Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes
Abstract
1. Introduction
2. Results
2.1. Cats with HER2-Positive or TN Normal-Like Mammary Carcinoma Showed Higher Serum PD-1 and PD-L1 Levels
2.2. Serum CTLA-4 and TNF-α Levels are Positively Correlated with Serum PD-1/PD-L1 Levels in Cats with HER2-Positive and TN Normal-Like Tumors
2.3. TN normal-like Mammary Carcinomas Showed Lower PD-L1 Expression than HER2-Positive Tumors
2.4. Elevated Serum PD-1/PD-L1 Levels Showed A Higher Concordance with IHC for PD-1 and PD-L1 in Cancer Cells than in TILs
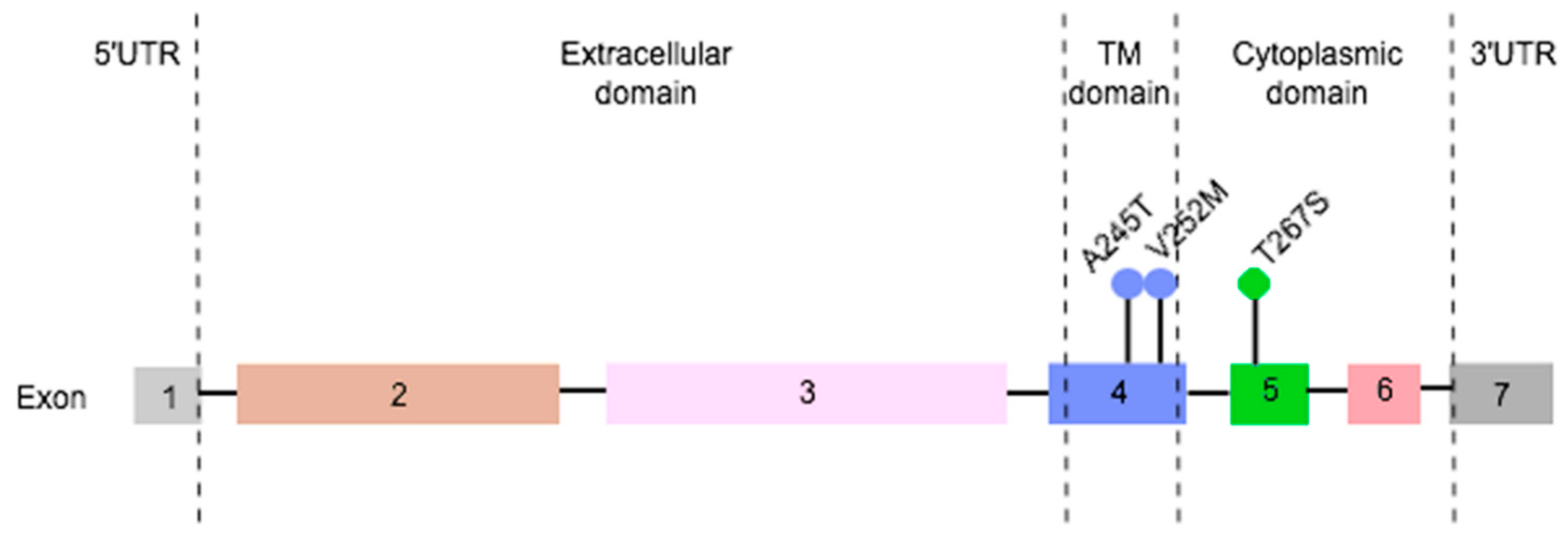
2.5. Three Non-Redundant Mutations Were Detected in Feline PD-L1 gene
3. Discussion
4. Material and Methods
4.1. Animal Population
4.2. Quantification of Serum PD-1, PD-L1, CTLA-4 and TNF-α Levels
4.3. Immunohistochemical Staining and Analysis
4.4. DNA Extraction, Amplification and Sequence Analysis of Feline PD-L1 Gene
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soare, G.R.; Soare, C.A. Immunotherapy for Breast Cancer: First FDA Approved Regimen. Discoveries 2019, 7, 4–6. [Google Scholar] [CrossRef]
- Solinas, C.; Carbognin, L.; De Silva, P.; Criscitiello, C.; Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast 2017, 35, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, R.; Kheraldine, H.S.; Meskin, N.; Vranic, S.; Al Moustafa, A.E. Crosstalk between HER2 and PD-1/PD-L1 in Breast Cancer: From Clinical Applications to Mathematical Models. Cancers 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C. Cats, Cancer and Comparative Oncology. Vet. Sci. 2015, 2, 111–126. [Google Scholar] [CrossRef]
- Adega, F.; Borges, A.; Chaves, R. Cat Mammary Tumors: Genetic Models for the Human Counterpart. Vet. Sci. 2016, 3, 17. [Google Scholar] [CrossRef]
- Hassan, B.B.; Elshafae, S.M.; Supsavhad, W.; Simmons, J.K.; Dirksen, W.P.; Sokkar, S.M.; Rosol, T.J. Feline Mammary Cancer: Novel Nude Mouse Model and Molecular Characterization of Invasion and Metastasis Genes. Vet. Pathol. 