Hereditary Breast and Ovarian Cancer in Families from Southern Italy (Sicily)—Prevalence and Geographic Distribution of Pathogenic Variants in BRCA1/2 Genes
Abstract
1. Introduction
2. Results
2.1. Clinical Features of Breast and/or Ovarian Cancer Patients Harbouring Germline BRCA1/2 Pathogenic Variants
2.2. Clinical Features of Germline BRCA1/2 PV Carriers
2.3. Typology and Distribution of BRCA1/2 PVs in the Sicilian Population
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Sample Collection and Next-Generation Sequencing Analysis for BRCA 1/2 Genes
4.3. Sanger Sequencing
4.4. CNV Analysis by Multiplex Ligation-Dependent Probe Amplification Analysis (MLPA)
4.5. Genetic Variant Classification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G.; et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.; Ding, W.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Yu, J.; Suenaga, M.; Fesharakizadeh, S.; Cho, C.; Macgregor-Das, A.; Siddiqui, A.; Witmer, P.D.; Tamura, K.; Song, T.J.; et al. Deleterious Germline Mutations in Patients with Apparently Sporadic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2017, 35, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. Jama 2018, 319, e2401. [Google Scholar] [CrossRef] [PubMed]
- Agalliu, I.; Gern, R.; Leanza, S.; Burk, R.D. Associations of High-Grade Prostate Cancer with BRCA1 and BRCA2 Founder Mutations. Clin. Cancer Res. 2009, 15, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Leongamornlert, D.; Mahmud, N.; Tymrakiewicz, M.; Saunders, E.; Dadaev, T.; Castro, E.; Goh, C.; Govindasami, K.; Guy, M.; O’Brien, L.; et al. Germline BRCA1 mutations increase prostate cancer risk. Br. J. Cancer 2012, 106, 1697–1701. [Google Scholar] [CrossRef]
- Pilarski, R. The Role of BRCA Testing in Hereditary Pancreatic and Prostate Cancer Families. Am. Soc. Clin. Oncol. Educ. Book 2019, 79–86. [Google Scholar] [CrossRef]
- Mavaddat, N.; Peock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Evans, D.G.; Izatt, L.; Eeles, R.A.; Adlard, J.; et al. Cancer Risks for BRCA1 and BRCA2 Mutation Carriers: Results from Prospective Analysis of EMBRACE. JNCI J. Natl. Cancer Inst. 2013, 105, 812–822. [Google Scholar] [CrossRef]
- Couch, F.J.; Shimelis, H.; Hu, C.; Hart, S.N.; Polley, E.C.; Na, J.; Hallberg, E.; Moore, R.; Thomas, A.; Lilyquist, J.; et al. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol. 2017, 3, e1190. [Google Scholar] [CrossRef]
- Marabelli, M.; Cheng, S.-C.; Parmigiani, G. Penetrance ofATMGene Mutations in Breast Cancer: A Meta-Analysis of Different Measures of Risk. Genet. Epidemiol. 2016, 40, 425–431. [Google Scholar] [CrossRef]
- Weischer, M.; Bojesen, S.E.; Ellervik, C.; Tybjærg-Hansen, A.; Nordestgaard, B.G. CHEK2*1100delC Genotyping for Clinical Assessment of Breast Cancer Risk: Meta-Analyses of 26,000 Patient Cases and 27,000 Controls. J. Clin. Oncol. 2008, 26, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Couch, F.J. Germline Genetic Testing for Breast Cancer Risk: The Past, Present and Future. Am. Soc. Clin. Oncol. Educ. Book 2019, 61–74. [Google Scholar] [CrossRef]
