Changes in DNA Damage Response Markers with Treatment in Advanced Ovarian Cancer
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Vergote, I.; Trope, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage iiic or iv ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Luck, H.J.; Meier, W.; Adams, H.P.; Mobus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schroder, W.; et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Karakasis, K.; Kohn, E.C.; Oza, A.M. Ovarian cancer treatment: The end of empiricism? Cancer 2015, 121, 3203–3211. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [CrossRef]
- Stover, E.H.; Konstantinopoulos, P.A.; Matulonis, U.A.; Swisher, E.M. Biomarkers of response and resistance to DNA repair targeted therapies. Clin. Cancer Res. 2016, 22, 5651–5660. [Google Scholar] [CrossRef]
- De Picciotto, N.; Cacheux, W.; Roth, A.; Chappuis, P.O.; Labidi-Galy, S.I. Ovarian cancer: Status of homologous recombination pathway as a predictor of drug response. Crit. Rev. Oncol. Hematol. 2016, 101, 50–59. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287. [Google Scholar] [CrossRef]
- Auguste, A.; Genestie, C.; De Bruyn, M.; Adam, J.; Le Formal, A.; Drusch, F.; Pautier, P.; Crosbie, E.J.; MacKay, H.; Kitchener, H.C.; et al. Refinement of high-risk endometrial cancer classification using DNA damage response biomarkers: A transportec initiative. Mod. Pathol. 2018, 31, 1851–1861. [Google Scholar] [CrossRef]
- Kubelac, P.; Auguste, A.; Genestie, C.; Mesnage, S.; Le Formal, A.; Achimas-Cadariu, P.; Leary, A. Changes in DNA damage response markers with treatment in advanced ovarian cancer. Gynecol. Oncol. 2018, 149, 164–165. [Google Scholar] [CrossRef]
- Choi, M.; Kipps, T.; Kurzrock, R. Atm mutations in cancer: Therapeutic implications. Mol. Cancer Ther. 2016, 15, 1781–1791. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. Atm and atr substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Ledermann, J.A.; Kohn, E.C. Parp inhibitors for brca1/2 mutation-associated and brca-like malignancies. Ann. Oncol. 2014, 25, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Sarangi, P.; D’Andrea, A.D. The fanconi anaemia pathway: New players and new functions. Nat. Rev. Mol. Cell Biol. 2016, 17, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Panier, S.; Boulton, S.J. Double-strand break repair: 53bp1 comes into focus. Nat. Rev. Mol. Cell Biol. 2014, 15, 7–18. [Google Scholar] [CrossRef]
- Schreiber, V.; Dantzer, F.; Ame, J.C.; de Murcia, G. Poly(adp-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef]