2017, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Michishita, M.; Ohtsuka, A.; Nakahira, R.; Tajima, T.; Nakagawa, T.; Sasaki, N.; Arai, T.; Takahashi, K. Anti-tumor effect of bevacizumab on a xenograft model of feline mammary carcinoma. J. Vet. Med. Sci. 2016, 78, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Ribeiro, R.; Najmudin, S.; Gameiro, A.; Rodrigues, R.; Cardoso, F.; Ferreira, F. Serum HER2 levels are increased in cats with mammary carcinomas and predict tissue HER2 status. Oncotarget 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Soares, M.; Correia, J.; Adega, F.; Ferreira, F.; Chaves, R. Assessment of ERBB2 and TOP2α gene status and expression profile in f eline mammary tumors: Findings and guidelines. Aging 2019, 11. [Google Scholar] [CrossRef]
- Urbano, A.C.; Nascimento, C.; Soares, M.; Correia, J.; Ferreira, F. Clinical Relevance of the serum CTLA-4 in Cats with Mammary Carcinoma. Sci. Rep. 2020, 10, 3822. [Google Scholar] [CrossRef]
- Bürtin, F.; Mullins, C.S.; Linnebacher, M. Mouse models of colorectal cancer: Past, present and future perspectives. World J. Gastroenterol. 2020, 26, 1394–1426. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Correia, J.; Adega, F.; Ferreira, D. Gene expression association study in feline mammary carcinomas. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Gatalica, Z.; Snyder, C.; Maney, T.; Ghazalpour, A.; Holterman, D.A.; Xiao, N.; Overberg, P.; Rose, I.; Basu, G.D.; Vranic, S.; et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2965–2970. [Google Scholar] [CrossRef]
- Kim, A.; Lee, S.J.; Kim, Y.K.; Park, W.Y.; Park, D.Y.; Kim, J.Y.; Lee, C.H.; Gong, G.; Huh, G.Y.; Choi, K.U. Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci. Rep. 2017, 7, 11671. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.M.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef]
- Cimino-Mathews, A.; Thompson, E.; Taube, J.M.; Ye, X.; Lu, Y.; Meeker, A.; Xu, H.; Sharma, R.; Lecksell, K.; Cornish, T.C.; et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 2016, 47, 52–63. [Google Scholar] [CrossRef]
- Gu, D.; Ao, X.; Yang, Y.; Chen, Z.; Xu, X. Soluble immune checkpoints in cancer: Production, function and biological significance. J. Immunother. Cancer 2018, 6, 1–14. [Google Scholar] [CrossRef]
