NDRG1 Expression Is an Independent Prognostic Factor in Inflammatory Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. IBC Tumor Microarrays and Immunohistochemical Staining
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, S.; Parker, S.L.; Pham, T.; Buzdar, A.U.; Hursting, S.D. Inflammatory Breast Carcinoma Incidence and Survival. Cancer 1998, 82, 2366–2372. [Google Scholar] [CrossRef]
- Hance, K.W.; Anderson, W.F.; Devesa, S.S.; Young, H.A.; Levine, P.H. Trends in inflammatory breast carcinoma incidence and survival: The surveillance, epidemiology, and end results program at the National Cancer Institute. J. Natl. Cancer Inst. 2005, 97, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L.Y.; Van Dam, P.; Prove, A.; Vermeulen, P.B. Inflammatory breast cancer: Current understanding. Curr. Opin. Oncol. 2006, 18, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, M.; Pan, J.; Wang, X.; Chen, X.S.; Shen, K.W. Pattern of distant metastases in inflammatory breast cancer—A large-cohort retrospective study. J. Cancer 2020, 11, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Valero, V.; Buzdar, A.U.; Kau, S.W.; Broglio, K.R.; Gonzalez-Angulo, A.M.; Sneige, N.; Islam, R.; Ueno, N.T.; Buchholz, T.A.; et al. Inflammatory breast cancer (IBC) and patterns of recurrence: Understanding the biology of a unique disease. Cancer 2007, 110, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Robertson, F.M.; Bondy, M.; Yang, W.; Yamauchi, H.; Wiggins, S.; Kamrudin, S.; Krishnamurthy, S.; Le-Petross, H.; Bidaut, L.; Player, A.N.; et al. Inflammatory breast cancer: The disease, the biology, the treatment. CA Cancer J. Clin. 2010, 60, 351–375. [Google Scholar] [CrossRef]
