Lipid–Saporin Nanoparticles for the Intracellular Delivery of Cytotoxic Protein to Overcome ABC Transporter-Mediated Multidrug Resistance In Vitro and In Vivo
Abstract
1. Introduction
2. Results
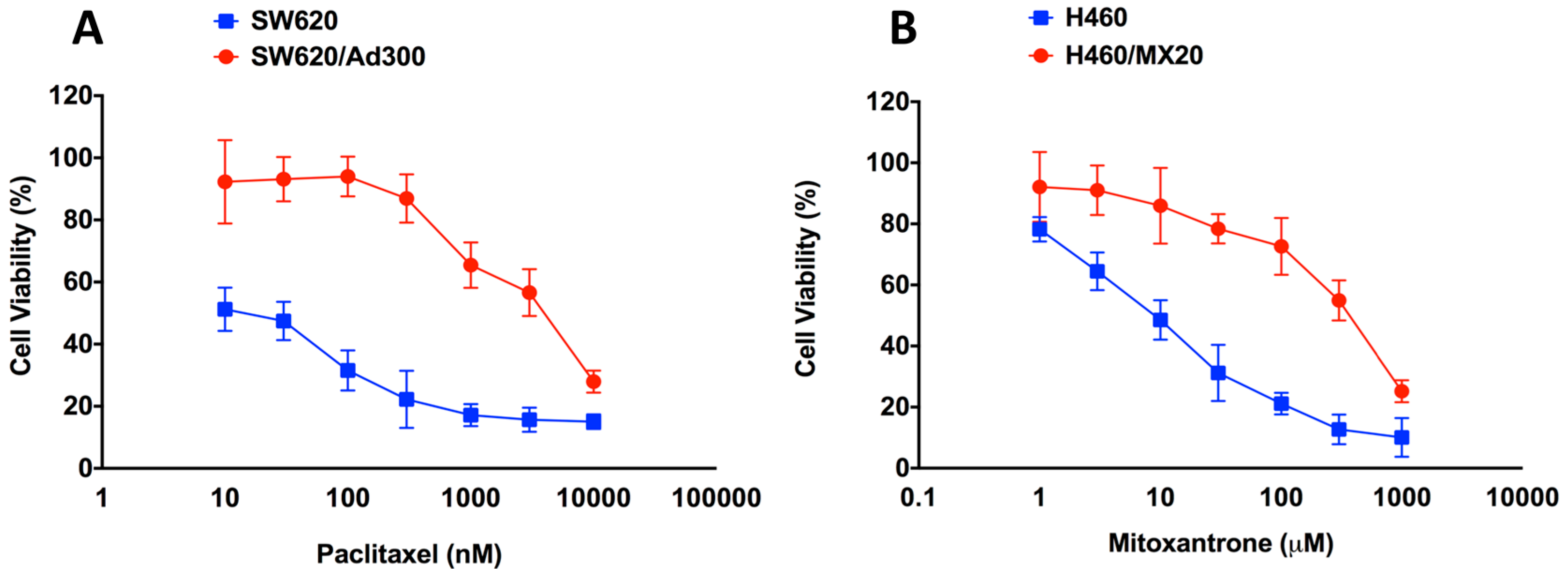
2.1. Assessment of the Magnitude of Resistance in ABCB1- and ABCG2-Overexpressing Cells
2.2. Effect of EC16-1/Saporin on the Cellular Proliferation of Parental and Resistant ABCB1- and ABCG2-Overexpressing Cells
2.3. Effect of EC16-1/Saporin on the Intracellular Localization of ABCB1 and ABCG2
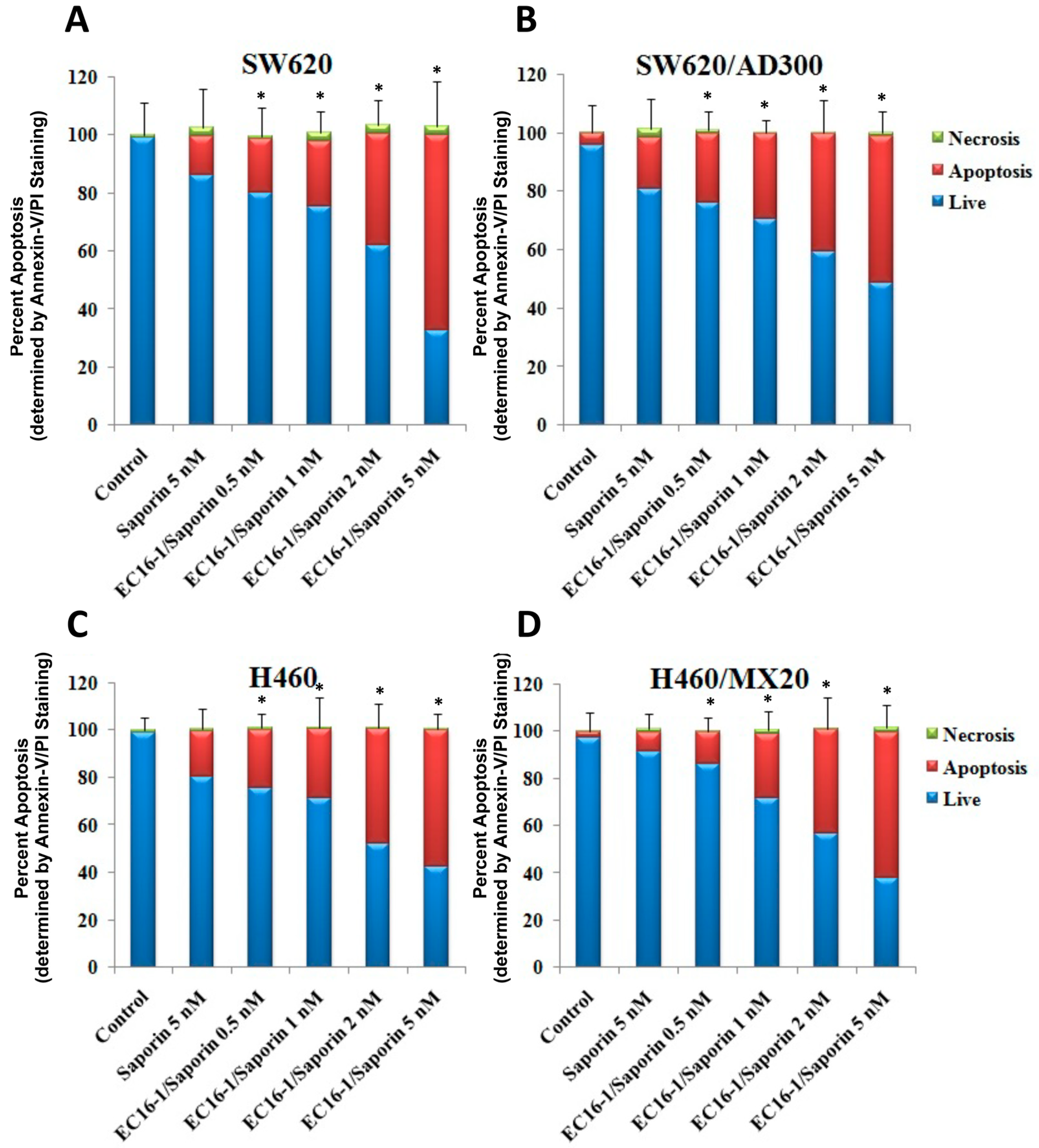
2.4. Effect of EC16-1/Saporin on the Apoptosis of ABCB1- or ABCG2-Overexpressing Cells
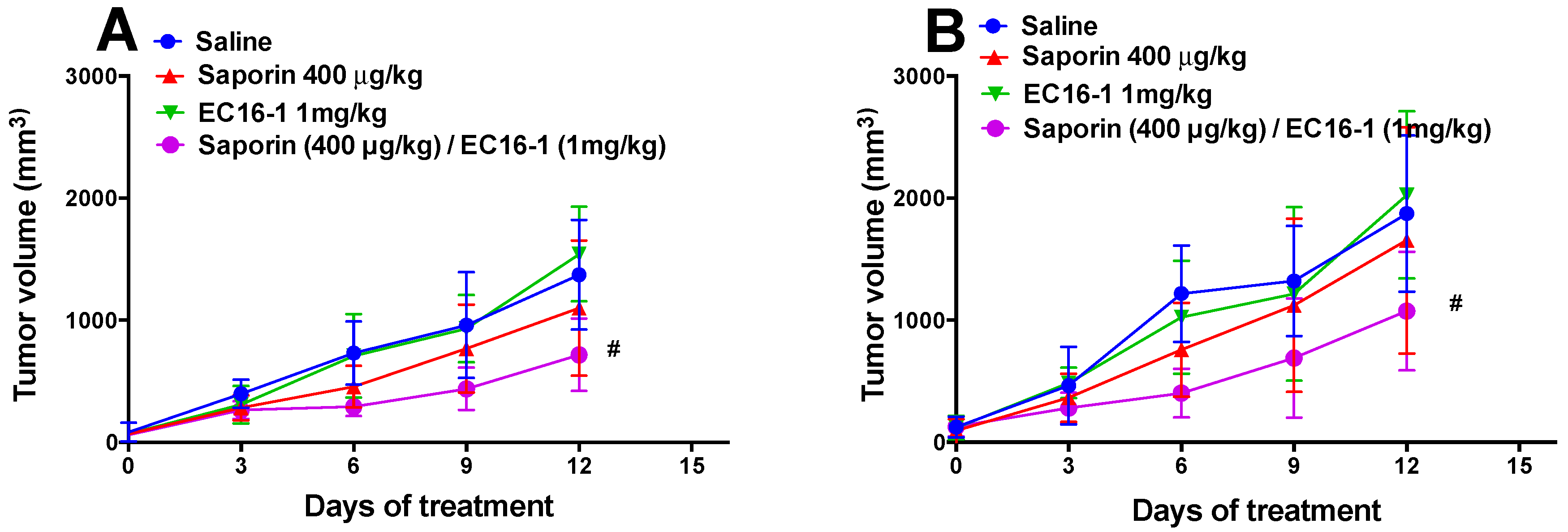
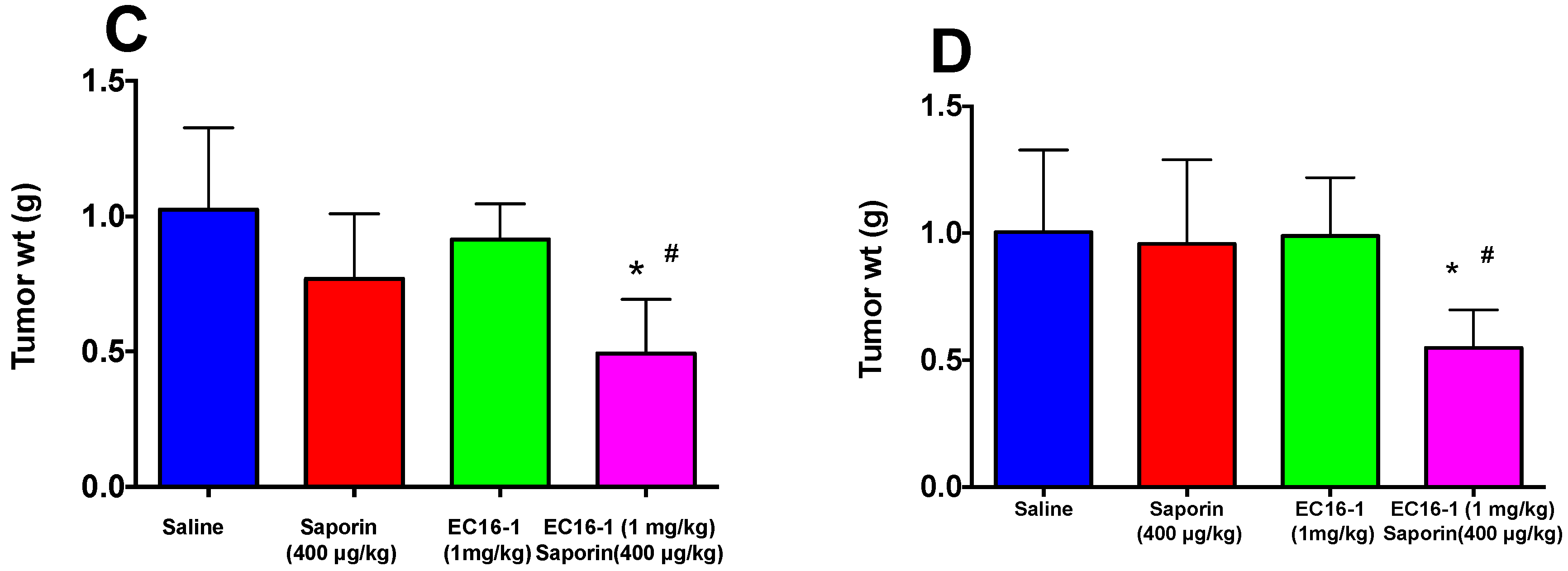
2.5. EC16-1/Saporin Decreases Tumor Volume and Weight in an ABCB1-Overexpressing Tumor Xenograft Model
2.6. EC16-1/Saporin Decreases Tumor Volume and Weight in ABCG2-Overexpressing Tumor Xenograft Models
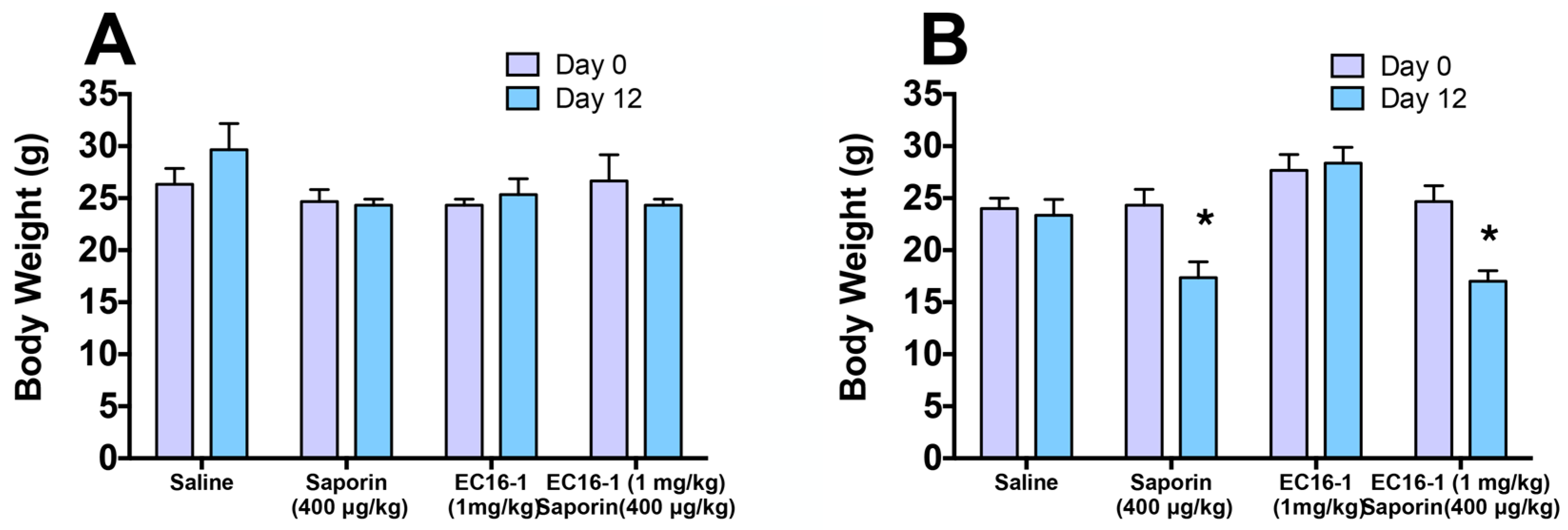
2.7. Assessment of Potential Toxicity of EC16-1/Saporin
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. EC16-1/Saporin Formulation Preparation
4.3. Cell Lines and Cell Culture
4.4. MTT Assay
4.5. Immunofluorescence
4.6. Apoptosis Assay
4.7. Experimental Animals
4.8. Methodology for Preclinical Antitumor Efficacy
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of Atp-Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Gottesman, M.M.A.; Mbudkar, S.V. Overview: Abc Transporters and Human Disease. J. Bioenerg. Biomembr. 2001, 33, 453–458. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming Abc Transporter-Mediated Multidrug Resistance: Molecular Mechanisms and Novel Therapeutic Drug Strategies. Drug Resist Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Chen, Z.S.; Ambudkar, S.V. Tyrosine Kinase Inhibitors as Modulators of Abc Transporter-Mediated Drug Resistance. Drug Resist Updat. 2012, 15, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Zeng, L.; Xu, M.; Wang, X.Q.; Yang, D.H.; Chen, Z.S. Osimertinib (Azd9291), a Mutant-Selective Egfr Inhibitor, Reverses Abcb1-Mediated Drug Resistance in Cancer Cells. Molecules 2016, 21, 1236. