Acute Conditioning of Antigen-Expanded CD8+ T Cells via the GSK3β-mTORC Axis Differentially Dictates Their Immediate and Distal Responses after Antigen Rechallenge
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
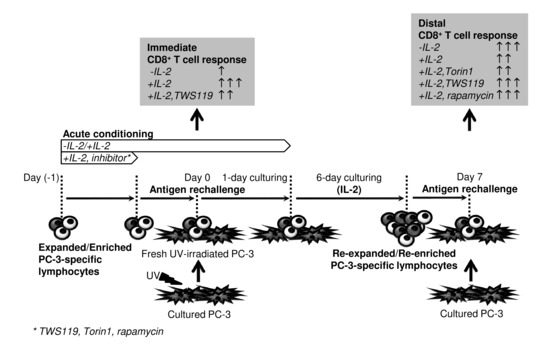
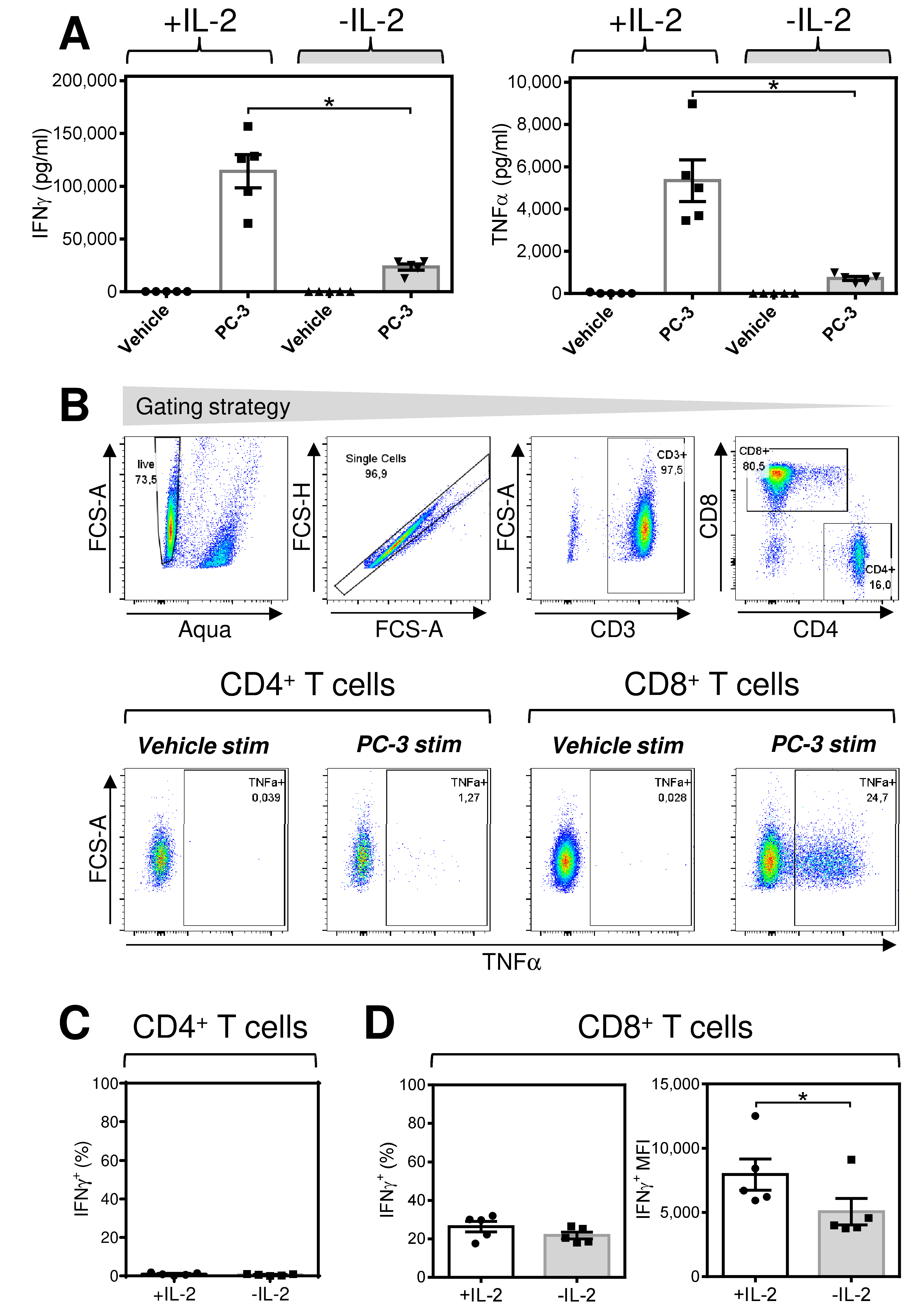
2.1. Acute Starvation of IL-2 Diminishes the Robustness of the Immediate Response of Antigen-Expanded CD8+ T Cells after Rechallenge with Antigen
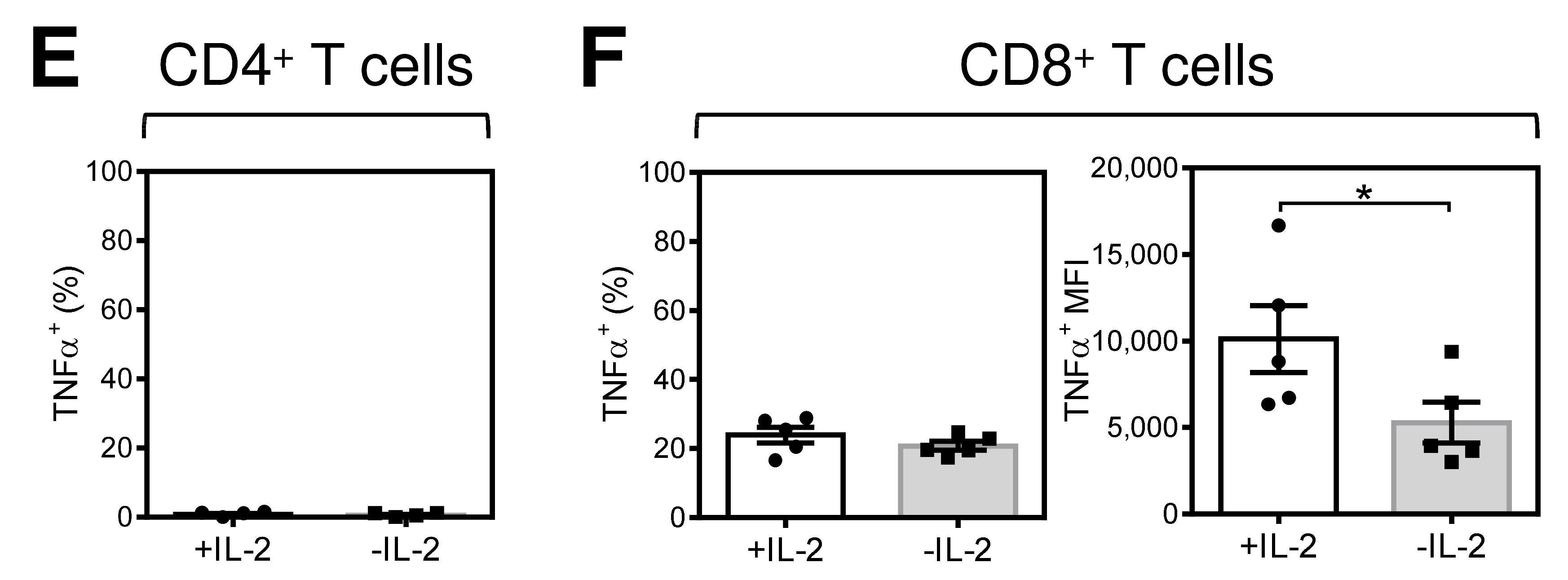
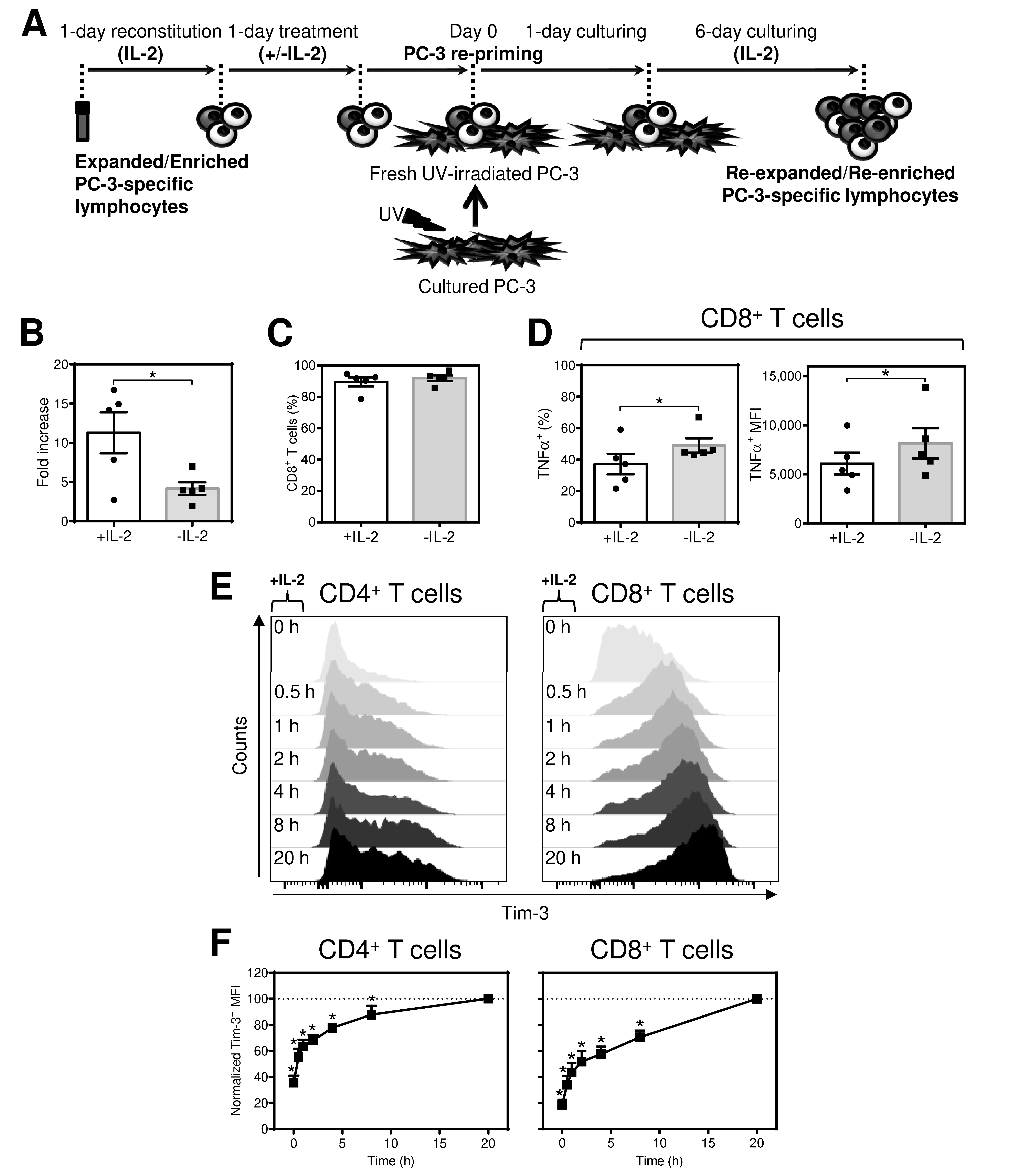
2.2. Acute Starvation of IL-2 before Antigen Rechallenge Promotes Re-Expansion of CD8+ T Cells with Enhanced Production of TNFα after Antigen Stimulation
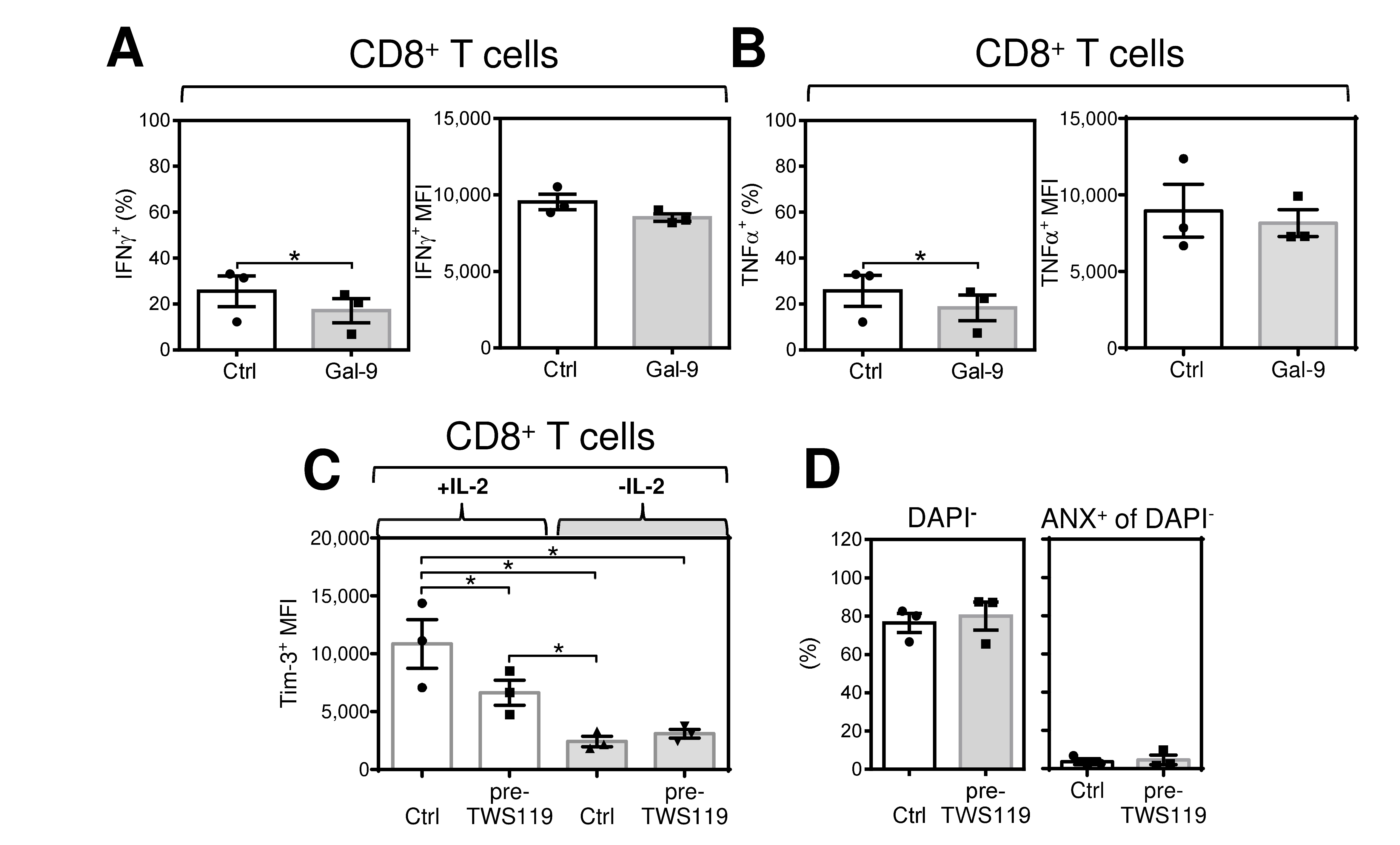
2.3. Tim-3 Is a Reversible Marker of the Acute Impact of IL-2, IL-7, and IL-15 in Antigen-Expanded CD8+ T Cells
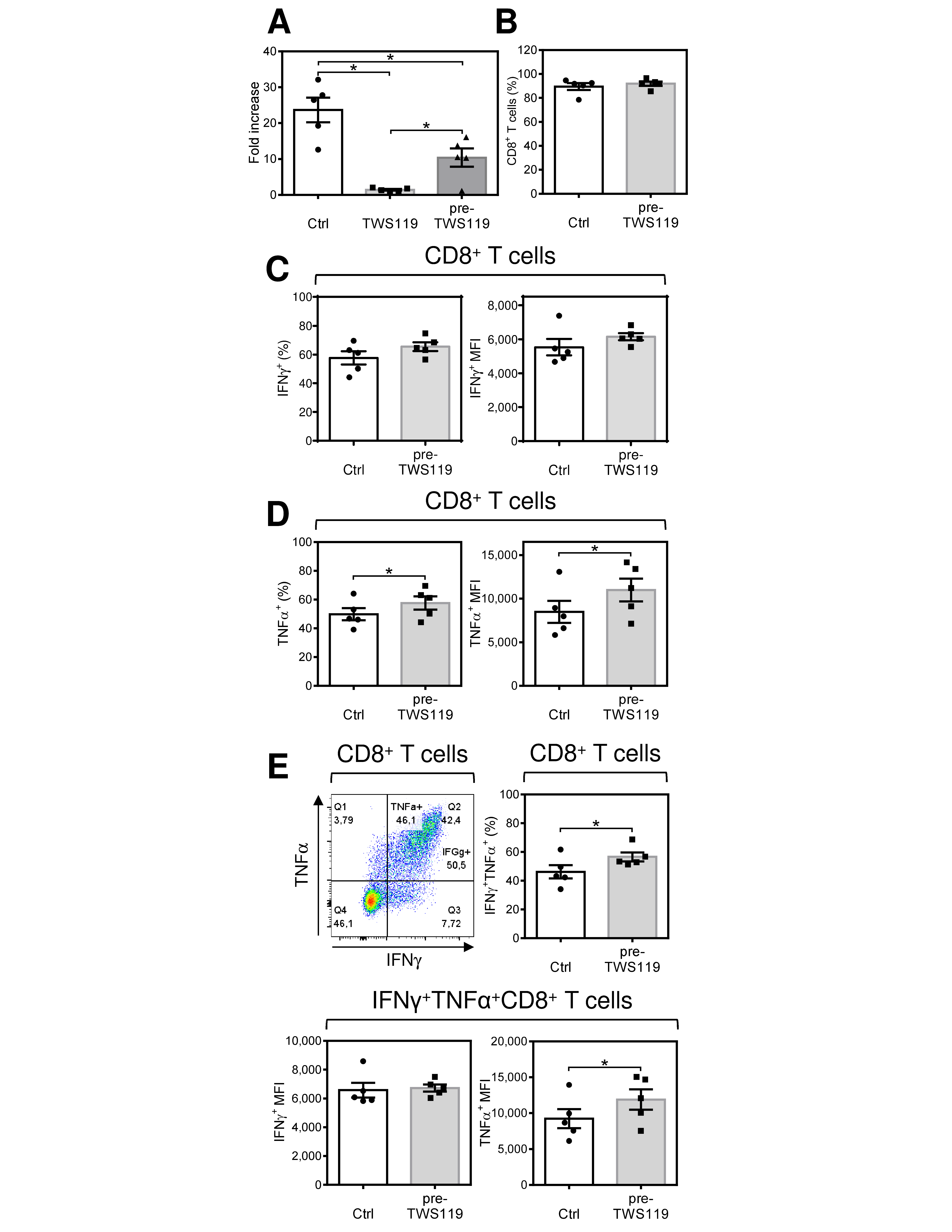
2.4. Acute Conditioning of Antigen-Expanded CD8+ T Cells with the GSK-3β Inhibitor, TWS119, Prior to Antigen Rechallenge Decreases IL-2-Mediated Tim-3 Expression and the Robustness of Their Immediate Response to Antigen Rechallenge
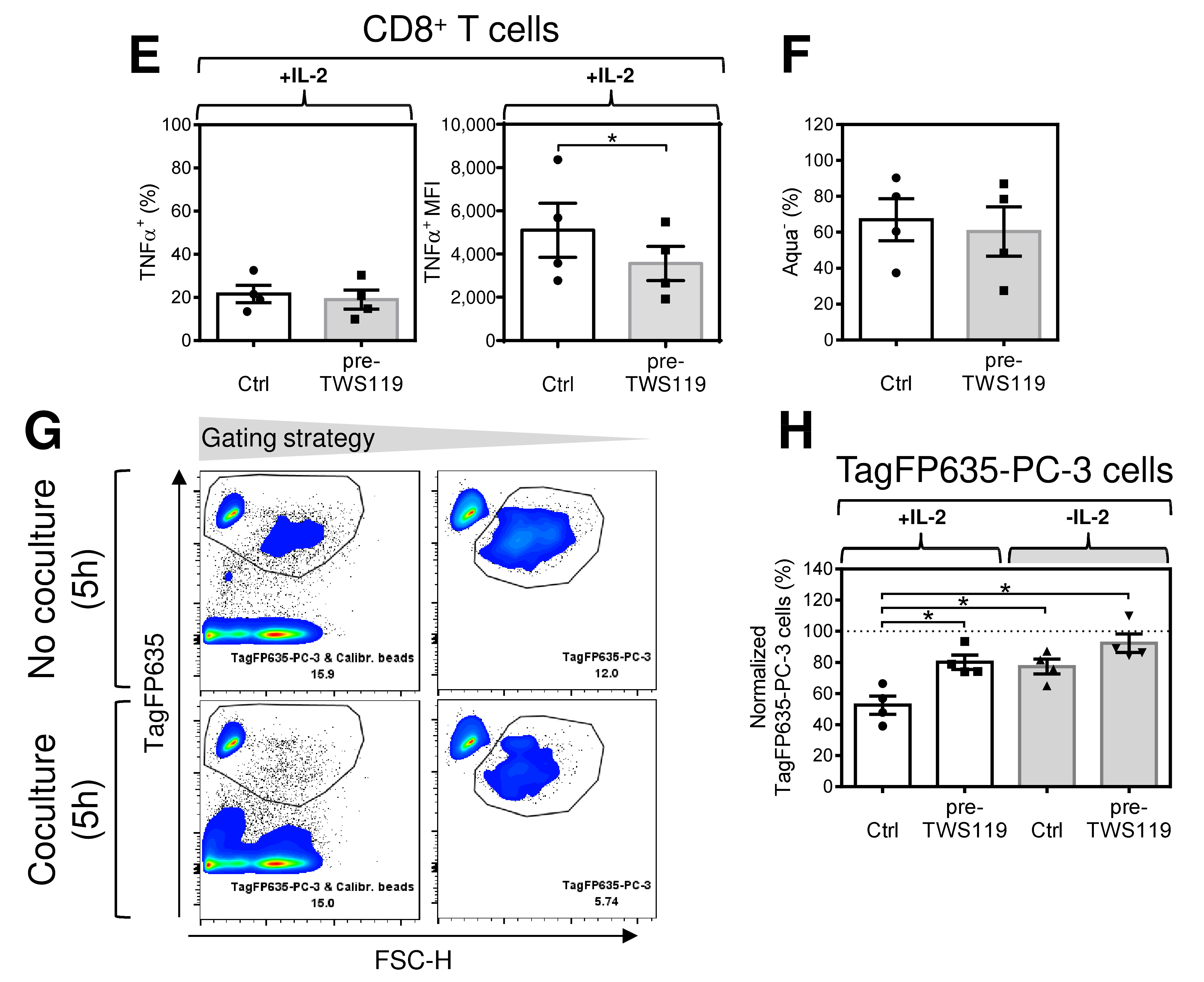
2.5. Acute Conditioning of Antigen-Expanded CD8+ T Cells with the GSK-3β Inhibitor TWS119 Prior to Antigen Rechallenge Promotes Re-Expansion of CD8+ T Cells with Enhanced Production of TNFα after Antigen Stimulation
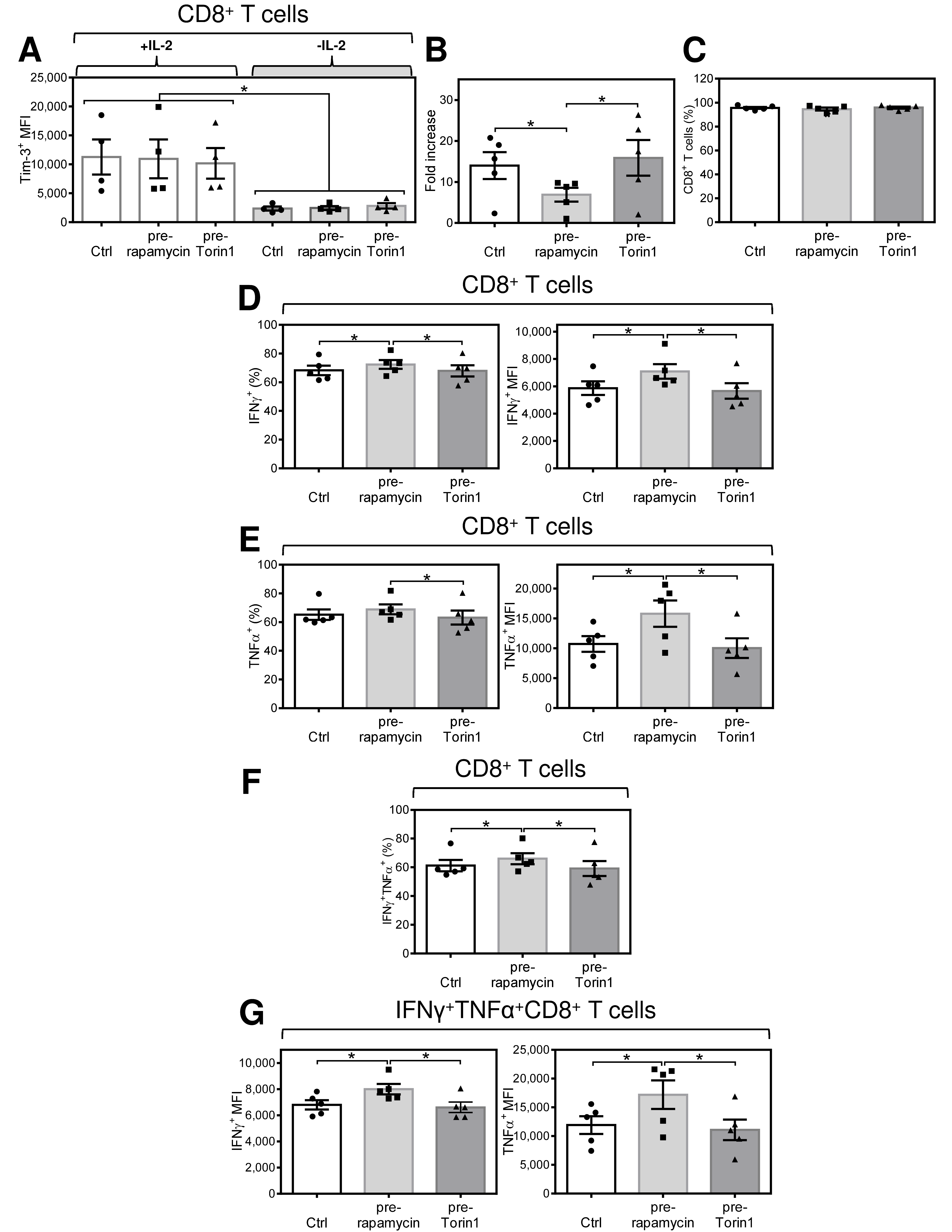
2.6. Acute Conditioning of Expanded CD8+ T Cells with Rapamycin, but Not with Torin1, Prior to Antigen Rechallenge Promotes Re-Expansion of CD8+ T Cells with Enhanced Production of TNFα and IFNγ after Antigen Stimulation
3. Discussion
4. Materials and Methods
4.1. Specimens
4.2. DC Preparation
4.3. Enrichment and Expansion of Antigen-Specific CD8+ T Cells
4.4. Tim-3 Kinetics, Reversion Analysis, and Annexin V Assay
4.5. Cell Stimulation, Intracellular Cytokine Staining, and Cytokine Release
4.6. β-Catenin Translocation Analysis and Phospho Flow
4.7. Flow Cytometry Cytotoxic Assay
4.8. Antigen Rechallenge and Re-Expansion of CD8+ T Cells
