1. Introduction
The complex physiology of tumor tissues, the coexistence of cell subpopulations with distinct phenotypes, and the development of intrinsic/acquired drug resistance are some of the causes of failure of conventional cancer therapies. In tumors, a large fraction of differentiated cells coexist with a small fraction of cancer stem cells (CSCs) that, similarly to normal stem cells, have the capability of self-renewal and differentiation into phenotypically diverse cancer cells [
1]. CSCs are highly tumorigenic and drive cancer initiation, progression, recurrence, and metastasis in a wide panel of tumor types, including breast cancers (BC). In fact, accumulating evidence has led to the concept of BC as a disease of BCSCs since the plasticity of BCSCs plays a pivotal role in determining the evolution and the prognosis of the disease [
2]. It is also clear that the concomitant killing of differentiated cells and targeting/killing of CSCs must be the driving goals in developing new therapeutic strategies aiming at assuring long-lasting disease remission and long-term survival of BC patients.
Currently, some clinical protocols are based on the administration of multiple drugs in a single cocktail or in separated doses as well as the combination of different treatment modalities [
3]. Among clinically approved cancer therapies, photodynamic therapy (PDT) is a compelling curative treatment for several solid tumors, able to efficiently reduce bulky cancer masses [
4]. PDT is based on three intrinsically non-toxic components that exert cytotoxicity only when combined together. These are a photosensitizer (PS), visible light, and molecular oxygen. The PS activation with appropriate wavelengths of light stimulates the production of highly reactive oxygen species (ROS), mainly singlet oxygen (
1O
2), that cause localized oxidative stress, cell death, and activation of immunological and inflammatory responses in the treated tissue [
5]. In comparison to conventional cancer treatments, PDT is selective because: (i) Owing to limited diffusion in the tissues of the produced ROS, the oxidative damage is restricted to the sites of PS localization in the tumor; (ii) light is delivered exclusively to desired areas using optical fibers; (iii) the PSs localize and are retained mainly in neoplastic lesions, especially if delivered as proteins/peptide-conjugates [
6,
7] or loaded in properly engineered nanoparticles (NPs) [
8]. In recent years, it has been reported that the combination of PDT with chemotherapy produced superior therapeutic efficacy over mono-therapies, in several cancer cell lines in vitro and in in vivo tumor models, including BC [
9,
10,
11,
12]. In addition, several studies highlighted that the therapeutic effects of the combined treatment is further improved if the chemotherapeutic and PS are co-encapsulated in nanometric delivery systems [
13,
14,
15]. In line with these observations, we have recently reported the successful synergic killing of docetaxel (DTX)-sensitive and -resistant cancer cells in vitro [
16], using biodegradable hyaluronic acid (HA)-decorated double-layered NPs co-loaded with DTX [
17] and the PS meso-tetraphenyl chlorine disulfonate (TPCS
2a) [
18]. NPs were conceived in order to: (i) Transport the hydrophobic DTX in the inner poly (D,L-lactide-
co-glycolide) (PLGA) core; (ii) favor electrostatic association of the amphiphilic PS to a positively charged polyethyleneimine (PEI) layer covering the core; (iii) exploit active targeting, via the HA receptor CD44 over-expressed on cancer cells, covering the NP surface with a layer of HA [
19]; (iv) guarantee synergic interaction of the transported drugs through the easy modification of drug ratio inside NPs [
20]. In combination therapies, the drug ratio is a crucial factor for obtaining synergism, but in previous work, we were able to synergistically kill CD44-overexpressing cervix cancer HeLa and triple negative BC MDA-MB-231 cells by loading NPs with an optimized DTX:TPCS
2a ratio that significantly improved treatment efficacy with respect to combination of chemotherapy and PDT using free drug formulations. The improvement was particularly important with DTX-resistant cells. Based on these results and on evidence that BCSCs eradication is particularly difficult because of slow proliferation rate, low sensitivity to antimitotic agents, and over-expression of different membrane efflux pumps causing resistance to chemotherapy [
21], we made the hypothesis that it might be possible to increase the killing of BCSCs with the combination of DTX-chemotherapy and TPCS
2a-PDT using HA-covered NPs for the targeting of BCSCs in addition to differentiated BC cells. In principle, the antimitotic DTX should be active against the bulky population of fast-proliferating cells while TPCS
2a-mediated PDT should be equally effective toward fast-proliferating and slow-proliferating BCSCs. Furthermore, the killing of BCSC might be potentiated by CD44 targeting through HA engrafted on NP surface, since BCSC population is given by cells with a high expression of CD44 and low expression of CD24 (CD44
high/CD24
low) [
22]. Thus, in the present work, we studied for the first time the capability of HA-targeted DTX and TPCS
2a-loaded NPs (HA@DTX/TPCS
2a-NPs) to kill proliferating BC cells, cultured as monolayers, as well as CSC-enriched tridimensional BC spheres (e.g., mammospheres) [
23] obtained from MCF-7 and triple negative MDA-MB-231 BC cells. The results showed that our HA-targeted DTX/TPCS
2a nanoformulation is more effective than monotherapies in: (i) killing proliferating BC cells; (ii) reducing their stemness and self-renewal capacity; (iii) reducing the total numbers of BCSCs.