- U.S.Food & Drug FDA Approves Atezolizumab for PD-L1 Positive Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative (accessed on 15 April 2019).
- Wimberly, H.; Brown, J.R.; Schalper, K.; Haack, H.; Silver, M.R.; Nixon, C.; Bossuyt, V.; Pusztai, L.; Lannin, D.R.; Rimm, D.L. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol. Res. 2015, 3, 326–332. [Google Scholar] [CrossRef]
- Solinas, C.; Garaud, S.; De Silva, P.; Boisson, A.; Van den Eynden, G.; de Wind, A.; Risso, P.; Vitória, J.R.; Richard, F.; Migliori, E.; et al. Immune checkpoint molecules on tumor-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer. Front. Immunol. 2017, 8, 1412. [Google Scholar] [CrossRef]
- Noske, A.; Möbus, V.; Weber, K.; Schmatloch, S.; Weichert, W.; Köhne, C.H.; Solbach, C.; Ingold Heppner, B.; Steiger, K.; Müller, V.; et al. Relevance of tumour-infiltrating lymphocytes, PD-1 and PD-L1 in patients with high-risk, nodal-metastasised breast cancer of the German Adjuvant Intergroup Node–positive study. Eur. J. Cancer 2019, 114, 76–88. [Google Scholar] [CrossRef]
- Vranic, S.; Cyprian, F.S.; Gatalica, Z.; Palazzo, J. PD-L1 status in breast cancer: Current view and perspectives. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, X.; Yang, Y.J.; Chen, Q.Q.; Zhong, L.; Zhang, T.; Cai, R.L.; Miao, J.Y.; Yu, S.C.; Zhang, F. Serum sPD-1 and sPD-L1 as biomarkers for evaluating the efficacy of neo-adjuvant chemotherapy in triple-negative breast cancer patients. Clin. Breast Cancer 2019, 326–332. [Google Scholar] [CrossRef]
- Zheng, Z.; Bu, Z.; Liu, X.; Zhang, L.; Li, Z.; Wu, A.; Wu, X.; Cheng, X.; Xing, X.; Du, H.; et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin. J. Cancer Res. 2014, 26, 104–111. [Google Scholar] [CrossRef]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, I.; Atiq, M.; Alwbari, A.; Kieber-Emmons, T. Breast Cancer Immunotherapy: An Update. Breast Cancer Basic Clin. Res. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulated PD-L1 expression in human prostate and colon cancer cells. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Lim, S.O.; Li, C.W.; Xia, W.; Cha, J.H.; Chan, L.C.; Wu, Y.; Chang, S.S.; Lin, W.C.; Hsu, J.M.; Hsu, Y.H.; et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 2016, 30, 925–939. [Google Scholar] [CrossRef]
