Prognostic Value of the Diversity of Nuclear Chromatin Compartments in Gynaecological Carcinomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohorts
2.2. Sample Preparation
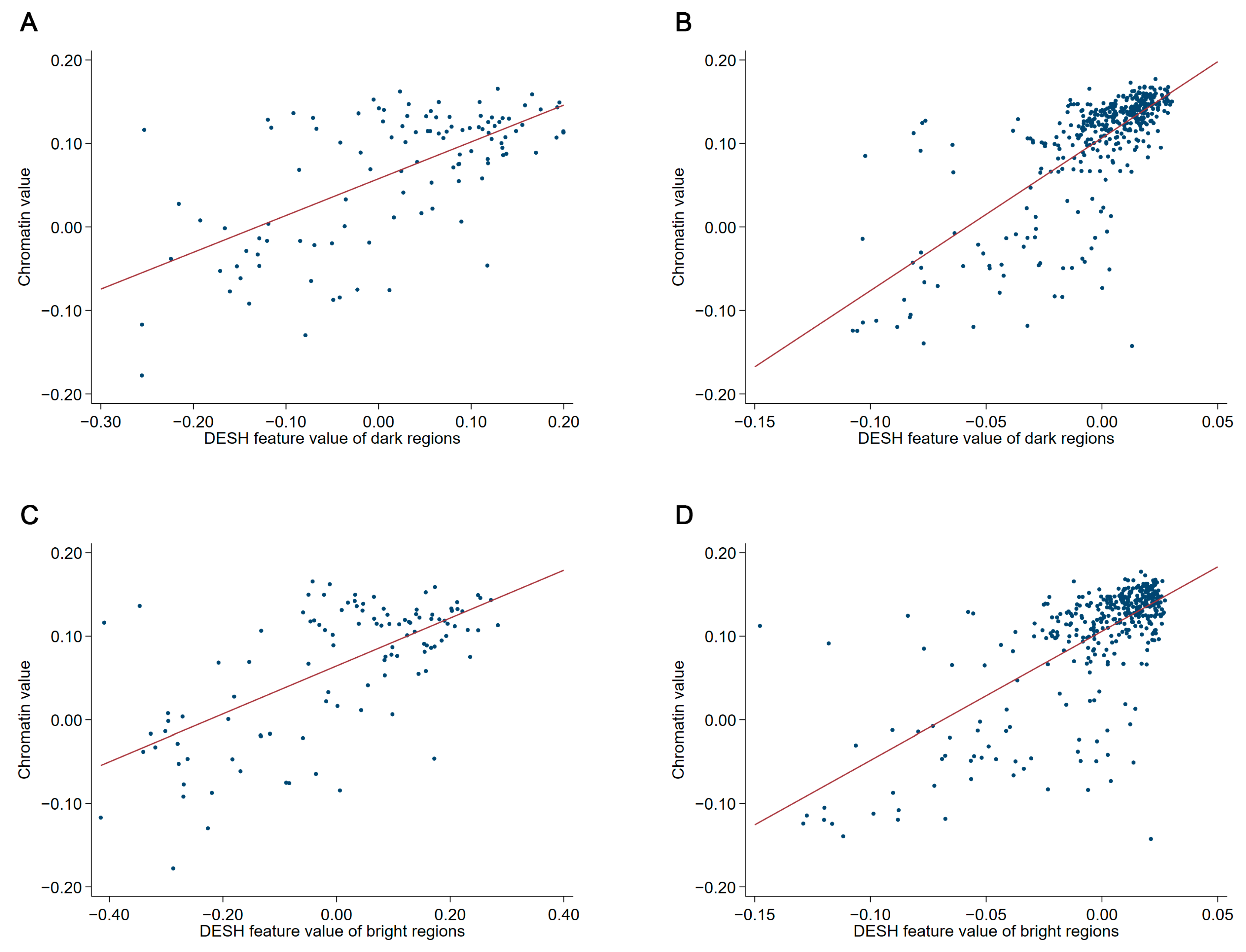
2.3. Quantification of Diversity of Chromatin Compartments
2.3.1. Segmentation of Chromatin Compartments
2.3.2. Analysis of Chromatin Compartments
2.3.3. Dual Entropy Sum Histogram
2.3.4. Marker of Diversity of Chromatin Compartments
2.4. Statistical Analyses
2.5. Data Availability Statement
3. Results
3.1. Patient Characteristics
3.2. Size and Number of Chromatin Compartments
3.3. Relative Prognostic Value of Markers of Chromatin Entropy
3.4. Classification Performance
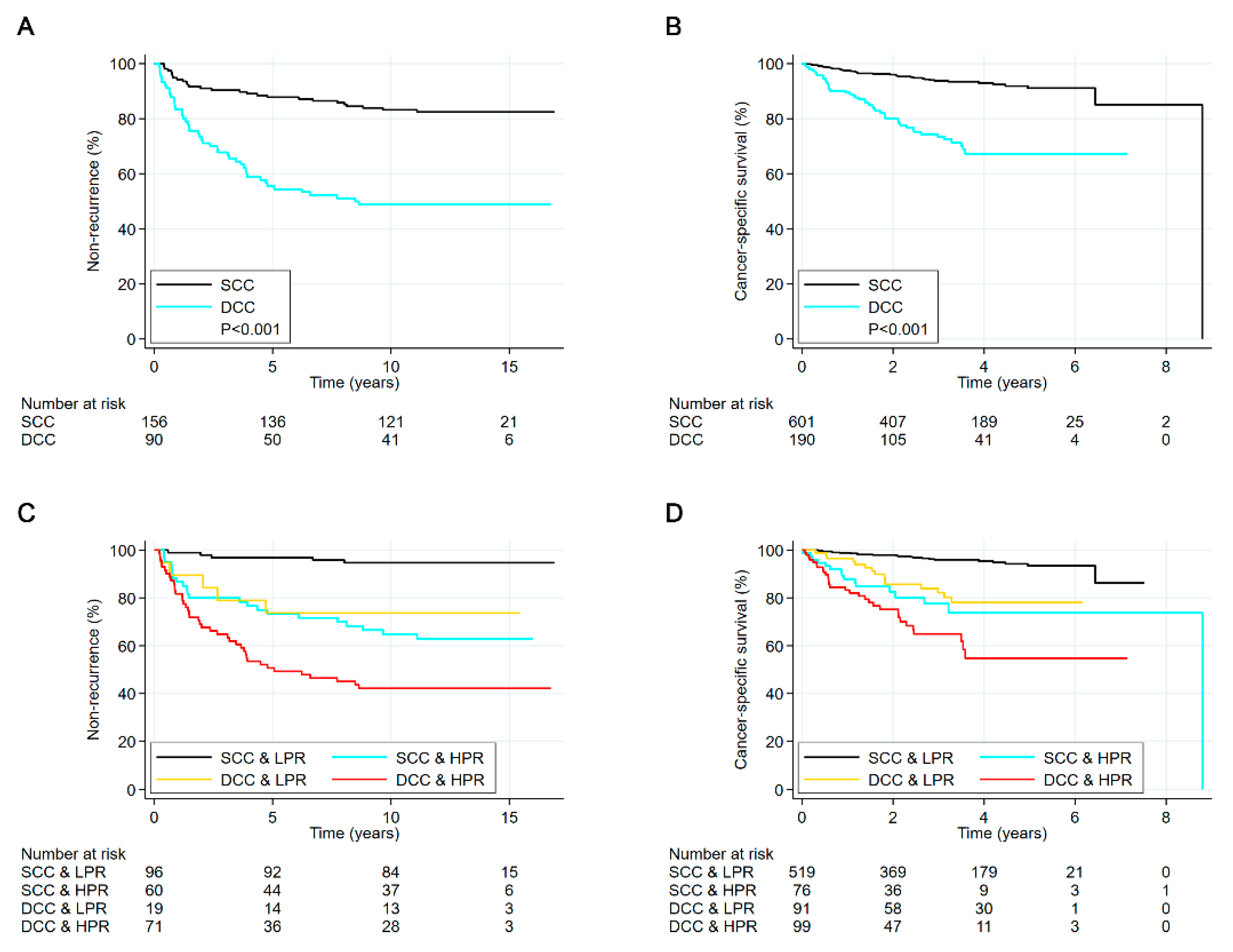
3.5. Survival Analyses of Entire Cohorts
3.6. Survival Analyses in Subgroups of Chromatin Heterogeneity
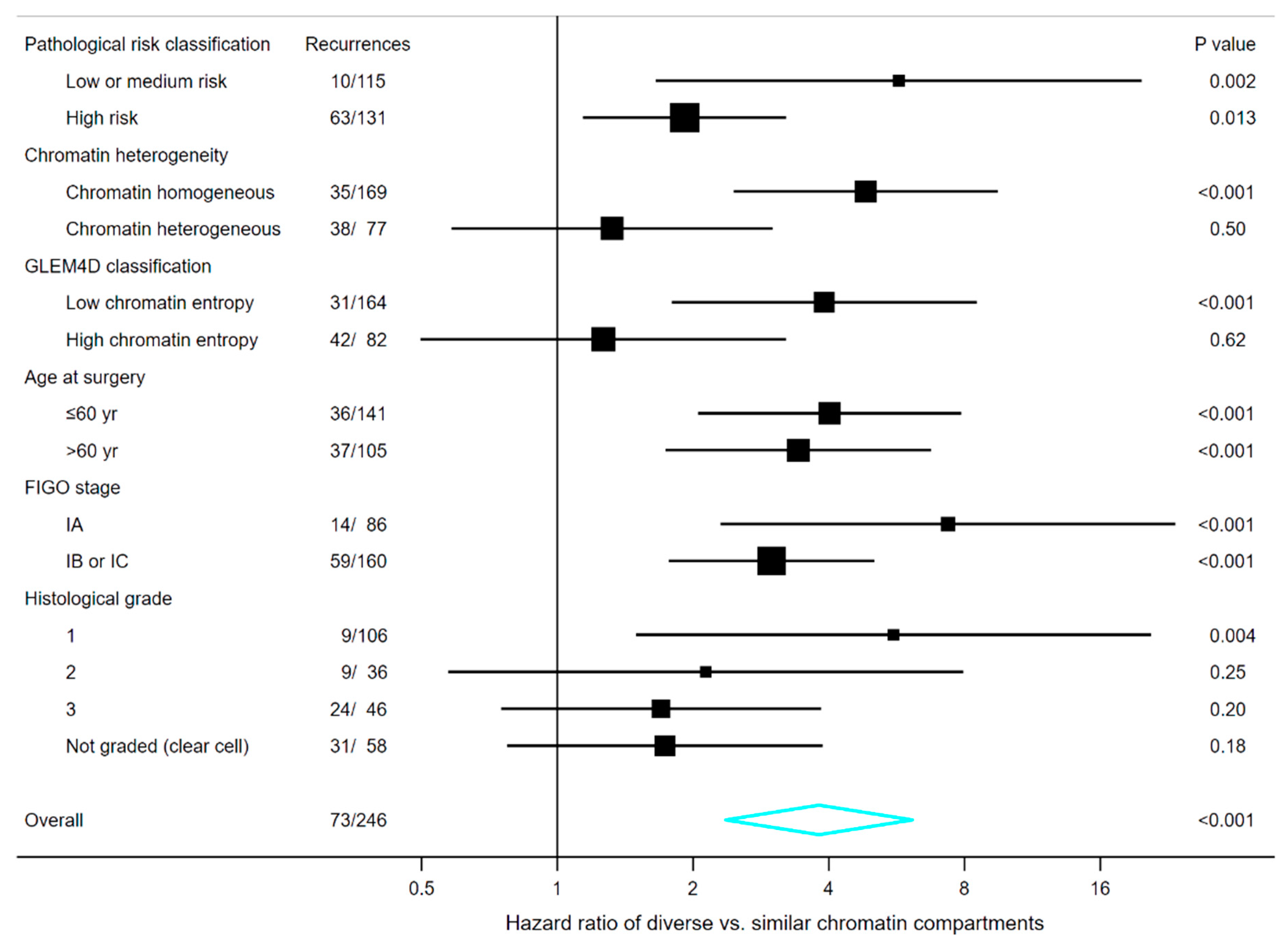
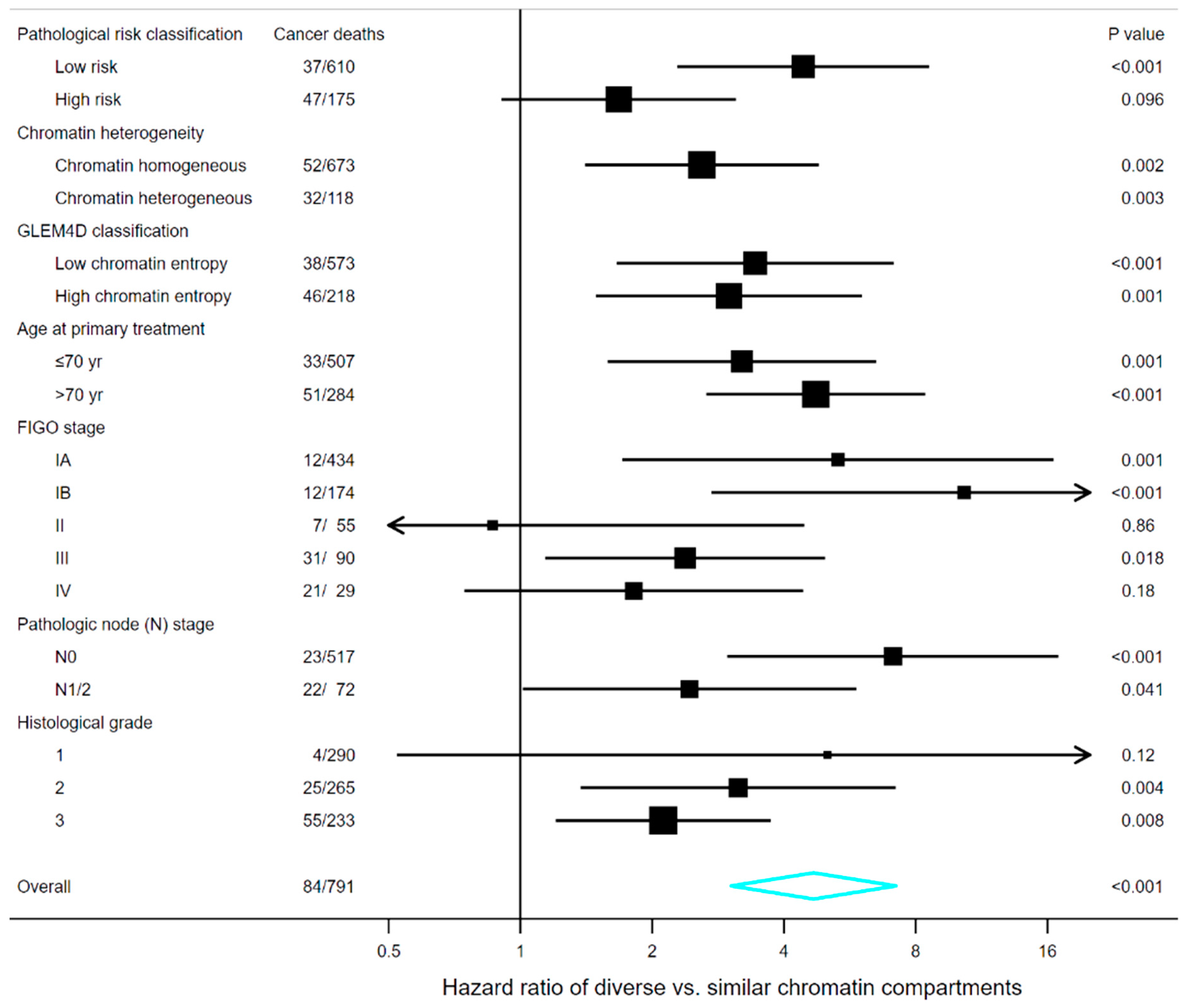
3.7. Survival Analyses in Subgroups of Clinical and Pathological Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dixon, J.R.; Jung, I.; Selvaraj, S.; Shen, Y.; Antosiewicz-Bourget, J.E.; Lee, A.Y.; Ye, Z.; Kim, A.; Rajagopal, N.; Xie, W.; et al. Chromatin architecture reorganization during stem cell differentiation. Nature 2015, 518, 331–336. [Google Scholar] [CrossRef]
- Zheng, H.; Xie, W. The role of 3D genome organization in development and cell differentiation. Nat. Rev. Mol. Cell Biol. 2019, 20, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Lupiáñez, D.G.; Spielmann, M.; Mundlos, S. Breaking TADs: How Alterations of Chromatin Domains Result in Disease. Trends Genet. 2016, 32, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Schuster-Böckler, B.; Lehner, B. Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature 2012, 488, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Polak, P.; Karlić, R.; Koren, A.; Thurman, R.; Sandstrom, R.; Lawrence, M.S.; Reynolds, A.; Rynes, E.; Vlahovicek, K.; Stamatoyannopoulos, J.A.; et al. Cell-of-origin chromatin organization shapes the mutational landscape of cancer. Nature 2015, 518, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kleppe, A.; Albregtsen, F.; Vlatkovic, L.; Pradhan, M.; Nielsen, B.; Hveem, T.S.; Askautrud, H.A.; Kristensen, G.B.; Nesbakken, A.; Trovik, J.; et al. Chromatin organisation and cancer prognosis: A pan-cancer study. Lancet Oncol. 2018, 19, 356–369. [Google Scholar] [CrossRef]
- Yang, L.; Chen, P.; Zhang, L.; Wang, L.; Sun, T.; Zhou, L.; Li, Z.; Wu, A. Prognostic value of nucleotyping, DNA ploidy and stroma in high-risk stage II colon cancer. Br. J. Cancer 2020, 123, 973–981. [Google Scholar] [CrossRef]
- Yogesan, K.; Jørgensen, T.; Albregtsen, F.; Tveter, K.J.; Danielsen, H.E. Entropy-based texture analysis of chromatin structure in advanced prostate cancer. Cytometry 1996, 24, 268–276. [Google Scholar] [CrossRef]
- Nielsen, B.; Albregtsen, F.; Kildal, W.; Abeler, V.M.; Kristensen, G.B.; Danielsen, H.E. The prognostic value of adaptive nuclear texture features from patient gray level entropy matrices in early stage ovarian cancer. Anal. Cell. Pathol. 2012, 35, 305–314. [Google Scholar] [CrossRef]
- Boone, C.W.; Sanford, K.K.; Frost, J.K.; Mantel, N.; Gill, G.W.; Jones, G.M. Cytomorphologic evaluation of the neoplastic potential of 28 cell culture lines by a panel of diagnostic cytopathologists. Int. J. Cancer 1986, 38, 361–367. [Google Scholar] [CrossRef]
- Danielsen, H.E. Premalignant Changes in DNA Organization in Mouse Liver After Diethylnitrosamine Treatment. Ph.D. Thesis, University of Oslo, Oslo, Norway, 1991; p. 40. [Google Scholar]
- Deligdisch, L.; Miranda, C.; Barba, J.; Gil, J. Ovarian Dysplasia: Nuclear Texture Analysis. Cancer 1993, 72, 3253–3257. [Google Scholar] [CrossRef]