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, A.; Pasare, C. Innate Control of Adaptive Immunity: Beyond the Three-Signal Paradigm. J. Immunol. 2017, 198, 3791–3800. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Sun, Z.; Chen, L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell 2020. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, C.J.; Knochelmann, H.M.; Smith, A.S.; Wyatt, M.M.; Rangel Rivera, G.O.; Arhontoulis, D.C.; Bartee, E.; Li, Z.; Rubinstein, M.P.; Paulos, C.M. Fueling Cancer Immunotherapy With Common Gamma Chain Cytokines. Front. Immunol. 2019, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Mahallawi, W.H.; Khabour, O.F.; Zhang, Q.; Makhdoum, H.M.; Suliman, B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 2018, 104, 8–13. [Google Scholar] [CrossRef]
- Wong, C.K.; Lam, C.W.; Wu, A.K.; Ip, W.K.; Lee, N.L.; Chan, I.H.; Lit, L.C.; Hui, D.S.; Chan, M.H.; Chung, S.S.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Younes, S.A.; Freeman, M.L.; Mudd, J.C.; Shive, C.L.; Reynaldi, A.; Panigrahi, S.; Estes, J.D.; Deleage, C.; Lucero, C.; Anderson, J.; et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J. Clin. Investig. 2016, 126, 2745–2756. [Google Scholar] [CrossRef]
- Kucera, R.; Topolcan, O.; Treskova, I.; Kinkorova, J.; Windrichova, J.; Fuchsova, R.; Svobodova, S.; Treska, V.; Babuska, V.; Novak, J.; et al. Evaluation of IL-2, IL-6, IL-8 and IL-10 in Malignant Melanoma Diagnostics. Anticancer. Res. 2015, 35, 3537–3541. [Google Scholar]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Somerville, R.P.T.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1803–1813. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef] [Green Version]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef] [Green Version]
- Granier, C.; Dariane, C.; Combe, P.; Verkarre, V.; Urien, S.; Badoual, C.; Roussel, H.; Mandavit, M.; Ravel, P.; Sibony, M.; et al. Tim-3 Expression on Tumor-Infiltrating PD-1(+)CD8(+) T Cells Correlates with Poor Clinical Outcome in Renal Cell Carcinoma. Cancer Res. 2017, 77, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- McKinney, E.F.; Lee, J.C.; Jayne, D.R.; Lyons, P.A.; Smith, K.G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015, 523, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Boll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Gattinoni, L.; Klebanoff, C.A.; Restifo, N.P. Pharmacologic induction of CD8+ T cell memory: Better living through chemistry. Sci. Transl. Med. 2009, 1, 11ps12. [Google Scholar] [CrossRef] [Green Version]
