Colorectal Cancer and Probiotics: Are Bugs Really Drugs? †
Abstract
1. Introduction
1.1. Epidemiology
1.2. Colon Cancer
1.2.1. Risk Factors and Potential Causes of Colon Cancer Carcinogenesis
Bacteria
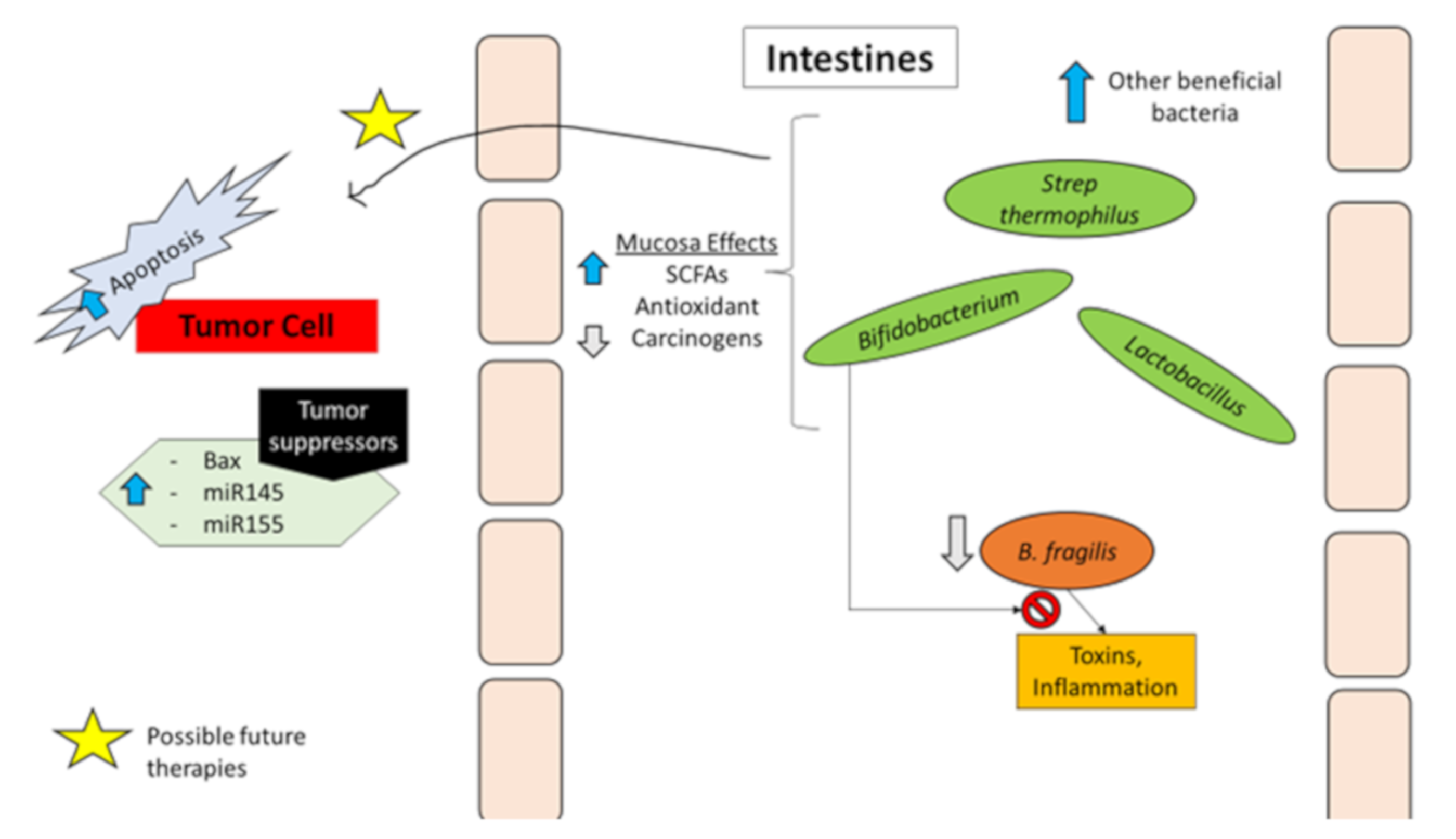
Bacteria and Protection against Colorectal Cancer
Genetic Factors
Patient Specific Risk Factors
| Bacterial Associations with CRC | |||
|---|---|---|---|
| Strain | Model/Samples | Key Findings/Associations | Possible Mechanism(s) |
| Bacteroides fragilis | Patient samples: Tissue [9], Stool [10] Murine model of microbe-induced colon tumorigenesis [12] | Increased abundance of ETBF in early-stage lesions [9] and stool samples [10] Bacteroides fragilis toxin (BFT) mediated increase in IL-17 dependent NF-κB activation, chemokines production and myeloid cell accumulation [12] | BFT mediated tumorigenesis of colonic epithelial cells through mechanisms dependent on STAT3 activation, and IL-17 signaling mediated NF-κB activation, production of C-X-C chemokines, and recruitment of CXCR2-expressing myeloid cells [12] |
| Streptococcus bovis/gallolyticus | CRC tissues from patients with or without bacteremia [17] In vitro with human colonic epithelial cell line Caco-2; and rat model of azoxymethane-induced colon carcinogenesis [15] | Concomitant colorectal tumors present in about 25 to 80% of patients with S. bovis/gallolyticus bacteremia [14] S. gallolyticus isolated from 20.5% and 17.3% tumorous and non-tumorous tissues, respectively, from CRC patients with bacteremia compared to 12.8% and 11.5%, respectively, of CRC patients without bacteremia [17] Increased expression of IL-1, IL-8, and COX-2 in tissues from S. gallolyticus- positive CRC patients compared to the bacteria negative and control samples [17] S. bovis wall extracted antigens (WEA) increased release of CXC chemokines and PGE2 and increased aberrant crypt formation in vivo. In vitro, WEA increased IL-8 and PGE2 release as well as increased COX-2 expression and MAPK activation in Caco-2 cells [15] | Increased MAPK activation, bacterial dysbiosis, and overall increased inflammatory responses [14] S. bovis WEA promoted formation of pre-neoplastic lesions through mechanisms dependent on increased release of IL-8 and PGE2, increased expression of COX-2, and increased activation of MAPK signals [15] |
| Fusobacterium nucleatum | Stool samples from patients and healthy controls [16] In vitro cell line (HCT116), xenograft mouse model, and human colon specimens [18] Human NK cells, tumor-infiltrating lymphocytes (TILs), various human cancer cell lines, and colon carcinoma tissues [48] | Higher levels of F. nucleatum detected in patients with adenoma and CRC compared to healthy controls [16] Increased FadA (virulence and attachment factor of F. nucleatum) gene expression detected in colon specimens of patients with precancerous adenomas or CRC. FadA increased CRC cell proliferation. [18] Decreased NK cell killing of tumor cells and decreased activity of TILs upon Fap2 (virulence factor of F. nucleatum) binding to TIGIT [48] | FadA promoted the E-cadherin/beta-catenin-mediated proliferation of CRC cells in vitro and E-cadherin-mediated growth of CRC and expression of pro-inflammatory cytokines in vivo [18] Immune evasion mediated by inhibition of NK and T-cell anti-tumor activity upon Fap2 binding to the inhibitory receptor TIGIT [48] |
| Enterococcus faecalis | Retroscopic study of patients with Enterococcus faecalis infective endocarditis [49] | 50.8% of patients with unknown source of E faecalis infective endocarditis were diagnosed with colorectal neoplasia upon colonoscopy [49] | |
| Escherichia coli | In vitro infection of murine enterocytes and colon carcinoma cell lines with E. coli [50] Human intestinal organoids exposed to polyketide-peptide genotoxin (Colibactin) expressing E. coli over a period of 5 months [51] Human monocytic THP-1 cell line differentiated into macrophages and infected with colon cancer-associated E. coli strain [52] | Infection of cells with polyketide-peptide genotoxin (Colibactin) expressing E. coli led to a significant increase in frequency of gene mutation and anchorage-independent colony formation [50] Exposure of intestinal organoids to colibactin-producing E. coli led to mutational signature which is similar to mutational structure found in two independent CRC cohorts [51] Survival of cancer-associated E. coli intracellularly in macrophages led to persistent increase in COX-2 expression [52] | Polyketide-peptide genotoxin-induced DNA double stranded breaks, incomplete DNA repair, and induced aneuploidy and tetraploidy [50] Colibactin dependent mutations likely through alkylation of DNA on adenine residues and subsequent double stranded DNA breaks [51] Infection by cancer-associated E coli. Increased COX-2 expression by macrophages in a p38 MAPK dependent manner [52] |
| Bacterial Associations with Protection from CRC | |||
|---|---|---|---|