- Zink, D.; Fischer, A.H.; Nickerson, J.A. Nuclear structure in cancer cells. Nat. Rev. Cancer 2004, 4, 677–687. [Google Scholar] [CrossRef]
- Nandakumar, V.; Kelbauskas, L.; Johnson, R.; Meldrum, D. Quantitative Characterization of Preneoplastic Progression Using Single-Cell Computed Tomography and Three-Dimensional Karyometry. Cytometry A 2011, 79, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Kelbauskas, L.; Hernandez, K.F.; Lintecum, K.M.; Senechal, P.; Bussey, K.J.; Davies, P.C.W.; Johnson, R.H.; Meldrum, D.R. Isotropic 3D Nuclear Morphometry of Normal, Fibrocystic and Malignant Breast Epithelial Cells Reveals New Structural Alterations. PLoS ONE 2012, 7, e29230. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, G.B.; Kildal, W.; Abeler, V.M.; Kaern, J.; Vergote, I.; Tropé, C.G.; Danielsen, H.E. Large-scale genomic instability predicts long-term outcome for women with invasive stage I ovarian cancer. Ann. Oncol. 2003, 14, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Njølstad, T.S.; Trovik, J.; Hveem, T.S.; Kjæreng, M.L.; Kildal, W.; Pradhan, M.; Marcickiewicz, J.; Tingulstad, S.; Staff, A.C.; Haugland, H.K.; et al. DNA ploidy in curettage specimens identifies high-risk patients and lymph node metastasis in endometrial cancer. Br. J. Cancer 2015, 112, 1656–1664. [Google Scholar] [CrossRef]
- Pecorelli, S.; FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Hveem, T.S.; Njølstad, T.S.; Nielsen, B.; Syvertsen, R.A.; Nesheim, J.A.; Kjæreng, M.L.; Kildal, W.; Pradhan, M.; Marcickiewicz, J.; Tingulstad, S.; et al. Changes in Chromatin Structure in Curettage Specimens Identifies High-Risk Patients in Endometrial Cancer. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 61–67. [Google Scholar] [CrossRef]