- Scott, C.L.; Swisher, E.M.; Kaufmann, S.H. Poly (adp-ribose) polymerase inhibitors: Recent advances and future development. J. Clin. Oncol. 2015, 33, 1397–1406. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Friedlander, M.; Matulonis, U.; Gourley, C.; du Bois, A.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br. J. Cancer 2018, 119, 1075–1085. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ariel3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Roques, C.; Magwood, A.C.; Masson, J.Y.; Baker, M.D. Recovery of deficient homologous recombination in brca2-depleted mouse cells by wild-type rad51 expression. DNA Repair (Amst) 2009, 8, 170–181. [Google Scholar] [CrossRef]
- Renaud, E.; Barascu, A.; Rosselli, F. Impaired tip60-mediated h4k16 acetylation accounts for the aberrant chromatin accumulation of 53bp1 and rap80 in fanconi anemia pathway-deficient cells. Nucleic Acids Res. 2016, 44, 648–656. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Cho, K.R.; Gilks, C.B.; Pearce, C.L.; Huntsman, D.G. The disparate origins of ovarian cancers: Pathogenesis and prevention strategies. Nat. Rev. Cancer 2016. [Google Scholar] [CrossRef]
- Schwarz, R.F.; Ng, C.K.; Cooke, S.L.; Newman, S.; Temple, J.; Piskorz, A.M.; Gale, D.; Sayal, K.; Murtaza, M.; Baldwin, P.J.; et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: A phylogenetic analysis. PLoS Med. 2015, 12, e1001789. [Google Scholar] [CrossRef] [PubMed]
- Paracchini, L.; Mannarino, L.; Craparotta, I.; Romualdi, C.; Fruscio, R.; Grassi, T.; Fotia, V.; Caratti, G.; Perego, P.; Calura, E.; et al. Regional and temporal heterogeneity of epithelial ovarian cancer tumor biopsies: Implications for therapeutic strategies. Oncotarget 2016. [Google Scholar] [CrossRef]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ariel2 part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef]
- Patel, J.; Sehouli, J.; Timms, K.; Solimeno, C.; Reid, J.; Lanchbury, J.; Braicu, I.; Darb-Esfahani, S.; Ganapathi, M.; Ganapathi, R. Characteristics of homologous recombination deficiency (hrd) in paired primary and recurrent high-grade serous ovarian cancer (hgsoc). Ann. Oncol. 2016, 27, 113P. [Google Scholar] [CrossRef]
- Kessous, R.; Octeau, D.; Klein, K.; Tonin, P.N.; Greenwood, C.M.T.; Pelmus, M.; Laskov, I.; Kogan, L.; Salvador, S.; Lau, S.; et al. Distinct homologous recombination gene expression profiles after neoadjuvant chemotherapy associated with clinical outcome in patients with ovarian cancer. Gynecol. Oncol. 