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Johnson, E.; Lewinson, O. Abc Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Peng, X.X.; Kim, I.W.; Shukla, S.; Si, Q.S.; Robey, R.W.; Bates, S.E.; Shen, T.; Ashby, C.R., Jr.; Fu, L.W.; et al. Erlotinib (Tarceva, Osi-774) Antagonizes Atp-Binding Cassette Subfamily B Member 1 and Atp-Binding Cassette Subfamily G Member 2-Mediated Drug Resistance. Cancer Res. 2007, 67, 11012–11020. [Google Scholar] [CrossRef] [PubMed]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The Modulation of Abc Transporter-Mediated Multidrug Resistance in Cancer: A Review of the Past Decade. Drug Resist Updat. 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Wang, Y.J.; Lei, Z.N.; Zhang, G.N.; Zhang, X.Y.; Wang, D.S.; Al-Rihani, S.B.; Shukla, S.; Ambudkar, S.V.; Kaddoumi, A.; et al. Regorafenib Antagonizes Bcrp-Mediated Multidrug Resistance in Colon Cancer. Cancer Lett. 2019, 442, 104–112. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhang, Y.-K.; Zhang, G.-N.; Al Rihani, S.B.; Wei, M.-N.; Gupta, P.; Zhang, X.-Y.; Shukla, S.; Ambudkar, S.V.; Kaddoumi, A.; et al. Regorafenib Overcomes Chemotherapeutic Multidrug Resistance Mediated by Abcb1 Transporter in Colorectal Cancer: In vitro and In vivo Study. Cancer Lett. 2017, 396, 145–154. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Chen, Z.S. Multidrug Resistance Proteins (Mrps) and Cancer Therapy. AAPS J. 2015, 17, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.K.; Wang, Y.J.; Kathawala, R.J.; Patel, A.; Zhu, H.; Sodani, K.; Talele, T.T.; Ambudkar, S.V.; Chen, Z.S.; et al. Whi-P154 Enhances the Chemotherapeutic Effect of Anticancer Agents in Abcg2-Overexpressing Cells. Cancer Sci. 2014, 105, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Anreddy, N.; Patel, A.; Zhang, Y.K.; Wang, Y.J.; Shukla, S.; Kathawala, R.J.; Kumar, P.; Gupta, P.; Ambudkar, S.V.; Wurpel, J.N.; et al. A-803467, a Tetrodotoxin-Resistant Sodium Channel Blocker, Modulates Abcg2-Mediated Mdr in Vitro and in Vivo. Oncotarget 2015, 6, 39276–39291. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ling, V. A Surface Glycoprotein Modulating Drug Permeability in Chinese Hamster Ovary Cell Mutants. Biochim. Biophys. Acta 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Ueda, K.; Cornwell, M.M.; Gottesman, M.M.; Pastan, I.; Roninson, I.B.; Ling, V.; Riordan, J.R. The Mdr1 Gene, Responsible for Multidrug-Resistance, Codes for P-Glycoprotein. Biochem. Biophys. Res. Commun. 1986, 141, 956–962. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Borgnia, M.J. Differential Reversal of Lipophilic Antifolate Resistance in Mammalian Cells with Modulators of the Multidrug Resistance Phenotype. Anticancer Drugs 1993, 4, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Sarkadi, B.; Homolya, L.; Szakacs, G.; Varadi, A. Human Multidrug Resistance Abcb and Abcg Transporters: Participation in a Chemoimmunity Defense System. Physiol. Rev. 2006, 86, 1179–1236. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Smith, M.M.; Muller, M.; Kerr, K.M.; Ambudkar, S.V. The Mechanism of Action of Multidrug-Resistance-Linked P-Glycoprotein. J. Bioenerg. Biomembr. 2001, 33, 481–491. [Google Scholar] [CrossRef]
- Chen, Z.S.; Tiwari, A.K. Multidrug Resistance Proteins (Mrps/Abccs) in Cancer Chemotherapy and Genetic Diseases. FEBS J. 2011, 278, 3226–3245. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Sodani, K.; Wang, S.R.; Kuang, Y.H.; Ashby, C.R., Jr.; Chen, X.; Chen, Z.S. Nilotinib (Amn107, Tasigna) Reverses Multidrug Resistance by Inhibiting the Activity of the Abcb1/Pgp and Abcg2/Bcrp/Mxr Transporters. Biochem. Pharmacol. 2009, 78, 153–161. [Google Scholar] [CrossRef]
- Sodani, K.; Patel, A.; Kathawala, R.J.; Chen, Z.S. Multidrug Resistance Associated Proteins in Multidrug Resistance. Chin. J. Cancer 2012, 31, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Cooray, H.C.; Blackmore, C.G.; Maskell, L.; Barrand, M.A. Localisation of Breast Cancer Resistance Protein in Microvessel Endothelium of Human Brain. Neuroreport 2002, 13, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Gujarati, N.A.; Zeng, L.; Gupta, P.; Chen, Z.S.; Korlipara, V.L. Design, Synthesis and Biological Evaluation of Benzamide and Phenyltetrazole Derivatives with Amide and Urea Linkers as Bcrp Inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 4698–4704. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A Multidrug Resistance Transporter from Human Mcf-7 Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the Breast Cancer Resistance Protein (Abcg2) in Drug Transport. AAPS J. 2005, 7, 118–133. [Google Scholar] [CrossRef]
- Natarajan, K.; Xie, Y.; Baer, M.R.; Ross, D.D. Role of Breast Cancer Resistance Protein (Bcrp/Abcg2) in Cancer Drug Resistance. Biochem. Pharmacol. 2012, 83, 1084–1103. [Google Scholar] [CrossRef]
- Olarte Carrillo, I.; Ramos Penafiel, C.; Miranda Peralta, E.; Rozen Fuller, E.; Kassack Ipina, J.J.; Centeno Cruz, F.; Garrido Guerrero, E.; Collazo Jaloma, J.; Nacho Vargas, K.; Martinez Tovar, A. Clinical Significance of the Abcb1 and Abcg2 Gene Expression Levels in Acute Lymphoblastic Leukemia. Hematology 2017, 22, 286–291. [Google Scholar] [CrossRef]
- Damiani, D.; Tiribelli, M.; Geromin, A.; Michelutti, A.; Cavallin, M.; Sperotto, A.; Fanin, R. Abcg2 Overexpression in Patients with Acute Myeloid Leukemia: Impact on Stem Cell Transplantation Outcome. Am. J. Hematol. 2015, 90, 784–789. [Google Scholar] [CrossRef]
- Gupta, P.; Jani, K.A.; Yang, D.H.; Sadoqi, M.; Squillante, E.; Chen, Z.S. Revisiting the Role of Nanoparticles as Modulators of Drug Resistance and Metabolism in Cancer. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 281–289. [Google Scholar] [CrossRef]
- Chen, Y.; Bathula, S.R.; Li, J.; Huang, L. Multifunctional Nanoparticles Delivering Small Interfering Rna and Doxorubicin Overcome Drug Resistance in Cancer. J. Biol. Chem. 2010, 285, 22639–22650. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L. Nanoparticle-Based Combination Therapy toward Overcoming Drug Resistance in Cancer. Biochem. Pharmacol. 2012, 83, 1104–1111. [Google Scholar] [CrossRef]
