MR Thermometry Data Correlate with Pathological Response for Soft Tissue Sarcoma of the Lower Extremity in a Single Center Analysis of Prospectively Registered Patients
Abstract
1. Introduction
2. Results
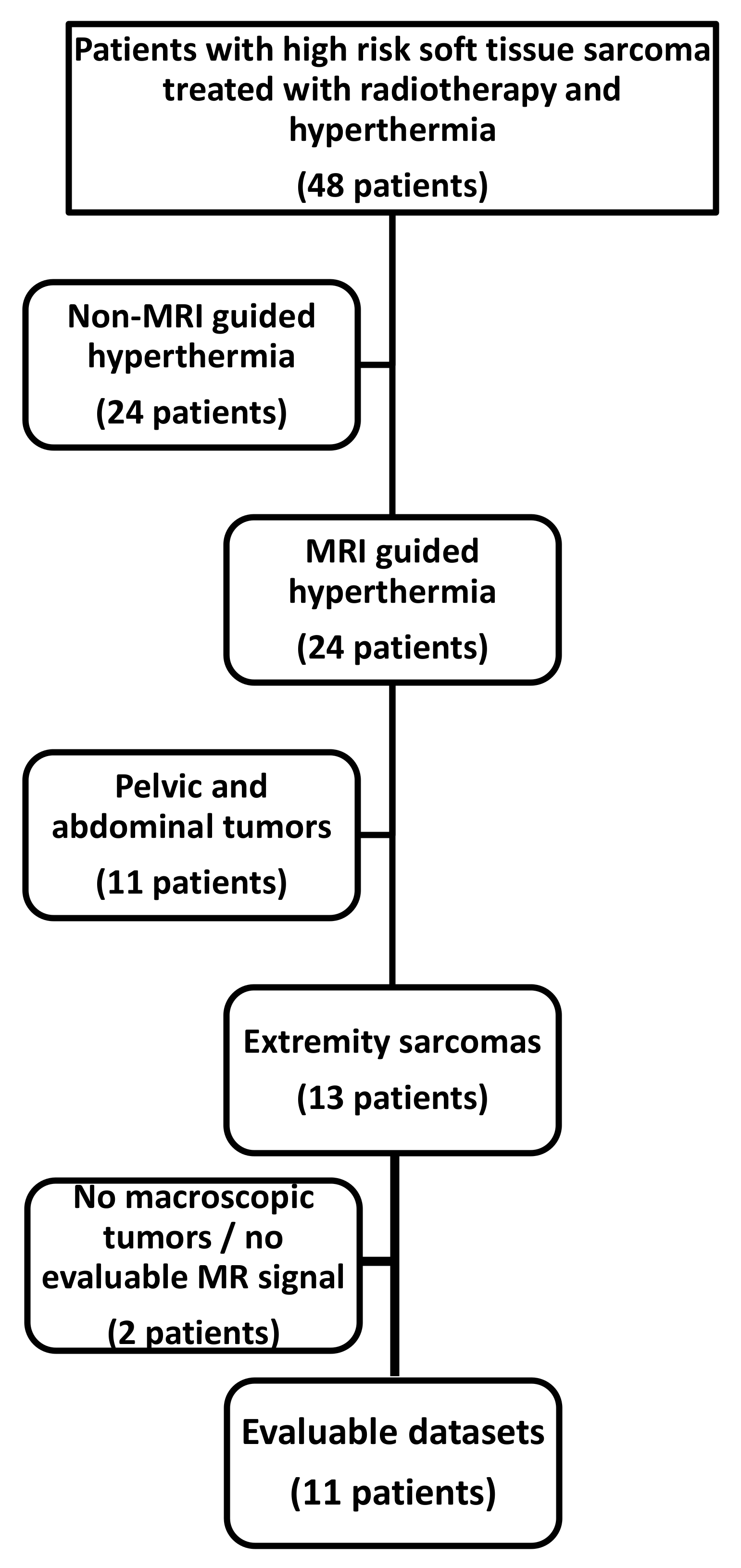
2.1. Patient Selection and Patient Characteristics
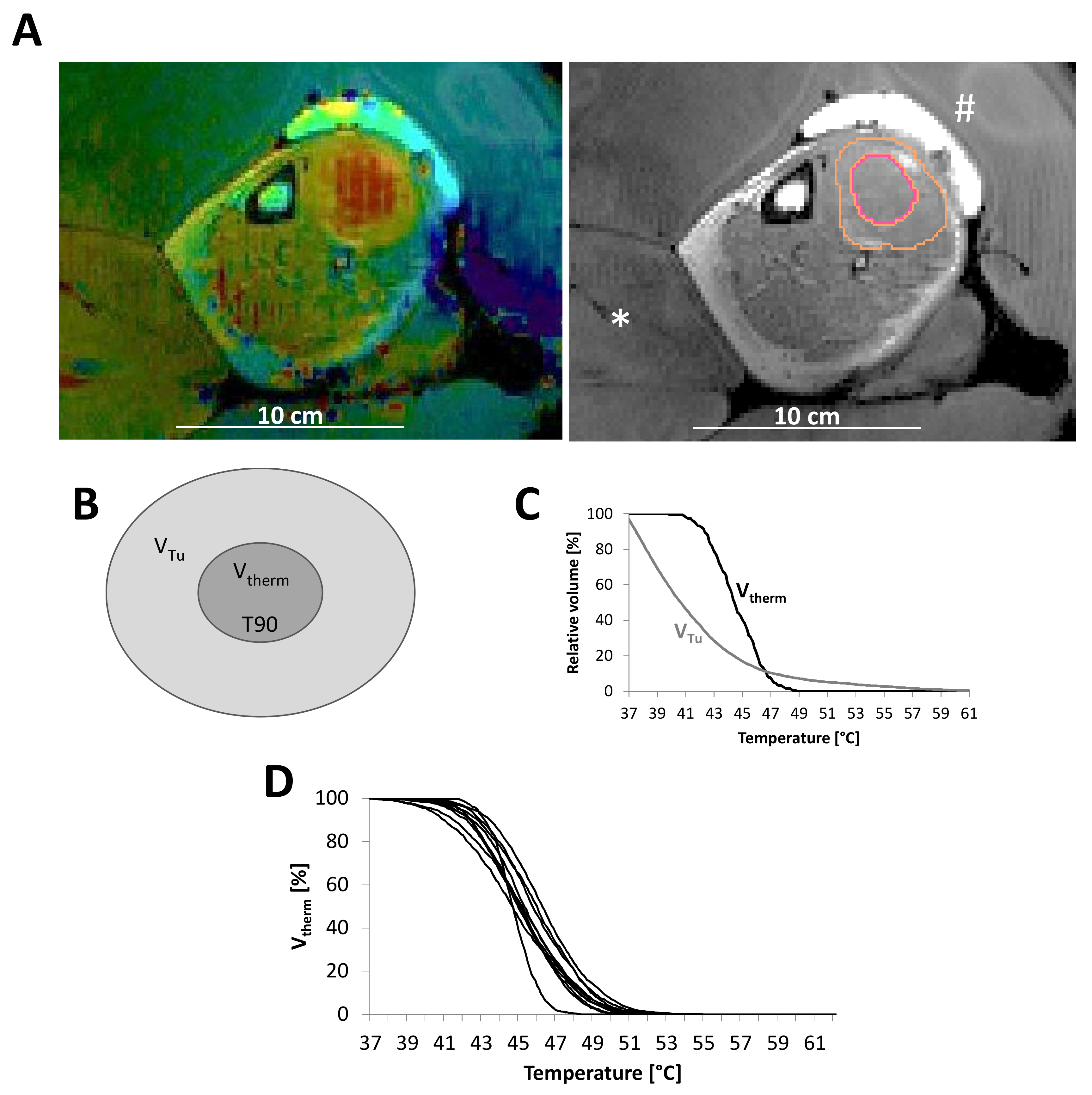
2.2. Contouring and Quality Assurance
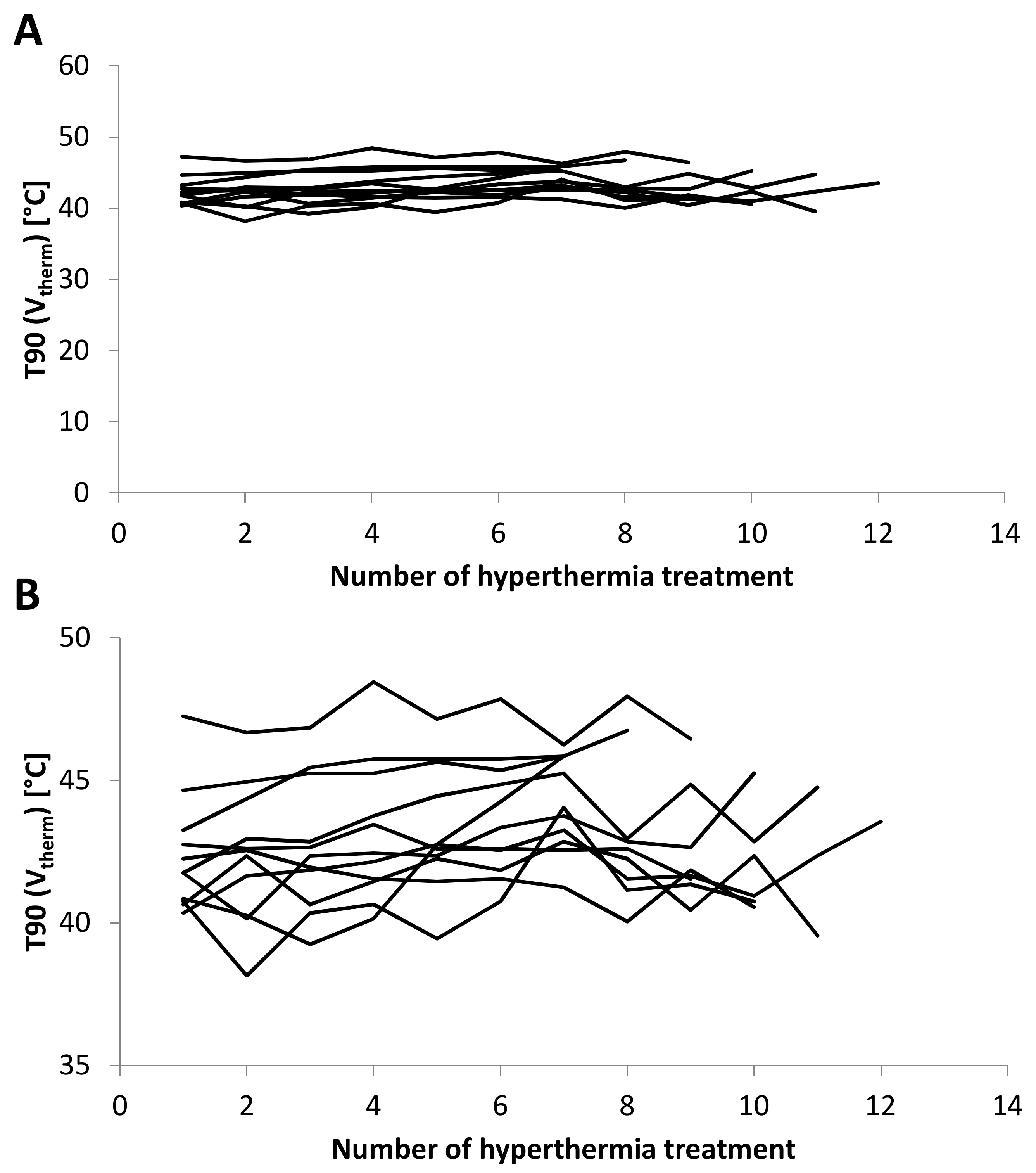
2.3. T90 and CEM43 during Course of Therapy
2.4. Correlation of Mean T90 and Cumulative CEM with Tumor Size
2.5. Correlation of Temperature with Pathologic Response
3. Discussion
4. Patients and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Issels, R.; Lindner, L.H. Regional hyperthermia for high-risk soft tissue sarcoma treatment: Present status and next questions. Curr. Opin. Oncol. 2016, 28, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Bell, R.S.; Turcotte, R.; Catton, C.N.; Wunder, J.S.; Chabot, P.; Hammond, A.; Benk, V.; Isler, M.; et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J. Clin. Oncol. 2002, 20, 4472–4477. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Hartmann, J.; Schutte, J. Neoadjuvante Chemotherapie des lokalisierten Weichteilsarkoms (Neoadjuvant chemotherapy of localised soft tissue sarcomas). Onkologe 2009, 15, 389–397. [Google Scholar] [CrossRef]
- Eckert, F.; Matuschek, C.; Mueller, A.C.; Weinmann, M.; Hartmann, J.T.; Belka, C.; Budach, W. Definitive radiotherapy and single-agent radiosensitizing ifosfamide in patients with localized, irresectable soft tissue sarcoma: A retrospective analysis. Radiat. Oncol. 2010, 5, 55. [Google Scholar] [CrossRef]
- Eckert, F.; Braun, L.H.; Traub, F.; Kopp, H.G.; Sipos, B.; Lamprecht, U.; Muller, A.C.; Paulsen, F.; Zips, D. Radiotherapy and Hyperthermia with Curative Intent in Recurrent High Risk Soft Tissue Sarcomas. Int. J. Hyperth. 2017, 1–24. [Google Scholar] [CrossRef]