- Tanke, H.J.; van Ingen, E.M. A reliable Feulgen-acriflavine-SO2 staining procedure for quantitative DNA measurements. J. Histochem. Cytochem. 1980, 28, 1007–1013. [Google Scholar] [CrossRef]
- Niblack, W. An Introduction to Digital Image Processing, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1986; pp. 115–116. [Google Scholar]
- Yanowitz, S.D.; Bruckstein, A.M. A New Method for Image Segmentation. Comput. Vis. Graph. Image Process. 1989, 46, 82–95. [Google Scholar] [CrossRef]
- Maître, H.; Bloch, I.; Sigelle, M. Spatial entropy: A tool for controlling contextual classification convergence. In Proceedings of the 1st International Conference on Image Processing, Austin, TX, USA, 13–16 November 1994; Volume 2, pp. 212–216. [Google Scholar] [CrossRef]
- Tupin, F.; Sigelle, M.; Maître, H. Definition of a spatial entropy and its use for texture discrimination. In Proceedings of the 2000 International Conference on Image Processing (Cat. No.00CH37101), Vancouver, BC, Canada, 10–13 September 2000; Volume 1, pp. 725–728. [Google Scholar] [CrossRef]
- Unser, M. Sum and Difference Histograms for Texture Classification. IEEE Trans. Pattern Anal. Mach. Intell. 1986, PAMI-8, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.F.; Jackway, P.T.; Longstaff, I.D. Recent Developments in the Use of the Co-occurrence Matrix for Texture Recognition. In Proceedings of the 13th International Conference on Digital Signal Processing, Santorini, Greece, 2–4 July 1997; Volume 1, pp. 63–65. [Google Scholar] [CrossRef]
- Albregtsen, F.; Nielsen, B.; Danielsen, H.E. Adaptive gray level run length features from class distance matrices. In Proceedings of the 15th International Conference on Pattern Recognition. ICPR-2000, Barcelona, Spain, 3–7 September 2000; Volume 3, pp. 738–741. [Google Scholar] [CrossRef]