- Yee, D. The Insulin-Like Growth Factor System as a Target in Breast Cancer. Breast Cancer Res. Treat. 1994, 32, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Criscitiello, C.; Esposito, A.; Intra, M.; Minucci, S. Pharmacokinetic Drug Evaluation of Ribociclib for the Treatment of Metastatic, Hormone-Positive Breast Cancer. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.F.; Corthay, A.; Koutsilieris, M. Aiming for the Insulin-Like Growth Factor-1 System in Breast Cancer Therapeutics. Cancer Treat Rev. 2017, 63, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Mercatelli, D.; Battelli, M.G.; Bolognesi, A. Saporin-S6: A Useful Tool in Cancer Therapy. Toxins 2013, 5, 1698–1722. [Google Scholar] [CrossRef]
- Walsh, M.J.; Dodd, J.E.; Hautbergue, G.M. Ribosome-Inactivating Proteins: Potent Poisons and Molecular Tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef]
- Fabbrini, M.S.; Katayama, M.; Nakase, I.; Vago, R. Plant Ribosome-Inactivating Proteins: Progesses, Challenges and Biotechnological Applications (and a Few Digressions). Toxins 2017, 9, 314. [Google Scholar] [CrossRef]
- Giansanti, F.; Flavell, D.J.; Angelucci, F.; Fabbrini, M.S.; Ippoliti, R. Strategies to improve the clinical utility of saporin-based targeted toxins. Toxins 2018, 10, 82. [Google Scholar] [CrossRef]
- Soria, M.R.; Benatti, L.; Nitti, G.; Ceriotti, A.; Solinas, M.; Lappi, D.A.; Lorenzetti, R. Studies on Ribosome-Inactivating Proteins from Saponaria Officinalis. Targeted Diagn. Ther. 1992, 7, 193–212. [Google Scholar]
- Roy, S.; Axup, J.Y.; Forsyth, J.S.; Goswami, R.K.; Hutchins, B.M.; Bajuri, K.M.; Kazane, S.A.; Smider, V.V.; Felding, B.H.; Sinha, S.C. Smi-Ribosome Inactivating Protein Conjugates Selectively Inhibit Tumor Cell Growth. Chem. Commun. 2017, 53, 4234–4237. [Google Scholar] [CrossRef]
- Wang, M.; Alberti, K.; Sun, S.; Arellano, C.L.; Xu, Q. Combinatorially Designed Lipid-Like Nanoparticles for Intracellular Delivery of Cytotoxic Protein for Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 2893–2898. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Ahmad, A.; Azmi, A.S.; Kong, D.; Banerjee, S.; Sarkar, F.H. Targeting Mirnas Involved in Cancer Stem Cell and Emt Regulation: An Emerging Concept in Overcoming Drug Resistance. Drug Resist Updat. 2010, 13, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Alberti, K.; Varone, A.; Pouli, D.; Georgakoudi, I.; Xu, Q. Enhanced Intracellular Sirna Delivery Using Bioreducible Lipid-Like Nanoparticles. Adv. Healthc. Mater. 