Advances in Targeting Cancer-Associated Genes by Designed siRNA in Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. siRNA as a Therapeutic Agent in Various Types of Cancers
3. siRNA-Mediated Cancer-Associated Gene Silencing in Prostate Cancer
4. Attenuating Drug Resistance in Prostate Cancer Using siRNA
5. Improving Antitumoral Immune Response by siRNA
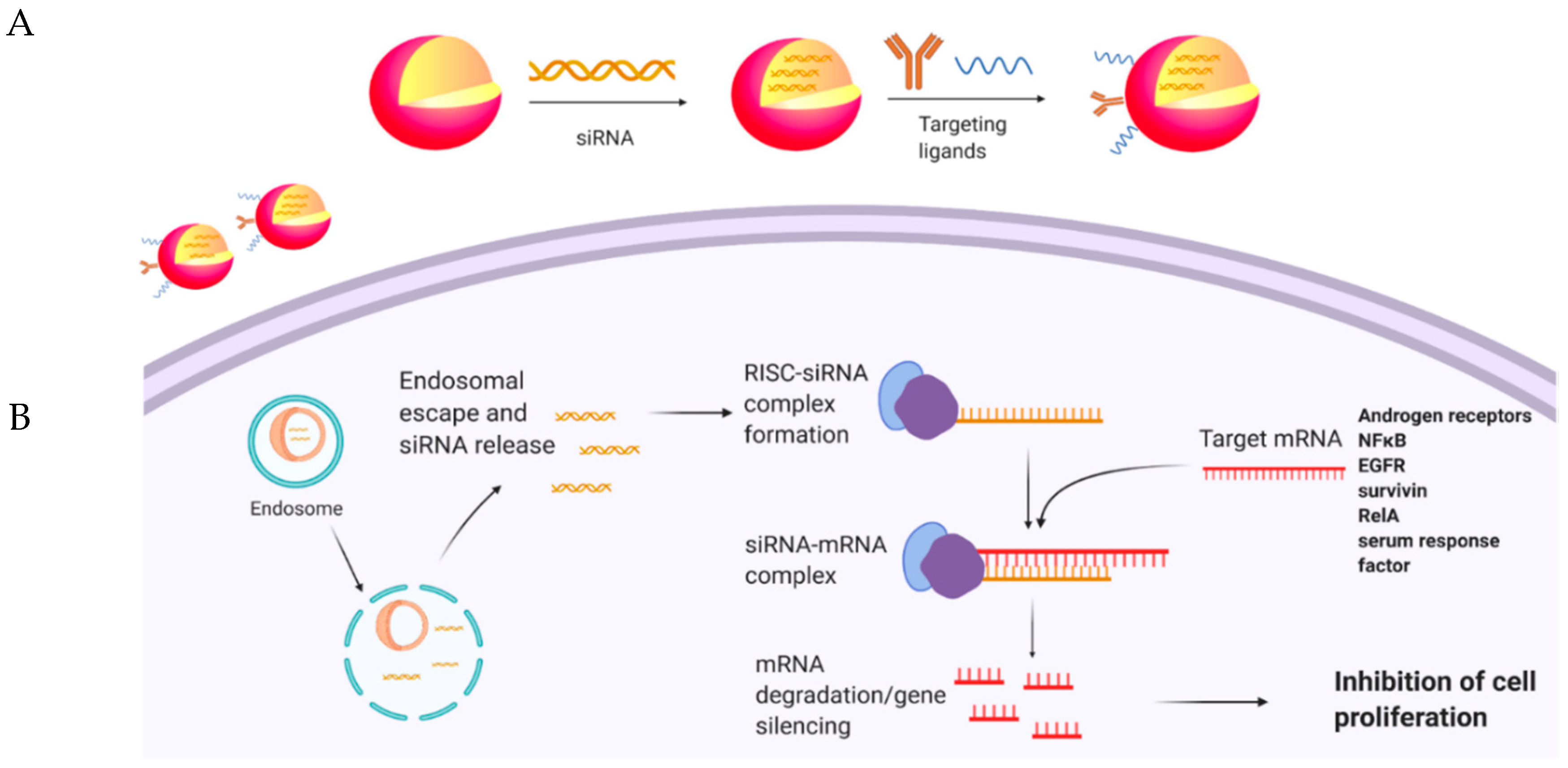
6. Targeted siRNA Delivery
7. Conclusions
Funding
Conflicts of Interest
References
- Gaudreau, P.-O.; Stagg, J.; Soulières, D.; Saad, F. The Present and Future of Biomarkers in Prostate Cancer: Proteomics, Genomics, and Immunology Advancements. Biomarkers Cancer 2016, 8, 15–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Siegel, R.L.; Khan, R.; Jemal, A. Cancer Statistics. Cancer Rehabilitation 2018, 69, 7–34. [Google Scholar] [CrossRef]
- Hoey, C.; Liu, S.K. Circulating blood miRNAs for prostate cancer risk stratification: miRroring the underlying tumor biology with liquid biopsies. Res. Rep. Urol. 2019, 11, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agochukwu, N.Q.; Skolarus, T.A.; Wittmann, D. Telemedicine and prostate cancer survivorship: A narrative review. mHealth 2018, 4, 45. [Google Scholar] [CrossRef]
- Lam, D.; Clark, S.; Stirzaker, C.; Pidsley, R. Advances in Prognostic Methylation Biomarkers for Prostate Cancer. Cancers 2020, 12, 2993. [Google Scholar] [CrossRef]
- Hayat, M.J.; Howlader, N.; Reichman, M.E.; Edwards, B.K. Cancer Statistics, Trends, and Multiple Primary Cancer Analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007, 12, 20–37. [Google Scholar] [CrossRef] [Green Version]
- Tucci, M.; Zichi, C.; Buttigliero, C.; Vignani, F.; Scagliotti, G.V.; Di Maio, M. Enzalutamide-resistant castration-resistant prostate cancer: Challenges and solutions. OncoTargets Ther. 2018, 11, 7353–7368. [Google Scholar] [CrossRef] [Green Version]
- Messner, E.A.; Steele, T.M.; Tsamouri, M.M.; Hejazi, N.; Gao, A.C.; Mudryj, M.; Ghosh, P.M. The Androgen Receptor in Prostate Cancer: Effect of Structure, Ligands and Spliced Variants on Therapy. Biomedicines 2020, 8, 422. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.J.; Kwon, I.C.; Roberts, T.M. Delivery strategies and potential targets for siRNA in major cancer types. Adv. Drug Deliv. Rev. 2016, 104, 2–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, M.E.; Kaneko, H.; Gil Cho, W.; Dridi, S.; Fowler, B.J.; Blandford, A.D.; Albuquerque, R.J.C.; Hirano, Y.; Terasaki, H.; Kondo, M.; et al. Short-interfering RNAs Induce Retinal Degeneration via TLR3 and IRF3. Mol. Ther. 2012, 20, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Fakhr, E.; Zare, F.; Teimoori-Toolabi, L. Precise and efficient siRNA design: A key point in competent gene silencing. Cancer Gene Ther. 2016, 23, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Parvani, J.G.; Jackson, M.W. Silencing the roadblocks to effective triple-negative breast cancer treatments by siRNA nanoparticles. Endocrine-Related Cancer 2017, 24, R81–R97. [Google Scholar] [CrossRef] [Green Version]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nat. Cell Biol. 2004, 431, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Patkaniowska, A.; Urlaub, H.; Lührmann, R.; Tuschl, T. Single-Stranded Antisense siRNAs Guide Target RNA Cleavage in RNAi. Cell 2002, 110, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Selvam, C.; Mutisya, D.; Prakash, S.; Ranganna, K.; Thilagavathi, R. Therapeutic potential of chemically modified siRNA: Recent trends. Chem. Biol. Drug Des. 2017, 90, 665–678. [Google Scholar] [CrossRef]