- Pearce, E.L.; Walsh, M.C.; Cejas, P.J.; Harms, G.M.; Shen, H.; Wang, L.S.; Jones, R.G.; Choi, Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009, 460, 103–107. [Google Scholar] [CrossRef]
- Marro, B.S.; Zak, J.; Zavareh, R.B.; Teijaro, J.R.; Lairson, L.L.; Oldstone, M.B.A. Discovery of Small Molecules for the Reversal of T Cell Exhaustion. Cell Rep. 2019, 29, 3293–3302 e3293. [Google Scholar] [CrossRef] [Green Version]
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy. Cell Metab. 2017, 26, 94–109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Romero, P. Metabolic Control of CD8(+) T Cell Fate Decisions and Antitumor Immunity. Trends Mol. Med. 2018, 24, 30–48. [Google Scholar] [CrossRef]
- Benczik, M.; Gaffen, S.L. The interleukin (IL)-2 family cytokines: Survival and proliferation signaling pathways in T lymphocytes. Immunol. Investig. 2004, 33, 109–142. [Google Scholar] [CrossRef]
- Taylor, H.E.; Calantone, N.A.; D’Aquila, R.T. mTOR signaling mediates effects of common gamma-chain cytokines on T cell proliferation and exhaustion: Implications for HIV-1 persistence and cure research. Aids 2018, 32, 2847–2851. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Avery, L.; Filderman, J.; Szymczak-Workman, A.L.; Kane, L.P. Tim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustion. Proc. Natl. Acad. Sci. USA 2018, 115, 2455–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, B.; Man, H.; Liu, J.; Wang, L.; Zhu, T.; Ma, M.; Xv, Z.; Chen, X.; Yang, X.; Li, P. TIM-3 promotes the metastasis of esophageal squamous cell carcinoma by targeting epithelial-mesenchymal transition via the Akt/GSK-3beta/Snail signaling pathway. Oncol. Rep. 2016, 36, 1551–1561. [Google Scholar] [CrossRef]
- Pallet, N.; Legendre, C. Adverse events associated with mTOR inhibitors. Expert Opin. Drug Saf. 2013, 12, 177–186. [Google Scholar] [CrossRef]
- Nabben, M.; Neumann, D. GSK-3 Inhibitors: Anti-Diabetic Treatment Associated with Cardiac Risk? Editorial to: “The Impact of Chronic Glycogen Synthase Kinase-3 Inhibition on Remodeling of Normal and Pre-Diabetic Rat Hearts.” by Barbara Huisamen et al. Cardiovasc. Drugs Ther. 2016, 30, 233–235. [Google Scholar] [CrossRef] [Green Version]