- Fouad, T.M.; Kogawa, T.; Liu, D.D.; Shen, Y.; Masuda, H.; El-Zein, R.; Woodward, W.A.; Chavez-MacGregor, M.; Alvarez, R.H.; Arun, B.; et al. Overall survival differences between patients with inflammatory and noninflammatory breast cancer presenting with distant metastasis at diagnosis. Breast Cancer Res. Treat. 2015, 152, 407–416. [Google Scholar] [CrossRef]
- Lim, B.; Woodward, W.A.; Wang, X.; Reuben, J.M.; Ueno, N.T. Inflammatory breast cancer biology: The tumour microenvironment is key. Nat. Rev. Cancer 2018, 18, 485–499. [Google Scholar] [CrossRef]
- Zhang, D.; LaFortune, T.A.; Krishnamurthy, S.; Esteva, F.J.; Cristofanilli, M.; Liu, P.; Lucci, A.; Singh, B.; Hung, M.C.; Hortobagyi, G.N.; et al. Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin. Cancer Res. 2009, 15, 6639–6648. [Google Scholar] [CrossRef]
- Kleer, C.G.; van Golen, K.L.; Braun, T.; Merajver, S.D. Persistent E-cadherin expression in inflammatory breast cancer. Mod. Pathol. 2001, 14, 458–464. [Google Scholar] [CrossRef]
- Van Golen, K.L.; Wu, Z.F.; Qiao, X.T.; Bao, L.W.; Merajver, S.D. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000, 60, 5832–5838. [Google Scholar] [PubMed]
- Costa, R.; Santa-Maria, C.A.; Rossi, G.; Carneiro, B.A.; Chae, Y.K.; Gradishar, W.J.; Giles, F.J.; Cristofanilli, M. Developmental therapeutics for inflammatory breast cancer: Biology and translational directions. Oncotarget 2017, 8, 12417–12432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Semba, T.; Phi, L.T.H.; Chainitikun, S.; Iwase, T.; Lim, B.; Ueno, N.T. Targeting Signaling Pathways in Inflammatory Breast Cancer. Cancers 2020, 12, 2479. [Google Scholar] [CrossRef] [PubMed]
- Cangul, H. Hypoxia upregulates the expression of the NDRG1 gene leading to its overexpression in various human cancers. BMC Genet. 2004, 5, 27. [Google Scholar] [CrossRef]