2018, 148, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Swisher, E.M.; Gonzalez, R.M.; Taniguchi, T.; Garcia, R.L.; Walsh, T.; Goff, B.A.; Welcsh, P. Methylation and protein expression of DNA repair genes: Association with chemotherapy exposure and survival in sporadic ovarian and peritoneal carcinomas. Mol. Cancer 2009, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Bunting, S.F.; Callen, E.; Wong, N.; Chen, H.T.; Polato, F.; Gunn, A.; Bothmer, A.; Feldhahn, N.; Fernandez-Capetillo, O.; Cao, L.; et al. 53bp1 inhibits homologous recombination in brca1-deficient cells by blocking resection of DNA breaks. Cell 2010, 141, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Castroviejo-Bermejo, M.; Gutierrez-Enriquez, S.; Llop-Guevara, A.; Ibrahim, Y.H.; Gris-Oliver, A.; Bonache, S.; Morancho, B.; Bruna, A.; Rueda, O.M.; et al. Rad51 foci as a functional biomarker of homologous recombination repair and parp inhibitor resistance in germline brca-mutated breast cancer. Ann. Oncol. 2018, 29, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Collis, S.J.; Adelman, C.A.; Silva, N.; Horejsi, Z.; Ward, J.D.; Martinez-Perez, E.; Boulton, S.J.; La Volpe, A. Preventing nonhomologous end joining suppresses DNA repair defects of fanconi anemia. Mol. Cell 2010, 39, 25–35. [Google Scholar] [CrossRef]
- Kim, H.; D’Andrea, A.D. Regulation of DNA cross-link repair by the fanconi anemia/brca pathway. Genes Dev. 2012, 26, 1393–1408. [Google Scholar] [CrossRef]
- Mesnage, S.J.L.; Auguste, A.; Genestie, C.; Dunant, A.; Pain, E.; Drusch, F.; Gouy, S.; Morice, P.; Bentivegna, E.; Lhomme, C.; et al. Neoadjuvant chemotherapy (nact) increases immune infiltration and programmed death-ligand 1 (pd-l1) expression in epithelial ovarian cancer (eoc). Ann. Oncol. 2017, 28, 651–657. [Google Scholar] [CrossRef]
- Parra, E.R.; Villalobos, P.; Behrens, C.; Jiang, M.; Pataer, A.; Swisher, S.G.; William, W.N., Jr.; Zhang, J.; Lee, J.; Cascone, T.; et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J. Immunother. Cancer 2018, 6, 48. [Google Scholar] [CrossRef]
- Garcia-Martinez, E.; Gil, G.L.; Benito, A.C.; Gonzalez-Billalabeitia, E.; Conesa, M.A.; Garcia Garcia, T.; Garcia-Garre, E.; Vicente, V.; Ayala de la Pena, F. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014, 16, 488. [Google Scholar] [CrossRef]
- Opzoomer, J.W.; Sosnowska, D.; Anstee, J.E.; Spicer, J.F.; Arnold, J.N. Cytotoxic chemotherapy as an immune stimulus: A molecular perspective on turning up the immunological heat on cancer. Front. Immunol. 2019, 10, 1654. [Google Scholar] [CrossRef]
- Zhang, A.W.; McPherson, A.; Milne, K.; Kroeger, D.R.; Hamilton, P.T.; Miranda, A.; Funnell, T.; Little, N.; de Souza, C.P.E.; Laan, S.; et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell 2018, 173, 1755–1769.e1722. [Google Scholar] [CrossRef] [PubMed]