| Strain | Model/Samples | Key Findings/Associations | Possible Mechanism(s) |
| Bifidobacterium longum | Feces from healthy persons taking or not taking B. longum and fructo-oligosaccharides (FOS); Human colon cancer cell lines [22] B. longum administration in colitis-induced murine model of CRC [23] Rat model of 2-Amino-3-methylimidazo[4,5-f]quinolone (IQ) induced colon cancer [24] | Increased amounts of short-chain fatty acids (SCFAs) and decreased Bacteroides fragilis enterotoxin in feces of individuals taking B. longum and FOS. In vitro, SCFAs, such as butyric acid, isobutyric acid, and acetic acid, had growth inhibitory activity against colon cancer cell lines [22] B. longum administration increased expression of tumor suppressor micro-RNAs (miRs) miR-145 and miR-155, decreased expression of miR-146a (regulator of interleukins 1β and 6), decreased nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) activation and resulted in decreased aberrant crypt foci numbers [23] Dietary supplementation with B. longum led to 100% inhibition of IQ-induced incidence of CRC [24] | Ingesting B. longum with FOS leads to decreased B. fragilis enterotoxin, increased production of SCFAs, and subsequent inhibition of colorectal carcinogenesis and cancer cell growth [22] Decreased expression of oncogenic miRNAs and increased expression of tumor suppressor miRNAs [23] |
| Lactobacillus | Rat model of 1,2-dimethylhydrazine (DMH)-induced precancerous growths in colon [53] Murine model of azoxymethane (AOM)-induced colon cancer [54] Rat model of 1, 2-dimethylhydrazine (DMH)-induced CRC [55] Rat model of 1,2-dimethyl hydrazine (DMH)-induced CRC [56] | Lactobacillus acidophilus administration decreased aberrant crypts formation in colon [53] Lactobacillus acidophilus decreased incidence of colonic lesions by about 57% (compared to 27% by Bifidobacterium bifidum) [54] Lactobacillus salivarius Ren treatment led to 40% decrease in aberrant crypt foci formation [55] Lactobacillus salivarius Ren treatment led to significant decrease in cancer incidence compared to controls (from 87.5% to 25%). Administration of Lactobacillus salivarius Ren reduced Ruminococcus sp, Clostridiales, and Bacteroides dorei, and increased Prevotella [56] | Lactobacillus acidophilus administration decreased number of E. coli in feces, decreased activities of DMH metabolizing enzymes β-glucosidase and β-glucuronidase, and decreased plasma triglyceride concentration [53] Lactobacillus acidophilus administration significantly increased number of CD4+ and CD8+ T-cells [54] Lactobacillus salivarius Ren treatment increased SCFA levels and decreased azoreductase activity [55] |
| Bacteroides fragilis | Murine model of azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colitis-associated CRC [13] Human CRC cell lines in vitro [57] | B. fragilis colonization decreased DSS-induced inflammation and colitis, and decreased size and numbers of AOM/DSS-induced colitis-associated CRC tumors [13] B. fragilis Polysaccharide A (PSA), in TLR2 dependent manner, inhibited proliferation of CRC cells by suppressing cell cycle progression (downregulation of CCND1 and CDK2, upregulation of CDKN1B). PSA suppressed EMT and decreased migration and invasion of CRC cells in vitro [57] | Protection against CRC was dependent on B. fragilis polysaccharide A production and toll-like receptor 2 (TLR2) signaling and associated with inhibition of C-C motif chemokine receptor5 (CCR5) in colon [13] Inhibition of cell cycle progression and inhibition of EMT and cancer cell migration and invasion in TLR2 dependent manner [57] |
| Clostridium | Murine model of 1,2-dimethylhydrazine dihydrochloride (DMH)-induced CRC and human colon cancer cell lines [58] Murine model of high-fat diet (HFD)-induced intestinal tumor [59] | Clostridium butyricum decreased DMH-induced colon cancer incidence from 90% to 30% [58] Oral administration of Clostridium butyricum led to significant decrease in numbers of HFD-induced intestinal tumors [59] | Clostridium butyricum inhibited proliferation of colorectal cancer cells, increased cell-cycle arrest and apoptosis of colon cancer cells, and modulated T-cells [58] Clostridium butyricum increased SCFAs and G-protein coupled receptor GPR43, suppressed tumor cell proliferation, increased tumor-cell apoptosis, and suppressed the Wnt/β-catenin signaling pathway [59] |
1.2.2. Therapeutic Targets in Colon Cancer
Cytokines
Prostaglandin G/H Synthase
Proto-Oncogene RAF
Vascular Endothelial Growth Factor (VEGF)
Fibronectin
1.2.3. Current Treatment and Its Limitations
1.3. Probiotics
1.3.1. History and Rationale behind the Use of Probiotics in Cancer
1.3.2. Types or Strains Used
1.3.3. Current Products on Market
1.3.4. Clinical Trials
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society. Key Statistics for Colorectal Cancer. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html (accessed on 27 April 2020).