- Eckert, F.; Gani, C.; Kluba, T.; Mayer, F.; Kopp, H.G.; Zips, D.; Bamberg, M.; Muller, A.C. Effect of concurrent chemotherapy and hyperthermia on outcome of preoperative radiotherapy of high-risk soft tissue sarcomas. Strahlenther. Onkol. 2013, 189, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Aiba, H.; Yamada, S.; Mizutani, J.; Yamamoto, N.; Okamoto, H.; Hayashi, K.; Kimura, H.; Takeuchi, A.; Miwa, S.; Higuchi, T.; et al. Preoperative evaluation of the efficacy of radio-hyperthermo-chemotherapy for soft tissue sarcoma in a case series. PLoS ONE 2018, 13, e0195289. [Google Scholar] [CrossRef] [PubMed]
- Aiba, H.; Yamada, S.; Mizutani, J.; Yamamoto, N.; Okamoto, H.; Hayashi, K.; Kimura, H.; Takeuchi, A.; Miwa, S.; Kawai, A.; et al. Clinical outcomes of radio-hyperthermo-chemotherapy for soft tissue sarcoma compared to a soft tissue sarcoma registry in Japan: A retrospective matched-pair cohort study. Cancer Med. 2018, 7, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Aiba, H.; Yamada, S.; Mizutani, J.; Yamamoto, N.; Okamoto, H.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Higuchi, T.; Abe, K.; et al. Efficacy of radio-hyperthermo-chemotherapy as salvage therapy for recurrent or residual malignant soft tissue tumors. Int. J. Hyperth. 2018, 35, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Cormier, J.N.; Patel, S.R.; Herzog, C.E.; Ballo, M.T.; Burgess, M.A.; Feig, B.W.; Hunt, K.K.; Raney, R.B.; Zagars, G.K.; Benjamin, R.S.; et al. Concurrent ifosfamide-based chemotherapy and irradiation. Analysis of treatment-related toxicity in 43 patients with sarcoma. Cancer 2001, 92, 1550–1555. [Google Scholar] [CrossRef]
- Li, M.; Andra, C.; Niyazi, M.; Issels, R.D.; Abdel-Rahman, S.; Oskan, F.; Manapov, F. Concomitant trimodality therapy of re-irradiation, chemotherapy and regional hyperthermia for a pretreated inoperable sarcoma recurrence. Tumori 2015, 101, e54–e56. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, F.; Agaimy, A.; Ott, O.; Lettmaier, S.; Vassos, N.; Croner, R.; Hohenberger, W.; Fietkau, R.; Semrau, S. Effective local control of advanced soft tissue sarcoma with neoadjuvant chemoradiotherapy and surgery: A single institutional experience. Cancer Radiother. 