2014, 3, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Bornmann, W.; Pellicena, P.; Miller, W.T.; Clarkson, B.; Kuriyan, J. Structural Mechanism for Sti-571 Inhibition of Abelson Tyrosine Kinase. Science 2000, 289, 1938–1942. [Google Scholar] [CrossRef]
- Shen, T.; Kuang, Y.H.; Ashby, C.R.; Lei, Y.; Chen, A.; Zhou, Y.; Chen, X.; Tiwari, A.K.; Hopper-Borge, E.; Ouyang, J.; et al. Imatinib and Nilotinib Reverse Multidrug Resistance in Cancer Cells by Inhibiting the Efflux Activity of the Mrp7 (Abcc10). PLoS ONE 2009, 4, e7520. [Google Scholar] [CrossRef]
- Fan, Y.F.; Zhang, W.; Zeng, L.; Lei, Z.N.; Cai, C.Y.; Gupta, P.; Yang, D.H.; Cui, Q.; Qin, Z.D.; Chen, Z.S.; et al. Dacomitinib Antagonizes Multidrug Resistance (Mdr) in Cancer Cells by Inhibiting the Efflux Activity of Abcb1 and Abcg2 Transporters. Cancer Lett. 2018, 421, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhao, R.Q.; Zhang, Y.K.; Gupta, P.; Fu, L.X.; Tang, A.Z.; Liu, B.M.; Chen, Z.S.; Yang, D.H.; Liang, G. Effect of Y6, an Epigallocatechin Gallate Derivative, on Reversing Doxorubicin Drug Resistance in Human Hepatocellular Carcinoma Cells. Oncotarget 2017, 8, 29760–29770. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.S. The Development of Anticancer Ruthenium (Ii) Complexes: From Single Molecule Compounds to Nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi. Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Dong, X.; Mumper, R.J. Nanomedicinal Strategies to Treat Multidrug-Resistant Tumors: Current Progress. Nanomedicine 2010, 5, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.F.; Lei, L.; Guo, S.R.; Huang, W.L. Recent Progress in Nanotechnology for Cancer Therapy. Chin. J. Cancer 2010, 29, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Jiang, K.; Song, X.; Yang, L.; Li, L.; Wan, Z.; Sun, X.; Gong, T.; Lin, Q.; Zhang, Z. Enhanced Antitumor and Anti-Metastasis Efficacy against Aggressive Breast Cancer with a Fibronectin-Targeting Liposomal Doxorubicin. J. Control. Release 2017, 271, 21–30. [Google Scholar] [CrossRef]