- NIH. Cancer Stat Facts: Colorectal Cancer. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 1 December 2019).
- CDC. Colorectal Cancer Statistics. 28 May 2019. Available online: https://www.cdc.gov/cancer/colorectal/statistics/index.htm (accessed on 1 December 2019).
- American Cancer Society. Colorectal Cancer Facts and Figures 2017–2019. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf (accessed on 1 December 2019).
- World Health Organization. Cancers Fact Sheets: Colorectal Cancer. Available online: http://gco.iarc.fr/today/data/pdf/fact-sheets/cancers/cancer-fact-sheets-6.pdf (accessed on 1 December 2019).
- Venook, A. Right-Sided vs Left-Sided Colorectal Cancer. Clin. Adv. Hematol. Oncol. 2017, 15, 22–24. [Google Scholar]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Fang, L.; Lee, M.H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Purcell, R.V.; Pearson, J.; Aitchison, A.; Dixon, L.; Frizelle, F.A.; Keenan, J.I. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 2017, 12, e0171602. [Google Scholar] [CrossRef] [PubMed]
- Toprak, N.U.; Yagci, A.; Gulluoglu, B.M.; Akin, M.L.; Demirkalem, P.; Celenk, T.; Soyletir, G. A possible role of Bacteroides fragilis enterotoxin in the etiology of colorectal cancer. Clin. Microbiol. Infect. 2006, 12, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C.; et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Chung, L.; Thiele Orberg, E.; Geis, A.L.; Chan, J.L.; Fu, K.; DeStefano Shields, C.E.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 2018, 23, 203–214. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mehrabian, P.; Boyajian, S.; Wu, W.L.; Selicha, J.; Vonderfecht, S.; Mazmanian, S.K. The Protective Role of Bacteroides fragilis in a Murine Model of Colitis-Associated Colorectal Cancer. mSphere 2018, 3, e00587-18. [Google Scholar] [CrossRef]
- Abdulamir, A.S.; Hafidh, R.R.; Abu Bakar, F. The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role. J. Exp. Clin. Cancer Res. 2011, 30, 11. [Google Scholar] [CrossRef]
- Biarc, J.; Nguyen, I.S.; Pini, A.; Gossé, F.; Richert, S.; Thiersé, D.; Van Dorsselaer, A.; Leize-Wagner, E.; Raul, F.; Klein, J.P.; et al. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis). Carcinogenesis 2004, 25, 1477–1484. [Google Scholar] [CrossRef]
- Suehiro, Y.; Sakai, K.; Nishioka, M.; Hashimoto, S.; Takami, T.; Higaki, S.; Shindo, Y.; Hazama, S.; Oka, M.; Nagano, H.; et al. Highly sensitive stool DNA testing of Fusobacterium nucleatum as a marker for detection of colorectal tumors in a Japanese population. Ann. Clin. Biochem. 2017, 54, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Rubinstei, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogesis by modulating E-cadherin/β-catenin signaling via its FadA adhesion. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Cur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Shang, F.M.; Liu, H.L. Fusobacterium nucleatum and colorectal cancer: A review. World J. Gastrointest. Oncol. 2018, 10, 71–81. [Google Scholar] [CrossRef]
- Kelly, D.; Yang, L.; Pei, Z. Gut Microbiota, Fusobacteria, and Colorectal Cancer. Diseases 2018, 6, 109. [Google Scholar] [CrossRef]
- Dahmus, J.D.; Kotler, D.L.; Kastenberg, D.M.; Kistler, C.A. The gut microbiome and colorectal cancer: A review of bacterial pathogenesis. J. Gastrointest. Oncol. 2018, 9, 769–777. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 2014, 20, 7878–7886. [Google Scholar] [CrossRef]
- Wei, H.; Chen, L.; Lian, G.; Yang, J.; Li, F.; Zou, Y.; Lu, F.; Yin, Y. Antitumor Mechanisms of Bifidobacteria. Oncol. Lett. 2018, 16, 3–8. [Google Scholar] [CrossRef]
- Ohara, T.; Suzutani, T. Intake of Bifidobacterium longum and Fructo-oligosaccharides prevents Colorectal Carcinogenesis. Euroasian. J. Hepatogastroenterol. 2018, 8, 11–17. [Google Scholar] [CrossRef]
- Fahmy, C.A.; Gamal-Eldeen, A.M.; El-Hussieny, E.A.; Raafat, B.M.; Mehanna, N.S.; Talaat, R.M.; Shaaban, M.T. Bifidobacterium longum Suppresses Murine Colorectal Cancer through the Modulation of oncomiRs and Tumor Suppressor miRNAs. Nutr. Cancer 2019, 71, 688–700. [Google Scholar] [CrossRef]
- Reddy, B.S.; Rivenson, A. Inhibitory effect of Bifidobacterium longum on colon, mammary, and liver carcinogenesis induced by 2-amino-3-methylimidazo[4,5-f]quinoline, a food mutagen. Cancer Res. 1993, 53, 3914–3918. [Google Scholar] [PubMed]
- Hendler, R.; Zhang, Y. Probiotics in the Treatment of Colorectal Cancer. Medicines 2018, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Vetizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Amersi, F.; Agustin, M.; Ko, C.Y. Colorectal Cancer: Epidemiology, Risk Factors, and Health Services. Clin. Colon Rectal Surg. 2005, 18, 133–140. [Google Scholar] [CrossRef]
- Valle, L. Genetic predisposition to colorectal cancer: Where we stand and future perspectives. World J. Gastroenterol. 2014, 20, 9828–9849. [Google Scholar] [CrossRef]
- Haggar, F.A.; Boushey, R.P. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef]
- Ahsan, H.; Neugut, A.I.; Garbowski, G.C.; Jacobson, J.S.; Forde, K.A.; Treat, M.R.; Waye, J.D. Family history of colorectal adenomatous polyps and increased risk for colorectal cancer. Ann. Int. Med. 1998, 128, 900–905. [Google Scholar] [CrossRef]
- Cancer.net. Lynch Syndrome. Available online: https://www.cancer.net/cancer-types/lynch-syndrome (accessed on 27 April 2020).
- Jang, E.; Chung, D.C. Hereditary Colon Cancer: Lynch Syndrome. Gut Liver 2010, 4, 151–160. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Lynch Syndrome. Available online: https://www.cdc.gov/genomics/disease/colorectal_cancer/lynch.htm (accessed on 4 November 2019).