- NaghiZadeh, S.; Mohammadi, A.; Baradaran, B.; Mansoori, B. Overcoming multiple drug resistance in lung cancer using siRNA targeted therapy. Gene 2019, 714, 143972. [Google Scholar] [CrossRef]
- Khalil, H.; El Maksoud, A.I.A.; Alian, A.; El-Hamady, W.A.; Daif, A.A.; Awad, A.M.; Guirgis, A.A. Interruption of Autophagosome Formation in Cardiovascular Disease, an Evidence for Protective Response of Autophagy. Immunol. Investig. 2020, 49, 249–263. [Google Scholar] [CrossRef]
- Riahi, A.; Radmanesh, H.; Schürmann, P.; Bogdanova, N.; Geffers, R.; Meddeb, R.; Kharrat, M.; Dork, T. Exome sequencing and case-control analyses identifyRCC1as a candidate breast cancer susceptibility gene. Int. J. Cancer 2018, 142, 2512–2517. [Google Scholar] [CrossRef] [Green Version]
- Altanerova, U.; Jakubechova, J.; Benejova, K.; Priscakova, P.; Pesta, M.; Pitule, P.; Topolcan, O.; Kausitz, J.; Zduriencikova, M.; Repiska, V.; et al. Prodrug suicide gene therapy for cancer targeted intracellular by mesenchymal stem cell exosomes. Int. J. Cancer 2019, 144, 897–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asik, E.; Akpinar, Y.; Caner, A.; Kahraman, N.; Guray, T.; Volkan, M.; Albarracin, C.; Pataer, A.; Arun, B.; Ozpolat, B. EF2-kinase targeted cobalt-ferrite siRNA-nanotherapy suppressesBRCA1-mutated breast cancer. Nanomedicine 2019, 14, 2315–2338. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yu, Q.; Ji, H.; Tian, D. Tip60-siRNA regulates ABCE1 acetylation to suppress lung cancer growth via activation of the apoptotic signaling pathway. Exp. Ther. Med. 2019, 17, 3195–3202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Lu, Y.; Li, P.; Liu, P.; Shi, X.; Liu, C.; Zhang, Y.; Liu, S.; Wang, J. The short interference RNA (siRNA) targeting NMUR2 relieves nociception in a bone cancer pain model of rat through PKC-ERK and PI3K-AKT pathways. Biochem. Biophys. Res. Commun. 2019, 512, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, J.; Yang, X.; Guo, J.; Wang, T.; Zhuo, W. ProNGF siRNA inhibits cell proliferation and invasion of pancreatic cancer cells and promotes anoikis. Biomed. Pharmacother. 2019, 111, 1066–1073. [Google Scholar] [CrossRef]
- Li, X.; Pei, B.; Wang, H.; Tang, C.; Zhu, W.; Jin, F. Effect of AQP-5 silencing by siRNA interference on chemosensitivity of breast cancer cells. OncoTargets Ther. 2018, 11, 3359–3368. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Tang, S.; Guo, X.; Yang, C.; Jia, K. CT45A1 siRNA silencing suppresses the proliferation, metastasis and invasion of lung cancer cells by downregulating the ERK/CREB signaling pathway. Mol. Med. Rep. 2017, 16, 6708–6714. [Google Scholar] [CrossRef] [Green Version]
- Salguero-Aranda, C.; Sancho-Mensat, D.; Canals-Lorente, B.; Sultan, S.; Reginald, A.; Chapman, L. STAT6 knockdown using multiple siRNA sequences inhibits proliferation and induces apoptosis of human colorectal and breast cancer cell lines. PLoS ONE 2019, 14, e0207558. [Google Scholar] [CrossRef]
- Alshaer, W.; Alqudah, D.A.; Wehaibi, S.; Abuarqoub, D.; Zihlif, M.; Hatmal, M.M.; Awidi, A. Downregulation of STAT3, β-Catenin, and Notch-1 by Single and Combinations of siRNA Treatment Enhance Chemosensitivity of Wild Type and Doxorubicin Resistant MCF7 Breast Cancer Cells to Doxorubicin. Int. J. Mol. Sci. 2019, 20, 3696. [Google Scholar] [CrossRef] [Green Version]