3. Discussion
Breast cancer is one of the major causes of death worldwide for women, especially in the case of triple negative BC (TNBC), characterized by poor prognosis and survival due to ineffectiveness of hormonal therapies. Moreover, the aggressive phenotype of this type of cancer is mainly attributed to drug-resistance phenomena and the presence of BCSCs, responsible for the high rate of metastasis formations and tumor relapse after treatments. While the origin and role of BCSC is not completely understood [
2], evidence suggests that CSCs derived from the transformation of normal stem cells are responsible for tumor’s aggressiveness higher than that of CSC derived from progenitor cells. Different therapeutic approaches are being developed and proposed, to precisely target and eradicate this small but difficult-to-kill cell population. We have previously demonstrated that TPCS
2a delivered through HA-functionalized polymeric NPs exerts markedly higher photosensitizing activity in TNBCs [
16]. Interestingly, when TPCS
2a-PDT was combined to DTX-chemotherapy, by encapsulating the two drugs at optimal ratio within the same NP, a high synergism was found, and TNBC MDA-MB-231 cells were efficiently killed with DTX and TPCS
2a doses, respectively, 11- and 6-fold lower than those used in monotherapies for obtaining comparable effects. Nevertheless, very few examples are reported on the use of DTX nanoformulations for the eradication of differentiated cancer and CSCs [
28,
29,
30]. To the best of our knowledge, this is the first time that DTX-chemotherapy is proposed in combination with PDT to fulfill this scope.
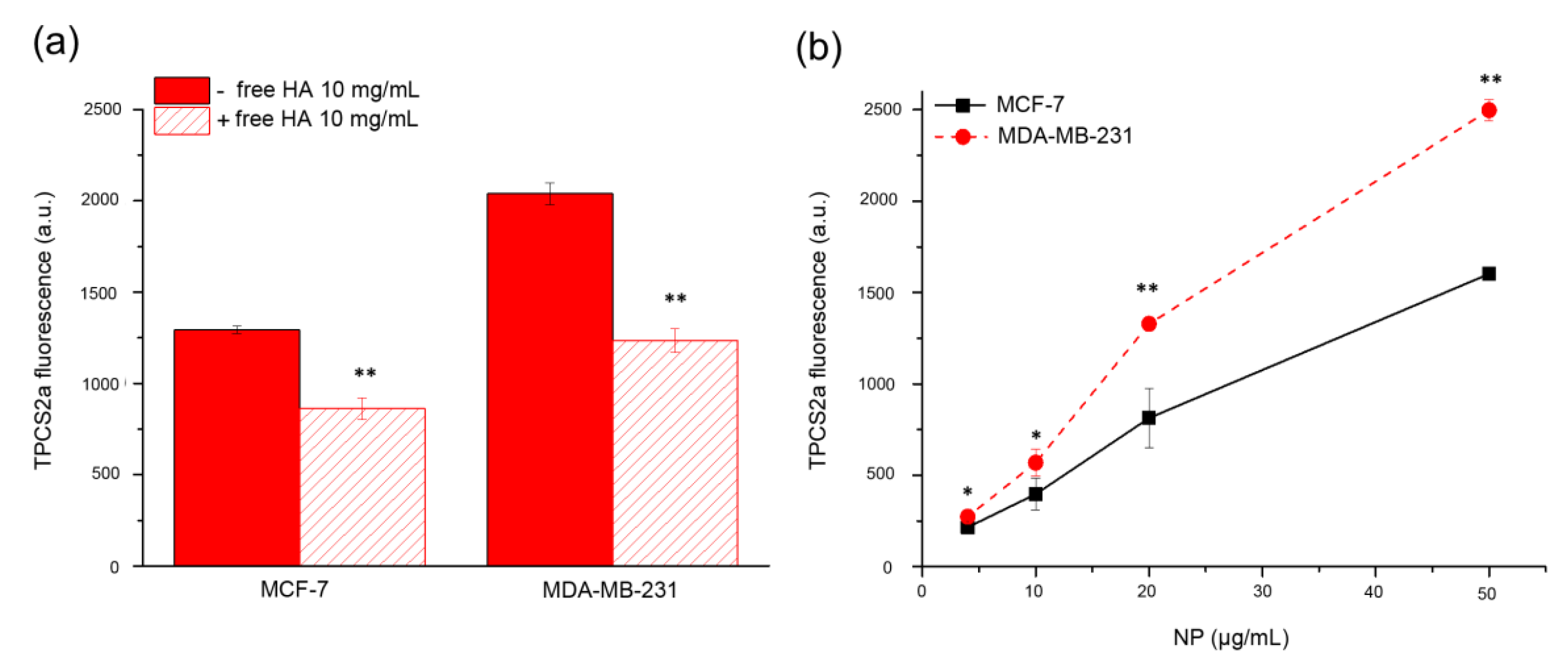
The increase of internalized drugs due to CD44 targeting by the HA coating of NPs coupled with the concomitant slow release inside the cells were identified as key factors for the increased performances of the nanoformulation with respect to free drugs. Since many BC cells and BCSCs are characterized by high expression of CD44 while others show low expression, we decided to select MDA-MB-231 as CD44