- Antoniou, A.C.; Casadei, S.; Heikkinen, T.; Barrowdale, D.; Pylkäs, K.; Roberts, J.; Lee, A.; Subramanian, D.; De Leeneer, K.; Fostira, F.; et al. Breast-Cancer Risk in Families with Mutations in PALB2. N. Engl. J. Med. 2014, 371, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Kaurah, P.; MacMillan, A.; Boyd, N.; Senz, J.; De Luca, A.; Chun, N.; Suriano, G.; Zaor, S.; Van Manen, L.; Gilpin, C.; et al. Founder and Recurrent CDH1 Mutations in Families with Hereditary Diffuse Gastric Cancer. Jama 2007, 297, e2360. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. Jama 2017, 317, e2402. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime Cancer Risks in Individuals with Germline PTEN Mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.L.; Best, A.F.; Peters, J.A.; DeCastro, R.M.; Khincha, P.P.; Loud, J.T.; Bremer, R.C.; Rosenberg, P.S.; Savage, S.A. Risks of first and subsequent cancers amongTP53mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 2016, 122, 3673–3681. [Google Scholar] [CrossRef]
- Thompson, D.; Easton, D.F. Cancer Incidence in BRCA1 Mutation Carriers. J. Natl. Cancer Inst. 2002, 94, 1358–1365. [Google Scholar] [CrossRef]
- Breast Cancer Linkage Consortium, T. Cancer Risks in BRCA2 Mutation Carriers. JNCI J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Massihnia, D.; Galvano, A.; Fanale, D.; Perez, A.; Castiglia, M.; Incorvaia, L.; Listì, A.; Rizzo, S.; Cicero, G.; Bazan, V.; et al. Triple negative breast cancer: Shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget 2016, 7. [Google Scholar] [CrossRef]
- Waddell, N.; Arnold, J.; Cocciardi, S.; da Silva, L.; Marsh, A.; Riley, J.; Johnstone, C.N.; Orloff, M.; Assie, G.; Eng, C.; et al. Subtypes of familial breast tumours revealed by expression and copy number profiling. Breast Cancer Res. Treat. 2009, 123, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Prokunina-Olsson, L.; Larsen, M.J.; Kruse, T.A.; Tan, Q.; Lænkholm, A.-V.; Bak, M.; Lykkesfeldt, A.E.; Sørensen, K.P.; Hansen, T.v.O.; Ejlertsen, B.; et al. Classifications within Molecular Subtypes Enables Identification of BRCA1/BRCA2 Mutation Carriers by RNA Tumor Profiling. PLoS ONE 2013, 8, e64268. [Google Scholar] [CrossRef]
- Lakhani, S.R.; Jacquemier, J.; Sloane, J.P.; Gusterson, B.A.; Anderson, T.J.; van de Vijver, M.J.; Farid, L.M.; Venter, D.; Antoniou, A.; Storfer-Isser, A.; et al. Multifactorial Analysis of Differences Between Sporadic Breast Cancers and Cancers Involving BRCA1 and BRCA2 Mutations. JNCI J. Natl. Cancer Inst. 1998, 90, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Benusiglio, P.R. Lobular breast cancer: Incidence and genetic and non-genetic risk factors. Breast Cancer Res. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Alsop, K.; Fereday, S.; Meldrum, C.; deFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA Mutation Frequency and Patterns of Treatment Response in BRCA Mutation–Positive Women with Ovarian Cancer: A Report From the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Rust, K.; Spiliopoulou, P.; Tang, C.Y.; Bell, C.; Stirling, D.; Phang, T.H.F.; Davidson, R.; Mackean, M.; Nussey, F.; Glasspool, R.M.; et al. Routine germline BRCA1 and BRCA2 testing in patients with ovarian carcinoma: Analysis of the Scottish real-life experience. BJOG J. Obstet. Gynaecol. 2018, 125, 1451–1458. [Google Scholar] [CrossRef]
- Gori, S.; Barberis, M.; Bella, M.A.; Buttitta, F.; Capoluongo, E.; Carrera, P.; Colombo, N.; Cortesi, L.; Genuardi, M.; Gion, M.; et al. Recommendations for the implementation of BRCA testing in ovarian cancer patients and their relatives. Crit. Rev. Oncol./Hematol. 2019, 140, 67–72. [Google Scholar] [CrossRef]