- Kilbas, P.O.; Akcay, I.M.; Doganay, G.D.; Arısan, E.D. Bag-1 silencing enhanced chemotherapeutic drug-induced apoptosis in MCF-7 breast cancer cells affecting PI3K/Akt/mTOR and MAPK signaling pathways. Mol. Biol. Rep. 2019, 46, 847–860. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Wang, L.; Peng, H.; Xiao, L.; Li, C.; Lin, D.; Yang, K. Silencing TTK expression inhibits the proliferation and progression of prostate cancer. Exp. Cell Res. 2019, 385, 111669. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, L.; Khelfat, L.; Qiu, G.; Wang, Y.; Mao, K.; Chen, W. Rho-Associated Protein Kinase (ROCK) Promotes Proliferation and Migration of PC-3 and DU145 Prostate Cancer Cells by Targeting LIM Kinase 1 (LIMK1) and Matrix Metalloproteinase-2 (MMP-2). Med Sci. Monit. 2019, 25, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Soofiyani, S.R.; Hoseini, A.M.; Mohammadi, A.; Shahgoli, V.K.; Baradaran, B.; Hejazi, M.S. siRNA-Mediated Silencing of CIP2A Enhances Docetaxel Activity Against PC-3 Prostate Cancer Cells. Adv. Pharm. Bull. 2017, 7, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konecny, G.E.; Kristeleit, R.S. PARP inhibitors for BRCA1/2-mutated and sporadic ovarian cancer: Current practice and future directions. Br. J. Cancer 2016, 115, 1157–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.; Kong, Z.; Zeng, T.; Xu, S.; Duan, X.; Li, S.; Cai, C.; Zhao, Z.; Wu, W. PARP1-siRNA suppresses human prostate cancer cell growth and progression. Oncol. Rep. 2018, 39, 1901–1909. [Google Scholar] [CrossRef] [Green Version]
- Pederzoli, F.; Bandini, M.; Marandino, L.; Ali, S.M.; Madison, R.; Chung, J.; Ross, J.S.; Necchi, A. Targetable gene fusions and aberrations in genitourinary oncology. Nat. Rev. Urol. 2020, 17, 613–625. [Google Scholar] [CrossRef]
- Umbreen, S.; Banday, M.M.; Jamroze, A.; Mansini, A.P.; Ganaie, A.A.; Ferrari, M.G.; Maqbool, R.; Beigh, F.H.; Murugan, P.; Morrissey, C.; et al. COMMD3:BMI1 Fusion and COMMD3 Protein Regulate C-MYC Transcription: Novel Therapeutic Target for Metastatic Prostate Cancer. Mol. Cancer Ther. 2019, 18, 2111–2123. [Google Scholar] [CrossRef] [Green Version]
- Urbinati, G.; De Waziers, I.; Slamiç, M.; Foussignière, T.; Ali, H.M.; Desmaele, D.; Couvreur, P.; Massaad-Massade, L. Knocking Down TMPRSS2-ERG Fusion Oncogene by siRNA Could be an Alternative Treatment to Flutamide. Mol. Ther.-Nucleic Acids 2016, 5, e301. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Zhang, X.; Liu, K.; Song, J.; Leng, X.; Ran, L.; Ran, L. Transmembrane Channel-Like 5 (TMC5) promotes prostate cancer cell proliferation through cell cycle regulation. Biochimie 2019, 165, 115–122. [Google Scholar] [CrossRef]
- Silvestri, R.; Pucci, P.; Venalainen, E.; Matheou, C.; Mather, R.; Chandler, S.; Aceto, R.; Rigas, S.; Wang, Y.; Rietdorf, K.; et al. T-type calcium channels drive the proliferation of androgen-receptor negative prostate cancer cells. Prostate 2019, 79, 1580–1586. [Google Scholar] [CrossRef]