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and Familial Colon Cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef]
- Hopkins Medicine. Familial Adenomatous Polyposis. Available online: https://www.hopkinsmedicine.org/gastroenterology_hepatology/_pdfs/small_large_intestine/familial_adenomatous_polyposis.pdf (accessed on 4 November 2019).
- NIH. APC Gene. Available online: https://ghr.nlm.nih.gov/gene/APC (accessed on 4 November 2019).
- Yang, J.; Zhang, W.; Evans, P.M.; Chen, X.; He, X.; Liu, C. Adenomatous Polyposis Coli (APC) Differentially Regulates β-Catenin Phosphorylation and Ubiquitination in Colon Cancer Cells. J. Biol. Chem. 2006, 281, 17751–17757. [Google Scholar] [CrossRef] [PubMed]
- Anaya, D.A.; Chang, G.J.; Rodriguez-Bigas, M.A. Extracolonic manifestations of hereditary colorectal cancer syndromes. Clin Colon Rectal Surg. 2008, 21, 263–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Centers for Disease Control and Prevention. Colorectal (Colon) Cancer. Available online: https://www.cdc.gov/cancer/colorectal/basic_info/screening/index.htm (accessed on 7 November 2019).
- Larsson, S.C.; Wolk, A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. Int. J. Cancer 2006, 119, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Diet and cancer: An evolving picture. JAMA 2005, 293, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Lee, K.-J.; Inoue, M.; Otani, T.; Iwasaki, M.; Sasazuki, S.; Tsugane, S.; JPHC Study Group. Physical activity and risk of colorectal cancer in Japanese men and women: The Japan Public Health Center-based prospective Study. Cancer Causes Control 2007, 19, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Poschl, G.; Seitz, H.K. Alcohol and Cancer. Alcohol Alcoholism 2004, 39, 155–165. [Google Scholar] [CrossRef]
- Abdulamir, A.S.; Hafidh, R.R.; Bakar, F.A. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: Inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer 2010, 9, 249. [Google Scholar] [CrossRef]
- Pericas, J.M.; Corredoira, J.; Moreno, A.; García-País, M.J.; Falces, C.; Rabuñal, R.; Mestres, C.A.; Alonso, M.P.; Marco, F.; Quintana, E.; et al. Relationship Between Enterococcus faecalis Infective Endocarditis and Colorectal Neoplasm: Preliminary Results from a Cohort of 154 Patients. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 451–458. [Google Scholar] [CrossRef]
- Cuevas-Ramos, G.; Petit, C.R.; Marcq, I.; Boury, M.; Oswald, E.; Nougayrède, J.P. Escheria coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Aca. Sci. USA 2010, 107, 11437–11542. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Huber, A.R.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E.coli. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Raisch, J.; Rolhion, N.; Dubois, A.; Darfeuille-Michaud, A.; Bringer, M.A. Intracellular colon cancer-associated Escherichia coli promote protumoral activities of human macrophages by inducing sustained COX-2 expression. Lab. Investig. 2015, 95, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Shim, C.; Cha, S.K.; Reaney, M.; Mahn Chee, K. Effect of Lactobacillus acidophilus KFRI34 on the development of chemically induced precancerous growths in the rat colon. J. Med. Microbiol. 2012, 61, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Agah, S.; Alizadeh, A.M.; Mosavi, M.; Ranji, P.; Khavari-Daneshvar, H.; Ghasemian, F.; Bahmani, S.; Tavassoli, A. More Protection of Lactobacillus acidophilus Than Bifidobacterium bifidum Probiotics on Azoxymethane-Induced Mouse Colon Cancer. Probiotics Antimicrob. Protein. 2019, 11, 857–864. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, C.; Ge, S.; Zhang, M.; Jiang, L.; Cui, J.; Ren, F. Lactobacillus salivarius Ren prevent the early colorectal carcinogenesis in 1,2-dimethylhydrazine-induced rate model. J. Appl. Microbiol. 2014, 117, 208–216. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, X.; Fang, B.; Zhu, C.; Zhu, J.; Ren, F. Effects of Lactobacillus salivarius Ren on cancer prevention and intestinal microbiota in 1, 2-dimethylhydrazine-induced rat model. J. Microbiol. 2015, 53, 398–405. [Google Scholar] [CrossRef]
- Sittipo, P.; Lobionda, S.; Choi, K.; Sari, I.N.; Kwon, H.Y.; Lee, Y.K. Toll-Like Receptor 2-Mediated Suppression of Colorectal Cancer Pathogenesis by Polysaccharide A from Bacteroides fragilis. Front. Microbiol. 2018, 9, 1588. [Google Scholar] [CrossRef]
- Chen, Z.F.; Al, L.Y.; Wang, J.L.; Ren, L.L.; Yu, Y.N.; Xu, J.; Chen, H.Y.; Yu, J.; Li, M.; Qin, W.X.; et al. Probiotics Clostridium butyridium and Bacillus subtilis ameliorate intestinal tumorigenesis. Future Microbiol. 2015, 10, 1433–1445. [Google Scholar] [CrossRef]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal humor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Xie, Z.; Qu, Y.; Leng, Y.; Sun, W.; Ma, S.; Wei, J.; Hu, J.; Zhang, X. Human Colon Carcinogenesis is Associated with Increased Interleukin-17 Driven Inflammatory Responses. Drug Des. Devel. Ther. 2015, 9, 1679–1689. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C.; Vallance, B.A.; Krogfelt, K.A.; Petersen, A.M. Escherichia coli pathobionts associated with inflammatory bowel disease. Clin. Microbiol. Rev. 2019, 32, e00060-18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jobin, C. Microbial imbalance and intestinal pathologies: Connections and contributions. Dis. Model Mech. 2014, 7, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Langowski, J.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumor incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defenses: Homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genomics Proteomics Bioinforma. 2018, 16, 33–49. [Google Scholar] [CrossRef]
- Bellam, N.; Pasche, B. Tgf-beta signaling alterations and colon cancer. Cancer Treats Res. 2010, 155, 85–103. [Google Scholar] [CrossRef]
- Villalba, M.; Evans, S.R.; Vidal-Vanaclocha, F.; Calvo, A. Role of TGF-β in metastatic colon cancer: It is finally time for targeted therapy. Cell Tissue Res. 2017, 370, 29–39. [Google Scholar] [CrossRef]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012, 22, 571–584. [Google Scholar] [CrossRef]