- Akbari, M.R.; Gojska, N.; Narod, S.A. Coming of age in Canada: A study of population-based genetic testing for breast and ovarian cancer. Curr. Oncol. 2017, 24, e282. [Google Scholar] [CrossRef]
- Manchanda, R.; Loggenberg, K.; Sanderson, S.; Burnell, M.; Wardle, J.; Gessler, S.; Side, L.; Balogun, N.; Desai, R.; Kumar, A.; et al. Population Testing for Cancer Predisposing BRCA1/BRCA2 Mutations in the Ashkenazi-Jewish Community: A Randomized Controlled Trial. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Rowley, S.M.; Mascarenhas, L.; Devereux, L.; Li, N.; Amarasinghe, K.C.; Zethoven, M.; Lee, J.E.A.; Lewis, A.; Morgan, J.A.; Limb, S.; et al. Population-based genetic testing of asymptomatic women for breast and ovarian cancer susceptibility. Genet. Med. 2018, 21, 913–922. [Google Scholar] [CrossRef]
- Manchanda, R.; Burnell, M.; Gaba, F.; Desai, R.; Wardle, J.; Gessler, S.; Side, L.; Sanderson, S.; Loggenberg, K.; Brady, A.F.; et al. Randomised trial of population-based BRCA testing in Ashkenazi Jews: Long-term outcomes. BJOG Int. J. Obstet. Gynaecol. 2019, 127, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Burnell, M.; Gaba, F.; Sanderson, S.; Loggenberg, K.; Gessler, S.; Wardle, J.; Side, L.; Desai, R.; Brady, A.F.; et al. Attitude towards and factors affecting uptake of population-based BRCA testing in the Ashkenazi Jewish population: A cohort study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 784–794. [Google Scholar] [CrossRef]
- Shkedi-Rafid, S.; Gabai-Kapara, E.; Grinshpun-Cohen, J.; Levy-Lahad, E. BRCA genetic testing of individuals from families with low prevalence of cancer: Experiences of carriers and implications for population screening. Genet. Med. 2012, 14, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Gaba, F. Population Based Testing for Primary Prevention: A Systematic Review. Cancers 2018, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Plon, S.E.; Eccles, D.M.; Easton, D.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.L.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 29, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Couch, F.J.; DeShano, M.L.; Blackwood, M.A.; Calzone, K.; Stopfer, J.; Campeau, L.; Ganguly, A.; Rebbeck, T.; Weber, B.L.; Jablon, L.; et al. BRCA1Mutations in Women Attending Clinics That Evaluate the Risk of Breast Cancer. N. Engl. J. Med. 1997, 336, 1409–1415. [Google Scholar] [CrossRef]
- Nedelcu, R.; Liede, A.; Aubé, J.; Finch, A.; Kwan, E.; Jack, E.; Narod, S.A.; Randall, S.; Hugel, L.; Clark, K. BRCA mutations in Italian breast/ovarian cancer families. Eur. J. Hum. Genet. 2002, 10, 150–152. [Google Scholar] [CrossRef]
- Janavičius, R. Founder BRCA1/2 mutations in the Europe: Implications for hereditary breast-ovarian cancer prevention and control. EPMA J. 2010, 1, 397–412. [Google Scholar] [CrossRef]
- Finch, A.; Wang, M.; Fine, A.; Atri, L.; Khalouei, S.; Pupavac, M.; Rosen, B.; Eisen, A.; Elser, C.; Charames, G.; et al. Genetic testing forBRCA1andBRCA2in the Province of Ontario. Clin. Genet. 2016, 89, 304–311. [Google Scholar] [CrossRef]
- Ottini, L.; D’Amico, C.; Noviello, C.; Lauro, S.; Lalle, M.; Fornarini, G.; Colantuoni, O.A.; Pizzi, C.; Cortesi, E.; Carlini, S.; et al. BRCA1 and BRCA2 mutations in central and southern Italian patients. Breast Cancer Res. 2000, 2. [Google Scholar] [CrossRef]