- Shia, J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part i. The utility of immunohistochemistry. J. Mol. Diagn. 2008, 10, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Loukola, A.; Eklin, K.; Laiho, P.; Salovaara, R.; Kristo, P.; Jarvinen, H.; Mecklin, J.P.; Launonen, V.; Aaltonen, L.A. Microsatellite marker analysis in screening for hereditary nonpolyposis colorectal cancer (hnpcc). Cancer Res. 2001, 61, 4545–4549. [Google Scholar] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (tils) in breast cancer: Recommendations by an international tils working group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase iii randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: Big 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting recommendations for tumor marker prognostic studies. J. Clin. Oncol. 2005, 23, 9067–9072. [Google Scholar] [CrossRef]
| DDR Biomarker | Relevance | Reference |
|---|---|---|
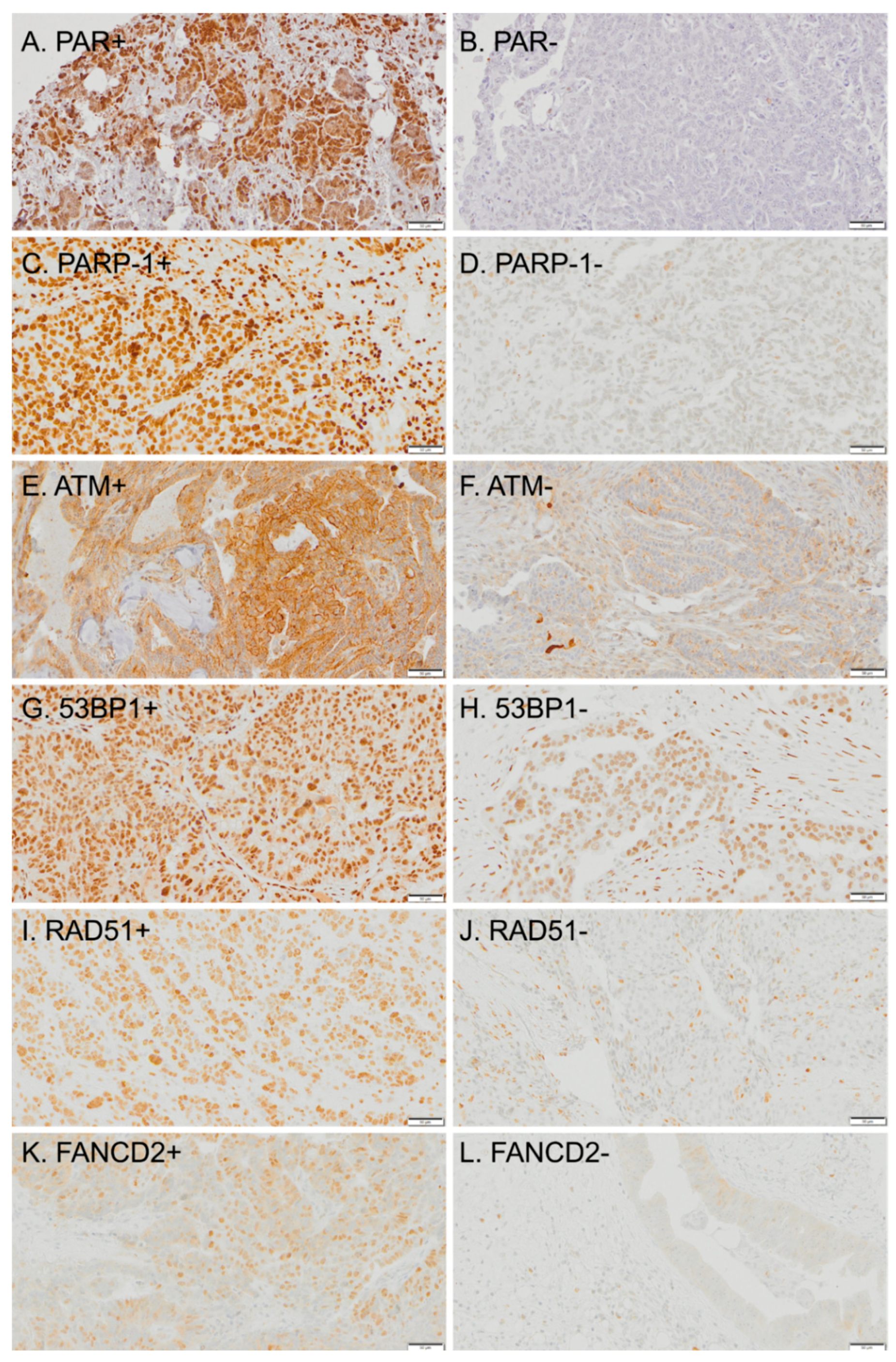
| PARP-1 | Polymerase inhibitors (PARP-1) binds to damaged DNA and synthetizes PAR chains, essential for base excision repair (BER) pathway | [17,18] |
| PAR | PARP-1 effector, PAR chains are a scaffold for the recruitment of DNA repair proteins, a measure of BER activity | [17,18] |
| ATM | DNA damage sensor and mediator of homologous recombination (HR). Also involved in DNA replication, cell cycle checkpoint arrest, and apoptosis, as means of coordinating several pathways during genomic stress | [12,13] |
| RAD51 | Initiates repair protein assembly in the HR pathway | [23] |
| FANCD2 | Member of the Fanconi anemia genes, involved in strand-annealing repair of DSB and in repairing inter-strand crosslinks Seems to limit the use of error-prone non-homologous end-joining (NHEJ), proposed inhibitory relationship with 53BP1. | [15,24] |
| 53BP1 | Promotes NHEJ and inhibits HR, favoring error-prone DNA repair | [16] |
| Variable | Number | % |
|---|---|---|
| Median age (range) | 60.9 (20.8–78.8) years | |
| The International Federation of Gynecology and Obstetrics (FIGO) stage (2014) | ||
| IIIC | 126 | 85.7 |
| IV | 21 | 14.3 |
| BRCA status | ||
| Wild-type | 66 | 44.9 |
| BRCA 1/2 mutation | 22 | 15.0 |
| Unknown | 59 | 40.1 |
| Outcome of IDS | ||
| Macroscopic complete resection | 103 | 70.1 |
| Not operated 1 | 44 | 29.9 |
| Histological type | ||
| High-grade serous | 108 | 73.5 |
| Low-grade serous | 11 | 7.5 |
| Clear cell | 2 | 1.4 |
| Mucinous | 2 | 1.4 |
| Other high-grade 2 | 24 | 16.3 |
| Relapse | 132 | 89.8 |
| Death | 92 | 62.6 |
| Median follow-up (95% CI) | 71.6 (63.8–79.3) months | |
| Median PFS (95% CI) | 21.7 (18.4–24.9) months | |