+ and adenocarcinoma MCF-7 as CD44
− cells, respectively (
Figure S4), to study receptor-mediated endocytosis of NPs. As shown in
Figure 1, the different level of CD44 expression in these two cell lines correlated with the different extent of cellular uptake of HA-NPs, indicating a clear contribution of HA targeting and CD44-mediated delivery, leading to increased PS internalization in MDA-MB-231 with respect to MCF-7 cells (
Figure 1b). This is well supported by the competition experiments (
Figure 1a) demonstrating that cell pre-incubation with an excess of free HA to saturate CD44 receptors inhibited TPCS
2a uptake to higher extent in MDA-MB-231 compared to MCF-7 as expected based on the expression level of the receptor. Similarly, Gao and co-workers reported the CD44-mediated uptake of the PS chlorin e6 loaded in HA nanocomplexes in CD44
+ HT-29 cells in vitro and in HT-29 tumor bearing mice [
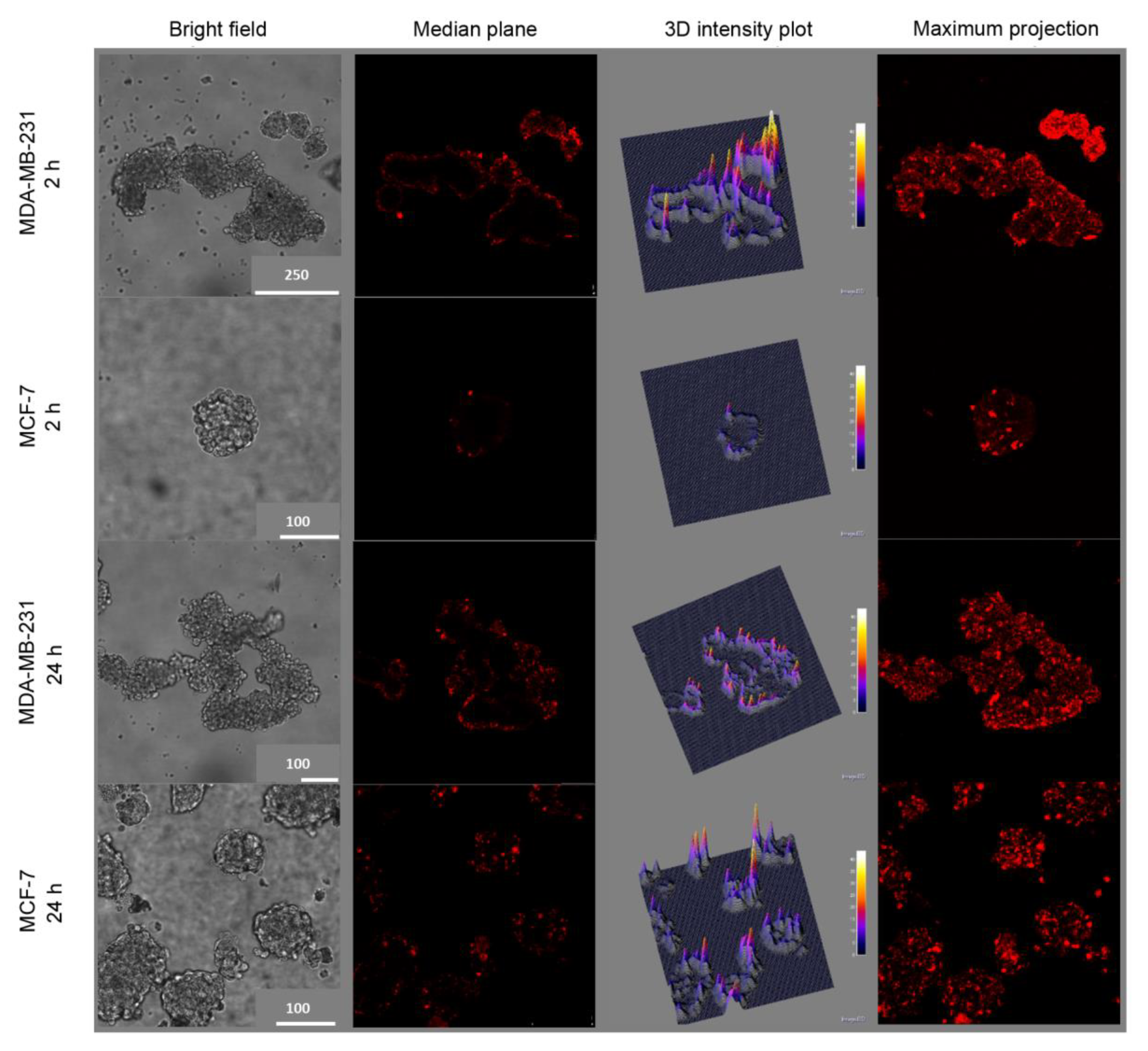
31]. The targeting capability of HA-NPs was observed also in mammospheres (
Figure 5), a 3D in vitro culture model enriched of BCSCs. In line with the fact that in the first generation of mammospheres about 97% of MDA-MB-231 cells were CD44
high/CD24
low while only approximately 6% of MCF-7 showed this expression profile (
Figure S2), after 2 h incubation, the uptake of HA@TPCS
2a in MDA-MB-231 was significantly higher than in MCF-7 cells (
Figure 5). As found with other tridimensional in vitro models and other PSs [
14,
32], TPCS
2a localized mainly in the more external cells layers of the mammospheres, and penetrated deeper at increasing incubation time. Weak penetration into the core of MCF-7 mammospheres was observed also with coumarin-loaded HA hybrid NPs [
33].