- Lee, J.C.; Chiang, K.C.; Feng, T.H.; Chen, Y.J.; Chuang, S.T.; Tsui, K.H.; Chung, L.C.; Juang, H.H. The Iron Chelator, Dp44mT, Effectively Inhibits Human Oral Squamous Cell Carcinoma Cell Growth In Vitro and In Vivo. Int. J. Mol. Sci. 2016, 17, 1435. [Google Scholar] [CrossRef]
- Fotovati, A.; Abu-Ali, S.; Kage, M.; Shirouzu, K.; Yamana, H.; Kuwano, M. N-myc downstream-regulated gene 1 (NDRG1) a differentiation marker of human breast cancer. Pathol. Oncol. Res. 2011, 17, 525–533. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Yue, F.; Zheng, M.; Kovacevic, Z.; Richardson, D.R. The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1). J. Biol. Chem. 2012, 287, 17016–17028. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Chikhani, S.; Lui, G.Y.; Sivagurunathan, S.; Richardson, D.R. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid. Redox Signal. 2013, 18, 874–887. [Google Scholar] [CrossRef]
- Sevinsky, C.J.; Khan, F.; Kokabee, L.; Darehshouri, A.; Maddipati, K.R.; Conklin, D.S. NDRG1 regulates neutral lipid metabolism in breast cancer cells. Breast Cancer Res. 2018, 20, 55. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Pai, S.K.; Hirota, S.; Hosobe, S.; Takano, Y.; Saito, K.; Piquemal, D.; Commes, T.; Watabe, M.; Gross, S.C.; et al. Role of the putative tumor metastasis suppressor gene Drg-1 in breast cancer progression. Oncogene 2004, 23, 5675–5681. [Google Scholar] [CrossRef]
- Liu, W.; Xing, F.; Iiizumi-Gairani, M.; Okuda, H.; Watabe, M.; Pai, S.K.; Pandey, P.R.; Hirota, S.; Kobayashi, A.; Mo, Y.Y.; et al. N-myc downstream regulated gene 1 modulates Wnt-β-catenin signalling and pleiotropically suppresses metastasis. EMBO Mol. Med. 2012, 4, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Godbole, M.; Togar, T.; Patel, K.; Dharavath, B.; Yadav, N.; Janjuha, S.; Gardi, N.; Tiwary, K.; Terwadkar, P.; Desai, S.; et al. Up-regulation of the kinase gene SGK1 by progesterone activates the AP-1-NDRG1 axis in both PR-positive and -negative breast cancer cells. J. Biol. Chem. 2018, 293, 19263–19276. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Fan, C.F.; Wei, J.; Liu, C.; Zheng, H.C.; Yao, F.; Jin, F. Increased N-myc downstream-regulated gene 1 expression is associated with breast atypia-to-carcinoma progression. Tumour Biol. 2011, 32, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, E.Y.; Huang, W.Y.; Chang, Y.C.; Tsai, M.H.; Chuang, E.Y.; Kuok, Q.Y.; Bai, S.T.; Chao, L.Y.; Sher, Y.P.; Lai, L.C. Aryl Hydrocarbon Receptor Activates NDRG1 Transcription under Hypoxia in Breast Cancer Cells. Sci. Rep. 2016, 6, 20808. [Google Scholar] [CrossRef] [PubMed]
- Villodre, E.S.; Hu, X.; Larson, R.; Eckhardt, B.L.; Gong, Y.; Huo, L.; Song, J.; Krishnamurthy, S.; Ibrahim, N.K.; Ueno, N.T.; et al. Abstract P3-01-10: Ndrg1-egfr axis in inflammatory breast cancer tumorigenesis and brain metastasis. Cancer Res. 2020, 80, P3-01-10. [Google Scholar] [CrossRef]
- Nagai, M.A.; Gerhard, R.; Fregnani, J.H.; Nonogaki, S.; Rierger, R.B.; Netto, M.M.; Soares, F.A. Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res. Treat. 2011, 126, 1–14. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yeh, C.N.; Chung, L.C.; Feng, T.H.; Sun, C.C.; Chen, M.F.; Jan, Y.Y.; Yeh, T.S.; Chen, S.C.; Juang, H.H. WNT-1 inducible signaling pathway protein-1 enhances growth and tumorigenesis in human breast cancer. Sci. Rep. 2015, 5, 8686. [Google Scholar] [CrossRef]
- Yip, C.H.; Rhodes, A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014, 10, 2293–2301. [Google Scholar] [CrossRef]
- Grann, V.R.; Troxel, A.B.; Zojwalla, N.J.; Jacobson, J.S.; Hershman, D.; Neugut, A.I. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer 2005, 103, 2241–2251. [Google Scholar] [CrossRef]