| Median OS (95% CI) | 44.9 (37.3–52.4) months |
| Temporal Setting | PAR % Negative (no. neg/total) | PARP-1 % Negative (no. neg/total) | ATM % Negative (no. neg/total) | 53BP1 % Negative (no. neg/total) | RAD51 % Negative (no. neg/total) | FANCD2 % Negative (no. neg/total) |
|---|---|---|---|---|---|---|
| Pre-NACT | 60 (40/80) | 3 (3/87) | 21 (16/78) | 15 (13/88) | 24 (16/68) | 60 (53/89) |
| Post-NACT | 82 (72/88) 1 | 6 (5/85) | 34 (28/82) | 18 (15/83) | 52 (37/71) 1 | 62 (53/86) |
| Relapse | 60 (9/15) | 0 (0/9) | 21 (3/14) | 27 (3/11) | 20 (2/10) | 50 (6/12) |
| Biomarker | Result | N | PFS | OS | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| POST-NACT ATM (PRE-NACT ATM+) | Negative | 9 | 1 | 0.003 | 1 | 0.008 |
| Positive | 22 | 0.21 (0.08–0.59) | 0.21 (0.07–0.66) | |||
| POST-NACT RAD51 (PRE-NACT RAD51+) | Negative | 12 | 1 | 0.029 | 1 | 0.008 |
| Positive | 15 | 2.55 (1.09–5.92) | 5.44 (1.56–18.92) | |||
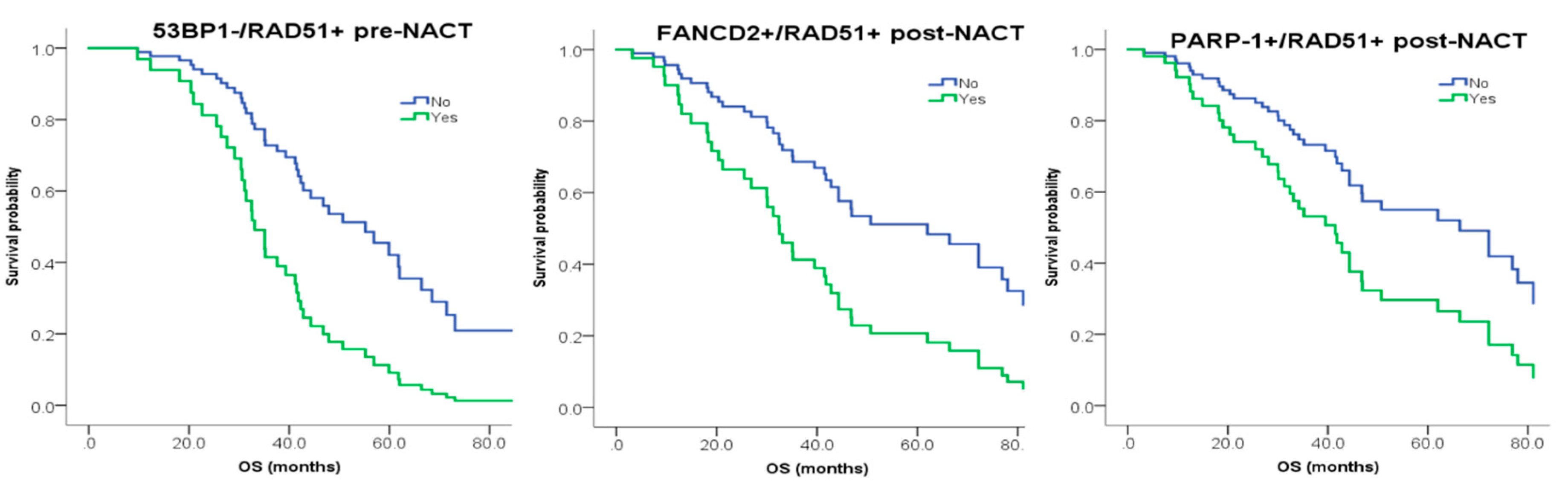
| PRE-NACT 53BP1−/RAD51+ | False | 57 | 1 | 0.009 | 1 | 0.024 |
| True | 7 | 3.13 (1.33–7.69) | 2.78 (1.15–7.14) | |||
| POST-NACT FANCD2+/RAD51+ | False | 50 | 1 | 0.05 | 1 | 0.02 |
| True | 14 | 1.89 (1–3.57) | 2.38 (1.15–5) | |||
| POST-NACT PARP-1+/RAD51+ | False | 36 | 1 | 0.038 | 1 | 0.034 |
| True | 29 | 1.79 (1.04–3.03) | 2.04 (1.06–4) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubelac, P.; Genestie, C.; Auguste, A.; Mesnage, S.; Le Formal, A.; Pautier, P.; Gouy, S.; Morice, P.; Bentivegna, E.; Maulard, A.; et al. Changes in DNA Damage Response Markers with Treatment in Advanced Ovarian Cancer. Cancers 2020, 12, 707. https://doi.org/10.3390/cancers12030707
Kubelac P, Genestie C, Auguste A, Mesnage S, Le Formal A, Pautier P, Gouy S, Morice P, Bentivegna E, Maulard A, et al. Changes in DNA Damage Response Markers with Treatment in Advanced Ovarian Cancer. Cancers. 2020; 12(3):707. https://doi.org/10.3390/cancers12030707
Chicago/Turabian StyleKubelac, Paul, Catherine Genestie, Aurelie Auguste, Soizick Mesnage, Audrey Le Formal, Patricia Pautier, Sebastien Gouy, Philippe Morice, Enrica Bentivegna, Amandine Maulard, and et al. 2020. "Changes in DNA Damage Response Markers with Treatment in Advanced Ovarian Cancer" Cancers 12, no. 3: 707. https://doi.org/10.3390/cancers12030707
APA StyleKubelac, P., Genestie, C., Auguste, A., Mesnage, S., Le Formal, A., Pautier, P., Gouy, S., Morice, P., Bentivegna, E., Maulard, A., Adam, J., Achimas-Cadariu, P., & Leary, A. (2020). Changes in DNA Damage Response Markers with Treatment in Advanced Ovarian Cancer. Cancers, 12(3), 707. https://doi.org/10.3390/cancers12030707