- Lawson, N.L.; Dix, C.I.; Scorer, P.W.; Stubbs, C.J.; Wong, E.; Hutchinson, L.; McCall, E.J.; Schimpl, M.; DeVries, E.; Walker, J.; et al. Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies. Mod. Pathol. 2019. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Jiang, J. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer: A systematic review and meta-analysis. Medicine 2019, 98. [Google Scholar] [CrossRef]
- Soares, M.; Madeira, S.; Correia, J.; Peleteiro, M.; Cardoso, F.; Ferreira, F. Molecular based subtyping of feline mammary carcinomas and clinicopathological characterization. Breast 2016, 27, 44–51. [Google Scholar] [CrossRef]
- Leitzel, K.; Ali, S.M.; Shepherd, L.E.; Parulekar, W.R.; Zhu, L.; Virk, S.; Nomikos, D.; Aparicio, S.; Gelmon, K.A.; Drabick, J.J.; et al. Serum PD-L1 and outcomes in CCTG MA.31 phase 3 trial of anti-HER2 therapy in first-line HER2+ metastatic breast cancer patients (trastuzumab arm only). J. Clin. Oncol. 2018, 36, 1031. [Google Scholar] [CrossRef]
- Ito, M.; Yajima, S.; Suzuki, T.; Oshima, Y.; Nanami, T.; Sumazaki, M.; Shiratori, F.; Funahashi, K.; Tochigi, N.; Shimada, H. High serum PD-L1 level is a poor prognostic biomarker in surgically treated esophageal cancer. Cancer Med. 2019, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Kruger, S.; Legenstein, M.L.; Rösgen, V.; Haas, M.; Modest, D.P.; Westphalen, C.B.; Ormanns, S.; Kirchner, T.; Heinemann, V.; Holdenrieder, S.; et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology 2017, 6, e1310358. [Google Scholar] [CrossRef]
- Abu Hejleh, T.; Furqan, M.; Ballas, Z.; Clamon, G. The clinical significance of soluble PD-1 and PD-L1 in lung cancer. Crit. Rev. Oncol. Hematol. 2019, 143, 148–152. [Google Scholar] [CrossRef]
- Ugurel, S.; Schadendorf, D.; Horny, K.; Sucker, A.; Schramm, S.; Utikal, J.; Pföhler, C.; Herbst, R.; Schilling, B.; Blank, C.; et al. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ward, F.J.; Dahal, L.N.; Wijesekera, S.K.; Abdul-Jawad, S.K.; Kaewarpai, T.; Xu, H.; Vickers, M.A.; Barker, R.N. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur. J. Immunol. 2013, 43, 1274–1285. [Google Scholar] [CrossRef]