- Munoz, D.F.; Plevritis, S.K. Estimating Breast Cancer Survival by Molecular Subtype in the Absence of Screening and Adjuvant Treatment. Med. Decis. Mak. 2018, 38, 32S–43S. [Google Scholar] [CrossRef]
- Daugherty, E.C.; Daugherty, M.R.; Bogart, J.A.; Shapiro, A. Adjuvant Radiation Improves Survival in Older Women Following Breast-Conserving Surgery for Estrogen Receptor-Negative Breast Cancer. Clin. Breast Cancer 2016, 16, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Speers, C.; Pierce, L.J. Postoperative Radiotherapy After Breast-Conserving Surgery for Early-Stage Breast Cancer: A Review. JAMA Oncol. 2016, 2, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Ohri, N.; Haffty, B.G. The evolution of adjuvant radiation therapy for early-stage and locally advanced breast cancer. Breast J. 2020, 26, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Shin, Y.K.; Kim, Y.A.; Jang, S.G.; Ku, J.L. Identification of genes inducing resistance to ionizing radiation in human rectal cancer cell lines: Re-sensitization of radio-resistant rectal cancer cells through down regulating NDRG1. BMC Cancer 2018, 18, 594. [Google Scholar] [CrossRef] [PubMed]
- Servant, N.; Bollet, M.A.; Halfwerk, H.; Bleakley, K.; Kreike, B.; Jacob, L.; Sie, D.; Kerkhoven, R.M.; Hupe, P.; Hadhri, R.; et al. Search for a gene expression signature of breast cancer local recurrence in young women. Clin. Cancer Res. 2012, 18, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Tramm, T.; Mohammed, H.; Myhre, S.; Kyndi, M.; Alsner, J.; Borresen-Dale, A.L.; Sorlie, T.; Frigessi, A.; Overgaard, J. Development and validation of a gene profile predicting benefit of postmastectomy radiotherapy in patients with high-risk breast cancer: A study of gene expression in the DBCG82bc cohort. Clin. Cancer Res. 2014, 20, 5272–5280. [Google Scholar] [CrossRef]