- Bauche, D.; Marie, J.C. Transforming growth factor β: A master regulator of the gut microbiota and immune cell interactions. Clin. Trans. Immunol. 2017, 6, e136. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J. Transforming Growth Factor-beta: A Therapeutic Target for Cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, K.; Jimenez-Martinez, M.; Jimenez-Segovia, A.; Chico-Calero, I.; Conde, E.; Galán-Martínez, J.; Ruiz, J.; Pascual, A.; Barrocal, B.; López-Pérez, R.; et al. Prostaglandins induce early growth response 1 transcription factor mediated microsomal prostaglandin E2 synthase up-regulation for colorectal cancer progression. Oncotarget 2015, 6, 39941–39959. [Google Scholar] [CrossRef] [PubMed]
- Makar, K.W.; Poole, E.M.; Resler, A.J.; Seufert, B.; Curtin, K.; Kleinstein, S.E.; Duggan, D.; Kulmacz, R.J.; Hsu, L.; Whitton, J.; et al. COX-1 (PTGS1) and COX-2 (PTGS2) Polymorphisms, NSAID Interactions, and Risk of Colon and Rectal Cancer in Two Independent Populations. Cancer Causes Control 2013, 24, 2059–2075. [Google Scholar] [CrossRef]
- Sanz-Garcia, E.; Argiles, G.; Tabernero, E.; Tabernero, J. BRAF mutation colorectal cancer: Prognosis, treatment, and new perspectives. Ann. Oncol. 2017, 28, 2648–2657. [Google Scholar] [CrossRef]
- Leicht, D.T.; Balan, V.; Kaplun, A.; Singh-Gupta, V.; Kaplun, L.; Dobson, M.; Tzivion, G. Raf Kinases: Function, Regulation, and Role in Human Cancer. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2007, 1773, 1196–1212. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Cane, G.; Moal, V.L.; Pagès, G.; Servin, A.L.; Hofman, P.; Vouret-Craviari, V. Up-regulation of intestinal vascular endothelial growth factor by Afa/Dr diffusely adhering Escherichia coli. PLoS ONE 2007, 2, e1359. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Fan, F.; Wang, R.; Ye, X.; Xia, L.; Boulbes, D.; Ellis, L.M. Intracrine VEGF Signaling Mediates Colorectal Cancer Cell Migration and Invasion. Br. J. Cancer 2017, 117, 848–855. [Google Scholar] [CrossRef]
- Schirbel, A.; Kessler, S.; Rieder, F.; West, G.; Rebert, N.; Asosingh, K.; McDonald, C.; Fiocchi, C. Pro-angiogenic activity of TLRs and NLRs: A novel link between gut microbiota and intestinal angiogenesis. Gastroenterology 2013, 144, 613–623. [Google Scholar] [CrossRef]
- Yi, W.; Xiao, E.; Ding, R.; Luo, P.; Yang, Y. High Expression of Fibronectin is Associated with Poor Prognosis, Cell Proliferation and Malignancy via the NF-kB/p53-Apoptosis Signaling Pathway in Colorectal Cancer. Oncol. Rep. 2016, 36, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hielscher, A. Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. J. Cancer 2017, 8, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Sherafat, S.; Somayeh, M.; Moghim, S.; Ahmadi Amoli, H.; Ghasemian-Safaei, H. Role of gut microbiota in the pathogenesis of colorectal cancer; a review article. Gastroenterol. Hepatol. Bed Bench 2018, 11, 101–109. [Google Scholar] [PubMed]
- NIH. Cancer Staging. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/staging (accessed on 5 November 2019).
- NCCN Guidelines for Patients. Colon Cancer. 2018. Available online: https://www.nccn.org/patients/guidelines/colon/files/assets/common/downloads/files/colon.pdf (accessed on 5 November 2019).
- Danenberg, P.V.; Malli, H.; Swenson, S. Thymidylate synthase inhibitors. Semin Oncol. 1999, 26, 621–631. [Google Scholar] [PubMed]
- Saif, M.W.; Makrilia, N.; Syrigos, K. CoFactor: Folate requirement for optimization of 5-fluouracil activity in anticancer chemotherapy. J. Oncol. 2010, 2010, 934359. [Google Scholar] [CrossRef]
- Drugs Approved for Colon and Rectal Cancer. National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/colorectal. (accessed on 27 April 2020).
- Syed, Y.Y.; McKeage, K. Aflibercept: A review in metastatic colorectal cancer. Drugs 2015, 75, 1435–1445. [Google Scholar] [CrossRef]
- Jaiswal, P.; Goel, A.; Mittal, R.D. Survivin: A Molecular Biomarker in Cancer. Indian J. Med. Res. 2015, 141, 389–397. [Google Scholar] [CrossRef]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The Relationship Between the Structural Characteristics of Lactobacilli-EPS and Its Ability to Induce Apoptosis in Colon Cancer Cells in vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef]
- Ding, L.; Lan, Z.; Xiong, X.; Ao, H.; Feng, Y.; Gu, H.; Yu, M.; Cui, Q. The Dual Role of MicroRNAs in Colorectal Cancer Progression. Int. J. Mol. Sci. 2018, 19, 2791. [Google Scholar] [CrossRef]
- Masuda, T.; Hayashi, N.; Kuroda, Y.; Ito, S.; Eguchi, H.; Mimori, K. MicroRNAs as Biomarkers in Colorectal Cancer. Cancers 2017, 9, 124. [Google Scholar] [CrossRef]
- Stattin, P.; Lukanova, A.; Biessy, C.; Soderberg, S.; Palmqvist, R.; Kaaks, R.; Olsson, T.; Jellum, E. Obesity and colon cancer: Does leptin provide a link? Int. J. Cancer 2004, 109, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Ranji, P.; Agah, S.; Heydari, Z.; Rahmati-yamchi, M.; Mohammad Alizadeh, A. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the serum biochemical parameters, and the vitamin D and leptin receptor genes on mice colon cancer. Iran. J. Basic Med. Sci. 2019, 22, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Richman, S. Deficient mismatch repair: Read all about it (Review). Int. J. Oncol. 2015, 47, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Bauer, T.M.; Lee, J.J.; Dowlati, A.; Brose, M.S.; Farago, A.F.; Taylor, M.; Shaw, A.T.; Montez, S.; Meric-Bernstam, F.; et al. Larotrectinib in adult patients with solid tumours: A multi-center, open-label, phase I dose-escalation study. Ann. Oncol. 2019, 30, 325–331. [Google Scholar] [CrossRef]
- Stenger, M. Larotrectinib for Solid Tumors with NTRK Gene Fusions. ASCO Post. Available online: https://www.ascopost.com/issues/december-25-2018/larotrectinib-for-solid-tumors-with-ntrk-gene-fusions/ (accessed on 22 November 2019).
- FDA. FDA Approves Larotrectinib for Solid Tumors with NTRK Gene Fusions. 26 November 2018. Available online: https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0 (accessed on 6 December 2019).