To study the efficacy of the combination of DTX-chemotherapy and TPCS
2a-PDT in reducing cell stemness and to effectively eradicate BCSCs, we used the mammosphere-forming assay, as a convenient in vitro approach to estimate BC cell potential to behave like “stem cells” or ”tumor initiating” cells (TICs) [
34]. Toward this aim, we have determined MFE of cells that had been treated as monolayers (protocol 1) or as mammospheres of first generation (protocol 2). As protocol 1 is concerned, we have preliminarily determined the impact of chemotherapy/PDT combination in CD44
low MCF-7 cells cultured as monolayers. Differently from monolayers of MDA-MB-231 cells previously studied [
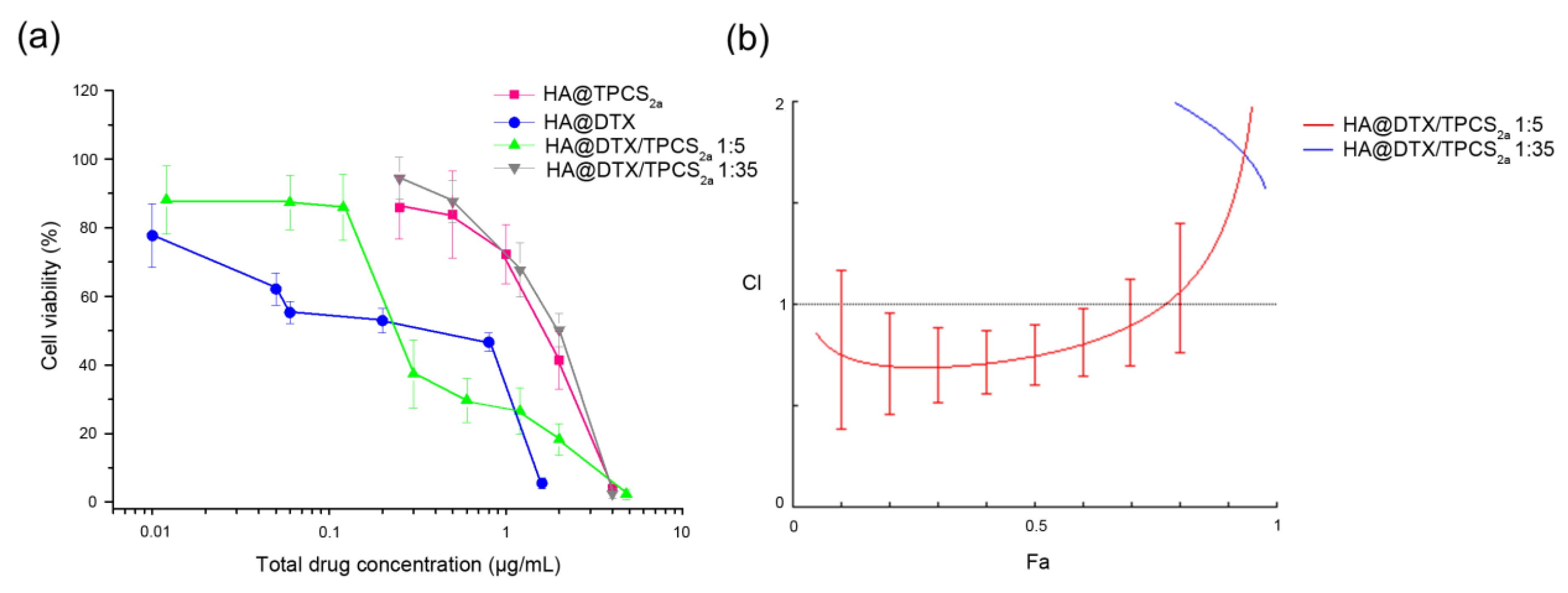
16], HA-NPs gave synergistic killing of MCF-7 exclusively if DTX loading inside NPs was increased (
Figure 2b), thus modifying DTX:TPCS
2a ratio from 1:35 to 1:5. It is worth to note, that the increased drug ratio did not affect NP stability and drug entrapment efficiency (>95% DTX and TPCS
2a, see
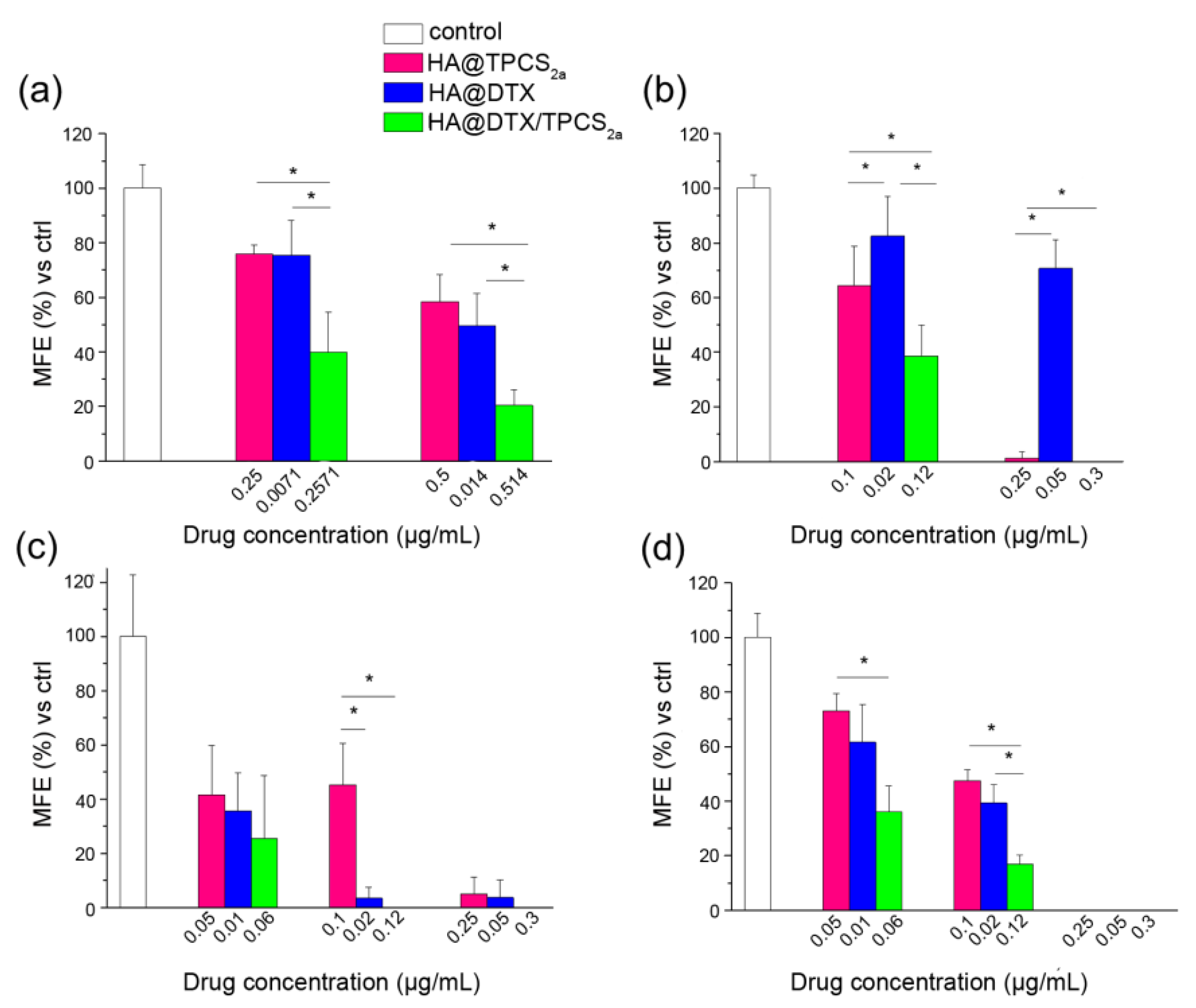
Table 1). When we treated cells cultured as monolayer and then re-propagated them as mammospheres, we observed a clear decrease of the MFEs in both cell lines and, especially in MDA-MB-231, the reduction with combined with respect to single treatments was remarkable (
Figure 3a,c). In fact, an MFE higher than 50% was maintained when MDA-MB-231 cells were exposed to monotherapies while the same drugs co-delivered through HA-NPs lowered MFE to less than 50%. Generally, the MFE measured in MCF-7 was significantly lower than that observed in MDA-MB-231, but lower was also the impact of combination therapy on MFE reduction. It can be speculated that a correlation occurs between the cytotoxicity induced in monolayered cells and the reduction of sphere-forming capacity. Interestingly, analyzing cell viability and MFE values in the two cell lines we observed that, while in MCF-7 sub lethal doses (e.g., 0.05 μg/mL TPCS
2a, 0.01 μg/mL DTX; cell viability around 90–80%) of drugs decreased MFE to less than 40%, in MDA-MB-231 cells the same extent of reduction of sphere-forming capacity was observed with drug doses close to IC
50 (
Table S2 and [
16]) (e.g., 0.5 μg/mL TPCS
2a, 0.014 μg/mL DTX). The higher stemness potential demonstrated by survived MDA-MB-231 cells very likely correlates with the intrinsic high metastatic potential of triple negative cells, while the adenocarcinoma MCF-7 cell line is considered non metastatic [