- Albregtsen, F.; Nielsen, B. Texture Classification based on Cooccurrence of Gray Level Run Length Matrices. Aust. J. Intell. Inf. Process. Syst. 2000, 6, 38–45. [Google Scholar]
- Nielsen, B.; Albregtsen, F.; Danielsen, H.E. Low Dimensional Adaptive Texture Feature Vectors From Class Distance and Class Difference Matrices. IEEE Trans. Med. Imaging 2004, 23, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.; Albregtsen, F.; Danielsen, H.E. Statistical Nuclear Texture Analysis in Cancer Research: A Review of Methods and Applications. Crit. Rev. Oncog. 2008, 14, 89–164. [Google Scholar] [CrossRef] [PubMed]
- Raudys, S.J.; Jain, A.K. Small sample size effects in statistical pattern recognition: Recommendations for practitioners. IEEE Trans. Pattern Anal. Mach. Intell. 1991, 13, 252–264. [Google Scholar] [CrossRef]
- Michiels, S.; Koscielny, S.; Hill, C. Prediction of cancer outcome with microarrays: A multiple random validation strategy. Lancet 2005, 365, 488–492. [Google Scholar] [CrossRef]
- Punt, C.J.A.; Buyse, M.; Köhne, C.-H.; Hohenberger, P.; Labianca, R.; Schmoll, H.J.; Påhlman, L.; Sobrero, A.; Douillard, J.-Y. Endpoints in Adjuvant Treatment Trials: A Systematic Review of the Literature in Colon Cancer and Proposed Definitions for Future Trials. J. Natl. Cancer Inst. 2007, 99, 998–1003. [Google Scholar] [CrossRef]
- Nielsen, B.; Kleppe, A.; Hveem, T.S.; Pradhan, M.; Syvertsen, R.A.; Nesheim, J.A.; Kristensen, G.B.; Trovik, J.; Kerr, D.J.; Albregtsen, F.; et al. Association Between Proportion of Nuclei with High Chromatin Entropy and Prognosis in Gynecological Cancers. J. Natl. Cancer Inst. 2018, 110, 1400–1408. [Google Scholar] [CrossRef]