- Buisseret, L.; Garaud, S.; De Wind, A.; Van Den Eynden, G.; Boisson, A.; Solinas, C. Tumor-infiltrating lymphocyte composition, organization and PD-1 / PD-L1 expression are linked in breast cancer. Oncoimmunology 2017, 6. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Polónia, A.; Pinto, R.; Cameselle-Teijeiro, J.F.; Schmitt, F.C.; Paredes, J. Prognostic value of stromal tumour infiltrating lymphocytes and programmed cell death-ligand 1 expression in breast cancer. J. Clin. Pathol. 2017, 70, 860–867. [Google Scholar] [CrossRef]
- Bottai, G.; Raschioni, C.; Losurdo, A.; Di Tommaso, L.; Tinterri, C.; Torrisi, R.; Reis-Filho, J.S.; Roncalli, M.; Sotiriou, C.; Santoro, A.; et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016, 18, 121. [Google Scholar] [CrossRef]
- Kuipers, H.; Muskens, F.; Willart, M.; Hijdra, D.; van Assema, F.B.J.; Coyle, A.J.; Hoogsteden, H.C.; Lambrecht, B.N. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ cell activation. Eur. J. Immunol. 2006, 36, 2472–2482. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Whiteside, T.L.; Gooding, W.E.; Yerneni, S.S.; Hoffmann, T.K. Clinical Significance of PD-L1 + Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2017, 24, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Stovgaard, E.S.; Dyhl-Polk, A.; Roslind, A.; Balslev, E.; Nielsen, D. PD-L1 expression in breast cancer: Expression in subtypes and prognostic significance: A systematic review. Breast Cancer Res. Treat. 2019, 174, 571–584. [Google Scholar] [CrossRef]
- Beckers, R.K.; Selinger, C.I.; Vilain, R.; Madore, J.; Wilmott, J.S.; Harvey, K.; Holliday, A.; Cooper, C.L.; Robbins, E.; Gillett, D.; et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016, 69, 25–34. [Google Scholar] [CrossRef]