35]. It has been also reported that tumor aggressiveness and metastatic potential are associated with elevated ALDH expression, especially in the case of TNBC [
36]. This evidence is confirmed in our models by the higher percentage of ALDH
+ cells counted in untreated mammospheres of MDA-MB-231 with respect to MCF-7 (
Figure 4) and, is in agreement with the observations of Laranjo and co-workers that reported lower ALDH expression in MCF-7 mammospheres with respect to those derived from TNBC HCC1806 cells [
37]. It must also be pointed out that the higher IC
50 for DTX measured in MCF-7 monolayers with respect to MDA-MB-231 (
Tables S1 and S2) does not correlate with a higher self-renewal/stemness capacity.
Using protocol 2, we treated first generation mammospheres and measured MFEs in the second generation. Basically, by adopting this protocol we tried to simulate the in vivo tumor environment by reproducing the tridimensional arrangement and the BCSC enrichment. As for the first generation mammospheres obtained from cells treated in monolayer, lower MFE values were measured for MCF-7 with respect to MDA-MB-231 cells, at the same drug doses (
Figure 3b,d). The combination therapy was clearly more efficient than each single therapy in MCF-7; this was less evident in MDA-MB-231 mammospheres because of high and low sensitivity of the CSC to PDT and DTX, respectively. The high MFE measured following HA@DTX-NP therapy is very likely in agreement with a more specific activity of taxanes toward non-BCSCs rather than toward BCSCs [
28,
29,
38]. In this context, Zhang and colleagues reported that in xenografts of MDA-MB-231Luc treated with DTX (dose approximately IC
50), the MFE of isolated cells was even higher than that of cells that had been treated with the vehicle alone, due to an increase of TICs after the treatment [
39]. The same DTX-treated cells re-implanted into mice gave rise to tumors whose size was increased by 50% with respect to those derived from cells treated with vehicle only. On the other hand, TPCS
2a-based PDT seems to target more indistinctly all BC populations. Accordingly, Usacheva and coworkers reported an efficient cytotoxicity of methylene blue (MB) NP-mediated PDT toward proliferating and BCSCs, under normoxic and hypoxic conditions [