- Casadei, B.; Pellegrini, C.; Tonialini, L.; Argnani, L.; Zinzani, P.L. Interesting Activity of Pegylated Liposomal Doxorubicin in Primary Refractory and Multirelapsed Hodgkin Lymphoma Patients: Bridge to Transplant. Hematol. Oncol. 2018. [Google Scholar] [CrossRef]
- Casagrande, N.; Celegato, M.; Borghese, C.; Mongiat, M.; Colombatti, A.; Aldinucci, D. Preclinical Activity of the Liposomal Cisplatin Lipoplatin in Ovarian Cancer. Clin. Cancer Res. 2014, 20, 5496–5506. [Google Scholar] [CrossRef]
- Wang, Y.J.; Huang, Y.; Anreddy, N.; Zhang, G.N.; Zhang, Y.K.; Xie, M.; Lin, D.; Yang, D.H.; Zhang, M.; Chen, Z.S. Tea Nanoparticle, a Safe and Biocompatible Nanocarrier, Greatly Potentiates the Anticancer Activity of Doxorubicin. Oncotarget 2016, 7, 5877–5891. [Google Scholar] [CrossRef]
- Dai, C.L.; Tiwari, A.K.; Wu, C.P.; Su, X.D.; Wang, S.R.; Liu, D.G.; Ashby, C.R.; Huang, Y.; Robey, R.W.; Liang, Y.J.; et al. Lapatinib (Tykerb, Gw572016) Reverses Multidrug Resistance in Cancer Cells by Inhibiting the Activity of Atp-Binding Cassette Subfamily B Member 1 and G Member 2. Cancer Res. 2008, 68, 7905–7914. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kathawala, R.J.; Zhang, Y.K.; Patel, A.; Kumar, P.; Shukla, S.; Fung, K.L.; Ambudkar, S.V.; Talele, T.T.; Chen, Z.S. Motesanib (Amg706), a Potent Multikinase Inhibitor, Antagonizes Multidrug Resistance by Inhibiting the Efflux Activity of the Abcb1. Biochem. Pharmacol. 2014, 90, 367–378. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.J.; Wang, Y.; Yi, S.; Fan, Z.; Sun, L.; Lin, D.; Anreddy, N.; Zhu, H.; Schmidt, M.; et al. Exploring Naturally Occurring Ivy Nanoparticles as an Alternative Biomaterial. Acta Biomater. 2015. [Google Scholar] [CrossRef]
- Bolognesi, A.; Tazzari, P.L.; Olivieri, F.; Polito, L.; Falini, B.; Stirpe, F. Induction of apoptosis by ribosome-inactivating proteins and related immunotoxins. Int. J. Cancer 1996, 68, 349–355. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Perfetti, V.; Tonon, L.; Novella, A.; Lucotti, C.; Danova, M.; Glennie, M.J.; Merlini, G.; Cazzola, M. Saporin, a ribosome-inactivating protein used to prepare immunotoxins, induces cell death via apoptosis. Br. J. Haematol. 1996, 93, 789–794. [Google Scholar] [CrossRef] [PubMed]