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of larotrectinib in trk fusion–positive cancers in adults and children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Edwards, M.S.; Chadda, S.D.; Zhao, Z.; Barber, B.L.; Sykes, D.P. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis. 2012, 14, e31–e47. [Google Scholar] [CrossRef]
- Caffrey, M. New Nccn Crc Guidelines include Updates in Testing, Treatment Options. Targeted Oncology. 25 March 2019. Available online: https://www.targetedonc.com/news/new-nccn-crc-guidelines-include-updates-in-testing-treatment-options (accessed on 6 December 2019).
- Food and Agriculture Organization of the United Nations. Probiotics in Food. Available online: http://www.fao.org/3/a-a0512e.pdf (accessed on 22 April 2020).
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The Infant Microbiome Development: Mom Matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef]
- Rapozo, D.C.M.; Bernardazzi, C.; de Souza, H.S.P. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J. Gastroenterol. 2017, 23, 2124–2140. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The Role of Probiotics in Colorectal Cancer Management. Evid Based Complement Alternat Med. 2020, 2020, 3535982. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e0000145. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, V.K.; Morrow, L.E.; Malesker, M.A. Probiotics: Mechanisms of action and clinical applications. J. Probiotics Health 2013, 1, 2. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 106–174. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The influence of gut microbiota dysbiosis to the efficacy of 5-fluorouracil treatment on colorectal cancer. Sci. Direct. 2018, 108, 184–193. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Healthy Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Walker, A.; Lawley, T. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 2013, 69, 75–86. [Google Scholar] [CrossRef]
- Weir, T.L.; Manter, D.K.; Sheflin, A.M.; Barnett, B.A.; Heuberger, A.L.; Ryan, E.P. Stool Microbiome and Metabolome Differences between Colorectal Cancer Patients and Healthy Adults. PLoS ONE 2013, 8, e70803. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Wu, W.; Qin, H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol. Med. Rep. 2015, 12, 6119–6127. [Google Scholar] [CrossRef]
- Isolauri, E.; Salminen, S.; Ouwehand, A. Probiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 8, 299–313. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, J.; Yao, H.; Hu, H. Fusobacterium and Colorectal Cancer. Front. Oncol. 2018, 8, 371. [Google Scholar] [CrossRef]
- Haghi, F.; Goli, E.; Mirzaei, B.; Zeighami, H. The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer 2019, 19, 879. [Google Scholar] [CrossRef] [PubMed]
- Raisch, J.; Buc, E.; Bonnet, M.; Sauvanet, P.; Vazeille, E.; de Vallée, A.; Déchelotte, P.; Darcha, C.; Pezet, D.; Bonnet, R.; et al. Colon Cancer-Associated B2 Escherichia Coli Colonize Gut Mucosa and Promote Cell Proliferation. World J. Gastroenterol. 2014, 20, 6560–6572. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic Insights into Probiotic Properties of Lactic Acid Bacteria Associated with Ethnic Fermented Dairy Products. Front. Microbiol. 2019, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A. GUMS | Dietary Importance, 2nd ed.; Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 3007–3012. [Google Scholar] [CrossRef]
- McNabney, S.; Henagan, T. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed]
- Daroqui, M.C.; Augenlicht, L.H. Transcriptional attenuation in colon carcinoma cells in response to butyrate. Cancer Prev. Res. (Phila) 2010, 3, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Alao, J. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef]
- Lee, J.H.; Choy, M.L.; Ngo, L.; Foster, S.S.; Marks, P.A. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc. Natl. Acad. Sci. USA 2010, 2010 107, 14639–14644. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and Regulation of the Mucus Barrier in the Gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef]
- Molska, M.; Reguła, J. Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 2019, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.; Lal, S. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed]
- Gosai, V.; Ambalam, P.; Raman, M.; Kothari, C.R.; Kothari, R.K.; Vyas, B.R.; Sheth, N.R. Protective Effect of Lactobacillus rhamnosus 231 Against N-Methyl-N’-Nitro-N-Nitrosoguanidine in Animal Model. Gut Microbes 2011, 2, 319–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Almeida, C.V.; de Camargo, M.R.; Russo, E.; Amedei, A. Role of diet and gut microbiota on colorectal cancer immunomodulation. World J Gastroenterol. 2019, 25, 151–162. [Google Scholar] [CrossRef]
- Azad, A.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Inte. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, O.; Takahashi, T.; Asahara, T.; Nomoto, K.; Onodera, H. Changes of the Intestinal Microbiota, Short Chain Fatty Acids, and Fecal pH in Patients with Colorectal Cancer. Dig. Dis. Sci. 2013, 58, 1717–1726. [Google Scholar] [CrossRef]
- Norouzi, Z.; Salimi, A.; Halabian, R.; Fahimi, H. Nisin, a potent bacteriocin and anti-bacterial peptide, attenuates expression of metastatic genes in colorectal cancer cell lines. Microbial pathogenesis. 2018, 123, 183–189. [Google Scholar] [CrossRef]
- Probiotics. National Institutes of Health. 26 June 2019. Available online: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/ (accessed on 6 December 2019).
- Bourrie, B.; Willing, B.; Cotter, P. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef]
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M. Kefir: A powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 183–189. [Google Scholar] [CrossRef]
- Leedy, R. As Probiotics Use Grows for Gut Health, Vsl#3 Has Designations for Specific Gi Issues Eurekaalert! Available online: https://www.eurekalert.org/pub_releases/2011-11/rla-apu111711.php (accessed on 18 November 2011).
- Appleyard, C.B.; Cruz, M.L.; Isidro, A.A.; Arthur, J.C.; Jobin, C.; De Simone, C. Pretreatment with the probiotic vsl#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am. J. Phyiol. Gastrointest. Liver Physiol. 2011, 301, G1004–G1013. [Google Scholar] [CrossRef]
- Arthur, J.C.; Raad, Z.; Gharaibeh, R.Z.; Uronis, J.M.; Perez-Chanona, E.; Sha, W.; Tomkovich, S.; Mühlbauer, M.; Fodor, A.A.; Jobin, C. VSL#3 Probiotic Modifies Mucosal Microbial Composition but does not Reduce Colitis-Associated Colorectal Cancer. Sci. Rep. 2013, 3, 2868. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, S. Probiotics for gastrointestinal conditions: A summary of the evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar] [PubMed]
- Gossard, C.M.; Pizano, J.M.; Burns, C.M.; Williamson, C.B.; Dolan, K.E.; Finley, H.J.; Gasta, M.G.; Parker, E.C.; Lipski, E.A. Probiotics and Disease: A Comprehensive Summary—Part 9, Cancer. Integr. Med. (Encinitas) 2018, 17, 34–46. [Google Scholar] [PubMed]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in Cancer Immunotherapy. OncoImmunology 2016, 5, e1163462. [Google Scholar] [CrossRef]
- Zaharauddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Gianotti, L.; Morelli, L.; Galbiati, F.; Rocchetti, S.; Coppola, S.; Beneduce, A.; Gilardini, C.; Zonenschain, D.; Nespoli, A.; Braga, M. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J. Gastroenterol. 2010, 16, 167–175. [Google Scholar] [CrossRef]
- Vastra Gotaland Region. Using Probiotics to Reactivate Tumor Suppressor Genes in Colon Cancer. NIH. Available online: https://clinicaltrials.gov/ct2/show/NCT03072641 (accessed on 28 November 2019).