- Russo, A.; Calò, V.; Bruno, L.; Schirò, V.; Agnese, V.; Cascio, S.; Foddai, E.; Fanale, D.; Rizzo, S.; Di Gaudio, F.; et al. Is BRCA1-5083del19, identified in breast cancer patients of Sicilian origin, a Calabrian founder mutation? Breast Cancer Res. Treat. 2008, 113, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Calò, V.; Augello, C.; Bruno, L.; Agnese, V.; Schirò, V.; Barbera, F.; Cascio, S.; Foddai, E.; Badalamenti, G.; et al. 4843delC of the BRCA1 gene is a possible founder mutation in Southern Italy (Sicily). Ann. Oncol. 2007, 18, vi99–vi102. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Capalbo, C.; Ristori, E.; Ricevuto, E.; Sidoni, T.; Buffone, A.; Cortesi, E.; Marchetti, P.; Scambia, G.; Tomao, S.; et al. Novel BRCA1 and BRCA2 germline mutations and assessment of mutation spectrum and prevalence in Italian breast and/or ovarian cancer families. Breast Cancer Res. Treat. 2006, 100, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Capalbo, C.; Ricevuto, E.; Vestri, A.; Ristori, E.; Sidoni, T.; Buffone, O.; Adamo, B.; Cortesi, E.; Marchetti, P.; Scambia, G.; et al. BRCA1 and BRCA2 genetic testing in Italian breast and/or ovarian cancer families: Mutation spectrum and prevalence and analysis of mutation prediction models. Ann. Oncol. 2006, 17, vii34–vii40. [Google Scholar] [CrossRef]
- Minucci, A.; Scambia, G.; Santonocito, C.; Concolino, P.; Canu, G.; Mignone, F.; Saggese, I.; Guarino, D.; Costella, A.; Molinario, R.; et al. Clinical impact on ovarian cancer patients of massive parallel sequencing forBRCAmutation detection: The experience at Gemelli hospital and a literature review. Expert Rev. Mol. Diagn. 2015, 15, 1383–1403. [Google Scholar] [CrossRef]
- Caux-Moncoutier, V.; Castéra, L.; Tirapo, C.; Michaux, D.; Rémon, M.-A.; Laugé, A.; Rouleau, E.; De Pauw, A.; Buecher, B.; Gauthier-Villars, M.; et al. EMMA, a cost- and time-effective diagnostic method for simultaneous detection of point mutations and large-scale genomic rearrangements: Application to BRCA1 and BRCA2 in 1,525 patients. Hum. Mutat. 2011, 32, 325–334. [Google Scholar] [CrossRef]
- Russo, A.; Calò, V.; Agnese, V.; Bruno, L.; Corsale, S.; Augello, C.; Gargano, G.; Barbera, F.; Cascio, S.; Intrivici, C.; et al. BRCA1 genetic testing in 106 breast and ovarian cancer families from southern Italy (Sicily): A mutation analyses. Breast Cancer Res. Treat. 2007, 105, 267–276. [Google Scholar] [CrossRef]
- Susswein, L.R.; Marshall, M.L.; Nusbaum, R.; Vogel Postula, K.J.; Weissman, S.M.; Yackowski, L.; Vaccari, E.M.; Bissonnette, J.; Booker, J.K.; Cremona, M.L.; et al. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet. Med. 2015, 18, 823–832. [Google Scholar] [CrossRef]
- Golmard, L.; Delnatte, C.; Laugé, A.; Moncoutier, V.; Lefol, C.; Abidallah, K.; Tenreiro, H.; Copigny, F.; Giraudeau, M.; Guy, C.; et al. Breast and ovarian cancer predisposition due to de novo BRCA1 and BRCA2 mutations. Oncogene 2015, 35, 1324–1327. [Google Scholar] [CrossRef]
- Dodova, R.I.; Mitkova, A.V.; Dacheva, D.R.; Hadjo, L.B.; Vlahova, A.I.; Hadjieva, M.S.T.; Valev, S.S.; Caulevska, M.M.; Popova, S.D.; Popov, I.E.; et al. Spectrum and frequencies of BRCA1/2 mutations in Bulgarian high risk breast cancer patients. BMC Cancer 2015, 15. [Google Scholar] [CrossRef]