2016, 20, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Le Cesne, A.; Ouali, M.; Leahy, M.G.; Santoro, A.; Hoekstra, H.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Van Hoesel, R.; Verweij, J.; et al. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: Pooled analysis of two STBSG-EORTC phase III clinical trials. Ann. Oncol. 2014, 25, 2425–2432. [Google Scholar] [CrossRef]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.B.; Ott, O.; et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther. Onkol. 2011, 187, 605–610. [Google Scholar] [CrossRef]
- Winter, L.; Oberacker, E.; Paul, K.; Ji, Y.; Oezerdem, C.; Ghadjar, P.; Thieme, A.; Budach, V.; Wust, P.; Niendorf, T. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int. J. Hyperth. 2016, 32, 63–75. [Google Scholar] [CrossRef]
- Curto, S.; Aklan, B.; Mulder, T.; Mils, O.; Schmidt, M.; Lamprecht, U.; Peller, M.; Wessalowski, R.; Lindner, L.H.; Fietkau, R.; et al. Quantitative, Multi-institutional Evaluation of MR Thermometry Accuracy for Deep-Pelvic MR-Hyperthermia Systems Operating in Multi-vendor MR-systems Using a New Anthropomorphic Phantom. Cancers 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.I.; Stauffer, P.R.; Soher, B.J.; Wyatt, C.R.; Arabe, O.; Maccarini, P.; Das, S.K.; Cheng, K.S.; Wong, T.Z.; Jones, E.L.; et al. Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med Phys. 2009, 36, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Gellermann, J.; Hildebrandt, B.; Issels, R.; Ganter, H.; Wlodarczyk, W.; Budach, V.; Felix, R.; Tunn, P.U.; Reichardt, P.; Wust, P. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer 2006, 107, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, D.; Van der Zee, J.; Notenboom, A.; van Rhoon, G.C. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther. Onkol. 