- Smrž, D.; Bandara, G.; Beaven, M.A.; Metcalfe, D.D.; Gilfillan, A.M. Prevention of F-actin assembly switches the response to SCF from chemotaxis to degranulation in human mast cells. Eur. J. Immunol. 2013, 43, 1873–1882. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Smrž, D.; Jung, M.Y.; Bandara, G.; Desai, A.; Smržová, S.; Kuehn, H.S.; Beaven, M.A.; Metcalfe, D.D.; Gilfillan, A.M. Stem Cell Factor Programs the Mast Cell Activation Phenotype. J. Immunol. 2012, 188, 5428–5437. [Google Scholar] [CrossRef]
- Jung, M.Y.; Smrž, D.; Desai, A.; Bandara, G.; Ito, T.; Iwaki, S.; Kang, J.H.; Andrade, M.V.; Hilderbrand, S.C.; Brown, J.M.; et al. IL-33 Induces a Hyporesponsive Phenotype in Human and Mouse Mast Cells. J. Immunol. 2013, 190, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Mujib, S.; Jones, R.B.; Lo, C.; Aidarus, N.; Clayton, K.; Sakhdari, A.; Benko, E.; Kovacs, C.; Ostrowski, M.A. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J. Immunol. 2012, 188, 3745–3756. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.Y.; Turka, L.A. Immunometabolism and PI(3)K Signaling As a Link between IL-2, Foxp3 Expression, and Suppressor Function in Regulatory T Cells. Front. Immunol. 2018, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.R.; Li, Q.; Odunsi, K.; Shrikant, P.A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 2010, 32, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macintyre, A.N.; Finlay, D.; Preston, G.; Sinclair, L.V.; Waugh, C.M.; Tamas, P.; Feijoo, C.; Okkenhaug, K.; Cantrell, D.A. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 2011, 34, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Azoulay-Alfaguter, I.; Elya, R.; Avrahami, L.; Katz, A.; Eldar-Finkelman, H. Combined regulation of mTORC1 and lysosomal acidification by GSK-3 suppresses autophagy and contributes to cancer cell growth. Oncogene 2015, 34, 4613–4623. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Harker, J.A.; Chanthong, K.; Stevenson, P.G.; Zuniga, E.I.; Rudd, C.E. Glycogen Synthase Kinase 3 Inactivation Drives T-bet-Mediated Downregulation of Co-receptor PD-1 to Enhance CD8(+) Cytolytic T Cell Responses. Immunity 2016, 44, 274–286. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Wu, T.Y.; Brinker, A.; Peters, E.C.; Hur, W.; Gray, N.S.; Schultz, P.G. Synthetic small molecules that control stem cell fate. Proc. Natl. Acad. Sci. USA 2003, 100, 7632–7637. [Google Scholar] [CrossRef] [Green Version]