- Kwong, A.; Wong, L.P.; Wong, H.N.; Law, F.B.F.; Ng, E.K.O.; Tang, Y.H.; Chan, W.K.; Ho, L.S.; Kwan, K.H.; Poon, M.; et al. A BRCA2 founder mutation and seven novel deleterious BRCA mutations in southern Chinese women with breast and ovarian cancer. Breast Cancer Res. Treat. 2009, 117, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Papi, L.; Putignano, A.L.; Congregati, C.; Zanna, I.; Sera, F.; Morrone, D.; Falchetti, M.; Turco, M.R.D.; Ottini, L.; Palli, D.; et al. Founder mutations account for the majority of BRCA1-attributable hereditary breast/ovarian cancer cases in a population from Tuscany, Central Italy. Breast Cancer Res. Treat. 2008, 117, 497–504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vézina, H.; Durocher, F.; Dumont, M.; Houde, L.; Szabo, C.; Tranchant, M.; Chiquette, J.; Plante, M.; Laframboise, R.; Lépine, J.; et al. Molecular and genealogical characterization of the R1443X BRCA1 mutation in high-risk French-Canadian breast/ovarian cancer families. Hum. Genet. 2005, 117, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudh, W.; Bouaouina, N.; Ahmed, S.B.; Gabbouj, S.; Shan, J.; Mathew, R.; Uhrhammer, N.; Bignon, Y.-J.; Troudi, W.; Elgaaied, A.B.A.; et al. Hereditary breast cancer in Middle Eastern and North African (MENA) populations: Identification of novel, recurrent and founder BRCA1 mutations in the Tunisian population. Mol. Biol. Rep. 2011, 39, 1037–1046. [Google Scholar] [CrossRef]
- Caputo, S.; Benboudjema, L.; Sinilnikova, O.; Rouleau, E.; Béroud, C.; Lidereau, R. Description and analysis of genetic variants in French hereditary breast and ovarian cancer families recorded in the UMD-BRCA1/BRCA2 databases. Nucleic Acids Res. 2012, 40, D992–D1002. [Google Scholar] [CrossRef]
- Hamel, N.; Feng, B.-J.; Foretova, L.; Stoppa-Lyonnet, D.; Narod, S.A.; Imyanitov, E.; Sinilnikova, O.; Tihomirova, L.; Lubinski, J.; Gronwald, J.; et al. On the origin and diffusion of BRCA1 c.5266dupC (5382insC) in European populations. Eur. J. Hum. Genet. 2010, 19, 300–306. [Google Scholar] [CrossRef]
- Campos, B.; Díez, O.; Odefrey, F.; Domènech, M.; Moncoutier, V.; Martínez-Ferrandis, J.I.; Osorio, A.; Balmaña, J.; Barroso, A.; Armengod, M.E.; et al. Haplotype analysis of theBRCA29254delATCAT recurrent mutation in breast/ovarian cancer families from Spain. Hum. Mutat. 2003, 21, 452. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Friebel, T.M.; Friedman, E.; Hamann, U.; Huo, D.; Kwong, A.; Olah, E.; Olopade, O.I.; Solano, A.R.; Teo, S.-H.; et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum. Mutat. 2018, 39, 593–620. [Google Scholar] [CrossRef]
- Ferla, R.; Calò, V.; Cascio, S.; Rinaldi, G.; Badalamenti, G.; Carreca, I.; Surmacz, E.; Colucci, G.; Bazan, V.; Russo, A. Founder mutations in BRCA1 and BRCA2 genes. Ann. Oncol. 2007, 18, vi93–vi98. [Google Scholar] [CrossRef]
- McClain, M.R.; Nathanson, K.L.; Palomaki, G.E.; Haddow, J.E. An evaluation of BRCA1 and BRCA2 founder mutations penetrance estimates for breast cancer among Ashkenazi Jewish women. Genet. Med. 2005, 7, 34–39. [Google Scholar] [CrossRef]
- Weitzel, J.N. Prevalence of BRCA Mutations and Founder Effect in High-Risk Hispanic Families. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Palomba, G.; Cossu, A.; Friedman, E.; Budroni, M.; Farris, A.; Contu, A.; Pisano, M.; Baldinu, P.; Sini, M.C.; Tanda, F.; et al. Origin and distribution of the BRCA2-8765delAG mutation in breast cancer. BMC Cancer 2007, 7. [Google Scholar] [CrossRef] [PubMed]