40]. In addition, in a very recent in vitro study, Abrahamse et al. reported the efficacy of aluminum phthalocyanine (AlPcS
mix)-based PDT for killing not only the bulk cervical cancer cell population but also the CSC population, isolated from HeLa cultured cells [
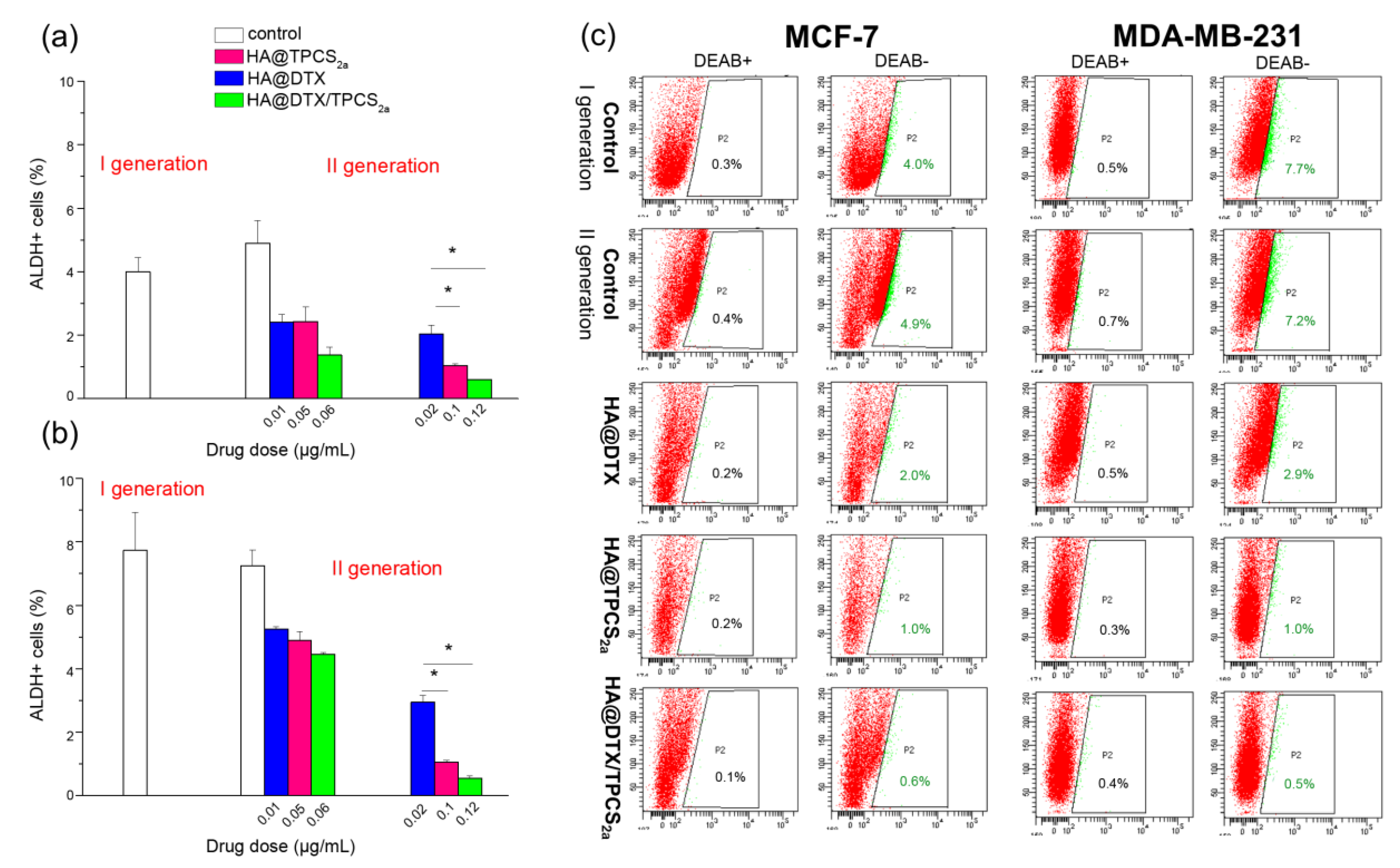
41]. The reduction of MFE that we observed in second generation mammospheres might correlate with a decrease in the numbers of BCSCs or BCSC-like cells in HA-NP treated first generation mammospheres. Accordingly, ALDEFLUOR assay results (
Figure 4) showed a significant correlation with MFE data, since a significant decrease of ALDH
+ (e.g., BCSCs) cells was observed. Of note, using HA@DTX/TPCS
2a−NPs we were able to completely eradicate the BCSC population in both cell lines, using the total drug dose of 0.12 μg/mL. Similarly, in MCF-7 cells, BCSC population identified by CD44 and CD24 immunostaining is reduced to the level of untreated first generation after treatments (
Figure S6). BCSC population decrease/eradication using HA-NPs loaded with DTX/TPCS
2a combination represents the key finding of the present work. In fact, we have demonstrated that DTX-resistance found especially in CSC-enriched MDA-MB-231 mammospheres could be eliminated by a combined PDT treatment. On the other hand, the same combination demonstrated superior efficacy than each single therapy also toward proliferating/differentiated BC cells. Of note, those BC cells that survived to combination therapy possess significantly lower self-renewal, stemness potential and sphere-forming capacity.
4. Materials and Methods
4.1. Chemicals and Reagents
TPCS2a was provided by PCI Biotech AS (Oslo, Norway). DTX was purchased from LC Laboratories (Woburn, MA, USA). Poly (D,L-lactide-co-glycolide) (PLGA) (50:50 Resomer RG 502H inherent viscosity 0.16−0.24 dL/g) was purchased from Boehringer Ingelheim (Ingelheim am Rhein, Germany). PEI (MW = 10–25 kDa branched) and Poloxamer 188 (Pluronic® F68) were purchased from Sigma-Aldrich (St. Louis, MI, USA). Acetonitrile and acetone were purchased from Carlo Erba Reagenti (Milan, Italy). Hyaluronic acid (HA, MW < 10 kDa) was a kind gift of Magaldi Life S.r.l. (Salerno, Italy). Dulbecco’s modified Eagle’s medium (DMEM), DMEM/F12 medium, fetal bovine serum (FBS) and B27 mix were purchased from Life Technologies (Milan, Italy).