- Speers, C.; Zhao, S.; Liu, M.; Bartelink, H.; Pierce, L.J.; Feng, F.Y. Development and Validation of a Novel Radiosensitivity Signature in Human Breast Cancer. Clin. Cancer Res. 2015, 21, 3667–3677. [Google Scholar] [CrossRef]
- Gong, Y.; Huo, L.; Liu, P.; Sneige, N.; Sun, X.; Ueno, N.T.; Lucci, A.; Buchholz, T.A.; Valero, V.; Cristofanilli, M. Polycomb group protein EZH2 is frequently expressed in inflammatory breast cancer and is predictive of worse clinical outcome. Cancer 2011, 117, 5476–5484. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, X.; Zhong, J.; Yuan, L.; Tao, X.; Zhang, S.; Zeng, Y.; He, G.; Tan, P.; Tao, Y. Prognostic value of biomarkers EpCAM and αB-crystallin associated with lymphatic metastasis in breast cancer by iTRAQ analysis. BMC Cancer 2019, 19, 831. [Google Scholar] [CrossRef]
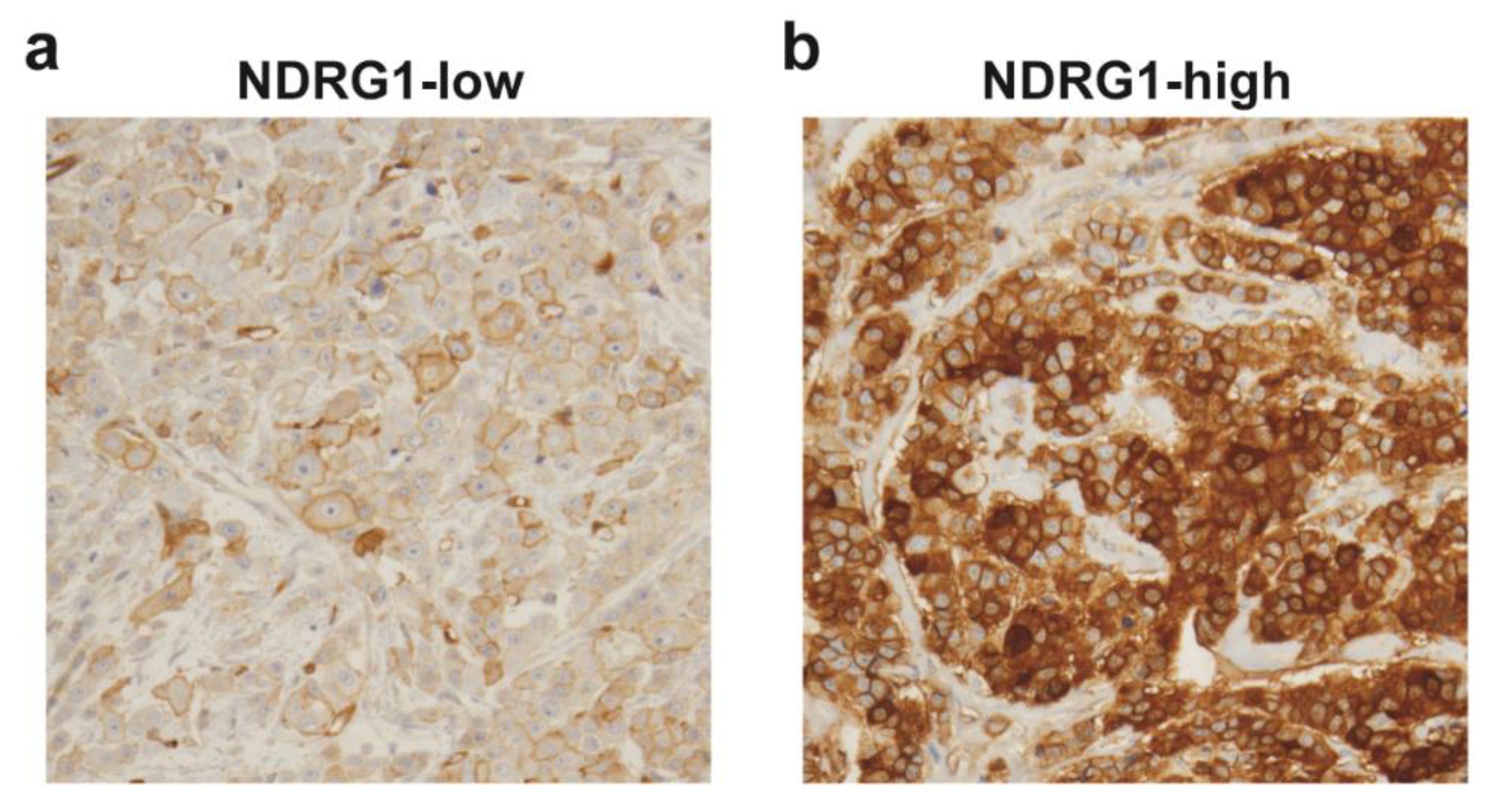
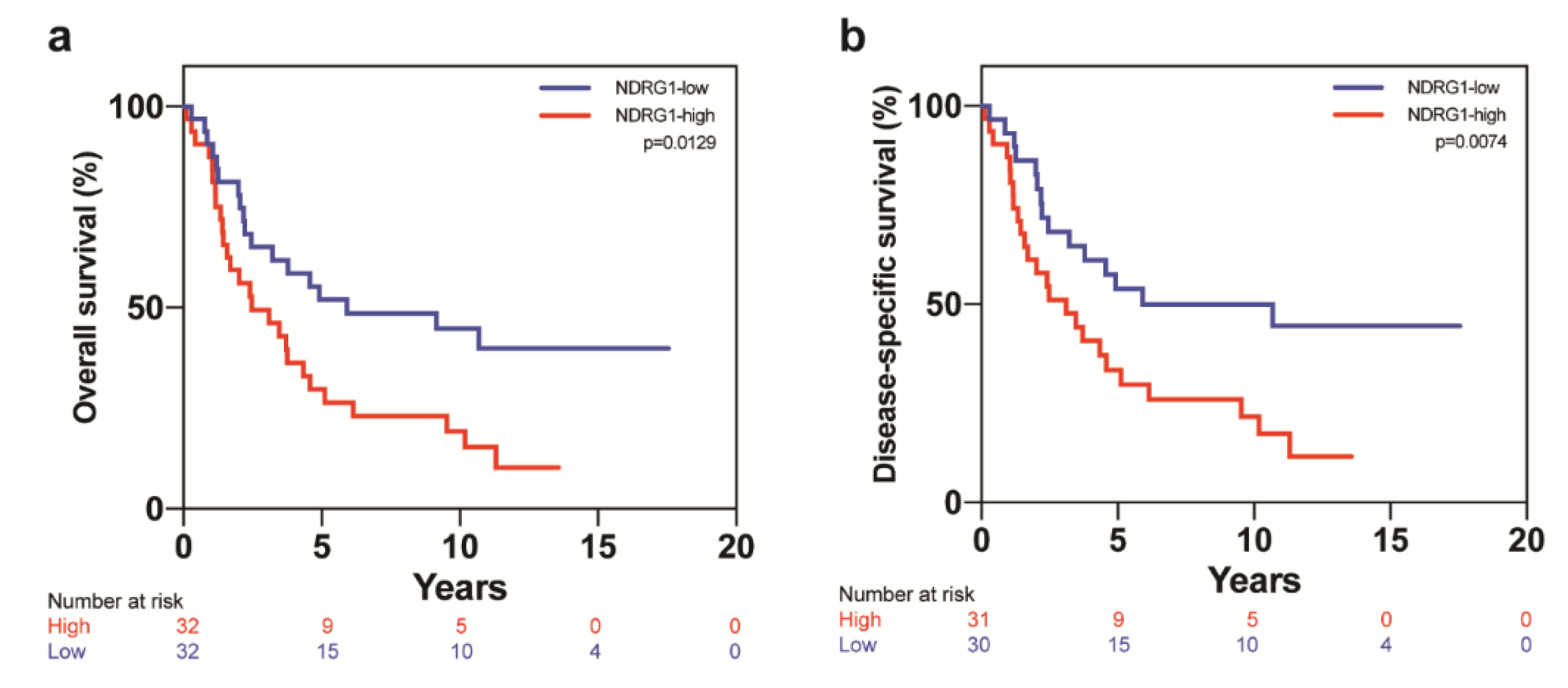
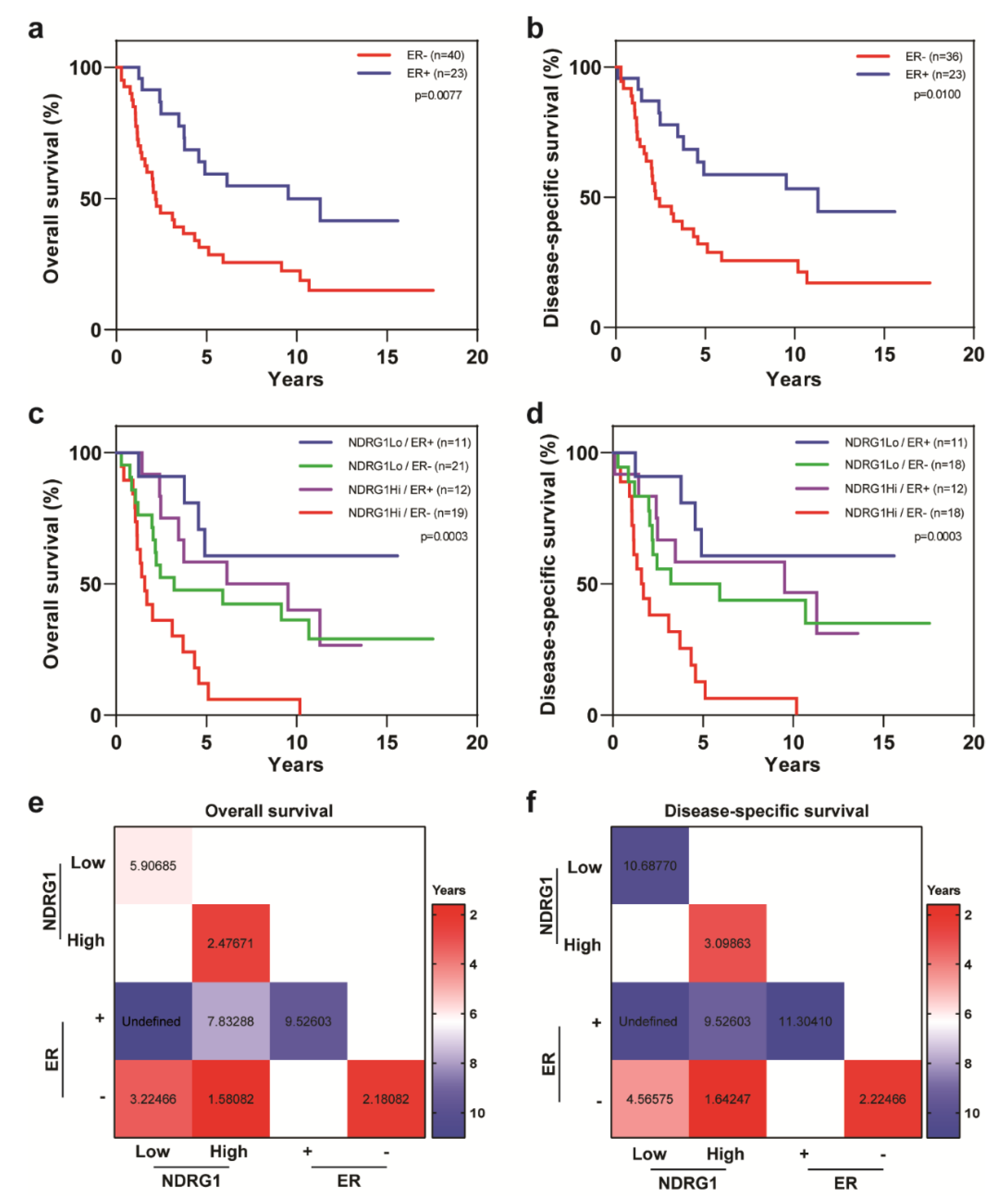
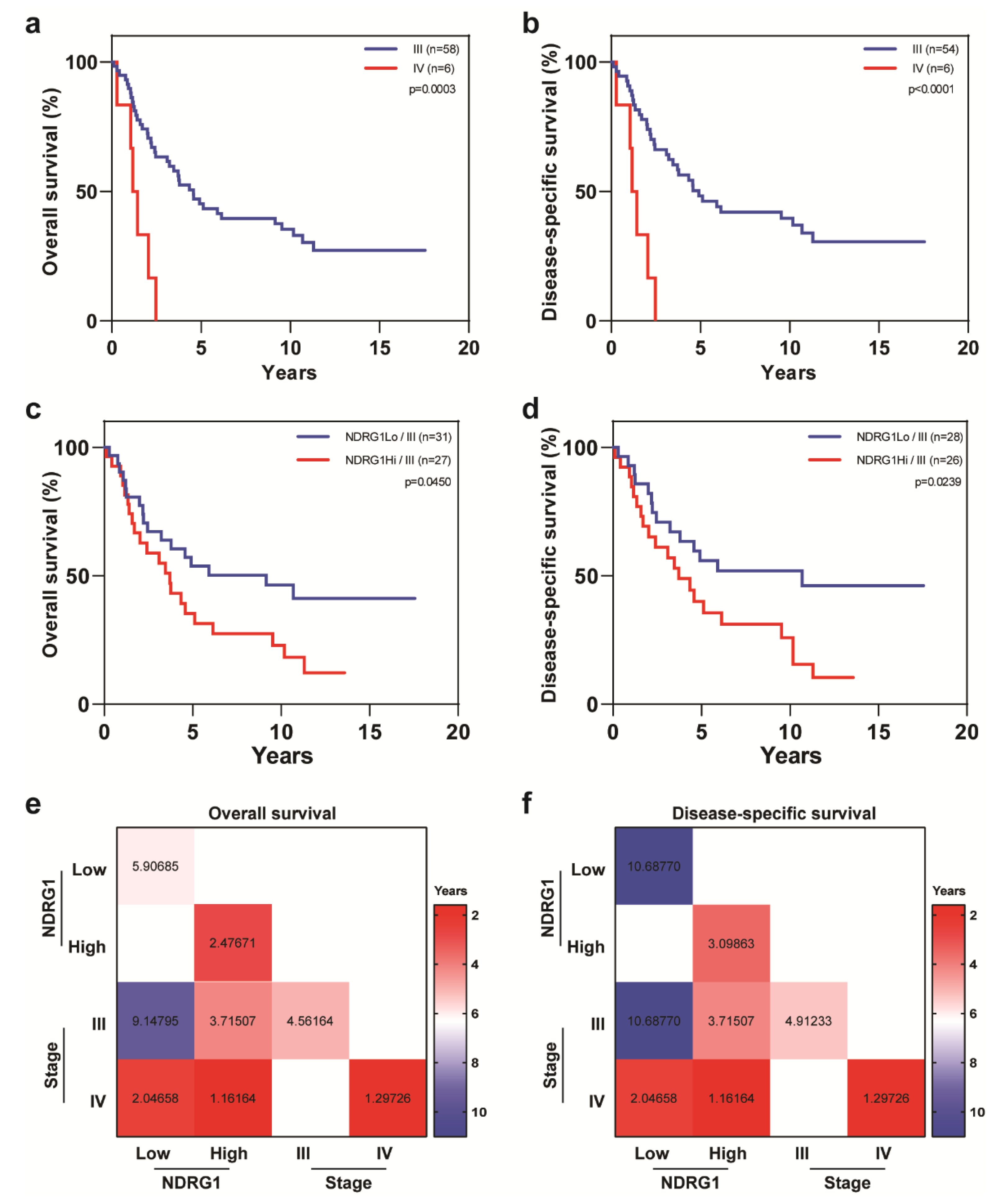
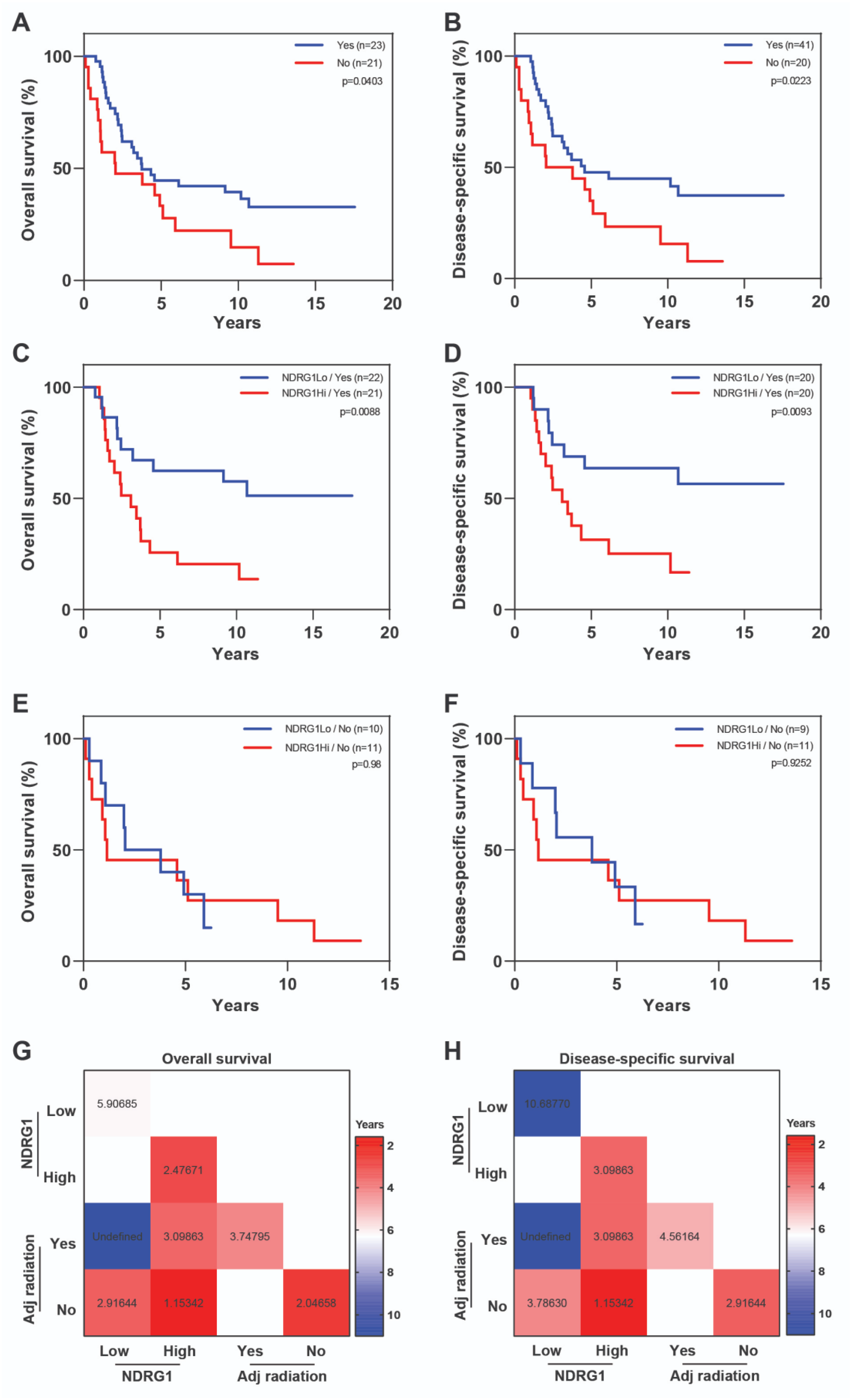
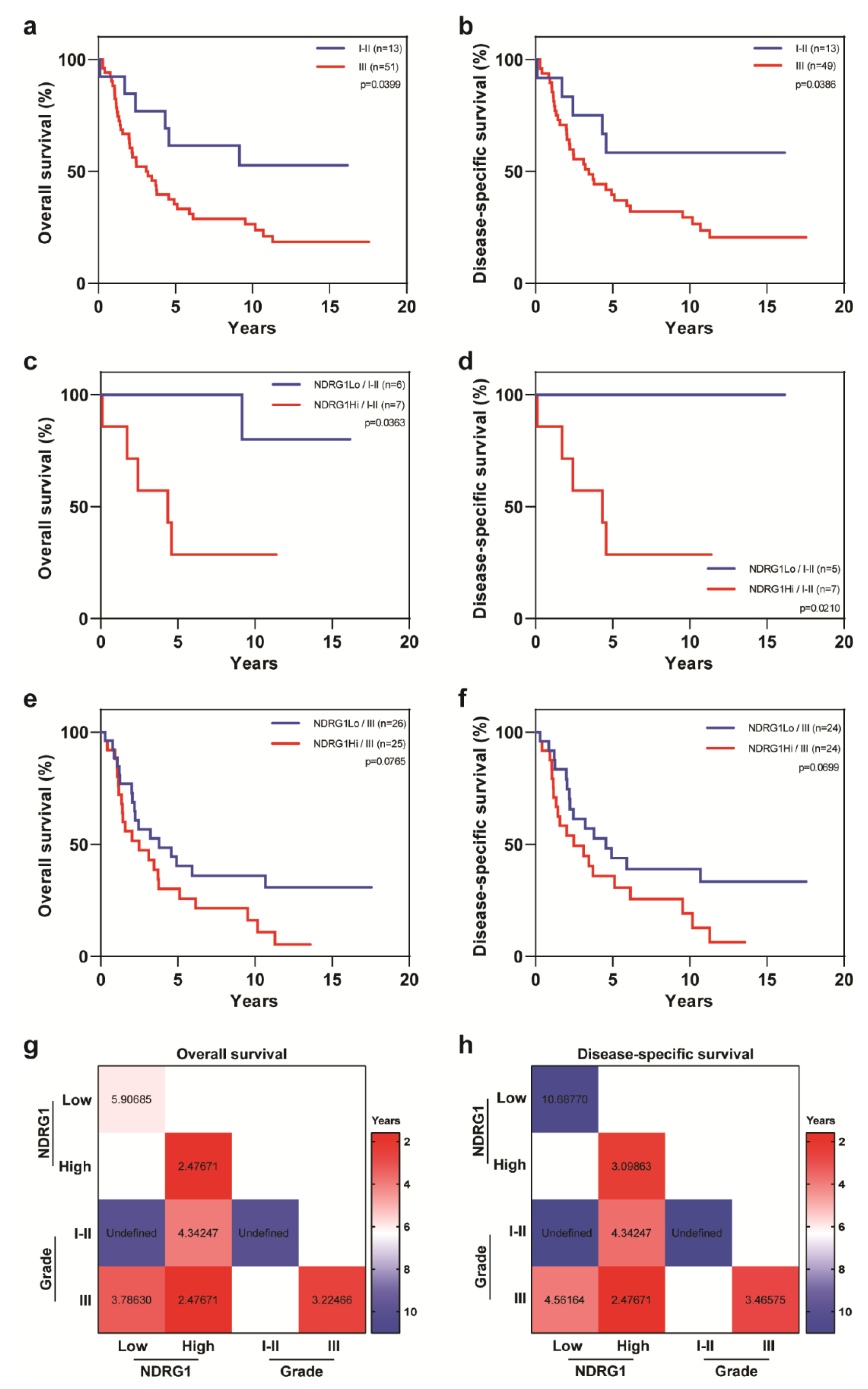
| Covariate | Level | NDRG1-Low (n = 32) | NDRG1-High (n = 32) | p-Value |
|---|---|---|---|---|
| Age | 51.5 ± 12.1 | 48.6 ± 12 | 0.3861 | |
| Race | Non-white | 8 (25%) | 5 (16%) | 0.5356 |
| White | 24 (75%) | 27 (84%) | ||
| Histologic type | Others | 2 (6%) | 4 (13%) | 0.6719 |
| Ductal | 30 (94%) | 28 (87%) | ||
| Grade | 1–2 | 6 (19%) | 7 (22%) | 0.7560 |
| 3 | 26 (81%) | 25 (78%) | ||
| Lymphovascular invasion | No | 4 (14%) | 3 (10%) | 0.7065 |
| Yes | 25 (86%) | 27 (90%) | ||
| Stage | III | 31 (97%) | 27 (84%) | 0.1961 |
| IV | 1 (3%) | 5 (16%) | ||
| Estrogen receptor | No | 21 (66%) | 19 (61%) | 0.7209 |
| Yes | 11 (34%) | 12 (39%) | ||
| Progesterone receptor | No | 24 (75%) | 19 (61%) | 0.2425 |