- Stakheev, D.; Taborska, P.; Strizova, Z.; Podrazil, M.; Bartunkova, J.; Smrz, D. The WNT/beta-catenin signaling inhibitor XAV939 enhances the elimination of LNCaP and PC-3 prostate cancer cells by prostate cancer patient lymphocytes in vitro. Sci. Rep. 2019, 9, 4761. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019, 72, 51–62. [Google Scholar] [CrossRef]
- Gibbons, J.J.; Abraham, R.T.; Yu, K. Mammalian target of rapamycin: Discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin. Oncol. 2009, 36 (Suppl. S3), S3–S17. [Google Scholar] [CrossRef] [PubMed]
- Thoreen, C.C.; Kang, S.A.; Chang, J.W.; Liu, Q.; Zhang, J.; Gao, Y.; Reichling, L.J.; Sim, T.; Sabatini, D.M.; Gray, N.S. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009, 284, 8023–8032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oubrahim, H.; Wong, A.; Wilson, B.A.; Chock, P.B. Mammalian target of rapamycin complex 1 (mTORC1) plays a role in Pasteurella multocida toxin (PMT)-induced protein synthesis and proliferation in Swiss 3T3 cells. J. Biol. Chem. 2013, 288, 2805–2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyuhas, O. Ribosomal Protein S6 Phosphorylation: Four Decades of Research. Int. Rev. Cell Mol. Biol. 2015, 320, 41–73. [Google Scholar] [CrossRef]
- Abdel-Hakeem, M.S.; Boisvert, M.; Bruneau, J.; Soudeyns, H.; Shoukry, N.H. Selective expansion of high functional avidity memory CD8 T cell clonotypes during hepatitis C virus reinfection and clearance. PLoS Pathog. 2017, 13, e1006191. [Google Scholar] [CrossRef]
- Pollizzi, K.N.; Patel, C.H.; Sun, I.H.; Oh, M.H.; Waickman, A.T.; Wen, J.; Delgoffe, G.M.; Powell, J.D. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J. Clin. Investig. 2015, 125, 2090–2108. [Google Scholar] [CrossRef] [Green Version]
- Hope, J.L.; Stairiker, C.J.; Bae, E.A.; Otero, D.C.; Bradley, L.M. Striking a Balance-Cellular and Molecular Drivers of Memory T Cell Development and Responses to Chronic Stimulation. Front. Immunol. 2019, 10, 1595. [Google Scholar] [CrossRef]
- Bucks, C.M.; Norton, J.A.; Boesteanu, A.C.; Mueller, Y.M.; Katsikis, P.D. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J. Immunol. 2009, 182, 6697–6708. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Rangachari, M.; Kuchroo, V.K. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019, 42, 101302. [Google Scholar] [CrossRef]