- Baudi, F.; Lavecchia, A.M.; Quaresima, B.; Faniello, M.C.; De Paola, L.; D’Amico, W.; Fabiani, F.; Cuda, G.; Costanzo, F.S.; Venuta, S. High prevalence of a BRCA1 gene founder mutation, 5083del19, in unselected breast–ovarian cancer patients from Southern Italy: Genotype–phenotype correlations. Breast Cancer Res. 2005, 7. [Google Scholar] [CrossRef][Green Version]
- Marroni, F.; Cipollini, G.; Peissel, B.; D’Andrea, E.; Pensabene, M.; Radice, P.; Caligo, M.A.; Presciuttini, S.; Bevilacqua, G. Reconstructing the Genealogy of a BRCA1 Founder Mutation by Phylogenetic Analysis. Ann. Hum. Genet. 2008, 72, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Giambona, A.; Vinciguerra, M.; Cannata, M.; Cassarà, F.; Fiorentino, G.; Leto, F.; Gioco, P.L.; Renda, D.; Passarello, C.; Maggio, A. The genetic heterogeneity of β-globin gene defects in Sicily reflects the historic population migrations of the island. Blood Cells Mol. Dis. 2011, 46, 282–287. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Mitra, N.; Wan, F.; Sinilnikova, O.M.; Healey, S.; McGuffog, L.; Mazoyer, S.; Chenevix-Trench, G.; Easton, D.F.; Antoniou, A.C.; et al. Association of Type and Location ofBRCA1andBRCA2Mutations With Risk of Breast and Ovarian Cancer. Jama 2015, 313, e1347. [Google Scholar] [CrossRef]
- Mazzola, E.; Blackford, A.; Parmigiani, G.; Biswas, S. Recent Enhancements to the Genetic Risk Prediction Model BRCAPRO. Cancer Inform. 2015, 14s2, CIN.S17292. [Google Scholar] [CrossRef]
- Hoheisel, J.D.; Simbolo, M.; Gottardi, M.; Corbo, V.; Fassan, M.; Mafficini, A.; Malpeli, G.; Lawlor, R.T.; Scarpa, A. DNA Qualification Workflow for Next Generation Sequencing of Histopathological Samples. PLoS ONE 2013, 8, e62692. [Google Scholar] [CrossRef]
- Fanale, D.; Iovanna, J.L.; Calvo, E.L.; Berthezene, P.; Belleau, P.; Dagorn, J.C.; Bronte, G.; Cicero, G.; Bazan, V.; Rolfo, C.; et al. Germline copy number variation in theYTHDC2gene: Does it have a role in finding a novel potential molecular target involved in pancreatic adenocarcinoma susceptibility? Expert Opin. Ther. Targets 2014, 18, 841–850. [Google Scholar] [CrossRef]
- Szabo, C.; Masiello, A.; Ryan, J.F.; Brody, L.C. The Breast Cancer Information Core: Database design, structure and scope. Hum. Mutat. 2000, 16, 123–131. [Google Scholar] [CrossRef]
- Den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.-F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E.M. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [PubMed]
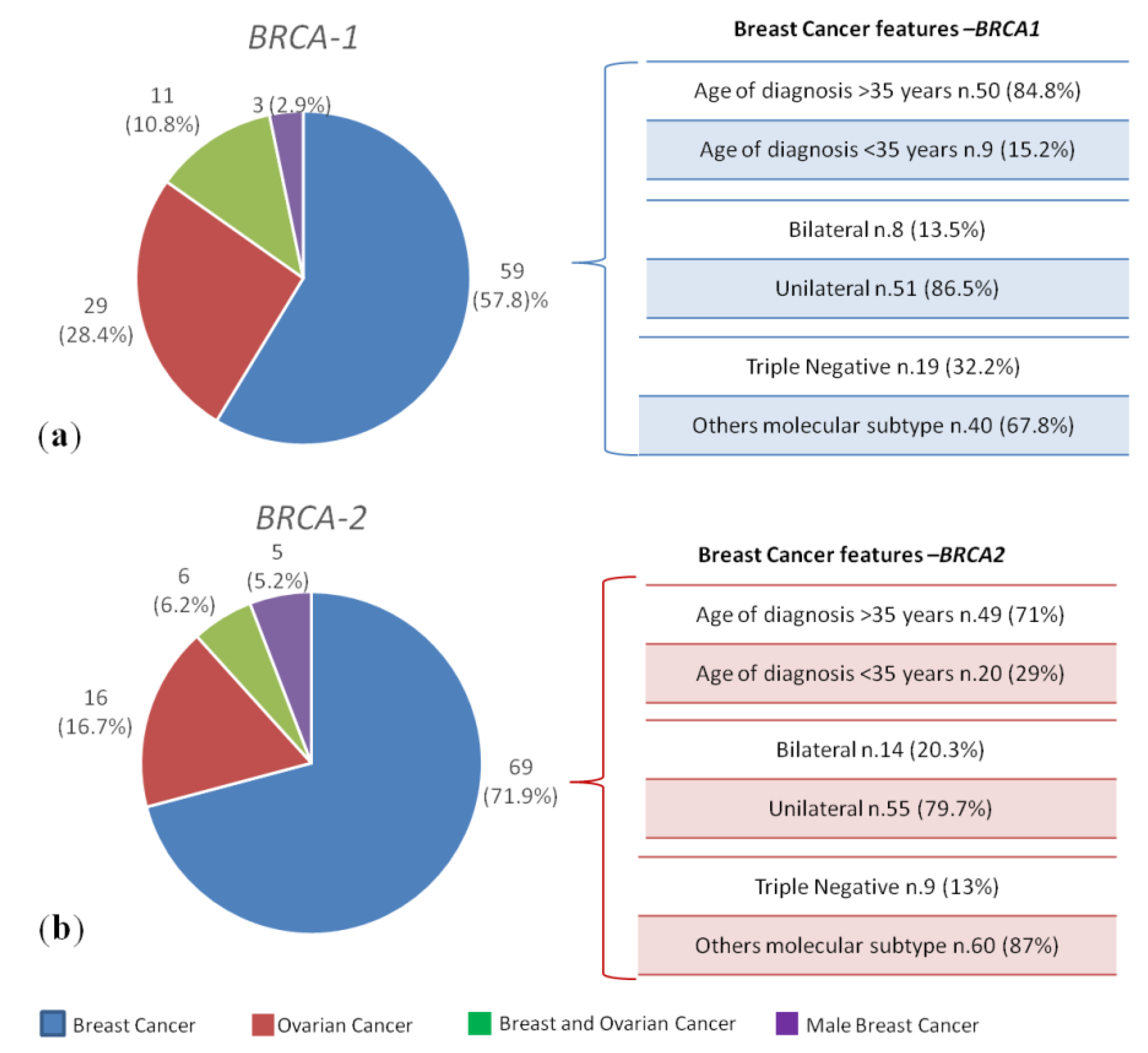


| BRCA1/2 PVs Positive/Total HBOC n = 200/1346 (14.8%) | BRCA1 PVs Positive n = 102 (51%) | BRCA2 PVs Positive n = 96 (48%) | DH BRCA1- BRCA2 PVs n = 2 (1%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of cancer | Breast cancer n = 59 (57.8%) | Ovarian cancer n = 29 (28.4%) | Breast and ovarian cancers n = 11 (10.8%) | Male breast cancer n = 3 (2.9%) | Breast cancer n = 69 (71.9%) | Ovarian cancer n = 16 (16.7%) | Breast and ovarian cancers n = 6 (6.2%) | Male breast cancer n = 5 (5.2%) | Bilateral Breast cancer n = 2 (100%) |
| Age at diagnosis (yr), median (range) | 41 (28–62) | 52 (39–69) | BC 48 (36–77) | 60 (67–73) | 41 (25–80) | 59 (40–81) | BC 50 (39–78) | 67 (62–68) | Patient 1 37,41 |