| Yes | 8 (25%) | 12 (39%) | ||
| HER2 | No | 12 (37%) | 22 (71%) | 0.0077 |
| Yes | 20 (63%) | 9 (29%) | ||
| Triple-negative breast cancer | No | 27 (84%) | 20 (64%) | 0.0879 |
| Yes | 5 (16%) | 11 (36%) | ||
| Adjuvant radiation | No | 10 (31%) | 11 (34%) | 0.7901 |
| Yes | 22 (69%) | 21 (66%) |
| Overall Survival | Disease-Specific Survival | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Level | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | 1 Unit Change | 1.007 | 0.980–1.035 | 0.6080 | 1.000 | 0.971–1.029 | 0.9904 |
| NDRG1 | Low | 1.000 | 1.000 | ||||
| High | 2.107 | 1.155–3.842 | 0.0150 | 2.354 | 1.235–4.485 | 0.0092 | |
| Race | Non-white | 1.000 | 1.000 | ||||
| White | 1.823 | 0.769–4.321 | 0.1724 | 1.940 | 0.757–4.973 | 0.1676 | |
| Histologic type | Others | 1.000 | 1.000 | ||||
| Ductal | 1.027 | 0.368–2.870 | 0.9591 | 1.153 | 0.410–3.243 | 0.7874 | |
| Grade | 1–2 | 1.000 | 1.000 | ||||
| 3 | 2.404 | 1.014–5.698 | 0.0463 | 2.612 | 1.020–6.688 | 0.0453 | |
| Lymphovascular invasion | No | 1.000 | 1.000 | ||||
| Yes | 1.684 | 0.601–4.717 | 0.3216 | 1.493 | 0.529–4.213 | 0.4486 | |
| Stage | III | 1.000 | 1.000 | ||||
| IV | 4.638 | 1.847–11.647 | 0.0011 | 5.485 | 2.138–14.069 | 0.0004 | |
| Estrogen receptor | No | 1.000 | 1.000 | ||||
| Yes | 0.414 | 0.212–0.808 | 0.0098 | 0.426 | 0.211–0.860 | 0.0173 | |
| Progesterone receptor | No | 1.000 | 1.000 | ||||
| Yes | 0.699 | 0.359–1.361 | 0.2919 | 0.816 | 0.412–1.616 | 0.5598 | |
| HER2 | No | 1.000 | 1.000 | ||||
| Yes | 0.748 | 0.409–1.366 | 0.3445 | 0.602 | 0.313–1.161 | 0.1300 | |
| Triple-negative breast cancer | No | 1.000 | 1.000 | ||||
| Yes | 1.557 | 0.810–2.992 | 0.1844 | 1.692 | 0.852–3.358 | 0.1328 | |
| Adjuvant radiation | No | 1.000 | 1.000 | ||||
| Yes | 0.538 | 0.295–0.982 | 0.0434 | 0.486 | 0.259–0.914 | 0.0252 | |