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo-controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362. [Google Scholar] [CrossRef]
- Bong-Hyeon, K. The Effects of Mechnikov Probiotics on Symptom and Surgical Outcome. NIH. Available online: https://clinicaltrials.gov/ct2/show/NCT03531606 (accessed on 3 December 2019).
- Theodorophoulos, G.E.; Memos, N.A.; Peitsidou, K.; Karantanos, T.; Spyropoulos, B.G.; Zografos, G. Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann. Gastroenterol. 2016, 29, 56–62. [Google Scholar]
- First Affiliated Hospital of Harbin Medical University. Gut Mucosal Microbiota is Associated with Colorectal Cancer Relapse. NIH. Available online: https://clinicaltrials.gov/ct2/show/NCT03385213 (accessed on 3 January 2020).
- Friederich, P.; Verschuur, J.; van Heumen, B.W.; Roelofs, H.M.; Berkhout, M.; Nagtegaal, I.D.; van Oijen, M.G.; van Krieken, J.H.; Peters, W.H.; Nagengast, F.M. Effects of intervention with sulindac and inulin/VSL#3 on mucosal and luminal factors in the pouch of patients with familial adenomatous polyposis. Int. J. Colorectal. Dis. 2011, 26, 575–582. [Google Scholar] [CrossRef]
- Cho, J.R.; Yoon, B.; Oh, H.K. Effect of Probiotics on Bowel Function Restoration After Ileostomy Reversal in Patients with Rectal Cancer: A Double-Blind Randomized Controlled Trial. Gastroenterology 2019, 156, S1421. [Google Scholar] [CrossRef]
- Helsinki University. Lactobacillus Rhamnosus in Prevention of Chemotherapy-related Diarrhea. NIH. Available online: https://clinicaltrials.gov/ct2/show/NCT00197873 (accessed on 6 December 2019).
- Zhejiang University. Chemotherapy w/wo WeiLeShu in Metastatic Colorectal Cancer. NIH. Available online: https://clinicaltrials.gov/ct2/show/NCT04021589 (accessed on 6 December 2019).
- AC Camargo Cancer Center. Prebiotics and Probiotics During Definitive Treatment with Chemotherapy-radiotherapy SCC of the Anal Canal (BISQUIT). NIH. Updated November 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03870607 (accessed on 3 January 2020).
- King Hussein Cancer Center. Effect of Probiotics Supplementation on the Side Effects of Radiation Therapy Among Colorectal Cancer Patients. NIH. Updated January 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03742596 (accessed on 3 January 2020).
- Golcic, M. Probiotics as Adjuvant Therapy in the Treatment of Metastatic Colorectal Cancer (Probat-tmccc-17). NIH. Updated October 218. Available online: https://clinicaltrials.gov/ct2/show/NCT03705442 (accessed on 3 January 2020).
| Nonspecific Physiological Mechanisms | Immunological Mechanisms |
|---|---|
| Initiate antiproliferative and apoptotic signaling in colorectal cancer cells | Modulate immune functions in gut mucosa |
| Bolster the intestinal mucosal barrier function | Induce natural killer cells |
| Inhibition of the enzymatic activity of pathogenic bacteria | Helps in immune maturation and maintenance |
| Inhibition of carcinogenic agents | Diversified gut flora positively modulates T-regulatory cells against tumor cells |
| Study Title | NCT # | Status * | Intervention | Study | Inclusion/Exclusion Criteria | Outcomes | Results | Published * |
|---|---|---|---|---|---|---|---|---|
| An Evaluation of Probiotic in the Clinical Course of Patients with Colorectal Cancer [145] | 03782428 | C | Probiotics (Lactobacillus, Bifidobacterium). Placebo | 52 patients Double blinded, randomized Duration: 6 months | >18 years old, non-pregnant/nursing, with CRC planned for resection. No antibiotic or pro/prebiotic use in past 2–4 weeks | Level of circulating inflammatory cytokines. Episodes of chemo-induced diarrhea. | Decrease in inflammatory cytokines (ILs) 4w after surgery. Modified micro-environment | Yes |
| Probiotics in Colorectal Cancer Patients [146] | 00936572 | C | Probiotics (La1, BB536). Placebo given twice a day. Given for 3 days preoperatively | 31 patients Triple blinded, randomized Duration: 1 year | 18–80 years old going for colorectal surgery, able to provide fecal sample after pre-operatively. No immunological disorders. | Microbiology of gut flora and gastrointestinal function | La1 effects intestinal microbiota & decreases pathogenic bacterial concentrations. Little effect from BB536. | Yes |
| Using Probiotics to Reactivate Tumor Suppressor Genes in Colon Cancer [147] | 03072641 | C | ProBion Clinica (Bifidobacterium, Lactobacillus, Inulin) | 20 participants Randomized Duration: 6 years | 1+ malignant tumor in colon. No recent antibiotics or probiotics | Microbiology of gut flora after surgery. Genetic expression after probiotics. | Unknown | No |
| Prevention of Irinotecan Induced Diarrhea by Probiotics [148] | 01410955 | C | Probiotics (Colon Dophilus™). Placebo | 46 patients Quadruple blinded, randomized Duration: 1 year | Life expectancy >3 months with CRC (with irinotecan therapy). No history of ileostomy, no active infections, no antibiotics | Incidence of diarrhea | Decrease in severe diarrhea episodes and decrease of diarrhea episodes. No infections caused by probiotics observed. | Yes |
| The Effects of Metchnikoff Probiotics on Symptom and Surgical Outcome [149] | 03531606 | C | Metchnik-off (Probiotics). Placebo | 68 patients Randomized Duration: 2 years | >20 years old with sigmoid CRC. No metastasis, no preoperative chemo/radiotherapy. No use of pre/probiotics in 7 days | Resection improvement (by questionnaire) | Unknown | No |
| Synbiotics and Gastrointestinal Function Related Quality of Life After Colectomy for Cancer [150] | 01479907 | C | Synbiotics. Placebo Given postoperative. | 100 patients Quadruple blinded, Randomized | CRC non-hereditary, non-metastatic. No history of IBD. Not pregnant patients. | Quality of life related to GI function ‘ | Better quality of life score over 3 months. Less episodes of diarrhea. Non-significant for constipation episodes. | Yes |