4.2. Preparation and Characterization of NPs
HA@DTX/TPCS
2a-NPs co-loaded with DTX and TPCS
2a were prepared by a layer-by-layer deposition method as previously described by us [
13,
16]. Briefly, DTX-NPs were prepared by solvent diffusion of an organic phase (10 mg of PLGA and 50 µg or 7.5 µg of DTX in 2 mL of acetone, for obtaining the DTX/TPCS
2a ratio 1:5 and 1:35, respectively) in an aqueous phase (4 mL of water added with Pluronic F68 0.1%). After solvent evaporation, the dispersion was split into four Eppendorf tubes and centrifuged at 5000×
g for 15 min. NPs in each Eppendorf were coated with 125 μL of a PEI water solution (1 mg/mL), re-centrifuged at 2800×
g for 15 min, and then 25 µL of a TPCS
2a water solution (0.4 mg/mL) was added. Thereafter, NPs were coated with a layer of HA through the addition of 100 µL of a HA water solution (1 mg/mL) in each Eppendorf tube maintaining a constant interval of 15 min between each addition. The NP preparations were freeze-dried for 24 h and the recovery yield of the production process was evaluated on an aliquot of NPs by weighting the solid residue. Results of the quantification are expressed as the ratio of the actual NP weight to the theoretical polymer plus drug weight × 100. Unloaded NPs, HA@DTX-NPs and HA@TPCS
2a-NPs were prepared for control experiments. NP characterization was carried out as described in ref. [
16].
4.3. In Vitro Release of TPCS2a and DTX
The release of TPCS
2a was followed at 37 °C; 1 mg of NPs was dissolved in 1 mL of DMEM added with 10% FBS. At predetermined time intervals, the sample was centrifuged at 17000×
g for 20 min. TPCS
2a release was assessed in the supernatant by spectrophotometry at wavelength of 414 nm. A TPCS
2a calibration curve in the range 0.05–5 μg/mL was constructed in the release medium. The results from three independent experiments are expressed as % release vs. time ± SD. Release of DTX was assessed in 10 mM phosphate buffer saline (PBS) at pH 7.4 containing NaCl (137 mM) and KCl (2.7 mM) by a dialysis method. Four mg of NPs were dispersed in 0.5 mL DMEM + 10% FBS and placed in a dialysis bag Spectra/Por® (MWCO = 3500 Da)(Thermo Fisher Scientific, Waltham, MA, USA). The sample was plunged in 3 mL of PBS (sink condition) and kept at 37 °C up to 72 h. At selected time intervals, 1 mL of release medium was withdrawn and replaced with an equal volume of fresh medium. As control, release profiles of DTX dissolved in EtOH and added of DMEM + 10% FBS were assessed by HPLC as previously reported in [
16]. Results are expressed as release % vs. time ± SD of three experiments.
4.4. Cell Lines
MCF-7 and MDA-MB-231 human breast cancer cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were grown in DMEM with GlutamaxTM (Life Technologies, Milan, Italy) supplemented with 10% heat inactivated FBS, 100 U/mL streptomycin, and 100 µg/mL penicillin G and maintained at 37 °C under a humidified atmosphere containing 5% CO2.
4.5. Uptake of HA@TPCS2a-NPs in Monolayered Cancer Cells
To study the role of CD44 in the uptake of NPs, 5 × 104 cells were grown in 24-well plates for 24 h and incubated for 2 h with 50 μg/mL of NPs. In competition experiments, some samples were pre-incubated for 1 h with 10 mg/mL of free HA in order to saturate the CD44 receptor binding sites before NP addition. At the end of the incubation time, the cells were washed twice with Versene, (Life Technologies, Milan, Italy) detached from the plates with trypsin that was neutralized by the addition of FBS. Cells were centrifuged and resuspended in Versene before measuring TPCS2a fluorescence using a BD FACSCantoTM II flow cytometer (Becton Dickinson, San Jose, CA, USA). A blue laser at 488 nm was used to excite TPCS2a and its fluorescence was detected at wavelengths longer than 670 nm (PerCP channel); for each sample 105 events were acquired and analyzed using the FACSDiva software. The uptake of TPCS2a as a function of NP concentration was measured in cells seeded and handled as described above and incubated for 24 h with increasing concentrations of HA@TPCS2a-NPs.
4.6. Cytotoxicity and Determination of Combination Index (CI)
Cytotoxicity experiments in MCF-7 cells were performed as described in [
16] for MDA-MB-231 cells and the reduction of cell viability was measured with the MTS assay after single or combined treatments. The treatments were carried out with HA@NP-loaded drugs and free drugs for comparison. Briefly, 8000 cells/well were seeded in 96-well plates and, after 24 h of cell growth, the medium was replaced with fresh medium containing increasing concentrations of TPCS
2a or DTX or their combination delivered in free form or entrapped in NPs (DTX/TPCS
2a ratio 1:35 and 1:5, w/w). Cell viability was measured after 24 h of drug incubation in the dark (time point 24 h) as well as after an additional 24 h in which the cells were kept in drug-free medium (time point 24 + 24 h). For photo-toxicity experiments (PDT), cells were seeded and treated as described above, and at the end of the 24 h of drug incubation, cells were washed twice with PBS Ca
2+ and Mg
2+ and irradiated in PBS with a total fluence of 1 J/cm
2 of red light (600−800 nm) emitted from a Waldmann PDT 1200 lamp (Waldmann Medizintechnik, Villingen-Schwenningen, Germany). The power density was 15 mW/cm
2 as measured with the radiometer IL 1700 (International Light, Newburyport, MA, USA). Immediately after irradiation, the cells were brought back to the incubator after the replacement of PBS with fresh medium. Cell viability was measured using the MTS test after additional 24 h (phototoxicity; time point 24 + 24 h). Furthermore, in order to assess if the interaction of DTX-chemotherapy and TPCS
2a-PDT resulted in a synergic effect, CI values were calculated using the CompuSyn software (ComboSyn Inc., Paramus, NJ, USA), based on the Chou and Talalay method [
25]. With the experimental data obtained from the cell viability curves, we calculated the fraction affected (Fa) values for each drug concentration tested and analyzed the data on the Compusyn software as already described [
16]. For each drug and drug combination, the software calculated also the drug concentration that inhibits cell survival by 50% (IC
50 or Dm value).