- Ghebeh, H.; Lehe, C.; Barhoush, E.; Al-Romaih, K.; Tulbah, A.; Al-Alwan, M.; Hendrayani, S.F.; Manogaran, P.; Alaiya, A.; Al-Tweigeri, T.; et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010, 12. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Koutsopoulos, A.V.; Tsoulfas, P.G.; Lagoudaki, E.; Aggouraki, D.; Monastirioti, A.; Koutoulaki, C.; Apostolopoulou, C.A.; Merodoulaki, A.C.; Papadaki, C.; et al. Clinical relevance of immune checkpoints on circulating tumor cells in breast cancer. Cancers 2020, 12, 376. [Google Scholar] [CrossRef]
- Lippi, G.; Avanzini, P.; Zobbi, V.; Ippolito, L. Influence of mechanical hemolysis of blood on two D-dimer immunoassays. Blood Coagul. Fibrinolysis 2012, 23, 461–463. [Google Scholar] [CrossRef]
- Soares, M.; Correia, J.; Peleteiro, M.C.; Ferreira, F. St Gallen molecular subtypes in feline mammary carcinoma and paired metastases—Disease progression and clinical implications from a 3-year follow-up study. Tumor Biol. 2016, 37, 4053–4064. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: Recommendations by an International TILS Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, T.; Xuan, Q.; Zhao, H.; Qin, L.; Zhang, Q. Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Lee, Y.K.; Koo, J.S. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J. Transl. Med. 2016, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Ács, B.; Madaras, L.; Tőkés, A.M.; Kovács, A.K.; Kovács, E.; Ozsvári-Vidákovich, M.; Karászi, Á.; Birtalan, E.; Dank, M.; Szász, A.M.; et al. PD-1, PD-L1 and CTLA-4 in pregnancy-related and in early-onset breast cancer: A comparative study. Breast 2017, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
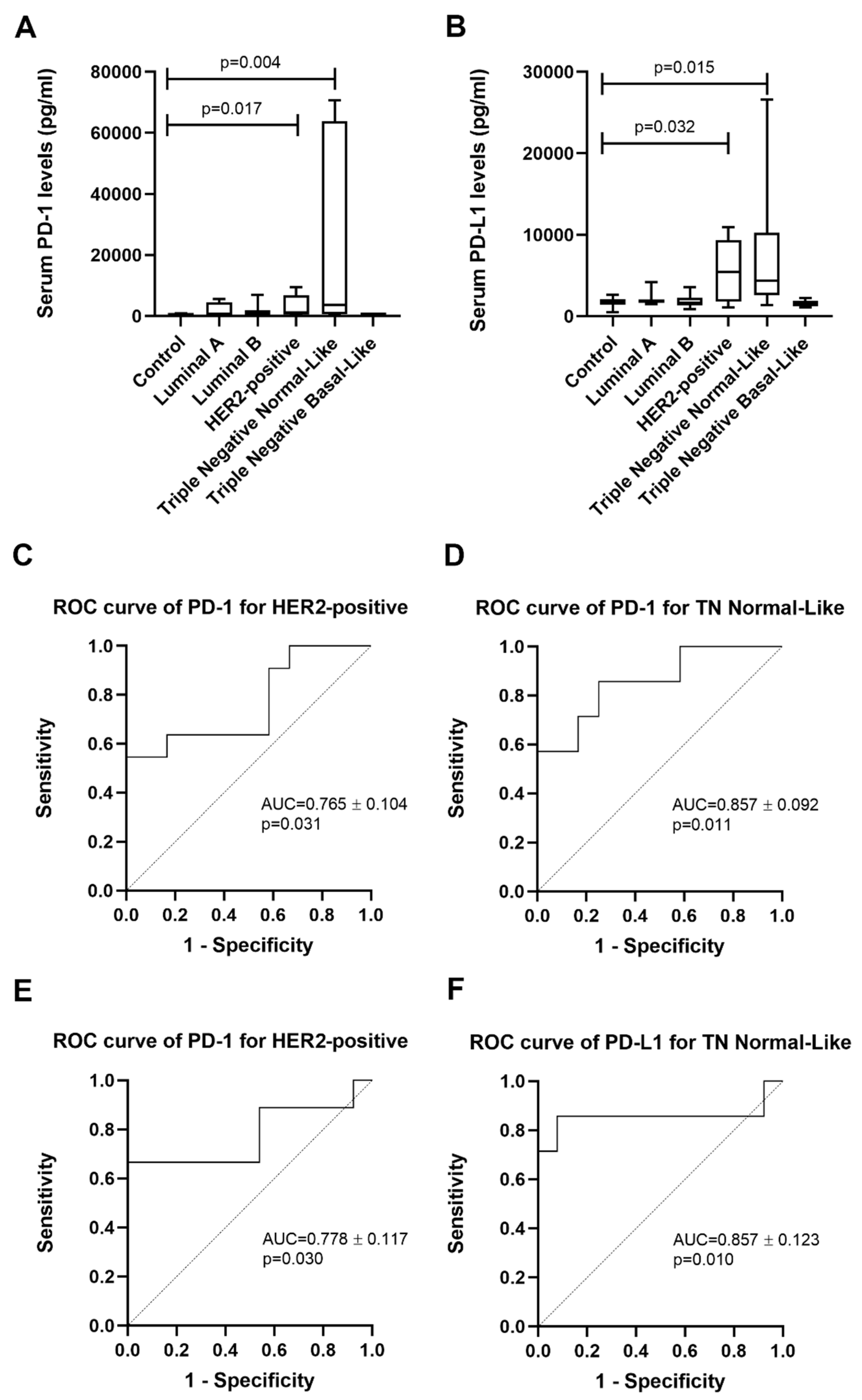
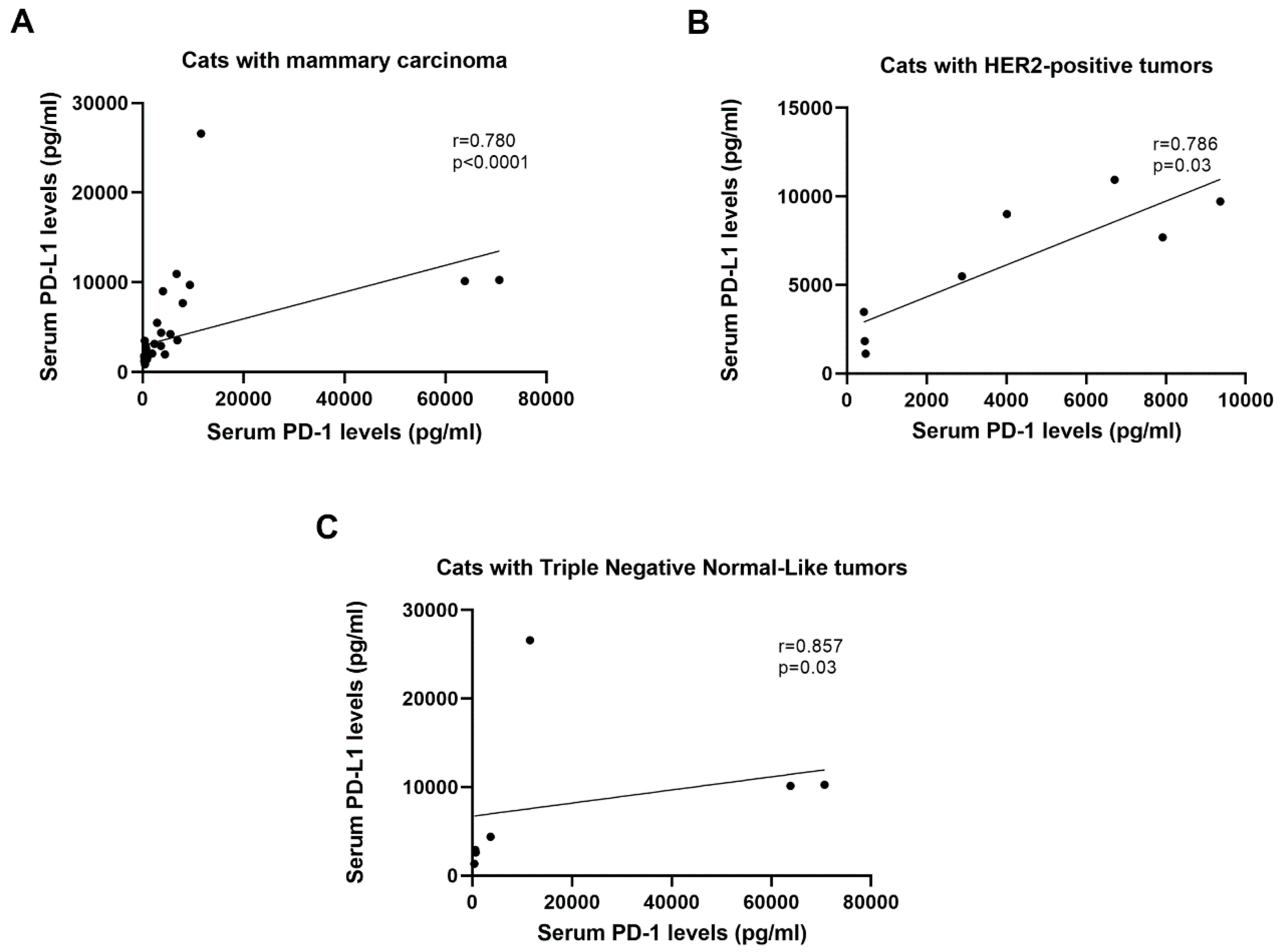
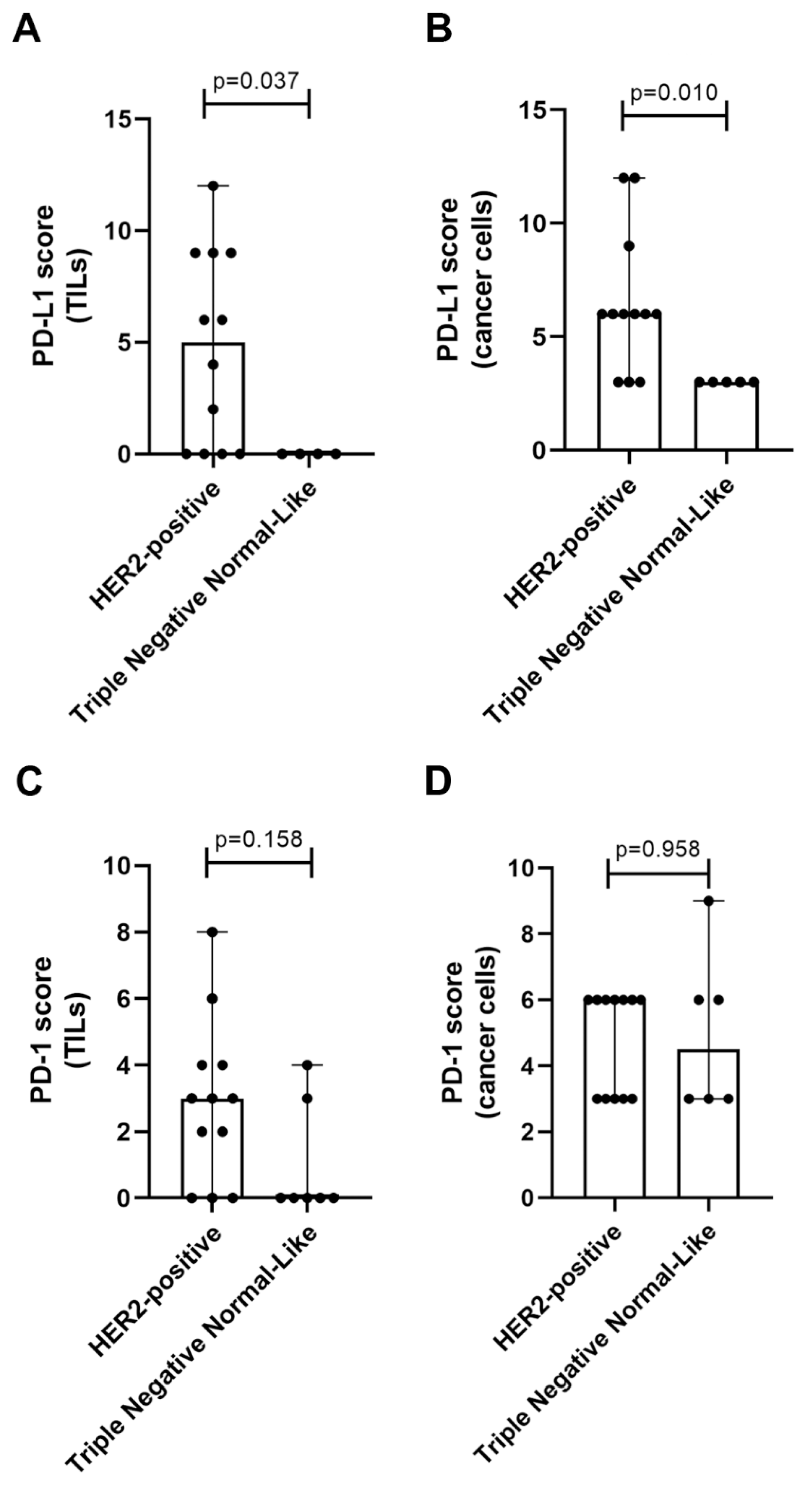
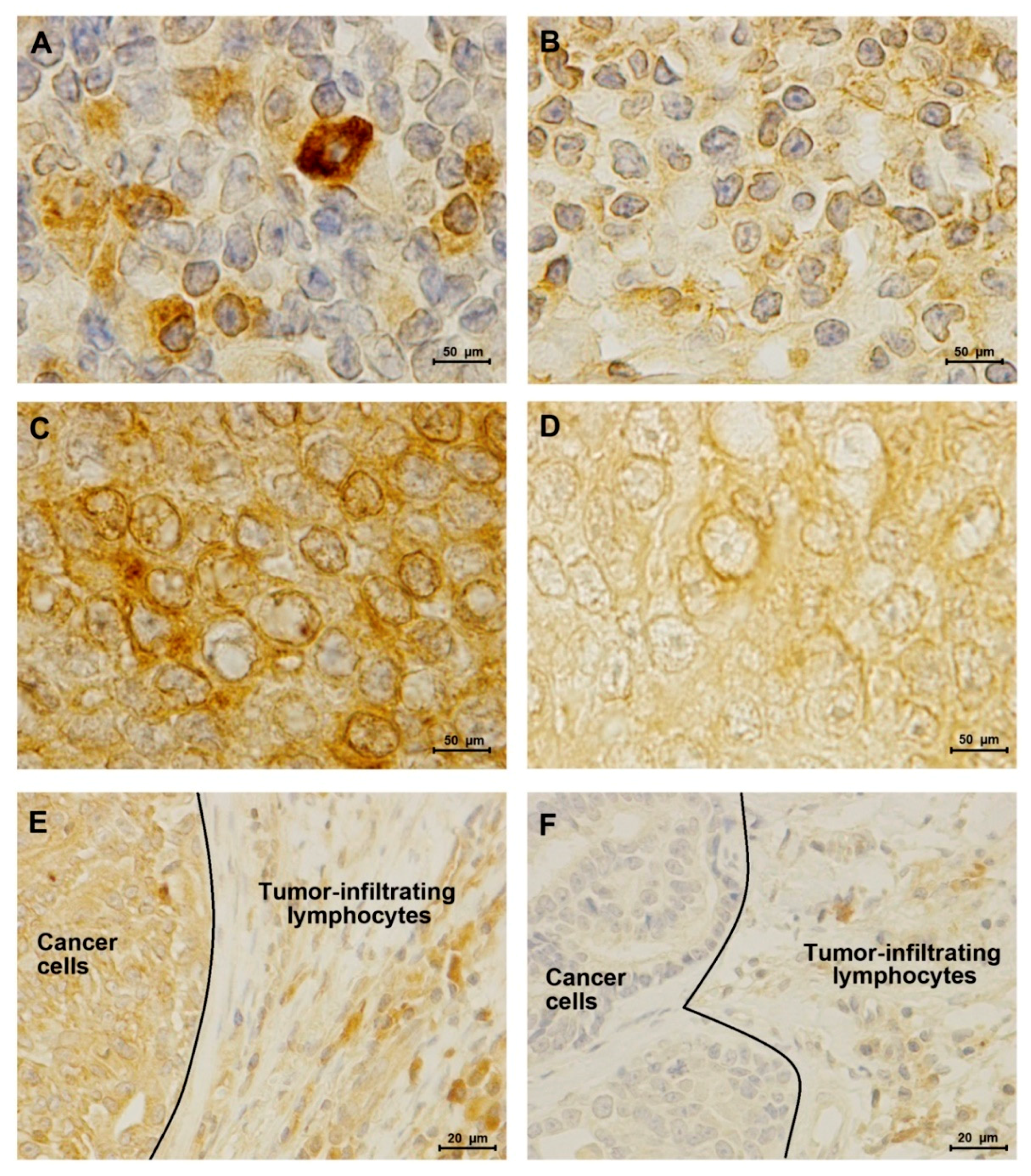
| Group | n | sPD-1 (pg/mL) Median ± SD | sPD-1 (pg/mL) Mean ± SEM |
|---|---|---|---|
| Control | 12 | 534.0 ± 253.2 | 459.0 ± 73.1 |
| Luminal A | 7 | 708.8 ± 2165.2 | 1826.5 ± 818.8 |
| Luminal B | 15 | 590.3 ± 1776.8 | 1386.3 ± 458.8 |
| HER2-positive | 11 | 1148.9 ± 3386.0 | 3129.6 ± 1020.9 |
| TN Normal-Like | 7 | 3655.1 ± 31,478.1 | 21,637.3 ± 11,897.6 |
| TN Basal-Like | 6 | 513.3 ± 145.0 | 519.3 ± 59.2 |
| Group | n | sPD-L1 (pg/mL) Median ± SD | sPD-L1 (pg/mL) Mean ± SEM |
|---|---|---|---|