- Mengus, C.; Le Magnen, C.; Trella, E.; Yousef, K.; Bubendorf, L.; Provenzano, M.; Bachmann, A.; Heberer, M.; Spagnoli, G.C.; Wyler, S. Elevated levels of circulating IL-7 and IL-15 in patients with early stage prostate cancer. J. Transl. Med. 2011, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, P.; Zheng, L.; Ishihara, S.; Reed, J.; Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007, 8, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Zago, C.A.; Bortoluci, K.R.; Sardinha, L.R.; Pretel, F.D.; Castillo-Mendez, S.I.; Freitas do Rosario, A.P.; Hiyane, M.I.; Muxel, S.M.; Rodriguez-Malaga, S.M.; Abrahamsohn, I.A.; et al. Anti-IL-2 treatment impairs the expansion of T(reg) cell population during acute malaria and enhances the Th1 cell response at the chronic disease. PLoS ONE 2012, 7, e29894. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.; Murray, S.; Divakar, P.; Burnaevskiy, N.; Pifer, R.; Forman, J.; Yarovinsky, F. Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J. Immunol. 2012, 188, 800–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sananez, I.; Raiden, S.; Erra-Diaz, F.; De Lillo, L.; Holgado, M.P.; Geffner, J.; Arruvito, L. Dampening of IL-2 Function in Infants With Severe Respiratory Syncytial Virus Disease. J. Infect. Dis. 2018, 218, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.; Martinez, A.C.; Gonzalez, A.; Gomez, J.; Rebollo, A. IL-2 deprivation triggers apoptosis which is mediated by c-Jun N-terminal kinase 1 activation and prevented by Bcl-2. Cell Death Differ. 1999, 6, 87–94. [Google Scholar] [CrossRef]
- Forsdyke, D.R. Metabolic optimization of adoptive T cell transfer cancer immunotherapy: A historical overview. Scand. J. Immunol. 2020, 92, e12929. [Google Scholar] [CrossRef]
- Richardson, N.H.; Luttrell, J.B.; Bryant, J.S.; Chamberlain, D.; Khawaja, S.; Neeli, I.; Radic, M. Tuning the performance of CAR T cell immunotherapies. BMC Biotechnol. 2019, 19, 84. [Google Scholar] [CrossRef]
- Yamamoto, T.N.; Lee, P.H.; Vodnala, S.K.; Gurusamy, D.; Kishton, R.J.; Yu, Z.; Eidizadeh, A.; Eil, R.; Fioravanti, J.; Gattinoni, L.; et al. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J. Clin. Investig. 2019, 129, 1551–1565. [Google Scholar] [CrossRef] [Green Version]