4.7. Generation and Treatment of Mammospheres
MCF-7 and MDA-MB-231 cells were trypsinized from T-75 cm2 flasks and then plated in 24-well ultralow attachment flat-bottom plates (Corning, New York, NY, USA) at a density of 5000 and 100,000 viable cells/mL, respectively. Seeding was performed in serum-free DMEM-F12 supplemented with B27 (1:50), 20 ng/mL epidermal growth factor (EGF), 20 ng/mL basic fibroblast growth factor (bFGF) (Peprotech, London, UK), and 5 µg/mL insulin (Sigma Aldrich). MCF-7 and MDA-MB-231 cells were grown for 7 and 4 days, respectively, to form the first generation of mammospheres. To re-propagate the second generation of mammospheres, the first generation mammospheres were collected, gently centrifuged (123× g, 10 min) and dissociated into single cells by using 0.25% trypsin/EDTA (Life Technologies). The dissociated cells were re-seeded as indicated for the first generation mammospheres in order to obtain the corresponding second generation. The number of mammospheres formed in each generation was evaluated by counting the number of spheres in the images acquired by bright field microscopy (DMI4000 Leica microscope, Wetzlar, Germany). The mean diameter of each mammosphere was calculated with the LAS AF Lite software, and spheres with a diameter below 100 µm were excluded from counting. For assessing the effects of single and combined therapies using HA-NPs toward BCSCs two different treatment protocols were used. In protocol 1, cells were seeded and treated in monolayer and, immediately after treatment, were re-seeded to form first generation mammospheres. Thus, according to this protocol, cells (5 × 105) were seeded in 60 mm tissue culture dishes and, after 24 h of growth, were treated with DTX or/and TPCS2a delivered in HA-NPs. At the end of the incubation, cells were irradiated in PBS (1 J/cm2 of red light) and, immediately after irradiation, were detached from plates and re-seeded in ultra-low attachment plates to allow the formation of the first generation mammospheres. Mammosphere formation efficiency (MFE) was calculated as the number of spheres counted in the first generation, divided by the number of seeded cells, and expressed as percentage means ± SD. In protocol 2, the first generation mammospheres were formed and incubated for 24 h, directly in ultra-low attachment plates, with DTX or/and TPCS2a delivered in HA-NPs. At the end of the incubation time, the spheres were irradiated with 1 J/cm2 of red light, when PDT was part of the treatment, and, immediately after irradiation, were dissociated and re-seeded in non-adherent condition to allow the formation of second-generation mammospheres.
Mammosphere formation efficiency (MFE) was calculated as the number of spheres counted in the second generation, divided by the number of cells seeded, and expressed as percentage (means ± SD).
4.8. Analysis of BCSC Population in Mammospheres
CD24 and CD44 immunostaining: First or second generation mammospheres were collected and enzymatically dissociated into single cells; 105 cells were collected, washed twice with cold PBS, and kept in ice. In parallel, the same number of cells was collected from monolayer cells routinely cultured on T-75 cm2 flasks. After a second step of washing, a combination of monoclonal antibodies against human CD44 (FITC-conjugated) and CD24 (PE-conjugated) (BD Biosciences, San Diego, CA, USA) was added to the cell suspension and incubated at 4 °C in the dark for 30–40 min following manufacturer’s protocol. Labeled cells were washed with PBS to eliminate unbound antibodies and analyzed by flow cytometry to count the percentage of CD44high/CD24low cells.
4.9. Aldehyde Dehydrogenase (ALDH) Activity Assay
ALDH enzymatic activity in first- and second-generation mammospheres was measured using AldeFluor Kit (StemCell Technologies, Durham, NC, USA), following the manufacturer’s protocol. Briefly, mammospheres were dissociated into single cells and cells were then re-suspended in Aldefluor Buffer and stained with activated Aldefluor Reagent. As control for background fluorescence, for each sample, 500 µL of cell suspension were transferred in a tube containing diethylaminobenzaldehyde (DEAB), a specific inhibitor of ALDH. After incubation for 40 min at 37 °C, cells were centrifuged at 250× g for 5 min, re-suspended in 500 µL of Aldefluor Buffer, and analyzed with the flow cytometer.
4.10. Uptake of HA@TPCS2a-NPs in Mammospheres
First generation mammospheres were generated as described above and incubated for 2 or 24 h with 1.25 µg/mL of TPCS2a loaded in HA@ NPs (50 µg/mL). The localization of TPCS2a was evaluated by confocal microscopy (Leica SP5) by transferring the mammospheres from 24-well/plates to 35 mm cell imaging dishes (Ibidi, Gräfelfing, Germany) and washing them twice with PBS before visualization. Images were acquired from the top to the bottom of the mammosphere in about 20 different focal planes (z-stack 10 µm). Furthermore, a 3D reconstruction of the distribution of the fluorescence signal in the equatorial plane of the mammospheres was obtained using the software ImageJ and a maximum projection image obtained by superimposition of 20 focal plains using the software LAS AF Lite (Leica).
4.11. Statistical Analysis
The Primer software for biostatistics (McGraw-Hill, Columbus, OH, USA) was used for statistical analysis of the data. The data are expressed as means ± standard deviations (SD) for at least two independent experiments, carried out in triplicate. The difference between two groups of treatments was evaluated by Student’s t test while the differences between more than two groups of treatment was evaluated with one-way ANOVA test with the Bonferroni’s correction and was considered significant for p < 0.05.