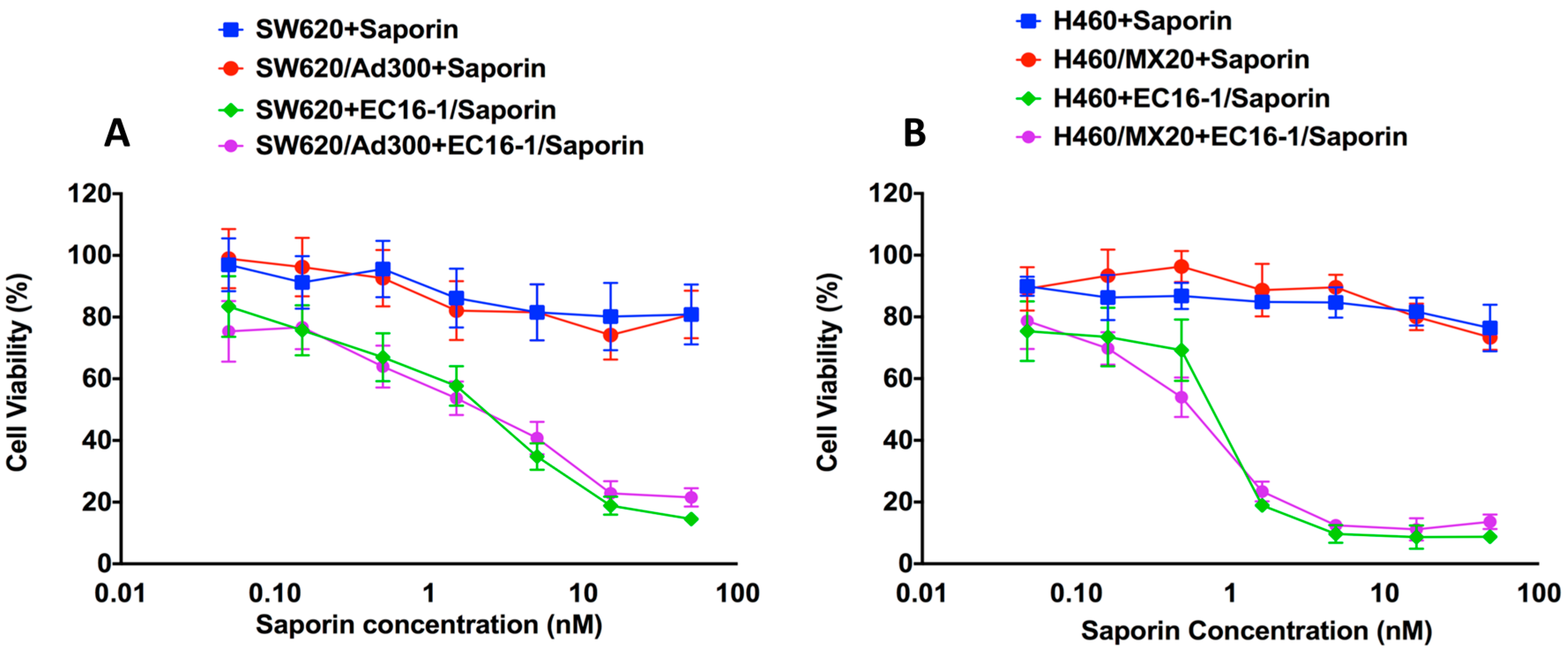




| SW620 | SW620/AD300 | |||||
|---|---|---|---|---|---|---|
| EC16-1 | Saporin | EC16-1/Saporin | EC16-1 | Saporin | EC16-1/Saporin | |
| IC50 a | >50 | >50 | 3.44 ± 0.63 | 34.45 ± 2.92 | >50 | 2.50 ± 0.43 |
| RF b | 1.00 | 1.00 | 1.00 | 0.69 | 1.00 | 0.74 |
| NCI-H460 | NCI-H460/MX20 | |||||
|---|---|---|---|---|---|---|
| EC16-1 | Saporin | EC16-1/Saporin | EC16-1 | Saporin | EC16-1/Saporin | |
| IC50 a | 19.57 ± 0.93 | >50 | 0.88 ± 0.12 | 17.51 ± 1.32 | >50 | 0.63 ± 0.07 |
| RF b | 1.00 | 1.00 | 1.00 | 0.89 | 1.00 | 0.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.-N.; Gupta, P.; Wang, M.; Barbuti, A.M.; Ashby, C.R., Jr.; Zhang, Y.-K.; Zeng, L.; Xu, Q.; Fan, Y.-F.; Chen, Z.-S. Lipid–Saporin Nanoparticles for the Intracellular Delivery of Cytotoxic Protein to Overcome ABC Transporter-Mediated Multidrug Resistance In Vitro and In Vivo. Cancers 2020, 12, 498. https://doi.org/10.3390/cancers12020498
Zhang G-N, Gupta P, Wang M, Barbuti AM, Ashby CR Jr., Zhang Y-K, Zeng L, Xu Q, Fan Y-F, Chen Z-S. Lipid–Saporin Nanoparticles for the Intracellular Delivery of Cytotoxic Protein to Overcome ABC Transporter-Mediated Multidrug Resistance In Vitro and In Vivo. Cancers. 2020; 12(2):498. https://doi.org/10.3390/cancers12020498
Chicago/Turabian StyleZhang, Guan-Nan, Pranav Gupta, Ming Wang, Anna Maria Barbuti, Charles R. Ashby, Jr., Yun-Kai Zhang, Leli Zeng, Qiaobing Xu, Ying-Fang Fan, and Zhe-Sheng Chen. 2020. "Lipid–Saporin Nanoparticles for the Intracellular Delivery of Cytotoxic Protein to Overcome ABC Transporter-Mediated Multidrug Resistance In Vitro and In Vivo" Cancers 12, no. 2: 498. https://doi.org/10.3390/cancers12020498
APA StyleZhang, G.-N., Gupta, P., Wang, M., Barbuti, A. M., Ashby, C. R., Jr., Zhang, Y.-K., Zeng, L., Xu, Q., Fan, Y.-F., & Chen, Z.-S. (2020). Lipid–Saporin Nanoparticles for the Intracellular Delivery of Cytotoxic Protein to Overcome ABC Transporter-Mediated Multidrug Resistance In Vitro and In Vivo. Cancers, 12(2), 498. https://doi.org/10.3390/cancers12020498