- Tektemur, A.; Ozaydin, S.; Onalan, E.E.; Kaya, N.; Kuloglu, T.; Ozercan, I.H.; Tekin, S.; Elyas, H.M. TRPM2 mediates distruption of autophagy machinery and correlates with the grade level in prostate cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Cruchaga, C.; Saccone, N.L.; Bertelsen, S.; Liu, P.; Budde, J.P.; Duan, W.; Fox, L.; Grucza, R.A.; Kern, J.; et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum. Mol. Genet. 2009, 18, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xue, W.; Zhang, Y.; Qu, C.; Lu, B.; Yin, Y.; Liu, K.; Wang, D.; Li, W.; Zhao, Z. Cholinergic α5 nicotinic receptor is involved in the proliferation and invasion of human prostate cancer cells. Oncol. Rep. 2019, 43, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, I.M.; Rocha, S.M.; Gaspar, C.; Alvelos, M.I.; Santos, C.R.; Socorro, S.; Baptista, C.J.M. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med. Oncol. 2018, 35, 40. [Google Scholar] [CrossRef]
- Dai, Y.; Siemann, D. c-Src is required for hypoxia-induced metastasis-associated functions in prostate cancer cells. OncoTargets Ther. 2019, 12, 3519–3529. [Google Scholar] [CrossRef] [Green Version]
- Dey, P.; Kundu, A.; Sachan, R.; Park, J.H.; Ahn, M.Y.; Yoon, K.; Lee, J.; Kim, N.D.; Kim, I.S.; Lee, B.M.; et al. PKM2 Knockdown Induces Autophagic Cell Death via AKT/mTOR Pathway in Human Prostate Cancer Cells. Cell. Physiol. Biochem. 2019, 52, 1535–1552. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Li, Y.; Zeng, J.; Wang, B.; Ji, K.; Tang, Y.; Sun, Q. Knockdown of HIF-1α by siRNA-expressing plasmid delivered by attenuated Salmonella enhances the antitumor effects of cisplatin on prostate cancer. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tong, D.; Liu, F.; Li, D.; Li, J.; Cheng, X.; Wang, Z. RPS7 inhibits colorectal cancer growth via decreasing HIF-1α-mediated glycolysis. Oncotarget 2015, 7, 5800–5814. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hou, J.; Lu, L.; Qi, Z.; Sun, J.; Gao, W.; Meng, J.; Wang, Y.; Sun, H.; Gu, H.; et al. Small Ribosomal Protein Subunit S7 Suppresses Ovarian Tumorigenesis through Regulation of the PI3K/AKT and MAPK Pathways. PLoS ONE 2013, 8, e79117. [Google Scholar] [CrossRef]
- Wen, Y.; An, Z.; Qiao, B.; Zhang, C.; Zhang, Z. RPS7 promotes cell migration through targeting epithelial-mesenchymal transition in prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 297.e1–297.e7. [Google Scholar] [CrossRef]
- Chen, T.; Tsang, J.Y.S.; Su, X.; Li, P.; Sun, W.; Wong, I.L.K.; Choy, K.; Yang, Q.; Tse, G.M.K.; Chan, T.H.; et al. SALL4 promotes tumor progression in breast cancer by targeting EMT. Mol. Carcinog. 2020, 59, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Shan, Y. Effects of siRNA-mediated silencing of Sal-like 4 expression on proliferation and apoptosis of prostate cancer C4-2 cells. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.S.; Henske, E.P.; Kwiatkowski, D.J.; Southwick, F.S. The Human Actin-Regulatory Protein Cap G: Gene Structure and Chromosome Location. Genomics 1994, 23, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, K.; Li, C.; Li, H.; Zhao, P.; Chen, K.; Liu, C. Influence of suppression of CapG gene expression by siRNA on the growth and metastasis of human prostate cancer cells. Genet. Mol. Res. 2015, 14, 15769–15778. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Park, J.-H.; Oh, N.; Cho, H.-J.; Park, K.-S. ELK3 expressed in lymphatic endothelial cells promotes breast cancer progression and metastasis through exosomal miRNAs. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Li, W.; Hua, B.; Gu, X.; Pan, W.; Chen, Q.; Xu, B.; Wang, Z.; Lu, C. Silencing of ELK3 Induces S-M Phase Arrest and Apoptosis and Upregulates SERPINE1 Expression Reducing Migration in Prostate Cancer Cells. BioMed Res. Int. 2020, 2020, 2406159–9. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Zhang, X.; Liu, P.; Yu, Q.; Li, Z.; Yu, Q.; Wei, X. Knockdown of anti-silencing function 1B histone chaperone induces cell apoptosis via repressing PI3K/Akt pathway in prostate cancer. Int. J. Oncol. 2018, 53, 2056–2066. [Google Scholar] [CrossRef]