| Control | 13 | 1835.5 ± 545.6 | 1762.5 ± 151.3 |
| Luminal A | 7 | 1789.6 ± 941.6 | 2121.5 ± 355.9 |
| Luminal B | 15 | 1628.0 ± 773.4 | 1877.6 ± 199.7 |
| HER2-positive | 9 | 5490.4 ± 3789.6 | 5672.6 ± 1263.2 |
| TN Normal-Like | 7 | 4377.5 ± 8819.4 | 8313.0 ± 3333.4 |
| TN Basal-Like | 6 | 1436.3 ± 425.6 | 1534.2 ± 173.8 |
| HER2-Positive | PD-1 | PD-L1 | CTLA-4 | TNF-α |
| PD-1 | − | 0.923** | 0.975 ** | 0.968 ** |
| PD-L1 | 0.923** | − | 0.947 ** | 0.922 ** |
| CTLA-4 | 0.975** | 0.947** | − | 0.947 ** |
| TNF-α | 0.968** | 0.922** | 0.947** | − |
| TN Normal-Like | PD-1 | PD-L1 | CTLA-4 | TNF-α |
| PD-1 | − | 0.857* | 0.927** | 0.893** |
| PD-L1 | 0.857* | − | 0.815* | 0.857* |
| CTLA-4 | 0.927** | 0.815* | − | 0.927** |
| TNF-α | 0.893** | 0.857* | 0.927** | − |
| Tumor subtype | sPD-1 | TILs | Concordance | sPD-1 | Cancer cells | Concordance |
| HER2-positive | 61.5% | 41.7% | 58.3% | 61.5% | 100% | 66.7% |
| Triple Negative Normal-Like | 57.1% | 33.3% | 0% | 57.1% | 100% | 66.7% |
| Tumor subtype | sPD-L1 | TILs | Concordance | sPD-L1 | Cancer cells | Concordance |
| HER2-positive | 76.9% | 66.7% | 41.7% | 76.9% | 100% | 75% |
| Triple Negative Normal-Like | 85.7% | 20% | 20% | 85.7% | 100% | 100% |
| Clinicopathological Feature | Number of Animals (%) | Clinicopathological Feature | Number of Animals (%) |
|---|---|---|---|
| Age | Tumor size | ||
| <8 years old | 3 (5.7%) | <2 cm | 19 (35.8%) |
| ≥ 8 years old | 50 (94.3%) | ≥ 2 cm | 34 (64.2%) |
| Breed | HP classification | ||
| Not determined | 39 (73.6%) | Tubulopapillary carcinoma | 21 (39.6%) |
| Siamese | 5 (9.4%) | Solid carcinoma | 6 (11.3%) |
| Persian | 6 (11.3%) | Cribriform carcinoma | 5 (9.4%) |
| Norwegian Forest Cat | 2 (3.8%) | Papillary-cystic carcinoma | 1 (1.9%) |
| Blue Russian | 1 (1.9%) | Tubular carcinoma | 12 (22.6%) |
| Spayed | Mucinous carcinoma | 8 (15.2%) | |
| No | 28 (52.8%) | Tumor malignancy grade | |
| Yes | 24 (45.3%) | I | 3 (5.7%) |
| Unknown | 1 (1.9%) | II | 7 (13.2%) |
| Contraceptive administration | III | 43 (81.1%) | |
| No | 19 (35.9%) | Tumor necrosis | |
| Yes | 28 (52.8%) | No | 13 (24.5%) |
| Unknown | 6 (11.3%) | Yes | 40 (75.5%) |