- Lozano, T.; Chocarro, S.; Martin, C.; Lasarte-Cia, A.; Del Valle, C.; Gorraiz, M.; Sarrion, P.; Ruiz de Galarreta, M.; Lujambio, A.; Hervas-Stubbs, S.; et al. Genetic Modification of CD8(+) T Cells to Express EGFR: Potential Application for Adoptive T Cell Therapies. Front. Immunol. 2019, 10, 2990. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhao, Y.L.; Lv, Y.P.; Cheng, P.; Chen, W.; Duan, M.; Teng, Y.S.; Wang, T.T.; Peng, L.S.; Mao, F.Y.; et al. Modulation of CD8(+) memory stem T cell activity and glycogen synthase kinase 3beta inhibition enhances anti-tumoral immunity in gastric cancer. Oncoimmunology 2018, 7, e1412900. [Google Scholar] [CrossRef] [Green Version]
- Langdon, S.; Hughes, A.; Taylor, M.A.; Kuczynski, E.A.; Mele, D.A.; Delpuech, O.; Jarvis, L.; Staniszewska, A.; Cosulich, S.; Carnevalli, L.S.; et al. Combination of dual mTORC1/2 inhibition and immune-checkpoint blockade potentiates anti-tumour immunity. Oncoimmunology 2018, 7, e1458810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostamzadeh, D.; Yousefi, M.; Haghshenas, M.R.; Ahmadi, M.; Dolati, S.; Babaloo, Z. mTOR Signaling pathway as a master regulator of memory CD8(+) T-cells, Th17, and NK cells development and their functional properties. J. Cell Physiol. 2019, 234, 12353–12368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiang, Y. mTOR Inhibitors at a Glance. Mol. Cell. Pharmacol. 2015, 7, 15–20. [Google Scholar] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef] [Green Version]
- Thoreen, C.C.; Sabatini, D.M. Rapamycin inhibits mTORC1, but not completely. Autophagy 2009, 5, 725–726. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Shaikenov, T.; Peterson, T.R.; Aimbetov, R.; Bissenbaev, A.K.; Lee, S.W.; Wu, J.; Lin, H.K.; Sarbassov dos, D. ER stress inhibits mTORC2 and Akt signaling through GSK-3beta-mediated phosphorylation of rictor. Sci. Signal. 2011, 4, ra10. [Google Scholar] [CrossRef]
- Kazyken, D.; Magnuson, B.; Bodur, C.; Acosta-Jaquez, H.A.; Zhang, D.; Tong, X.; Barnes, T.M.; Steinl, G.K.; Patterson, N.E.; Altheim, C.H.; et al. AMPK directly activates mTORC2 to promote cell survival during acute energetic stress. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Yin, Y.; Mitson-Salazar, A.; Wansley, D.L.; Singh, S.P.; Prussin, C. Rapamycin preferentially inhibits human IL-5(+) TH2-cell proliferation via an mTORC1/S6 kinase-1-dependent pathway. J. Allergy Clin. Immunol. 2017, 139, 1701–1704 e1710. [Google Scholar] [CrossRef] [Green Version]