- Jin, H.; Rugira, T.; Ko, Y.S.; Park, S.W.; Yun, S.; Kim, H.J. ESM-1 Overexpression is Involved in Increased Tumorigenesis of Radiotherapy-Resistant Breast Cancer Cells. Cancers 2020, 12, 1363. [Google Scholar] [CrossRef]
- Rebollo, J.; Geliebter, J.; Reyes, N. ESM-1 siRNA Knockdown Decreased Migration and Expression of CXCL3 in Prostate Cancer Cells. Int. J. Biomed. Sci. IJBS 2017, 13, 35–42. [Google Scholar]
- Liu, L.; Yan, L.; Liao, N.; Wu, W.-Q.; Shi, J.-L. A Review of ULK1-Mediated Autophagy in Drug Resistance of Cancer. Cancers 2020, 12, 352. [Google Scholar] [CrossRef] [Green Version]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nat. Cell Biol. 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; He, Z.; Xiang, L.; Li, L.; Zhang, H.; Lin, F.; Cao, H. Codelivery of GRP78 siRNA and docetaxel via RGD-PEG-DSPE/DOPA/CaP nanoparticles for the treatment of castration-resistant prostate cancer. Drug Des. Dev. Ther. 2019, 13, 1357–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Zhang, C.; Wang, F.; Zhang, W.; Shi, X.; Zhu, Y.; Fang, Z.; Yang, B.; Sun, Y. Deubiquitinating enzyme USP33 restrains docetaxel-induced apoptosis via stabilising the phosphatase DUSP1 in prostate cancer. Cell Death Differ. 2019, 27, 1938–1951. [Google Scholar] [CrossRef] [PubMed]
- Furuta, H.; Yoshihara, H.; Fukushima, T.; Yoneyama, Y.; Ito, A.; Worrall, C.; Girnita, A.; Girnita, L.; Yoshida, M.; Asano, T.; et al. IRS-2 deubiquitination by USP9X maintains anchorage-independent cell growth via Erk1/2 activation in prostate carcinoma cell line. Oncotarget 2018, 9, 33871–33883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Shen, Z.; Liu, H.; Yang, M.; Lin, J.; Luo, L.; Liu, L.; Chen, H. Upregulation of GRIM-19 augments the sensitivity of prostate cancer cells to docetaxel by targeting Rad23b. Clin. Exp. Pharmacol. Physiol. 2020, 47, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hour, T.-C.; Chung, S.-D.; Kang, W.-Y.; Lin, Y.-C.; Huang, C.-Y.; Huang, A.-M.; Wu, W.-J.; Huang, S.-P.; Pu, Y.-S. EGFR mediates docetaxel resistance in human castration-resistant prostate cancer through the Akt-dependent expression of ABCB1 (MDR1). Arch. Toxicol. 2015, 89, 591–605. [Google Scholar] [CrossRef]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [Green Version]
- Sullenger, B.A.; Nair, S. From the RNA world to the clinic. Science 2016, 352, 1417–1420. [Google Scholar] [CrossRef] [Green Version]
- Ghafouri-Fard, S.; Ghafouri-Fard, S. siRNA and cancer immunotherapy. Immunotherapy 2012, 4, 907–917. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Yantasee, W. siRNA therapeutics for breast cancer: Recent efforts in targeting metastasis, drug resistance, and immune evasion. Transl. Res. 2019, 214, 105–120. [Google Scholar] [CrossRef]
- Ahn, Y.-H.; Hong, S.-O.; Kim, J.H.; Noh, K.H.; Song, K.-H.; Lee, Y.-H.; Jeon, J.-H.; Kim, D.-W.; Seo, J.H.; Kim, T.W. The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-β receptor on dendritic cells potentiates tumour antigen-specific CD8 + T cell immunity. Clin. Exp. Immunol. 2015, 181, 164–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef] [PubMed]