2007, 183, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Gani, C.; Lamprecht, U.; von Weyhern, C.H.; Weinmann, M.; Bamberg, M.; Berger, B. Pathological complete response and sphincter-sparing surgery after neoadjuvant radiochemotherapy with regional hyperthermia for locally advanced rectal cancer compared with radiochemotherapy alone. Int. J. Hyperth. 2012, 28, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.W.; Deheshi, B.M.; Ferguson, P.C.; Wunder, J.S.; Griffin, A.M.; Catton, C.N.; Bell, R.S.; White, L.M.; Kandel, R.A.; O’Sullivan, B. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: A comparison with other soft tissue sarcomas. Cancer 2009, 115, 3254–3261. [Google Scholar] [CrossRef]
- Raaphorst, G.P.; Szekely, J.G. Thermal enhancement of cellular radiation damage: A review of complementary and synergistic effects. Scanning Microsc. 1988, 2, 513–535. [Google Scholar]
- Tamura, T.; Sugiyama, M.; Uekawa, M.; Nakai, Y.; Onoyama, Y.; Nakajima, T. Synergistic effects of irradiation and hyperthermia on established cell lines derived from maxillary carcinoma. Report II. Acta Oto-Laryngol. Suppl. 1991, 486, 234–244. [Google Scholar] [CrossRef]
- Tur, G.E.; Sato, Y.; Fukuoka, T.; Andoh, H.; Kotanagi, H.; Koyama, K. Effect of the combination of hyperthermia and irradiation on human colon cancer cells. J. Surg. Oncol. 1994, 56, 128–131. [Google Scholar] [CrossRef]
- Yuguchi, T.; Saito, M.; Yokoyama, Y.; Saito, T.; Nagata, T.; Sakamoto, T.; Tsukada, K. Combined use of hyperthermia and irradiation cause antiproliferative activity and cell death to human esophageal cell carcinoma cells—Mainly cell cycle examination. Hum. Cell 2002, 15, 33–42. [Google Scholar] [CrossRef]
- Mivechi, N.F.; Hofer, K.G.; Hofer, M.G. Influence of hypoxia and acidity on thermal radiosensitization and direct heat-induced death of BP-8 sarcoma cells. Radiology 1981, 138, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, G.; Wu, W.; Wang, M. DNA double strand break repair inhibition as a cause of heat radiosensitization: Re-evaluation considering backup pathways of NHEJ. Int. J. Hyperth. 