.; Takagi, T. A next-generation beneficial microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018, 63, 33–35. [Google Scholar] [CrossRef]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Xu, P.; Xue, T.; Wang, J.; Guo, Y.; Jia, L.; Qiao, X.; Li, L.; Zhai, Y. Melatonin Orchestrates Lipid Homeostasis through the Hepatointestinal Circadian Clock and Microbiota during Constant Light Exposure. Cells 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, N.; Heylmann, D.; Hasselwander, S.; Fahrer, J. Mechanism of colorectal carcinogenesis triggered by heme iron from red meat. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188334. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves Metab.olism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 2020. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus casei. Cancers 2020, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metab.olism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid Metab.olism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar]
- Angelin, B.; Einarsson, K.; Hellstrom, K.; Leijd, B. Bile acid kinetics in relation to endogenous tryglyceride Metab.olism in various types of hyperlipoproteinemia. J. Lipid Res. 1978, 19, 1004–1016. [Google Scholar] [PubMed]
- Ma, H.; Patti, M.E. Bile acids, obesity, and the Metab.olic syndrome. Best Pract Res. Clin. Gastroenterol. 2014, 28, 573–583. [Google Scholar] [PubMed]
- La Frano, M.R.; Hernandez-Carretero, A.; Weber, N.; Borkowski, K.; Pedersen, T.L.; Osborn, O.; Newman, J.W. Diet-induced obesity and weight loss alter bile acid concentrations and bile acid-sensitive gene expression in insulin target tissues of C57BL/6J mice. Nutr. Res. 2017, 46, 11–21. [Google Scholar] [PubMed]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of Bile Acids and Gut Microbiota in Obesity Induced by High Fat Diet in Rat Model. J. Agric. Food Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Ochsenkuhn, T.; Bayerdorffer, E.; Meining, A.; Schinkel, M.; Thiede, C.; Nussler, V.; Sackmann, M.; Hatz, R.; Neubauer, A.; Paumgartner, G. Colonic mucosal proliferation is related to serum deoxycholic acid levels. Cancer 1999, 85, 1664–1669. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ung, T.T.; Kim, N.H.; Jung, Y.D. Role of bile acids in colon carcinogenesis. World J. Clin. Cases 2018, 6, 577–588. [Google Scholar]
- Gadaleta, R.M.; Garcia-Irigoyen, O.; Moschetta, A. Bile acids and colon cancer: Is FXR the solution of the conundrum? Mol. Aspects Med. 2017, 56, 66–74. [Google Scholar] [CrossRef]
- Fang, S.; Suh, J.M.; Reilly, S.M.; Yu, E.; Osborn, O.; Lackey, D.; Yoshihara, E.; Perino, A.; Jacinto, S.; Lukasheva, Y.; et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 2015, 21, 159–165. [Google Scholar] [CrossRef]
- Cully, M. Obesity and diabetes: FXR and JAK step up to BAT. Nat. Rev. Drug Discov. 2015, 14, 91. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, C.; Lv, Y.; Li, J.; Krausz, K.W.; Shi, J.; Brocker, C.N.; Desai, D.; Amin, S.G.; Bisson, W.H.; et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related Metab.olic dysfunction. Nat. Commun. 2015, 6, 10166. [Google Scholar] [CrossRef] [PubMed]
- Modica, S.; Murzilli, S.; Salvatore, L.; Schmidt, D.R.; Moschetta, A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008, 68, 9589–9594. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Coulter, S.; Yoshihara, E.; Oh, T.G.; Fang, S.; Cayabyab, F.; Zhu, Q.; Zhang, T.; Leblanc, M.; Liu, S.; et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell 2019, 176, 1098–1112.e18. [Google Scholar] [PubMed]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Aspects Med. 2019, 69, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, P.; Xi, Y.; Huang, Z.; Xu, P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers 2020, 12, 1408. https://doi.org/10.3390/cancers12061408
Ye P, Xi Y, Huang Z, Xu P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers. 2020; 12(6):1408. https://doi.org/10.3390/cancers12061408
Chicago/Turabian StyleYe, Pengfei, Yue Xi, Zhiying Huang, and Pengfei Xu. 2020. "Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights" Cancers 12, no. 6: 1408. https://doi.org/10.3390/cancers12061408
APA StyleYe, P., Xi, Y., Huang, Z., & Xu, P. (2020). Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers, 12(6), 1408. https://doi.org/10.3390/cancers12061408