- Oweida, A.J.; Mueller, A.C.; Piper, M.; Milner, D.; Van Court, B.; Bhatia, S.; Phan, A.; Bickett, T.; Jordan, K.; Proia, T.; et al. Response to radiotherapy in pancreatic ductal adenocarcinoma is enhanced by inhibition of myeloid-derived suppressor cells using STAT3 anti-sense oligonucleotide. Cancer Immunol. Immunother. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hossain, D.M.S.; Pal, S.K.; Moreira, D.; Duttagupta, P.; Zhang, Q.; Won, H.; Jones, J.; D’Apuzzo, M.; Forman, S.; Kortylewski, M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin. Cancer Res. 2015, 21, 3771–3782. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.-D.; Li, X.; Hao, L.; Zhao, Y.; Wang, Y.-X.; Dong, B.-Z.; Chen, W.-H.; Zhang, Z.-G.; Wang, Y.-M.; Fu, Q.; et al. Cbl-b gene silencing in splenic T lymphocytes as a therapeutic strategy to target the prostate cancer RM-1 cell tumors in immune competent mice. Eur. Rev. Med Pharmacol. Sci. 2014, 18, 3819–3830. [Google Scholar]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef]
- Subhan, M.A.; Torchilin, V.P. Efficient nanocarriers of siRNA therapeutics for cancer treatment. Transl. Res. 2019, 214, 62–91. [Google Scholar] [CrossRef]
- Shi, S.J.; Wang, L.J.; Han, D.H.; Wu, J.H.; Jiao, D.; Zhang, K.L.; Chen, J.W.; Li, Y.; Yang, F.; Zhang, J.L.; et al. Therapeutic effects of human monoclonal PSMA antibody-mediated TRIM24 siRNA delivery in PSMA-positive castration-resistant prostate cancer. Theranostics 2019, 9, 1247–1263. [Google Scholar] [CrossRef]
- Lee, J.B.; Zhang, K.; Tam, Y.Y.; Quick, J.; Tam, Y.K.; Lin, P.J.; Chen, S.; Liu, Y.; Nair, J.K.; Zlatev, I.; et al. A Glu-urea-Lys ligand-conjugated lipid nanoparticle/siRNA system inhibits androgen receptor expression in vivo. Mol. Ther. Nucleic Acids 2016, 5, e348. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, T.; Ding, L.; Laurini, E.; Huang, Y.; Zhang, M.; Weng, Y.; Lin, S.; Chen, P.; Marson, D.; et al. A dual targeting dendrimer-mediated siRNA delivery system for effective gene silencing in cancer therapy. J. Am. Chem. Soc. 2018, 140, 16264–16274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Laurini, E.; Posocco, P.; Pricl, S.; Qu, F.; Rocchi, P.; Peng, L. Efficient delivery of sticky siRNA and potent gene silencing in a prostate cancer model using a generation 5 triethanolamine-core PAMAM dendrimer. Mol. Pharm. 2012, 9, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163. [Google Scholar] [CrossRef] [Green Version]
- Apartsin, E.; Buyanova, M.; Novopashina, D.; Venyaminova, A. Hybrids of siRNA with Carbon Nanotubes as RNA Interference Instruments. Nanobiophysics 2015, 33–57. [Google Scholar] [CrossRef]
- Luan, X.; Rahme, K.; Cong, Z.; Wang, L.; Zou, Y.; He, Y.; Yang, H.; Holmes, J.D.; O’Driscoll, C.M.; Guo, J. Anisamide-targeted PEGylated gold nanoparticles designed to target prostate cancer mediate: Enhanced systemic exposure of siRNA, tumour growth suppression and a synergistic therapeutic response in combination with paclitaxel in mice. Eur. J. Pharm. Biopharm. 2019, 137, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.T.; Lin, F.W.; Chuang, C.K.; Yang, H.W. Co-Delivery of docetaxel and p44/42 MAPK siRNA using PSMA antibody-conjugated BSA-PEI layer-by-layer nanoparticles for prostate cancer target therapy. Macromol. Biosci. 2017, 17, 1600421. [Google Scholar] [CrossRef]