2008, 24, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Sreenivasa, G.; Plotkin, M.; Pietsch, H.; Wust, P.; Ludemann, L. Tumour perfusion assessment during regional hyperthermia treatment: Comparison of temperature probe measurement with H(2)(15)O-PET perfusion. Int. J. Hyperth. 2010, 26, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Ludemann, L.; Sreenivasa, G.; Amthauer, H.; Michel, R.; Gellermann, J.; Wust, P. Use of H(2) (15)O-PET for investigating perfusion changes in pelvic tumors due to regional hyperthermia. Int. J. Hyperth. 2009, 25, 299–308. [Google Scholar] [CrossRef]
- Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Dodge, R.K.; Charles, H.C.; Samulski, T.V.; Prosnitz, L.R.; Dewhirst, M.W. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996, 56, 5347–5350. [Google Scholar]
- Milani, V.; Noessner, E. Effects of thermal stress on tumor antigenicity and recognition by immune effector cells. Cancer Immunol. Immunother. CII 2006, 55, 312–319. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef]
- Kelly, M.; McNeel, D.; Fisch, P.; Malkovsky, M. Immunological considerations underlying heat shock protein-mediated cancer vaccine strategies. Immunol. Lett. 2018, 193, 1–10. [Google Scholar] [CrossRef]
- Shevtsov, M.; Huile, G.; Multhoff, G. Membrane heat shock protein 70: A theranostic target for cancer therapy. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef]
- Schmidtner, J.; Distel, L.V.; Ott, O.J.; Nkenke, E.; Sprung, C.N.; Fietkau, R.; Lubgan, D. Hyperthermia and irradiation of head and neck squamous cancer cells causes migratory profile changes of tumour infiltrating lymphocytes. Int. J. Hyperth. 2009, 25, 347–354. [Google Scholar] [CrossRef]
- DeFillipo, A.M.; Dai, J.; Li, Z. Heat shock-induced dendritic cell maturation is coupled by transient aggregation of ubiquitinated proteins independently of heat shock factor 1 or inducible heat shock protein 70. Mol. Immunol. 2004, 41, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Knippertz, I.; Stein, M.F.; Dorrie, J.; Schaft, N.; Muller, I.; Deinzer, A.; Steinkasserer, A.; Nettelbeck, D.M. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int. J. Hyperth. 2011, 27, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Chen, Y.; Wang, S.; Yu, L.; Shen, Y.; Zhong, H.; Yang, Y. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology 2018, 154, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.L.; MacFall, J.R.; Clegg, S.T.; Wan, X.; Prescott, D.M.; Charles, H.C.; Samulski, T.V. Magnetic resonance thermometry during hyperthermia for human high-grade sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 815–822. [Google Scholar] [CrossRef]
- Wirth, V.J.; Van Lunen, B.L.; Mistry, D.; Saliba, E.; McCue, F.C. Temperature changes in deep muscles of humans during upper and lower extremity exercise. J. Athl. Train. 1998, 33, 211–215. [Google Scholar]
- Gregoire, V.; Mackie, T.R. State of the art on dose prescription, reporting and recording in Intensity-Modulated Radiation Therapy (ICRU report No. 83). Cancer Radiother. 2011, 15, 555–559. [Google Scholar] [CrossRef]
- Van Rhoon, G.C.; Samaras, T.; Yarmolenko, P.S.; Dewhirst, M.W.; Neufeld, E.; Kuster, N. CEM43 degrees C thermal dose thresholds: A potential guide for magnetic resonance radiofrequency exposure levels? Eur. Radiol. 2013, 23, 2215–2227. [Google Scholar] [CrossRef]


| Age at Diagnosis (years) | Localisation | Side | T Stage at Diagnosis | Size (cm) | Histologic Subtype | Histologic Grading (FNCLCC) | Concomitant Ifosfamide | T90 (Vtherm) (°C) | Vtherm/VTu | Path Response (% Viable Tumor) | Status at Last follow Up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 36 | Calf | right | 2b | 7.2 | synovial sarcoma | 2 | Yes | 41.5 | 0.26 | 40% | Local recurrence and distant metastases |
| 65 | Calf | right | 2b | 9 | MPNST | 3 | Yes | 40.7 | 0.08 | n.a. | Distant metastases |
| 60 | Thigh | left | 2b | 8 | sarcoma with myogenic differentiation | 3 | Yes | 42.7 | 0.26 | 30% | Distant metastases |
| 70 | Thigh | right | 2b | 6.6 | leiomyosarcoma | 3 | Yes | 45.3 | 0.08 | 2% | Distant metastases |
| 55 | Thigh | left | 2b | 11 | NOS | 3 | Yes | 41.9 | 0.03 | 0% | NED |