| Overall Survival | Disease-Specific Survival | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Level | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| NDRG1 | Low | 1.000 | 1.000 | ||||
| High | 2.449 | 1.302–4.607 | 0.0274 | 2.727 | 1.380–5.389 | 0.0039 | |
| Estrogen receptor | No | 1.000 | 1.000 | ||||
| Yes | 0.318 | 0.158–0.641 | 0.0014 | 0.316 | 0.151–0.665 | 0.0024 | |
| Stage | III | 1.000 | 1.000 | ||||
| IV | 4.350 | 1.685–11.229 | 0.0024 | 5.070 | 1.908–13.473 | 0.0011 | |
| Adjuvant radiation | No | 1.000 | 1.000 | ||||
| Yes | 0.620 | 0.336–1.145 | 0.1269 | 0.575 | 0.301–1.097 | 0.0930 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villodre, E.S.; Gong, Y.; Hu, X.; Huo, L.; Yoon, E.C.; Ueno, N.T.; Woodward, W.A.; Tripathy, D.; Song, J.; Debeb, B.G. NDRG1 Expression Is an Independent Prognostic Factor in Inflammatory Breast Cancer. Cancers 2020, 12, 3711. https://doi.org/10.3390/cancers12123711
Villodre ES, Gong Y, Hu X, Huo L, Yoon EC, Ueno NT, Woodward WA, Tripathy D, Song J, Debeb BG. NDRG1 Expression Is an Independent Prognostic Factor in Inflammatory Breast Cancer. Cancers. 2020; 12(12):3711. https://doi.org/10.3390/cancers12123711
Chicago/Turabian StyleVillodre, Emilly S., Yun Gong, Xiaoding Hu, Lei Huo, Esther C. Yoon, Naoto T. Ueno, Wendy A. Woodward, Debu Tripathy, Juhee Song, and Bisrat G. Debeb. 2020. "NDRG1 Expression Is an Independent Prognostic Factor in Inflammatory Breast Cancer" Cancers 12, no. 12: 3711. https://doi.org/10.3390/cancers12123711
APA StyleVillodre, E. S., Gong, Y., Hu, X., Huo, L., Yoon, E. C., Ueno, N. T., Woodward, W. A., Tripathy, D., Song, J., & Debeb, B. G. (2020). NDRG1 Expression Is an Independent Prognostic Factor in Inflammatory Breast Cancer. Cancers, 12(12), 3711. https://doi.org/10.3390/cancers12123711