- Yan, X.; Shen, H.; Jiang, H.; Hu, D.; Wang, J.; Wu, X. YXQ-EQ induces apoptosis and inhibits signaling pathways important for metastasis in non-small cell lung carcinoma cells. Cell. Physiol. Biochem. 2018, 49, 911–919. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yu, X.; Liu, H.; Wu, D.; She, J.X. Co-targeting EGFR and survivin with a bivalent aptamer-dual siRNA chimera effectively suppresses prostate cancer. Sci. Rep. 2016, 6, 30346. [Google Scholar] [CrossRef] [Green Version]
- Onuh, J.O.; Qiu, H. Serum response factor-cofactor interactions and their implications in disease. FEBS J. 2020. [Google Scholar] [CrossRef]
- Evans, J.C.; McCarthy, J.; Torres-Fuentes, C.; Cryan, J.F.; Ogier, J.; Darcy, R.; Watson, R.W.; O’Driscoll, C.M. Cyclodextrin mediated delivery of NF-kappaB and SRF siRNA reduces the invasion potential of prostate cancer cells in vitro. Gene Ther. 2015, 22, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Panday, R.; Abdalla, A.; Yu, M.; Li, X.; Ouyang, C.; Yang, G. Functionally modified magnetic nanoparticles for effective siRNA delivery to prostate cancer cells in vitro. J. Biomater. Appl. 2019, 34, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Yoo, W.; Park, J.H.; Kim, S. Simultaneous delivery of electrostatically complexed multiple gene-targeting siRNAs and an anticancer drug for synergistically enhanced treatment of prostate cancer. Mol. Pharm. 2018, 15, 3777–3785. [Google Scholar] [CrossRef] [PubMed]
- Eloy, J.O.; Petrilli, R.; Raspantini, G.L.; Lee, R.J. Targeted liposomes for siRNA delivery to cancer. Curr. Pharm. Des. 2018, 24, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Kahraman, N.; Yan, G.; Wang, J.; Ozpolat, B.; Ittmann, M. Targeting the TMPRSS2/ERG fusion mRNA using liposomal nanovectors enhances docetaxel treatment in prostate cancer. Prostate 2020, 80, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, Q. Systemic delivery of siRNA: Challenging but promising. Recent Pat. Drug Deliv. Formul. 2012, 6, 19–30. [Google Scholar] [CrossRef]
- Kim, H.J.; Yi, Y.; Kim, A.; Miyata, K. Small delivery vehicles of siRNA for enhanced cancer targeting. Biomacromolecules 2018, 19, 2377–2390. [Google Scholar] [CrossRef]
- Rahme, K.; Guo, J.; Holmes, J.D. Bioconjugated gold nanoparticles enhance siRNA delivery in prostate cancer cells. Methods Mol. Biol. 2019, 1974, 291–301. [Google Scholar]
- Chen, J.; Wu, Z.; Ding, W.; Xiao, C.; Zhang, Y.; Gao, S.; Gao, Y.; Cai, W. SREBP1 siRNA enhance the docetaxel effect based on a bone-cancer dual-targeting biomimetic nanosystem against bone metastatic castration-resistant prostate cancer. Theranostics 2020, 10, 1619–1632. [Google Scholar] [CrossRef]
| SiRNA-Target Gene | Knockdown Consequences | Ref. |
|---|---|---|
| Dual specificity protein kinase TTK | Reduces proliferation, invasion, and migration, as well as initiates cell death process in PC-3 and DU145 PCa cells. | [31] |
| BMI1: COMMD3 fusion gene | Diminishes c-MYC expression in PC-3 cells resistant to BRD/BET-inhibitor and suppresses metastasis of tumor in xenograft mouse models. | [37] |
| TMPRSS2:ERG fusion gene | Declines cell viability and inhibits tumor growth of VCaP PCa cells. | [38] |
| Transmembrane channel-like 5 (TMC5) | Inhibits cell proliferation and enhances cell sensitivity to 5-fluorouracil in PC-3 and DU145 cells. | [39] |
| T-type calcium channels | Lessens cell survival and proliferation of PC-3 cells. | [40] |