- Vergote, I.; Amant, F. Time to include high-risk early ovarian cancer in randomized phase III trials of advanced ovarian cancer. Gynecol. Oncol. 2006, 102, 415–417. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Kleppe, A.; Danielsen, H.E. Clinical utility of chromatin analysis. Oncotarget 2018, 9, 32406–32407. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Collinson, F.; Qian, W.; Fossati, R.; Lissoni, A.; Williams, C.; Parmar, M.; Ledermann, J.; Colombo, N.; Swart, A. Optimal treatment of early-stage ovarian cancer. Ann. Oncol. 2014, 25, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, H.B.; Haldorsen, I.S.; Trovik, J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012, 13, e353–e361. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.A.; Bohlke, K.; Powell, M.A.; Fader, A.N.; Franklin, G.E.; Lee, L.J.; Matei, D.; Coallier, L.; Wright, A.A. Postoperative Radiation Therapy for Endometrial Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J. Clin. Oncol. 2015, 33, 2908–2913. [Google Scholar] [CrossRef] [PubMed]

| Ovarian Carcinoma | Endometrial Carcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Marker | Set | BCCR | CCR | Sens. | Spec. | BCCR | CCR | Sens. | Spec. |
| Diversity of chromatin compartments | |||||||||
| Train | 71.7% | 72.4% | 70.3% | 73.2% | 67.5% | 77.6% | 54.5% | 80.5% | |
| Test | 66.8% | 69.9% | 57.1% | 76.5% | 66.9% | 76.3% | 55.0% | 78.8% | |
| All | 69.2% | 71.3% | 63.9% | 74.5% | 67.2% | 77.0% | 54.8% | 79.6% | |
| Chromatin heterogeneity | |||||||||
| Train | 65.7% | 70.9% | 54.1% | 77.3% | 64.0% | 81.9% | 40.9% | 87.0% | |
| Test | 63.2% | 67.0% | 51.4% | 75.0% | 61.8% | 83.2% | 35.0% | 88.7% | |
| All | 64.6% | 69.2% | 52.8% | 76.4% | 63.0% | 82.6% | 38.1% | 87.8% | |
| GLEM4D (for specific cancer type) | |||||||||
| Train | 70.1% | 72.4% | 64.9% | 75.3% | 66.0% | 74.9% | 54.5% | 77.4% | |
| Test | 63.9% | 68.0% | 51.4% | 76.5% | 64.5% | 72.0% | 55.0% | 73.9% | |
| All | 67.0% | 70.5% | 58.3% | 75.8% | 65.2% | 73.5% | 54.8% | 75.7% | |