| Gut Mucosal Microbiota is Associated with Colorectal Cancer Relapse [151] | 03385213 | C | No intervention post CRC treatment, including surgery | 200 patients Case Control, retrospective Completion: December 2022 | 18–75 years old, normal weight (BMI 18.5–23.9 kg/m2) with CRC. No renal/liver impairment. No antibiotics or probiotics within 3 months. No history of IBD or chronic diarrhea | Microbiology of gut. Genetic expression changes. | Unknown | No |
| Influence of Sundilac and Probiotics on the Development of Pouch Adenomas in Patients with Familial Adenomatous Polyposis [152] | 00319007 | U | Sundilac. Probiotics (VSL #3). Prebiotic (Inulin) | 30 patients Randomized Duration: 9 months | Proven FAP, restorative proctocolectomy with ileal pouch anal anastomosis. No renal/liver impairment, no history of ulcers, no aspirin within 3 months. No probiotics | Mucosal proliferation | Non-significant decrease in cell proliferation in any groups. | Yes |
| The Effects of Probiotics on Bowel Function Restoration After Ileostomy Closure in Patients with Rectal Cancer [153] | 02751736 | U | Probiotics (CJLP 243). Placebo Given daily for 3 weeks before & after ileostomy surgery. | 40 patients Quadruple blinded, randomized Duration: 1 year | 20–75 years old with CRC lower anterior resection, non-metastatic. Not pregnant, no valvular heart disease | Bowel function. MSKCC & LARS questionnaire scores. | Non-significant effect on improving bowel function. Non-significant use of questionnaires | Yes |
| VSL #3 Versus Placebo in Increasing the Pathological Major Response Rate in Patients with Rectal Cancer | 01579591 | U | Probiotics (VSL#3). Placebo | 160 patients Double blinded, randomized Duration: 1 year | >18 years old with CRC, expected to live >6 months. No antibiotics or probiotics in 1–2 weeks to registration | TRG1-2 rate. SCFA expression. Adverse effects. Immune system changes. | Unknown | U |
| Intestinal Microflora in Colorectal Cancer (CRC) After Chemotherapy | 02169388 | U | Probiotic (Clostridium Butyricum). Placebo. Given twice a day for 4 weeks. | 30 patients Triple blinded, randomized Duration: 4 months | 18–80 years old, non-pregnant/lactating scheduled for chemotherapy. No renal/liver impairment. No use of antibiotic or pre/probiotics for 1 month | Microbiology and SCFAs in feces, adverse reactions during chemotherapy | Unknown | U |
| Lactobacillus Rhamnous in Prevention of Chemotherapy-related Diarrhea [154] | 00197873 | U | Probiotic (Lactobacillus Rhamnosus). Placebo. During chemotherapy. | 84 patients Quadruple blinded, Randomized, Cross over Duration: 12 years | >18 years old with CRC expected to live >3 months. No diarrhea, no treatment with bevacizumab (within 12 months) | Number of bowel movements. Chemotherapy tolerability. | Unknown | U |
| Study Title | NCT # | Status * | Intervention | Study | Inclusion/Exclusion Criteria | Outcomes | Expected End Date |
|---|---|---|---|---|---|---|---|
| Chemotherapy w/wo WeiLeShu in Metastatic Colorectal Cancer [155] | 04021589 | R | Probiotics (WeiLeShu™ from Tongchuang Biotechnology) with Chemotherapy | 50 patients Randomized, Open label. Phase II | >18 years old with CRC with good renal function (creatinine > 2.9 mg/dl) | Progression free survival | July 2022 |
| Prebiotics and Probiotics During Definitive Treatment with Chemotherapy-radiotherapy SCC of the Anal Canal (BISQUIT) [156] | 03870607 | R | Synbiotics. Normal nutrition. | 75 patients Double blinded, randomized | >18 years old with squamous CRC, non-metastatic. No infections requiring antibiotics | Response to chemotherapy | February 2024 |
| Effect of Probiotics Supplementation on the Side Effects of Radiation Therapy Among Colorectal Cancer Patients [157] | 03742596 | R | Probiotics. Placebo | 40 patients Quadruple blinded, randomized Phase II | 35–65 years old with stage I–III CRC. No antibiotic or pre/probiotic use recently. | Level of immunoglobulins (A, F, M), interleukins (6, 1, 1), tumor necrosis factor, C-reactive protein | December 2022 |
| Probiotics as Adjuvant Therapy in the Treatment of Metastatic Colorectal Cancer [158] | 03705442 | R | Omni-Biotic 10. Loperamide. Given twice a day for 84 days. | 76 patients Assessor blinded, randomized. Phase II | > 18 years old with mitotic CRC (with FOLFIRI), not terminally ill (<6 months to live), not using probiotics | Incidence of diarrhea | February 2020 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamichhane, P.; Maiolini, M.; Alnafoosi, O.; Hussein, S.; Alnafoosi, H.; Umbela, S.; Richardson, T.; Alla, N.; Lamichhane, N.; Subhadra, B.; et al. Colorectal Cancer and Probiotics: Are Bugs Really Drugs? Cancers 2020, 12, 1162. https://doi.org/10.3390/cancers12051162
Lamichhane P, Maiolini M, Alnafoosi O, Hussein S, Alnafoosi H, Umbela S, Richardson T, Alla N, Lamichhane N, Subhadra B, et al. Colorectal Cancer and Probiotics: Are Bugs Really Drugs? Cancers. 2020; 12(5):1162. https://doi.org/10.3390/cancers12051162
Chicago/Turabian StyleLamichhane, Purushottam, Morgan Maiolini, Omar Alnafoosi, Sedra Hussein, Hasan Alnafoosi, Stewart Umbela, Tayanna Richardson, Nevien Alla, Narottam Lamichhane, Bobban Subhadra, and et al. 2020. "Colorectal Cancer and Probiotics: Are Bugs Really Drugs?" Cancers 12, no. 5: 1162. https://doi.org/10.3390/cancers12051162
APA StyleLamichhane, P., Maiolini, M., Alnafoosi, O., Hussein, S., Alnafoosi, H., Umbela, S., Richardson, T., Alla, N., Lamichhane, N., Subhadra, B., & Deshmukh, R. R. (2020). Colorectal Cancer and Probiotics: Are Bugs Really Drugs? Cancers, 12(5), 1162. https://doi.org/10.3390/cancers12051162