| Transient receptor potential melastatin 2 (TRPM2) | Induces autophagy in PC-3 cells. | [41] |
| Src | Impairs hypoxia-induced metastasis of PC-3ML and C4-2B cells. | [45] |
| Pyruvate kinase M2 (PKM2) | Inhibits cell viability and the ability of colony formation, as well as induces autophagic cell death in DU145 cells. | [46] |
| Rho-associated protein kinase (ROCK) | Reduces migration and invasion of PC-3 and DU145 cells. | [32] |
| Protein phosphatase 2A (PP2A) | Elicits sensitivity of PC-3 cells to docetaxel. | [33] |
| Poly (ADP-ribose) polymerase 1 (PARP-1) | Reduces PC-3 cell migration and invasion, and decreases xenograft tumor size. | [35] |
| Endothelial cell-specific molecule-1 (ESM-1) | Diminishes cell migration with no impact on proliferation of PC-3 cells. | [59] |
| Small ribosomal protein subunit 7 | Attenuates PCa growth and migration of PC-3 cells. | [50] |
| Sal-like 4 (SALL4) | Decreases proliferation and colony formation capacity of C4-2 cells. | [52] |
| Macrophage-capping protein (CAPG) | Reduces proliferatory, migratory, and invasive capacities of DU145 cells | [54] |
| Nicotinic acetylcholine receptor (nAChR) | Decreases cell migratory and invasive activities, and induces apoptosis of DU145 and PC-3 cells. | [43] |
| Six transmembrane epithelial antigen of the prostate 1 (STEAP1) | Declines cell viability and proliferation whilst promoting apoptosis of LnCap PCa cells. | [44] |
| siRNA Target | Delivery Platform | Effects | Ref. |
|---|---|---|---|
| Tripartite motif-containing 24 | PSMAab | Suppresses proliferation, colony formation, and invasion of PSMA+ CRPC cells in vitro, and inhibits tumor growth of PSMA+ CRPC xenografts and bone loss in a PSMA+ CRPC bone metastasis model. | [79] |
| Androgen receptor | Glu-urea-Lys PSMA-lipid nanoparticle | Inhibits serum prostate-specific antigen, tumor cellular proliferation, and androgen receptor levels. | [80] |
| NFκB | Gold nanoparticle-PEI PEGylated anisamide | Suppresses tumor growth in a PC-3 xenograft mouse model. Its combination with paclitaxel leads to a synergistic therapeutic response in terms of tumor growth inhibition. | [86] |
| p44/42 mitogen-activated protein kinase | PSMAab-Bovine Serum Albumin branched polyethylenimine | Inhibits cancer cell proliferation. | [87] |
| EGFR and survivin | RNA-based aptamer-siRNA chimera | Induces apoptosis both in vitro and in vivo, and diminishes tumor growth and angiogenesis in the C4-2 PCa xenograft model. | [89] |
| RelA and serum response factor | Non-viral modified cyclodextrin vector | Reduces metastatic potential of PC-3 cells without noticeable impacts on cell viability. | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahreyni, A.; Luo, H. Advances in Targeting Cancer-Associated Genes by Designed siRNA in Prostate Cancer. Cancers 2020, 12, 3619. https://doi.org/10.3390/cancers12123619
Bahreyni A, Luo H. Advances in Targeting Cancer-Associated Genes by Designed siRNA in Prostate Cancer. Cancers. 2020; 12(12):3619. https://doi.org/10.3390/cancers12123619
Chicago/Turabian StyleBahreyni, Amirhossein, and Honglin Luo. 2020. "Advances in Targeting Cancer-Associated Genes by Designed siRNA in Prostate Cancer" Cancers 12, no. 12: 3619. https://doi.org/10.3390/cancers12123619
APA StyleBahreyni, A., & Luo, H. (2020). Advances in Targeting Cancer-Associated Genes by Designed siRNA in Prostate Cancer. Cancers, 12(12), 3619. https://doi.org/10.3390/cancers12123619