| Treatment | Tumor lymphatic invasion | ||
| None | 1 (1.9%) | No | 48 (90.5%) |
| Mastectomy | 48 (90.6%) | Yes | 5 (9.5%) |
| Mastectomy + Chemo | 4 (7.5%) | Lymphocytic infiltration | |
| Multiple tumors | No | 17 (32.0%) | |
| No | 19 (35.8%) | Yes | 34 (64.2%) |
| Yes | 34 (64.2%) | Unknown | 2 (3.8%) |
| Lymph node status | Tumor ulceration | ||
| Negative | 32 (60.4%) | No | 46 (86.8%) |
| Positive | 17 (32.1%) | Yes | 7 (13.2%) |
| Unknown | 4 (7.5%) | Ki-67 index | |
| TNM classification | Low (<14%) | 36 (67.9%) | |
| I | 13 (24.5%) | High (>14%) | 17 (32.1%) |
| II | 6 (11.3%) | Progesterone status | |
| III | 29 (54.7%) | Negative | 28 (52.8%) |
| IV | 5 (9.5%) | Positive | 25 (47.2%) |
| Tumor localization | Estrogen status | ||
| M1 | 8 (15.1%) | Negative | 37 (69.8%) |
| M2 | 10 (18.9%) | Positive | 16 (30.2%) |
| M3 | 20 (37.7%) | HER2 status | |
| M4 | 15 (28.3%) | Negative | 41 (77.4%) |
| Positive | 12 (22.6%) |
| Exons | Forward 5’–3’ | Reverse 5’–3’ |
|---|---|---|
| 2 | TTTGGGGACAGCAGCTTGTT | TGAACAGACTGACACCGTGG |
| 3 | TCTGAGAACCAGCCAGAATTGA | ACTGGAACATAGGGCGTGTT |
| 4 | GTCGAAGGCATCTCGCTGT | AGAGCCACTGTGACAACAACA |
| 5 | AATTGACCTCAGGGGTTGGAA | GAGGTAAGGAGGAGCCCGTT |
| 6 | TACTGCAGAGGTAACTGGACA | GGCCTCTCACATCCGACATC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, C.; Urbano, A.C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes. Cancers 2020, 12, 1386. https://doi.org/10.3390/cancers12061386
Nascimento C, Urbano AC, Gameiro A, Ferreira J, Correia J, Ferreira F. Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes. Cancers. 2020; 12(6):1386. https://doi.org/10.3390/cancers12061386
Chicago/Turabian StyleNascimento, Catarina, Ana Catarina Urbano, Andreia Gameiro, João Ferreira, Jorge Correia, and Fernando Ferreira. 2020. "Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes" Cancers 12, no. 6: 1386. https://doi.org/10.3390/cancers12061386
APA StyleNascimento, C., Urbano, A. C., Gameiro, A., Ferreira, J., Correia, J., & Ferreira, F. (2020). Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes. Cancers, 12(6), 1386. https://doi.org/10.3390/cancers12061386