| 68 | Thigh | left | 2b | 11 | NOS | 3 | Yes | 41.9 | 0.10 | 40% | NED |
| 61 | Thigh | left | 2b | 9.3 | myxoid sarcoma | 2 | No | 45.4 | 0.52 | 0% | NED |
| 56 | Thigh | right | 2b | 12 | NOS | 3 | No | 43.8 | 0.20 | 0% | NED |
| 73 | Thigh | right | 2b | 14 | NOS | 3 | No | 41.5 | 0.12 | 20% | NED |
| 61 | Calf | left | 2b | 17.8 | myxoid liposarcoma | 2 | No | 46.9 | 0.33 | <5% | Distant metastases |
| 46 | Thigh | right | 2b | 8 | myxoid liposarcoma | 2 | No | 42.0 | 0.32 | 0% | NED |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unsoeld, M.; Lamprecht, U.; Traub, F.; Hermes, B.; Scharpf, M.; Potkrajcic, V.; Zips, D.; Paulsen, F.; Eckert, F. MR Thermometry Data Correlate with Pathological Response for Soft Tissue Sarcoma of the Lower Extremity in a Single Center Analysis of Prospectively Registered Patients. Cancers 2020, 12, 959. https://doi.org/10.3390/cancers12040959
Unsoeld M, Lamprecht U, Traub F, Hermes B, Scharpf M, Potkrajcic V, Zips D, Paulsen F, Eckert F. MR Thermometry Data Correlate with Pathological Response for Soft Tissue Sarcoma of the Lower Extremity in a Single Center Analysis of Prospectively Registered Patients. Cancers. 2020; 12(4):959. https://doi.org/10.3390/cancers12040959
Chicago/Turabian StyleUnsoeld, Michaela, Ulf Lamprecht, Frank Traub, Barbara Hermes, Marcus Scharpf, Vlatko Potkrajcic, Daniel Zips, Frank Paulsen, and Franziska Eckert. 2020. "MR Thermometry Data Correlate with Pathological Response for Soft Tissue Sarcoma of the Lower Extremity in a Single Center Analysis of Prospectively Registered Patients" Cancers 12, no. 4: 959. https://doi.org/10.3390/cancers12040959
APA StyleUnsoeld, M., Lamprecht, U., Traub, F., Hermes, B., Scharpf, M., Potkrajcic, V., Zips, D., Paulsen, F., & Eckert, F. (2020). MR Thermometry Data Correlate with Pathological Response for Soft Tissue Sarcoma of the Lower Extremity in a Single Center Analysis of Prospectively Registered Patients. Cancers, 12(4), 959. https://doi.org/10.3390/cancers12040959