| OC 53 (39–78) | OC 57 (41–79) | Patient 2 54,54 | |||||||
| Family history Yes, n = 53 (26.5%) No, n = 147 (73.5%) | 20 (33.8%) 39 (66.2%) | 7 (24.1%) 22 (75.9%) | 2 (18%) 9 (82%) | 0 (0%) 3 (100%) | 16 (23.2%) 53 (76.8%) | 4 (25%) 12 (75%) | 2 (33.3%) 4 (66.7%) | 0 (0%) 5 (100%) | 2 (100%) 0 (0%) |
| BRCA1/2 PVs Positive n = 485/1967 (24.6%). | BRCA1 PVs Positive n = 259/485 (53.4%) | BRCA2 PVs Positive n = 222/485 (45.8%) | DH BRCA1-2 PVs n = 4/485 (0.8%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No evidence of cancer n.244 | 133 (51.3%) | 109 (49.1%) | 2 (50%) | ||||||
| Lack of information n.18 | 10 (3.9%) | 8 (3.6%) | -- | ||||||
| Diagnosis of cancer n.223 | 116 (44.8%) | 105 (47.3%) | 2 (50%) | ||||||
| Type of cancer | BC 71 (61.2%) | OC 30 (25.9%) | BC and OC 12(10.3%) | MBC 3 (2.6%) | BC 78 (74.2%) | OC 17 (16.2%) | BC and OC 5(4.8%) | MBC 5 (4.8%) | BC 2 (100%) |
| BIC NOMENCLATURE | HGVS NOMENCLATURE | TYPE OF BRCA1 PV | BRCA1 PV CARRIERS (Patients and Family Members) | BRCA1 PV Cancer Patients | ALLELE FREQUENCY (ExAC */GnomAD **) | ALLELE FREQUENCY (Sicilian Population) | GEOGRAPHICAL AREA |
|---|---|---|---|---|---|---|---|
| 5083del19 (S551fs) | c.4964_4982del (p.Ser1655fs) | Deletion | 63 (13.0%) | 25 (39.68%) | ExAC 0.000008 | 0.00928 | Palermo and Agrigento |
| 633delC (Q172fs) | c.514del (p.Gln172fs) | Deletion | 38 (7.83%) | 15 (39.47%) | ExAC 0.000008 GnomAD 0.000004 | 0.00557 | Palermo and Messina |
| 1479delAG | c.1356_1357AG [2] (p.Glu453_Ser454insTer) | Deletion | 14 (2.87%) | 6 (42.86%) | ExAC 0.000016 GnomAD 0.000008 | 0.00222 | Deletion |
| 4446C > T (R1443X) | c.4327C > T (p.Arg1443Ter) | SNV | 12 (2.48%) | 6 (50.0%) | - | 0.00222 | Palermo and Trapani |
| 2841G > T (E908X) | c.2722G > T (p.Glu908Ter) | SNV | 11 (2.28%) | 4 (36.36%) | - | 0.00148 | Palermo, Agrigento, Caltanissetta and Enna |
| 5382insC | c.5266dupC (p.Gln1756Profs) | Duplication | 11 (2.28%) | 6 (54.54%) | ExAC 0.000156 GnomAD 0.00018 | 0.00222 | Messina |
| 5149del4 (T1677fs) | c.5030_5033del (p.Thr1677fs) | Deletion | 11 (2.28%) | 2 (18.18%) | - | 0.00074 | Caltanissetta and Palermo |
| 3347delAG | c.3226_3227AG [1] (p.Gly1077fs) | Deletion | 10 (2.07%) | 4 (40.0%) | GnomAD 0.000004 | 0.00148 | Palermo |
| 916delTT (S220fs) | c.798_799del (p.Ser267fs) | Deletion | 10 (2.07%) | 5 (50.0%) | - | 0.00185 | Palermo |
| 4023G > T (E1302X) | c.3904G > T (p.Glu1302Ter) | SNV | 8 (1.66%) | 6 (75.0%) | - | 0.00222 | Caltanissetta |
| 184T > C (L22S) | c.65T > C (p.Leu22Ser) | SNV | 8 (1.66%) | 2 (25.0%) | - | 0.00074 | Palermo |
| 300T > G (C61G)*** | c.181T > G (p.Cys61Gly)*** | SNV | 8 (1.66%) | 2 (25.0%) | ExAC 0.000067 GnomAD 0.000032 | 0.00074 | Ragusa and Palermo |
| 4843delC (P1596fs) | c.4583del (p.Pro1528fs) | Deletion | 7 (1.45%) | 2 (28.57%) | - | 0.00074 | Palermo |
| 3519G > T (E1134X) | c.3400G > T (p.Glu1134Ter) | SNV | 5 (1.03%) | 2 (40.0%) | GnomAD 0.000004 | 0.00074 | Palermo |
| 3372insA | c.3253dupA (p.Arg1085Lysfs) | Duplication | 5 (1.03%) | 1 (20.0%) | - | 0.00037 | Catania |
| BIC NOMENCLATURE | HGVS NOMENCLATURE | TYPE OF BRCA2 PV | BRCA2 PV CARRIERS (Patients and Family Members) | BRCA2 PV Cancer Patients | Allele Frequency (ExAC */GnomAD **) | Allele Frequency (Sicilian Population) | GEOGRAPHICAL AREA |
|---|---|---|---|---|---|---|---|