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Marker | Variable Treatment | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Diversity of chromatin compartments | DCC vs. SCC | 3.8 (2.4–6.1) | <0.001 | 2.1 (1.3–3.5) | 0.004 |
| FIGO stage | IB or IC vs. IA | 2.6 (1.5–4.7) | 0.001 | 2.1 (1.2–3.8) | 0.014 |
| Histological grade | Grade 3 or clear cell vs. Grade 1 or 2 | 5.6 (3.3–9.6) | <0.001 | 4.0 (2.2–7.1) | <0.001 |
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Marker | Variable Treatment | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Diversity of chromatin compartments | DCC vs. SCC | 4.7 (3.0–7.2) | <0.001 | 2.4 (1.5–3.9) | 0.001 |
| Pathological risk classification | High risk vs. Low risk | 5.8 (3.8–9.0) | <0.001 | 3.3 (2.0–5.4) | <0.001 |
| Age at primary treatment | 1-year increment | 1.06 (1.04–1.09) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleppe, A.; Albregtsen, F.; Trovik, J.; Kristensen, G.B.; Danielsen, H.E. Prognostic Value of the Diversity of Nuclear Chromatin Compartments in Gynaecological Carcinomas. Cancers 2020, 12, 3838. https://doi.org/10.3390/cancers12123838
Kleppe A, Albregtsen F, Trovik J, Kristensen GB, Danielsen HE. Prognostic Value of the Diversity of Nuclear Chromatin Compartments in Gynaecological Carcinomas. Cancers. 2020; 12(12):3838. https://doi.org/10.3390/cancers12123838
Chicago/Turabian StyleKleppe, Andreas, Fritz Albregtsen, Jone Trovik, Gunnar B. Kristensen, and Håvard E. Danielsen. 2020. "Prognostic Value of the Diversity of Nuclear Chromatin Compartments in Gynaecological Carcinomas" Cancers 12, no. 12: 3838. https://doi.org/10.3390/cancers12123838
APA StyleKleppe, A., Albregtsen, F., Trovik, J., Kristensen, G. B., & Danielsen, H. E. (2020). Prognostic Value of the Diversity of Nuclear Chromatin Compartments in Gynaecological Carcinomas. Cancers, 12(12), 3838. https://doi.org/10.3390/cancers12123838