- Taffs, R.E.; Redegeld, F.A.; Sitkovsky, M.V. Modulation of cytolytic T lymphocyte functions by an inhibitor of serine/threonine phosphatase, okadaic acid. Enhancement of cytolytic T lymphocyte-mediated cytotoxicity. J. Immunol. 1991, 147, 722–728. [Google Scholar]
- Taborska, P.; Bartunkova, J.; Smrz, D. Simultaneous in vitro generation of human CD34(+)-derived dendritic cells and mast cells from non-mobilized peripheral blood mononuclear cells. J. Immunol. Methods 2018, 458, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Smrž, D.; Dráberová, L.; Dráber, P. Non-apoptotic phosphatidylserine externalization induced by engagement of glycosylphosphatidylinositol-anchored proteins. J. Biol. Chem. 2007, 282, 10487–10497. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taborska, P.; Stakheev, D.; Svobodova, H.; Strizova, Z.; Bartunkova, J.; Smrz, D. Acute Conditioning of Antigen-Expanded CD8+ T Cells via the GSK3β-mTORC Axis Differentially Dictates Their Immediate and Distal Responses after Antigen Rechallenge. Cancers 2020, 12, 3766. https://doi.org/10.3390/cancers12123766
Taborska P, Stakheev D, Svobodova H, Strizova Z, Bartunkova J, Smrz D. Acute Conditioning of Antigen-Expanded CD8+ T Cells via the GSK3β-mTORC Axis Differentially Dictates Their Immediate and Distal Responses after Antigen Rechallenge. Cancers. 2020; 12(12):3766. https://doi.org/10.3390/cancers12123766
Chicago/Turabian StyleTaborska, Pavla, Dmitry Stakheev, Hana Svobodova, Zuzana Strizova, Jirina Bartunkova, and Daniel Smrz. 2020. "Acute Conditioning of Antigen-Expanded CD8+ T Cells via the GSK3β-mTORC Axis Differentially Dictates Their Immediate and Distal Responses after Antigen Rechallenge" Cancers 12, no. 12: 3766. https://doi.org/10.3390/cancers12123766
APA StyleTaborska, P., Stakheev, D., Svobodova, H., Strizova, Z., Bartunkova, J., & Smrz, D. (2020). Acute Conditioning of Antigen-Expanded CD8+ T Cells via the GSK3β-mTORC Axis Differentially Dictates Their Immediate and Distal Responses after Antigen Rechallenge. Cancers, 12(12), 3766. https://doi.org/10.3390/cancers12123766