| 1466delT (L413fs) | c.1238del (p.Leu413fs) | Deletion | 49 (10.10%) | 20 (40.82%) | GnomAD 0.000004 | 0.00742 | Palermo |
| 6079del4 (S1951fs) | c.5851_5854del (p.Ser1951fs) | Deletion | 17 (3.50%) | 8 (47.06%) | - | 0.00297 | Trapani and Palermo |
| 6310del5 (E2028fs) | c.6082_6086del (p.Glu2028fs) | Deletion | 16 (3.30%) | 5 (31.25%) | ExAC 0.000008 GnomAD 0.000004 | 0.00185 | Trapani |
| IVS19 + 1G > A | c.8487 + 1G > A | SNV | 10 (2.07%) | 4 (40.0%) | - | 0.00148 | Messina, Palermo and Caltanissetta |
| 9326insA | c.9098_9099insA (p.Gln3034fs) | Duplication | 9 (1.85%) | 2 (22.22%) | - | 0.00074 | Palermo and Agrigento |
| L2865X | c.8594T > A (p.Leu2865Ter) | SNV | 8 (1.66%) | 3 (37.5%) | - | 0.00111 | Palermo |
| V211I | c.631G > A (p.Val211Ile) | SNV | 8 (1.66%) | 4 (50.0%) | - | 0.00148 | Agrigento, Siracusa and Ragusa |
| 6714del4 | c.6482_6485ACAA [1] (p.Lys2162fs) | Deletion | 6 (1.24%) | 1 (16.66%) | ExAC 0.000017 GnomAD 0.000009 | 0.00037 | Messina |
| 9254del5 (Y3009fs) | c.9026_9030del (p.Tyr3009fs) | Deletion | 6 (1.24%) | 3 (50.0%) | GnomAD 0.000004 | 0.00111 | Palermo |
| 6265A > T (K2013X) | c.6037A > T (p.Lys2013Ter) | SNV | 5 (1.03%) | 1 (20.0%) | GnomAD 0.000004 | 0.00037 | Erice |
| Q2561X | c.7681C > T (p.Gln2561Ter) | SNV | 5 (1.03%) | 1 (20.0%) | - | 0.00037 | Palermo and Messina |
| 3036del4 (A938fs) | c.2808_2811del (p.Ala938Profs) | Deletion | 5 (1.03%) | 2 (40.0%) | ExAC 0.000017 GnomAD 0.000008 | 0.00074 | Caltanissetta |
| 4512insT | c.4284dup (p.Gln1429fs) | Duplication | 4 (0.82%) | 3 (75.0%) | GnomAD 0.000004 | 0.00111 | Palermo |
| 2070insT | c.1842dupT (p.Asn615Terfs) | Duplication | 4 (0.82%) | 1 (25.0%) | - | 0.00037 | Agrigento |
| IVS21 + 4A > G | c.8754 + 4A > G | SNV | 4 (0.82%) | 2 (50.0%) | - | 0.00074 | Catania |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Incorvaia, L.; Fanale, D.; Badalamenti, G.; Bono, M.; Calò, V.; Cancelliere, D.; Castiglia, M.; Fiorino, A.; Pivetti, A.; Barraco, N.; et al. Hereditary Breast and Ovarian Cancer in Families from Southern Italy (Sicily)—Prevalence and Geographic Distribution of Pathogenic Variants in BRCA1/2 Genes. Cancers 2020, 12, 1158. https://doi.org/10.3390/cancers12051158
Incorvaia L, Fanale D, Badalamenti G, Bono M, Calò V, Cancelliere D, Castiglia M, Fiorino A, Pivetti A, Barraco N, et al. Hereditary Breast and Ovarian Cancer in Families from Southern Italy (Sicily)—Prevalence and Geographic Distribution of Pathogenic Variants in BRCA1/2 Genes. Cancers. 2020; 12(5):1158. https://doi.org/10.3390/cancers12051158
Chicago/Turabian StyleIncorvaia, Lorena, Daniele Fanale, Giuseppe Badalamenti, Marco Bono, Valentina Calò, Daniela Cancelliere, Marta Castiglia, Alessia Fiorino, Alessia Pivetti, Nadia Barraco, and et al. 2020. "Hereditary Breast and Ovarian Cancer in Families from Southern Italy (Sicily)—Prevalence and Geographic Distribution of Pathogenic Variants in BRCA1/2 Genes" Cancers 12, no. 5: 1158. https://doi.org/10.3390/cancers12051158
APA StyleIncorvaia, L., Fanale, D., Badalamenti, G., Bono, M., Calò, V., Cancelliere, D., Castiglia, M., Fiorino, A., Pivetti, A., Barraco, N., Cutaia, S., Russo, A., & Bazan, V. (2020). Hereditary Breast and Ovarian Cancer in Families from Southern Italy (Sicily)—Prevalence and Geographic Distribution of Pathogenic Variants in BRCA1/2 Genes. Cancers, 12(5), 1158. https://doi.org/10.3390/cancers12051158








